Abstract
The gonadal steroid estrogen exerts an important modulatory influence on the activity of multiple neuronal networks. In addition to classical genomic mechanisms of action, estrogen also exerts poorly understood rapid, nongenomic effects on neurons. To examine whether estrogen may exert rapid actions on intracellular signaling within gonadotropin-releasing hormone (GnRH) neurons in vivo,we examined the phosphorylation status of cAMP response element-binding protein (CREB) in these cells after the administration of 17-β-estradiol to ovariectomized (OVX) mice. The percentage of GnRH neurons expressing phosphorylated CREB was increased more than sixfold (p < 0.05) in a time- and dose-dependent manner by estrogen, with the increase first observed 15 min after estrogen administration. A series of in vitro studies demonstrated that estrogen acted directly on native GnRH neurons to phosphorylate CREB, but that estrogen conjugated to bovine serum albumin was without effect. The role of classical estrogen receptors (ERs) was evaluated using ER knock-out mice in vivo. The effect of estrogen on CREB phosphorylation in GnRH neurons was normal in ERα knock-out mice but completely absent in ERβ knock-out mice. Finally, studies in intact female mice revealed levels of CREB phosphorylation within GnRH neurons that were equivalent to those of estrogen-treated OVX mice. These observations demonstrate that ERβ mediates the rapid, direct effects of estrogen on the GnRH neuronal phenotype, and that these actions persist under physiological conditions. They also provide the first evidence for a role of ERβ in nongenomic estrogen signaling within the brain in vivo.
Keywords: CREB, estrogen, estrogen receptor β, GnRH, LHRH, nongenomic, rapid, transgenics
Introduction
The activity of multiple neuronal networks is influenced by the ovarian hormone estrogen. Whereas early studies implicated estrogen as a classical feedback molecule acting to coordinate the activity of networks associated with reproductive control (Pfaff et al., 1994), more recent studies have documented actions of estrogen throughout the nervous system (McEwen and Alves, 1999). In addition to the established physiological roles of estrogen, recent studies are also highlighting its importance in the amelioration of neurodegenerative and ischemic insults to the nervous system (Henderson, 2000; Wise et al., 2001). Although it remains clear that many of the effects of estrogen occur through the estrogen receptor (ER) in a classic genomic manner, rapid actions of estrogen that are considered not to involve transcriptional regulation have also been demonstrated (Kelly and Wagner, 1999; McEwen and Alves, 1999). The nongenomic actions of estrogen have been shown to involve multiple different intracellular signaling pathways (Kelly and Levin, 2001), although the roles of ERα and ERβ in this process remain controversial.
In the mammalian brain, the gonadotropin-releasing hormone (GnRH) neurons represent the final output neuron of the network controlling gonadal function. Not surprisingly, their activity is regulated strongly by the fluctuating levels of plasma estrogen that exist during the ovarian cycle in the female (Herbison, 1998). Precisely how estrogen regulates the biosynthetic and secretory activity of the GnRH neurons is not clear, although the recent discovery of low levels of ERβ mRNA (Skynner et al., 1999; Hrabovszky et al., 2000) and protein (Hrabovszky et al., 2001; Kallo et al., 2001) in these cells has suggested a mechanism for estrogen-dependent transcriptional control in GnRH neurons (Herbison and Pape, 2001). However, the functional significance of these low levels of ERβ in GnRH neurons remains unknown.
A concerted understanding of the impact of estrogen on any neuronal phenotype such as the GnRH neuron requires information on the complete spectrum of genomic and nongenomic estrogenic effects. A previous study by Lagrange et al. (1995) demonstrated rapid effects of estrogen on the electrical excitability of GnRH neurons in the guinea pig. Using the phosphorylation of cAMP response element-binding protein (CREB) as an index of changes in intracellular signaling (Shaywitz and Greenberg, 1999; West et al., 2001), we used multiple in vivo and in vitro approaches to explore the mechanisms of rapid estrogen signaling in GnRH neurons. Previous studies have shown that acute estrogen elevates CREB phosphorylation in the brain (Gu et al., 1996; Zhou et al., 1996; Carlstrom et al., 2001). Our findings demonstrate that estrogen exerts rapid and direct actions on the GnRH neuronal phenotype, and that this requires estrogen to pass through the cell membrane and interact with ERβ. These studies provide the first evidence for a functional ER in the GnRH neuron and show that ERβ is involved in nongenomic estrogen signaling within the brain.
Materials and Methods
Animals
All of the mice were bred and housed at The Babraham Institute according to the United Kingdom Home Office requirements under Project license 80/1475, and the experiments were approved by The Babraham Institute Animal Welfare and Ethics Committee. Mice were maintained under a 12 hr light/dark cycle (lights on at 7:00 A.M.) with food and water available ad libitum.
Adult female wild-type mice (CBA/Ca × C57BL/6J) were ovariectomized (OVX) at 40–54 d of age under Avertin anesthesia and used for experiments 2–3 weeks later. Mice (∼20 gm) were administered 1 or 10 μg of 17-β-estradiol (E2) (Sigma, Poole, UK) (in 0.1 ml of ethyl oleate vehicle, s.c.) or vehicle alone between 10:00 and 11:00 A.M., killed 15 min, 1 hr, or 4 hr later by an overdose of Avertin (0.3 ml/20 gm of body weight), and perfused through the heart with ice-cold 4% paraformaldehyde (PFA) in phosphate buffer (PB), pH 7.6. Brains were removed, postfixed for 2 hr, and placed into 30% sucrose Tris-buffered saline (TBS) solution overnight at 4°C. The following day, a 1:4 series of 30 μm-thick coronal sections were cut through the septum and hypothalamus on a sliding microtome. Female homozygous ERα knock-out (KO) mice (Lubahn et al., 1993), ERβKO mice (Krege et al., 1998), and wild-type siblings (C57BL/6J) were identified by PCR and treated in the same manner as detailed above, with the exception that these mice were treated with only the lower 1 μg E2 dose or vehicle and killed 1 hr later, with blood taken before perfusion for luteinizing hormone (LH) radioimmunoassay. In the final study, mice were OVX or given sham surgery and then anesthetized and perfused 2 weeks later. The estrous stage of sham-treated mice was assessed by vaginal smear, and those in diestrous were selected for perfusion alongside the OVX mice. This represents a time when normal circulating levels of estrogen are ∼30–40 pg/ml (Bronson, 1981). Plasma samples were assayed for LH in duplicate, using reagents provided by National Institute of Diabetes and Digestive and Kidney Diseases in a single assay. The assay sensitivity was 0.16 ng/ml, and the intra-assay coefficient of variation was 8.6%.
Acute brain slice preparation for assessing CREB phosphorylation in vitro
An acute brain slice protocol was developed for in vitro investigations on the basis of our electrophysiological brain slice preparation (Han et al., 2002). Two weeks after ovariectomy, wild-type female mice were decapitated at ∼10:00 A.M., and their brains were rapidly removed and placed in oxygenated, ice-cold, cutting artificial CSF (ACSF) containing (in mm): 118 NaCl, 3 KCl, 0.5 CaCl2, 6 MgCl2,11 d-glucose, 10 HEPES, and 25 NaHCO3, pH 7.4 when bubbled with 95% O2–5% CO2. Coronal slices (400 μm thick) were then cut from each brain through the rostral preoptic area (rPOA) on a vibratome (Campden Instruments, Loughborough, UK), and slices were preincubated at 30°C for 30 min in oxygenated normal ACSF consisting of (in mm): 118 NaCl, 3 KCl, 2.5 CaCl2, 1.2 MgCl2, 11 d-glucose, 10 HEPES, and 25 NaHCO3. In the first experiment, slices were transferred into ACSF containing 100 nm E2 or vehicle (<0.01% ethyl alcohol) with or without 0.5 μm tetrodotoxin (TTX) (Tocris Cookson, Bristol, UK) for 1 hr. Those slices treated with TTX also had TTX included in the preincubation ACSF. Estrogen concentrations of 10–100 nm are used routinely to investigate rapid estrogen actions on cells in vitro (Kelly and Levin, 2001). In the second experiment, slices were transferred into either ACSF alone, 100 nm E2, or 100 nm E2–BSA (Sigma, Poole, UK) for 1 hr. Free or disassociated E2 was removed from the E2–BSA solution immediately before application by filtration following the protocol of Stevis et al. (1999). At the end of the 1 hr treatments, all of the slices were fixed in 4% PFA in PB at 4°C overnight. The next day, slices were transferred into 30% sucrose–TBS for 3 hr, and two sets of 30 μm-thick coronal sections were cut on a freezing microtome.
Immunocytochemistry
CREB immunocytochemistry. Free-floating, double-labeling, peroxidase immunocytochemistry was performed in the same manner as reported previously (Pape et al., 1999). In brief, after a 0.1% H2O2–40% methanol–TBS wash, all of the sections from one set were incubated in one of the primary antibodies [phosphorylated CREB (pCREB), 1:100; CREB, 1:100; Cell Signaling Technology, New England Biolabs, Hitchin, UK] for 48 hr at 4°C. This was followed by biotinylated goat anti-rabbit IgGs (1:200 for 2 hr; Vector Laboratories, Peterborough, UK) and the Vector Elite avidin–biotin–HRP complex (1:200 for 2 hr). Peroxidase labeling was then visualized with nickel-diaminobenzidine tetrahydrocloride (DAB) using glucose oxidase. Sections were then processed further for GnRH immunoreactivity with the LR1 antibody (1:20,000) followed by peroxidase-labeled anti-rabbit IgGs and revealed using DAB only. The specificities of the CREB antibodies have been reported previously in multiple rodent species (McNulty et al., 1998) (Cell Signaling Technology data), including the mouse (von Gall et al., 1998). The omission of primary antibodies in these studies resulted in a complete absence of immunoreactivity.
Analysis. Sections were examined under a Leica (Nussloch, Germany) DM-RB microscope at 10–40× objective magnification. GnRH neurons located in the medial septum (MS), rPOA, and anterior hypothalamus (AH) were examined in in vivo experiments, while sections from the rPOA were analyzed in in vitro experiments. Two sections representing each level were selected from each animal, and the number of single (GnRH) and double-labeled (GnRH plus pCREB or GnRH plus CREB) neurons was determined by an investigator blind to the experimental groupings. We only considered a GnRH neuron to be expressing CREB–pCREB if the nucleus displayed a uniform, dense-black immunoreactivity product. Although likely to underrepresent absolute CREB–pCREB expression in the GnRH neurons, this ensured consistency in our counting analysis. The mean values from each mouse were used to provide group means (+SEMs). The CREB and pCREB expression in GnRH neurons was calculated as a percentage of GnRH neurons at each level and also as a percentage of all of the GnRH neurons examined. Analysis of CREB and pCREB immunoreactivity in the anteroventral periventricular nucleus (AVPV) and CA1 region of the hippocampus was performed using a computer-assisted AIS 6.0 image analyzer (Imaging Research, St. Catharines, Ontario, Canada) with images digitized using a Sony (Tokyo, Japan) charge-coupled device camera. Cell counts were performed bilaterally within the defined area (AVPV, 0.03 mm 2; CA1, 1.5 mm 2) on two sections from each mouse. The anterioposterior levels for each region were plate 29 for the AVPV and plate 50 for the CA1 according to Paxinos and Franklin (2001).
To examine the differences between groups in the E2 dose–response study and in E2–BSA, a statistical analysis was performed by one-way ANOVA with Student–Newman–Keuls post hoc tests. Two-way ANOVA with Student–Newman–Keuls post hoc test was used to determine differences between groups in all of the other experiments.
Results
Estrogen rapidly phosphorylates CREB in GnRH neurons in vivo
Time course
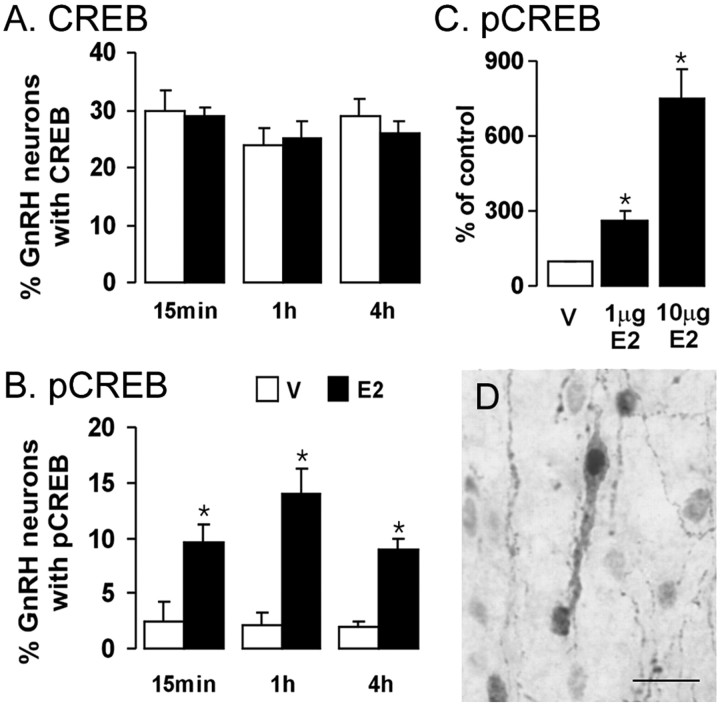
Immunoreactivity for CREB and pCREB was restricted to cell nuclei and readily detected in GnRH neurons with double-labeling immunocytochemistry (Fig. 1). The administration of 10 μg of E2 to OVX wild-type mice resulted in a significant (p < 0.05) sixfold to sevenfold increase in pCREB expression by GnRH neurons 15 min, 1 hr, and 4 hr later, with an apparent maximal increase observed at 1 hr (Fig. 1B). The expression of CREB by GnRH neurons was not changed (Fig. 1A). The same patterns of CREB and pCREB expression were observed in GnRH neurons irrespective of their location within the MS, rPOA, or AH (data not shown). The total numbers of GnRH neurons detected varied according to anatomical location (MS, 7.3 ± 1; rPOA, 19 ± 1.2; AH, 5.6 ± 0.6 GnRH neurons/section) but were not altered by estrogen treatment or time (data not shown).
Figure 1.
Estrogen rapidly phosphorylates CREB within GnRH neurons in a time- and dose-dependent manner in vivo. Histograms show the percentage of GnRH neurons expressing CREB (A) and pCREB (B) immunoreactivity 15 min, 1 hr, and 4 hr after the administration of vehicle (V) (open bars) or 10μg of E2 (filled bars) to ovariectomized mice. C, The percentage increment in the numbers of GnRH neurons expressing pCREB immunoreactivity 1 hr after 1 or 10μg of E2 (filled bars) compared with vehicle-treated mice (V) (open bars). D, Photomicrograph shows nuclear pCREB immunoreactivity (black) and a double-labeled GnRH neuron (brown cytoplasm). Scale bar, 20 μm. *p < 0.05; n = 5–9 in all of the groups. Histograms show mean ± SEM.
Dose dependence
The administration of 1 and 10 μg doses of E2 to OVX mice evoked a clear dose-dependent increase in pCREB expression within GnRH neurons at 1 hr (Fig. 1C) with no change in CREB (data not shown). Again, this response was observed in GnRH neurons located in the MS, rPOA, and AH, and the numbers of GnRH neurons were not altered by E2 treatment (data not shown).
Estrogen acts directly on GnRH neurons to phosphorylate CREB
An acute brain slice paradigm was developed to further evaluate the mechanism of CREB phosphorylation in GnRH neurons by estrogen. Immunocytochemical analysis of these brain slices showed them to have numbers of GnRH neurons equivalent to those found in vivo (e.g., GnRH neurons/rPOA section, 18 ± 1 in vivo vs 17 ± 1 in vitro).
Are the effects of E2 direct on the GnRH neurons?
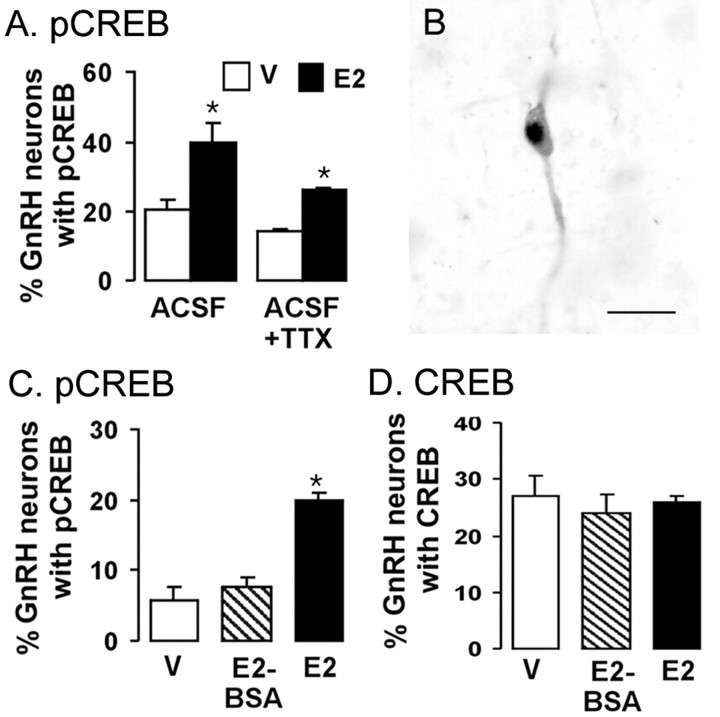
The treatment of brain slices with 100 nm E2 for 1 hr significantly (p < 0.05) elevated pCREB expression in GnRH neurons (Fig. 2A,B) in a manner similar to that found in vivo (Fig. 1). Although reduced in magnitude, this response was maintained in the presence of TTX (Fig. 2A), which electrically isolates GnRH neurons. Levels of CREB in GnRH neurons were not changed by E2 or TTX (data not shown).
Figure 2.
In vitro studies. Estrogen-dependent phosphorylation of CREB in GnRH neurons is direct and requires estrogen to pass into the cell. A, Histograms show the percentage of GnRH neurons expressing pCREB 1 hr after vehicle (V) (open bars) or 100 nm E2 (filled bars) in the presence of normal ACSF or with the addition of 0.5 μm TTX. B, Photomicrograph showing a single double-labeled GnRH plus pCREB neuron from the in vitro slice preparation. Scale bar, 20 μm. C, D, Histograms show the percentage of GnRH neurons expressing pCREB (C) or CREB (D) 1 hr after administration of vehicle (open bars), 100 nm E2–BSA (hatched bars), or 100 nm E2 (filled bars). *p < 0.05; n = 4–6 in all of the groups. Histograms show mean ± SEM.
Does E2 act at the cell membrane?
The cell-impermeant E2–BSA compound is commonly used to define E2 actions that occur through estrogen-binding receptors that exist on the outer surface of the cell membrane (Kelly and Levin, 2001). Whereas 100 nm E2 significantly (p < 0.05) increased pCREB expression in GnRH neurons, 100 nm E2–BSA was not found to have any effect (Fig. 2C). The levels of CREB in GnRH neurons were not altered by E2 or E2–BSA (Fig. 2D).
Estrogen requires ERβ to phosphorylate CREB in GnRH neurons
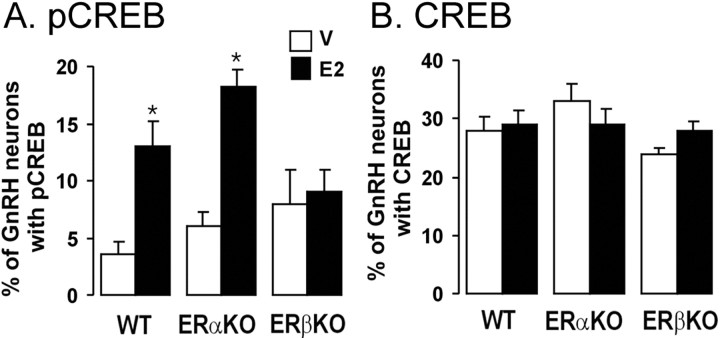
To evaluate whether the estrogen-dependent phosphorylation of CREB in GnRH neurons requires one of the classical ERs, we undertook experiments using ERαKO and ERβKO mice. The previous study showed that pCREB levels were likely to be maximal at 1 hr, so we used the lower 1 μg E2 dose at 1 hr to evaluate ERKO mice. As found in wild-type C57BL/6J × CBA/Ca mice (Fig. 1C), the treatment of OVX wild-type (C57BL/6J) littermates of the knock-out mice with 1 μg of E2 significantly (p < 0.05) increased pCREB expression in GnRH neurons at 1 hr (Fig. 3A). The same effect was observed in OVX ERαKO mice (p < 0.05), but E2 failed to change pCREB expression in GnRH neurons in OVX ERβKO mice (Fig. 3A). The levels of pCREB in GnRH neurons of vehicle-treated mice were not significantly different in the three genotypes. In all three experimental groups, the levels of CREB immunoreactivity in GnRH neurons were not altered by E2 treatment (Fig. 3B). The patterns of CREB and pCREB expression in GnRH neurons were the same regardless of their location in the MS, rPOA, or AH, and the numbers of GnRH neurons detected in all three genotypes were identical and not changed by E2 treatment (data not shown). To evaluate the effect of E2 on the activity of the hypothalamopituitary axis, plasma LH levels were determined in these mice. E2 significantly (p < 0.05) suppressed LH levels in wild-type mice (vehicle, 9 ± 1.4 ng/ml; E2, 2.6 ± 0.2 ng/ml) but had no effect on LH levels in ERαKO (vehicle, 3.0 ± 1.0 ng/ml; E2, 2.3 ± 0.5 ng/ml) or ERβKO (vehicle, 2.1 ± 0.5 ng/ml; E2, 2.9 ± 0.6 ng/ml) mice.
Figure 3.
Estrogen requires ERβ to phosphorylate CREB in GnRH neurons in vivo. Histograms show the percentage of GnRH neurons expressing pCREB (A) and CREB (B) in wild-type littermates (WT), ERαKO mice, and ERβKO mice 1 hr after vehicle (V) (open bars) or 1μg of E2 (filled bars). *p < 0.05, n = 5–6 in all of the groups. Histograms show mean ± SEM.
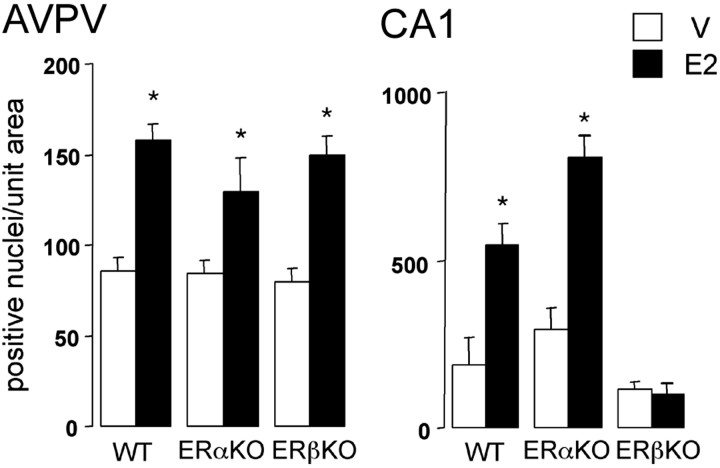
To evaluate further the role of ERβ in estrogen-dependent CREB phosphorylation, we performed an analysis of CREB and pCREB expression in the AVPV and CA1 hippocampus of the sections used above for analysis of the GnRH neurons. In rats, the AVPV expresses both ERα and ERβ, whereas ERβ is the predominant ER subtype in the CA1 (Shughrue et al., 1997). As shown previously in rats (Gu et al., 1996; Carlstrom et al., 2001), estrogen was found to acutely elevate CREB phosphorylation in the AVPV and CA1 of wild-type mice (Fig. 4). Whereas this effect persisted in the AVPV, the pattern of CREB phosphorylation in the CA1 was blocked completely in the ERβKO but not ERαKO mouse (Fig. 4). CREB expression was not altered significantly in either area by estrogen treatment (data not shown).
Figure 4.
Differential dependence on ERβ for rapid CREB phosphorylation in brain. Histograms show the numbers (mean + SEM) of pCREB immunoreactive nuclei counted in the AVPV and CA1 hippocampus in wild-type littermates (WT), ERαKO mice, and ERβKO mice 1 hr after vehicle (V) (open bars) or 1 μg of E2 (filled bars). *p < 0.05; n = 5–6 in all of the groups. Histograms show mean ± SEM.
Levels of pCREB remain elevated in intact female mice
To examine whether the rapid increase in the phosphorylation of CREB by estrogen in GnRH neurons may be maintained under normal physiological conditions of estrogen exposure, we compared OVX mice with intact females. As observed in previous experiments, GnRH neurons in OVX mice (n = 6) displayed low levels of pCREB. However, a fivefold greater number of GnRH neurons were found to express pCREB in intact (diestrous; n = 6) females (p < 0.05) (Fig. 5A). The increased numbers of pCREB plus GnRH neurons were observed throughout the MS, rPOA, and AHA (not shown). Intact mice displayed similar numbers of GnRH neurons to those found in 2 week OVX mice (Fig. 5C), and, similarly, CREB expression was not significantly different between the two groups (Fig. 5B).
Figure 5.
CREB phosphorylation is elevated in intact female mice in vivo. Histograms show the percentage of GnRH neurons expressing pCREB (A) and CREB (B) and the numbers of GnRH-immunoreactive neurons (C) detected per section through the rPOA of OVX (open bars) and intact diestrous female mice (filled bars). *p < 0.05; n = 6 in all of the groups. Histograms show mean ± SEM.
Discussion
We report here that GnRH neurons in the mouse respond to estrogen in a rapid and direct manner through an ERβ-dependent mechanism. The rapidity of the actions of estrogen on CREB phosphorylation (<15 min) indicates a nongenomic mechanism of action. Because the phosphorylation of CREB by estrogen occurs directly at the GnRH neuron and is absent in ERβKO mice, it is very likely that estrogen interacts with ERβ expressed by GnRH neurons. Such a scenario would be in good agreement with evidence for the expression of ERβ, but not ERα, in GnRH neurons (Herbison and Pape, 2001). A common theme in the investigation of the GnRH neuronal phenotype is that of marked heterogeneity (Sim et al., 2001), and once again, we find here that only a subpopulation (25–40%) of all GnRH neurons, irrespective of location, respond to estrogen. Studies in mice have shown that ∼20% of adult female GnRH neurons express ERβ transcripts (Skynner et al., 1999), whereas work in the rat has demonstrated 50–80% of GnRH neurons to contain ERβ immunoreactivity (Hrabovszky et al., 2001; Kallo et al., 2001). Although the reasons for the relatively low numbers of GnRH neurons expressing CREB and pCREB immunoreactivity in this study are at least in part methodological, it remains that not all of the GnRH neurons respond in the same manner. The physiological basis for this heterogeneity is not established.
Role of ERs in nongenomic effects of estrogen on the brain
Previous studies have identified rapid, nongenomic effects of estrogen on the phosphorylation status and activity of multiple different signaling pathways in the brain (Gu and Moss, 1996; Gu et al., 1996; Mermelstein et al., 1996; Zhou et al., 1996; Kelly and Wagner, 1999; Kelly et al., 1999; Singer et al., 1999; Bi et al., 2000; Singh et al., 2000; Cardona-Gomez et al., 2002; Ivanova et al., 2002). Although the role of established and novel estrogen-binding receptors in these effects is currently under investigation, at least two distinct mechanisms of nongenomic estrogen action appear to exist. In the first, the binding of estrogen to an ER located within the cytoplasm appears to enable direct activational protein–protein interactions with key upstream signaling molecules such as Src kinase (Migliaccio et al., 2000; Kousteni et al., 2001) and PI3K (phosphatidylinositol 3-kinase) (Simoncini et al., 2000). In the brain, this mechanism is typified by the neuroprotective actions of estrogen, which nongenomically modify the MAPK (mitogen-activated protein kinase) pathway to alter glutamatergic transmission (Singer et al., 1999; Bi et al., 2000; Nethrapalli et al., 2001). Most, but not all (Singh et al., 2000), in vitro studies indicate that classical ERs are required for the nongenomic activation of MAPK by estrogen (Migliaccio et al., 2000; Kousteni et al., 2001; Wade et al., 2001; Song et al., 2002), and in vivo, estrogenic protection from ischemic injury is known to depend on ERα specifically (Wise et al., 2001). The second mechanism involves the ability of cell membrane-located classical and nonclassical ERs to signal estrogen status to the cell (Razandi et al., 1999; Valverde et al., 1999; Nadal et al., 2000; Benten et al., 2001; Watson et al., 2002). Within the brain, this membrane-dependent mode of action is thought to underlie the ability of estrogen to modulate G-protein-coupled receptors using PKA (protein kinase A)- and PKC (protein kinase C)-dependent pathways (Kelly and Wagner, 1999).
Although liganded ERβ had been demonstrated to be capable of activating Src kinase (Migliaccio et al., 2000; Kousteni et al., 2001) and the MAPK pathway (Wade et al., 2001) in vitro, no information existed on its potential to mediate rapid nongenomic effects of estrogen in vivo in the brain or elsewhere. Indeed, when examined selectively, ERβ was found to be ineffective compared with ERα in mediating rapid estrogen actions in specific signaling pathways (Hisamoto et al., 2001). Using the GnRH neuronal phenotype, we now show that the rapid phosphorylation of CREB by estrogen is critically dependent on ERβ in vivo. Although we can be less certain about the mechanisms through which estrogen phosphorylates CREB in the CA1 hippocampus, it appears that ERβ is also responsible for mediating acute estrogen actions in this region. This is clearly not the case in the AVPV where the phosphorylation of CREB was maintained in the ERβKO mouse. Thus, it seems likely that ERβ may have a global role to play in mediating acute estrogen actions and, in this regard, may be of particular importance to brain regions and neuronal phenotypes that preferentially express ERβ.
The inability of cell-impermeant E2–BSA to replicate the effect of E2 alone in GnRH neurons suggests that either the coupling of estrogen to BSA may alter its receptor binding or estrogen needs to pass through the GnRH neuron cell membrane to influence signaling cascades. Because the E2–BSA preparation has been shown previously to activate classical ERs expressed in the cell membrane (Razandi et al., 1999), E2–BSA in our experiments should have been able to interact with ERβ had it been located within the membrane of the GnRH neuron. Because E2–BSA elicited no response, we favor the concept that estrogen must pass through the membrane and interact with ERβ within the cytoplasm of the GnRH neuron to regulate CREB phosphorylation. The precise intracellular pathway involved in CREB phosphorylation by estrogen–ERβ is unknown and may involve cAMP–PKA, calcium–calmodulin protein kinases, and/or MAPKs (Shaywitz and Greenberg, 1999; West et al., 2001).
Role of ERβ in estrogen feedback on the GnRH neuron
The mechanisms through which estrogen exerts critical feedback actions on the GnRH neurons are poorly understood, and the presence of ERs in these neurons has long been controversial (Herbison and Pape, 2001). Recent studies have indicated that GnRH neurons express low levels of ERβ transcript and protein (Skynner et al., 1999; Hrabovszky et al., 2000, 2001; Kallo et al., 2001), the functional significance of which has been in doubt. Together, the present series of experiments provide evidence that ERβ expressed by GnRH neurons is functional in mediating rapid estrogen actions. Whether ERβ is also active in a classic transcriptional manner in these cells has yet to be established. Although both rapid and classical genomic mechanisms of estrogen signaling clearly exist within the brain (Kelly and Levin, 2001), the relationship between the two is unclear. In this study, we evaluated whether the rapid estrogenic activation of CREB phosphorylation uncovered here in GnRH neurons may also exist under normal physiological conditions. Our results show that the GnRH neurons in diestrous female mice display levels of CREB phosphorylation that are the same as those of OVX mice given estrogen acutely. This suggests that the rapid estrogen signaling to phosphorylate CREB may be ongoing in a persistent manner within the GnRH neurons. Recently proposed models for the coordination of nongenomic and genomic estrogen actions have suggested that classical genomic actions are set on a background of rapid, nongenomic signaling (Kousteni et al., 2001; Vasudevan et al., 2001). The current observations indicate that a continuous background of rapid ERβ-dependent signaling, with genomic consequences, is likely to exist in GnRH neurons.
From a physiological perspective, the role of ERβ in GnRH neuron function is not yet established. It seems very likely that estrogen uses indirect transsynaptic and glial cell-dependent mechanisms, probably involving ERα, to influence the activity of the GnRH neuron (Herbison, 1998). However, ERβ expressed by these cells may also be of physiological relevance. Homozygous ERβKO female mice exhibit subnormal fertility (Krege et al., 1998) and have modestly elevated basal LH levels (A. E. Herbison and A. DorlingA. E., unpublished observations) suggestive of defective estrogen negative feedback. Furthermore, despite the observation here that LH levels were not markedly elevated in ERKO mice after ovariectomy, acute estrogen was not found to suppress LH in ERβKO mice. Together, these findings suggest that ERβ expressed by GnRH neurons and/or other cells is at least partly involved in the negative feedback influence of estrogen on GnRH neurons. The genes regulated by ERβ in GnRH neurons, either directly through E2 response elements or indirectly through cAMP response elements, are not yet established. However, estrogen-dependent CREB phosphorylation has been shown previously to have an important role in synaptic plasticity (Murphy and Segal, 1997), and, interestingly, mice with a brain-specific creb1 knock-out are known to be infertile (Mantamadiotis et al., 2002). However, as for ERβ itself, the precise definition of the role of CREB in the GnRH neurons awaits the production of GnRH-specific, ERβ- and CREB-deficient mice.
In summary, these studies provide the first demonstration that ERβ can underlie rapid, nongenomic effects of estrogen within the brain. The ability of ERβ to act as a classical transcription factor as well as a molecule enabling rapid, nongenomic effects of estrogen on intracellular signaling pathways makes it pivotal in orchestrating the impact of estrogen impact on neuronal function. This will likely be of physiological importance to the GnRH neuron and of particular relevance to neuronal phenotypes in regions such as the cerebral cortex and hippocampus where ERβ is the predominant ER.
Footnotes
This research was supported by the Biotechnology and Biological Sciences Research Council and a Marie Curie Fellowship of the European Community Human Potential Programme under contract number HPMF-CT-2000-00512. We thank Dr. R. Benoit for the LR1 antiserum, Amber Dorling for radioimmunoassay work, and Sandra Dye and members of SABU at The Babraham Institute for assistance with mice.
Correspondence should be addressed to Dr. Allan E. Herbison, Centre for Neuroendocrinology, Department of Physiology, University of Otago School of Medical Sciences, P.O. Box 913, Dunedin, New Zealand. E-mail address: allan.herbison@stonebow.otago.ac.nz.
I. M. Ábrahám's present address: Hungarian Academy of Sciences, Neurobiology Research Group at Eötvös Loránd University, Páymány P. st. 1/C, 1117, Budapest, Hungary.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235771-07$15.00/0
References
- Benten WPM, Stephan C, Lieberherr M, Wunderlich F ( 2001) Estradiol signaling via sequestrable surface receptors. Endocrinology 142: 1669-1677. [DOI] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M ( 2000) The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci USA 97: 3602-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH ( 1981) The regulation of lutienizing hormone secretion by estrogen: relationship among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology 108: 506-516. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez G, Mendez P, Garcia-Segura LM ( 2002) Synergistic interaction of estradiol and insulin-like growth factor-1 in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Mol Brain Res 107: 80-88. [DOI] [PubMed] [Google Scholar]
- Carlstrom L, Ke ZJ, Unnerstall JR, Cohen RS, Pandey SC ( 2001) Estrogen modulation of the cyclic AMP response element-binding protein pathway. Effects of long-term and acute treatments. Neuroendocrinology 74: 227-243. [DOI] [PubMed] [Google Scholar]
- Gu G, Moss RL ( 1996) 17β-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci 16: 3620-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Rojo AA, Zee MC, Yu J, Simerly RB ( 1996) Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci 16: 3035-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE ( 2002) Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143: 1459-1466. [DOI] [PubMed] [Google Scholar]
- Henderson VW ( 2000) Oestrogens and dementia. Novartis Found Symp 230: 254-265. [DOI] [PubMed] [Google Scholar]
- Herbison AE ( 1998) Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19: 302-330. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR ( 2001) New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 22: 292-308. [DOI] [PubMed] [Google Scholar]
- Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, Taniguchi N, Murata Y ( 2001) Estrogen induces the Akt-dependent activation of endothelial nitricoxide synthase in vascular endothelial cells. J Biol Chem 276: 3459-3467. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL ( 2000) Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 141: 3506-3509. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z ( 2001) Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142: 3261-3264. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Mendez P, Garcia-Segura LM, Beyer C ( 2002) Rapid stimulation of the PI3-kinase/Akt signalling pathway in developing midbrain neurones by oestrogen. J Neuroendocrinol 14: 73-79. [DOI] [PubMed] [Google Scholar]
- Kallo I, Butler JA, Barkovics-Kallo M, Goubillon ML, Coen CW ( 2001) Oestrogen receptor β-immunoreactivity in gonadotropin releasing hormone-expressing neurones: regulation by oestrogen. J Neuroendocrinol 13: 741-748. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER ( 2001) Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab 12: 152-156. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Wagner EJ ( 1999) Estrogen modulation of G-protein-coupled receptors. Trends Endocrinol Metab 10: 369-374. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Lagrange AH, Wagner EJ, Rønnekleiv OK ( 1999) Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroids 64: 64-75. [DOI] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodmenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Robertson PK, Weinstein RS, Jilka RL, Mangolagas SC ( 2001) Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104: 719-730. [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Magler JF, Sar M, Korach KS, Gustafsson J-A, Smithies O ( 1998) Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95: 15677-15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Rønnekleiv OK, Kelly MJ ( 1995) Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback. Endocrinology 136: 2341-2344. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O ( 1993) Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90: 11162-11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Villalba AM, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schutz G ( 2002) Disruption of CREB function in brain leads to neurodegeneration. Nat Genet 31: 47-54. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE ( 1999) Estrogen actions in the central nervous system. Endocr Rev 20: 279-307. [DOI] [PubMed] [Google Scholar]
- McNulty S, Schurov IL, Sloper PJ, Hastings MH ( 1998) Stimuli which entrain the circadian clock of the neonatal Syrian hamster in vivo regulate the phosphorylation of the transcription factor CREB in the suprachiasmatic nucleus in vitro. Eur J Neurosci 10: 1063-1072. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ ( 1996) Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci 16: 595-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F ( 2000) Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J 19: 5406-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Segal M ( 1997) Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci USA 94: 1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B ( 2000) Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc Natl Acad Sci USA 97: 11603-11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethrapalli IS, Singh M, Guan X, Guo Q, Lubahn DB, Korach KS, Toran-Allerand CD ( 2001) Estradiol (E2) elicits Src phosphorylation in the mouse neocortex: the initial event in E2 activation of the MAPK cascade? Endocrinology 142: 5145-5148. [DOI] [PubMed] [Google Scholar]
- Pape J-R, Skynner MJ, Allen ND, Herbison AE ( 1999) Transgenics identify distal 5′- and 3′ sequences specifying gonadotropin-releasing hormone expression in adult mice. Mol Endocrinol 13: 2203-2211. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ ( 2001) The mouse brain in stereotaxic coordinates. San Diego: Academic.
- Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow L-M ( 1994) Cellular and molecular mechanisms of female reproductive behaviors. In: The physiology of reproduction (Knobil E, Neill JD, eds). New York: Raven.
- Razandi M, Pedram A, Greene GL, Levin ER ( 1999) Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13: 307-319. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME ( 1999) CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68: 821-861. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I ( 1997) Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388: 507-525. [DOI] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Herbison AE ( 2001) Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J Neurosci 21: 1067-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Llao JK ( 2000) Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407: 538-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM ( 1999) The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci 19: 2455-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Setalo GJ, Guan X, Frail DE, Toran-Allerand CD ( 2000) Estrogen-induced activation of the mitogen-activated protein kinase cascade in the cerebral cortex of estrogen receptor-α knock-out mice. J Neurosci 20: 1694-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skynner MJ, Sim JS, Herbison AE ( 1999) Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology 140: 5195-5201. [DOI] [PubMed] [Google Scholar]
- Song RX-D, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ ( 2002) Linkage of rapid estrogen action to MAPK activation by ERα-Shc association and Shc pathway activation. Endocrinology 16: 116-127. [DOI] [PubMed] [Google Scholar]
- Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE ( 1999) Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology 140: 5455-5458. [DOI] [PubMed] [Google Scholar]
- Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R ( 1999) Acute activation of maxi-K channels (hSlo) by estradiol binding to the β subunit. Science 285: 1929-1931. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff DW ( 2001) Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci USA 98: 12267-12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gall C, Duffield GE, Hastings MH, Kopp MD, Dehghani F, Korf HW, Stehle JH ( 1998) CREB in the mouse SCN: a molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access. J Neurosci 18: 10389-10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM ( 2001) Estrogen receptor (ER)α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology 142: 2336-2342. [DOI] [PubMed] [Google Scholar]
- Watson CS, Campbell CH, Gametchu B ( 2002) The dynamic and elusive membrane estrogen receptor-α. Steroids 67: 429-437. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME ( 2001) Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 98: 11024-11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M, Rosewell KL ( 2001) Estradiol is a protective factor in the adult and aging brain: understanding of mechanisms derived from in vivo and in vitro studies. Brain Res Rev 37: 313-319. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM ( 1996) Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology 137: 2163-2166. [DOI] [PubMed] [Google Scholar]