Abstract
Substantia nigra (SN) dopamine neurons express D2 and D3 dopamine autoreceptors. A physiological role for the D3 receptor has not been identified, but an activation of G-protein-gated inwardly rectifying potassium (GIRK; also known as Kir3) channels is strongly implicated because D3 receptors activate channels composed of GIRK2 subunits in cell lines. We confirmed that acutely dissociated SN dopamine neurons indeed contain D3 and GIRK2 subunit mRNA using single-cell RT-PCR. We then tested whether D3 receptors activate GIRK currents in SN dopamine neurons by comparing acutely dissociated neurons from D2–/– receptor knock-out and congenic wild-type mice. In nearly all (14 of 15) wild-type SN dopamine neurons, the D2/D3 agonist quinpirole activated GIRK currents that were blocked by cesium. Quinpirole, however, elicited no GIRK currents in any SN dopamine neuron (0 of 13) derived from D2–/– receptor knock-out mice. The absence of quinpirole response was not caused by a lack of GIRK activity, because the GABAB receptor agonist baclofen continued to elicit these currents in the mutant neurons. Thus, it appears that D3 activation of GIRK currents in SN neurons does not occur or is exceedingly rare.
Keywords: GABA, inwardly rectifying potassium channel, Kir3, single-cell RT-PCR, weaver mouse mutation, ventral tegmental area
Introduction
Although various autoreceptor-mediated responses in substantia nigra (SN) dopamine neurons are mediated by D2 receptors (Usiello et al., 2000), the physiological roles, distribution, and even existence of D3 autoreceptors in the SN have been intensely debated. A recent study, however, reports D3 immunoreactivity in all midbrain dopamine neurons (Diaz et al., 2000). Various pharmacological studies using ligands with a preferential binding affinity for D3 receptors (Lejeune and Millan, 1995; Aretha and Galloway, 1996; Gainetdinov et al., 1996) or oligonucleotides that inhibit D3 transcription (Tepper et al., 1997) suggest possible physiological roles for D3 autoreceptors. Yet, SN neurons from D3–/– mice display normal basal firing rates, rates of dopamine synthesis, basal dopamine levels, and reuptake kinetics (Koeltzow et al., 1998; L'Hirondel et al., 1998; Dickinson et al., 1999; Zapata et al., 2001). In summary, there is as yet no clear evidence of a physiological role for D3 autoreceptors.
A strong candidate for such a role is suggested by studies in transfected cell lines expressing D3 receptors and GIRK2, a subunit of the G-protein coupled inwardly rectifying potassium channels (GIRKs; also known as Kir3) that is selectively expressed in SN neurons (Inanobe et al., 1999; Liss et al., 1999). Cells cotransfected with D3 receptors and GIRK2 exhibit GIRK activation by the D2/D3 receptor agonist quinpirole (Kuzhikandathil et al., 1998; Kuzhikandathil and Oxford, 2000). A D3 autoreceptor activation of GIRK currents, however, has yet to be tested directly in neurons. Here, we used SN neurons derived from D2 null mutant (–/–) mice, which express ventral midbrain D3 receptor at higher levels than wild-type littermates (Jung et al., 1999), to determine whether D3 autoreceptors activate GIRK currents.
Materials and Methods
Preparation of acutely dissociated neurons. Experiments were performed in accordance with the Columbia University Institutional Animal Care and Use Committee. Except where noted, reagents were obtained from Sigma (St. Louis, MO). Neurons were derived from C57BL6 mice (Taconic, Germantown, NY), or, in the case of D2 receptor –/– knock-out mutants, their wild-type siblings and wild-type non-siblings from the same mixed 129 Sr/C57BL6 background (Jung et al., 1999). Mice were anesthetized on postnatal day 18–29 using ketamine/xylazine (5 mg/ml and 3.4 mg/ml, respectively) by intraperitoneal injection, (0.1 ml/30 gm). The SN was dissected from 300 μm vibratome slices and dissociated at 34°C in continuously oxygenated solution with 1.34 mg/ml protease (type XIV; Sigma) in HBSS (containing in mm): 10 HEPES, 2 MgCl2, 1 CaCl2, pH 7.3–4), for 30 min, and cells were plated on poly-d-lysinecoated (40 mg/ml in dH2O) coverslips. Cultures were superfused with oxygenated physiological saline (in mm): 150 NaCl, 2 KCl, 1 MgCl2, 1.2 CaCl2, 10 HEPES, and 25 glucose, pH 7.3–7.4, throughout the recordings, and recordings were completed within 6 hr after dissociation.
cDNA synthesis. Cytosol was harvested by aspiration into a patch pipette filled with 5 μl of DEPC-treated water followed by expiration into 5.3 μl of DEPC-treated water, 7.1 mm DTT, 7 U RNAsin, 0.5 μg of oligo-dT, maintained in dry ice. Five microliters of the saline surrounding the cells were collected to provide negative controls. The cytosol/primer or saline/primer mixtures (12 μl of each) were incubated for 10 min at 70°C and placed on ice. To the cytosol/oligo-dT primer mixtures, 8 μl of reverse transcriptase reaction mix (1× PCR buffer, 2.5 mm MgCl2, 0.5 mm dNTP mix, 7.5 mm DTT, 7 U RNasin, 10 U Superscript II RT, and DEPC H2O) was added. The RT reaction mixture was incubated for 50 min at 42°C, followed by 15 min at 70°C, and then chilled on ice. To remove RNA, 2 U of RNaseH was added to each tube, incubated for 20 min at 37°C, and stored at –80°C.
Trizol reagent (Invitrogen) used for whole-tissue RNA extraction was used according to the manufacturer's instructions. To produce cDNA from RNA isolated from mouse striatum, 10 μl of RNA isolate was mixed with 0.5 μg of oligo-dT and heated to 65°C for 10 min. The RNA/oligo-dT reaction was then used for PCR amplification. The PCR reaction mix contained 1× PCR buffer, 2.5 mm MgCl2, 0.5 mm dNTP mix, 7.5 mm DTT, 14 U RNasin, and 10 U Superscript II RT. The RT-PCR reaction was performed with the following heating cycle: 42°C for 50 min, 70°C for 15 min, 4°C for 1 min. RNaseH (3.4 U) was added to the reaction and incubated for 20 min at 37°C followed by 1 min at 4°C. Mouse striatal cDNA was stored at –70°C and used in all PCR reactions as a control because tyrosine hydroxylase (TH), D2, D3, and GIRK1–4 are each expressed in this tissue.
PCR amplification. Reagents for PCR amplification of DNA were obtained from Promega (Madison, WI).
The analysis of single neurons used the following TH primer sequence: TH sense: 5′-CAGGACATTGGACTTGCATCTCTG; TH antisense: 5′-ATAGTTCCTGAGCTTGTCCTTGGC (296 bp).
The PCR reaction mixture for amplification of the TH transcript contained 1× PCR buffer, 2.5 mm MgCl2, 0.5 mm dNTP, 0.8 μm sense primer, 0.8 μm antisense primer, 2 μl of single-cell cDNA (or 1 μl (0.58 μg) of striatal cDNA), and 0.5 μlof Taq in 50 μl. Amplification of TH was performed as follows: (1) 4 min at 94°C, (2) 45 cycles of 1 min at 94°C, 1 min at 56°C, and 1.5 min at 72°C, and (3) 5 min at 72°C, held at 4°C.
To determine whether mice expressed the D2 mutation, we used a primer sequence designed to amplify exon 1 of D2, which is deleted in this mutant: mD2 sense: 5′-CAATGGATCCACTGAAC; mD2 antisense: 5′-ACCACCTCCAGATAGAC (290 bp).
The PCR reaction mixture for amplification of this transcript contained 1× PCR buffer, 2.5 mm MgCl2, 0.5 mm dNTP, 40 pm of sense primer, 40 pm of antisense primer, 3 μl of single-cell cDNA (or 1 μl (0.58 μg) of striatal cDNA), 0.2 μl of Taq in 20 μl. Amplification of the D2-exon1 transcript was performed as follows: (1) 4 min at 94°C, (2) 35 cycles of 1 min at 94°C, 1 min at 52°C, and 1.3 min at 72°C, (3) 5 min at 72°C, and held at 4°C.
The following nested primer sequences for D2 (Yan et al., 1997), D3, and GIRK subunits Girk1–4 were used for those neurons that were identified as dopaminergic: D2-external sense: 5′-GCAGTCGAGCTTTCAGAGCC; D2-external antisense: 5′-TCTGCGGCTCATCGTCTTAAG (404/317bp); D2-internal sense: 5′-AGAGCCAACCTGAAGACACCAC; D2-internal antisense: 5′-CTTAAGGGAGGTCCGGGTTTTG (375/288 bp); D3-external sense: 5′-GATCCCAGCATCTGCTCCATCTC; D3-external antisense: 5′-ATCTTGAGGAAGGCTTTGCGGAA (757/694 bp); D3-internal sense: 5′-CCATCTCCAACCCTGATTTTGTC; D3-internal antisense: 5′-TCTAAGCTGAGCTTGGGTGCCAT (483/420 bp); mGirk1,3,4 external sense: 5′-TTCACYACSCTGGTGGAYCT; mGirk1,3,4 external antisense: 5′-GCTTGRCAMGTCATWCCYGT; mGirk1 internal sense: 5′-AACAAAGCCCATGTCGGCAACTAC; mGirk1 internal antisense CTGTTCAGTTTGCATGCTTCGCTG (539 bp); mGirk3 internal sense: 5′-CTCAGACTGCTCTTCTTCGTGCTC; mGirk3 internal antisense: 5′-GTCGGTCTGGTGCAAAGGGATGAA (506 bp); mGirk4 internal sense: 5′-TCACCATGGTCTACACCATCACCT; mGirk4 internal antisense: 5′-CTCGTTGATCTCGTGGGAGATGAT (568 bp); mGirk2 external sense: 5′-TTCACCACCCTGGTGGACCT; Girk2 external antisense: 5′-GCTTGGCACGTCATTCCTGT; mGirk2 internal sense: 5′-TGTCATGGTCTACACAGTGACGTG; mGirk2 internal antisense: 5′-TTCCTCTTTAGGCAGCTGCGCTTT (615 bp).
Amplification of wild-type D2, D3, and the four GIRK subunits, Girk1–4, was performed with two rounds of cycling. The PCR reaction mixture for the first round of amplification of transcripts using nested primer sequences contained 1× PCR buffer, 3 mm MgCl2, 0.5 mm dNTP, 0.3 μm each of external sense and antisense primer, 2.5 μl of single-cell cDNA, (or 1 μl (0.58 μg) of striatal cDNA), and 0.2 μl of Taq in 20 μl. First round was as follows: (1) 4 min at 94°C, (2) 35 cycles of 1 min at 94°C, 1 min at 50°C, and 1.5 min at 72°C, (3) 5 min at 72°C, and then (4) held at 4°C.
The PCR reaction mixtures for the second round of amplification of wild-type D2, D3, and mGirk1–4 contained 1× PCR buffer, 2.5 mm MgCl2, 0.5 mm dNTP, 0.6 μm each of internal sense and antisense primer, 0.5 μl of first-reaction product, 0.2 μl of Taq and DEPC-treated water in 20 μl. Second round was as follows: (1) 4 min at 94°C, (2) 45 cycles of 1 min at 94°C, 1 min at 56°C, and 1.5 min at 72°C, (3) 5 min at 72°C, and then held at 4°C.
Electrophysiology. The patch pipette resistance was 6–15 MΩ and access resistances were <10 MΩ. Current traces were acquired using an Instrunet board and Axopatch 200 B amplifier and sampled at 20 kHz. The average whole-cell capacitance was 7.5 ± 0.6 pF (range: 3.9–10.3 pF). Input resistances for the three genotypes were not significantly different [C57BL6, 0.9 ± 0.11 GΩ (mean ± SEM); D2–/– knock-outs, 0.9 ± 0.02 GΩ; mixed 129/C57l6, 1.2 ± 0.37 GΩ]. Cells were recorded for no more than 11 min, and normal leak currents and input resistances were maintained during that period. Cells with leak currents >82 pA were not included in the analysis.
The internal recording solution contained (in mm): 55 K2SO4, 30 KF/2H2O, 60 sucrose, 5 HEPES, 5 BAPTA (K4 salt), 3 MgCl2, 2.8 CaCl2, 3.8 Mg-ATP, 0.2 Na2-GTP, 3.1 mg/ml phospocreatine, pH 7.36, ∼296 mOsm. The external control recording solution contained (in mm): 20 potassium gluconate, 5 HEPES, 10 glucose, 140 N-methylgluconate, 2 MgCl2, 0.5 CaCl2, pH 7.41, with 156 mm HCl and 10 mm KOH, 313 mOsm. The liquid junction potential between the external and internal solution was ∼1.7 mV.
Quinpirole or baclofen (Research Biochemicals, Natick, MA) was applied by local perfusion concurrently with a series of voltage changes controlled by locally written software (V. Davila) in Igor. In brief, (1) a trigger stimulated perfusion of control solution (external solution, no drug) while voltage was held at –50 mV for 1 sec; (2) a trigger stimulated perfusion of the experimental solution (external solution plus drug) while the neuronal voltage was held at –50 mV for 1 sec; (3) perfusion of the same experimental solution was maintained during a voltage ramp from –20 to –120 mV within 1.3 sec; and 4) a trigger stimulated perfusion of control solution while the voltage was held at –50 mV, 1 sec. At the end of the protocol, the voltage remained at –50 mV. Traces were 25× box smoothed, and the corresponding cesium current was subtracted from control and experimental currents from the same cell. Signal slopes were derived from a best-fit line of the cesium-subtracted inward rectifying current corresponding to the voltage ramp between –70 and –110 mV.
Immunocytochemistry. Postnatally derived ventral midbrain cultures were processed for TH peroxidase immunocytochemistry as published (Burke et al., 1998). For fluorescent immunolabel, cultures were fixed in methanol at –20°C for 15 min and exposed to rabbit primary anti-GIRK2 polyclonal antibodies (APC006; Alomone Labs, Jerusalem, Israel) at a dilution of 1:400 and monoclonal anti-TH antibody (Boehringer Mannheim, Mannheim, Germany) at a dilution of 1:1000, in 10% normal goat serum in PBS + 1:1000 dilution of Tween-20 at 4°C for 12 hr. After washing, the cultures were incubated with secondary fluorescent antibodies [Alexa Fluor 568–goat anti-rabbit IgG and Alexa Fluor 488–goat anti-mouse IgG (Molecular Probes, Eugene, OR)], each at a dilution of 1:1000, for 1 hr at room temperature.
Results
Kir3 channel activation
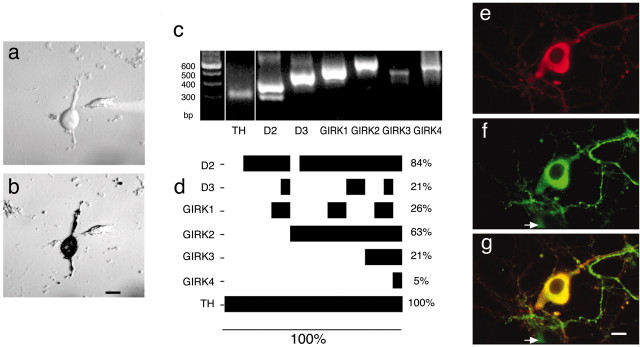
We adapted procedures used previously to characterize striatal neurons (Surmeier et al., 1998) to obtain acutely dissociated SN neurons from prepared midbrain slices. We chose this preparation because it is possible to quickly exchange media and drugs to test multiple responses by a given neuron and because mRNA detection may be more accurate in comparison with more intact systems. Most acutely dissociated neurons retained portions of their proximal dendrites (Fig. 1a), so our recordings reflect currents in the cell body and proximal dendrites with no components from axons or distal dendrites. The dopaminergic identity of the neurons was confirmed by TH immunolabel (Fig. 1b).
Figure 1.
a, b, An example of an acutely dissociated SN neuron before whole-cell patch-clamp recording and cytosol extraction (a). The same neuron is shown after recording and DAB immunolabeling for TH (b). Scale bar, 10 μm. c, Electrophoretic separation of amplified RT-PCR products. In striatal tissue, used as a positive control, amplified products are present for TH, D2 (long and short isoforms), D3, and all GIRK1–4 subunits. Thus, all of the transcripts of interest can be detected with the primers used. The leftmost lane indicates standards with numbers of base pairs as indicated. d, Coexpression profiles of mRNA for GIRK subunits and dopamine autoreceptors in single TH-expressing SN neurons (n = 19). The horizontal bars indicate the fraction of neurons that express a specific mRNA, which is also reported as a percentage of total neurons at right. In this manner of display, the extent of coexpression of the various mRNAs can be determined by examining the vertical overlap of the bars. e–g, Immunofluorescence label for GIRK2 and TH. Examples from an SN neuronal culture immunolabeled for anti-TH (e), anti-GIRK2 (f), and colocalization of the antigens (g) (in yellow) are shown. The arrow indicates an example of a nondopaminergic neuron that expresses GIRK2. Scale bar, 5 μm.
To confirm the presence of GIRK subunits, cytosol from individual neurons was screened by RT-PCR amplification for GIRK subunit mRNA. Parallel PCR amplification reactions were run using cDNA produced from mouse striatal tissue to control for proper amplification of desired gene products with our primers (Fig. 1c). We chose to use striatal tissue because all of the genes in question, including TH, are present in this region, whereas GIRK4 may not be present in substantia nigra compacta (Murer et al., 1997). Individual neurons were first identified as dopaminergic by PCR amplification with primers for TH and selected for further amplification with primers that specifically amplify mRNA for D2, D3, and the mammalian GIRK subunits (GIRK1–4). D2 mRNA was expressed in 84% and D3 in 21% of TH-expressing SN neurons (n = 19) (Fig. 1d). Each neuron that expressed D3 mRNA also expressed D2 mRNA. Of the 16 neurons that coexpressed D2 and TH, 11 neurons expressed GIRK2, confirming the abundance of this subunit in SN dopamine neurons.
GIRK mRNA expression levels are likely underestimated because only a fraction of the total cDNA was used for PCR amplification and possibly because of cellular damage that occurred during the preparation. We thus used immunolabel for TH and GIRK2 to label SN neurons after 9–10 d in vitro using our previously established culture methods for postnatally derived SN neurons (Burke et al., 1998). GIRK2 protein was excluded from the nucleus but was otherwise located throughout the cell, including the soma, dendrites, and axon (Fig. 1e–g). GIRK2 immunoreactivity was present in 89% of TH-expressing neurons in the SN and in 33% of TH-negative SN neurons (460 neurons rated). Thus, GIRK2 is preferentially expressed in dopaminergic neurons (p < 0.0001; Fisher's exact test). Unfortunately, the commercially available GIRK1 antibodies that we examined nonspecifically labeled both control and GIRK1-transfected cell lines (data not shown).
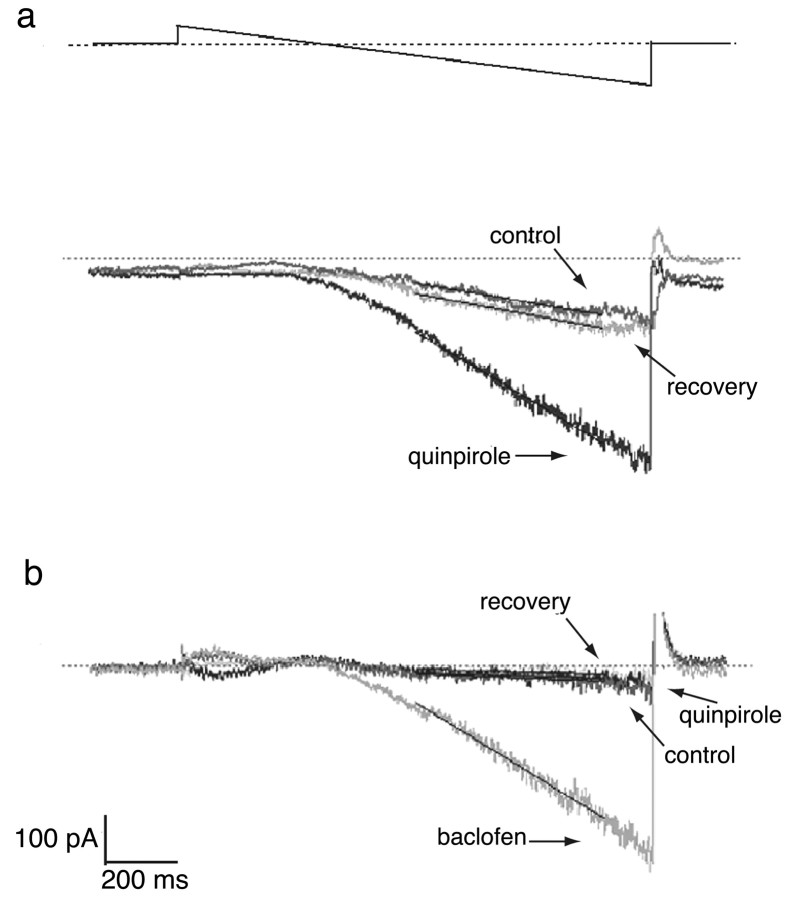
In SN dopamine neurons derived from wild-type animals, voltage-ramp recordings in the presence of TTX induced a small inwardly rectifying current at voltages below the expected Nernst potential for potassium. Application of the D2/D3 agonist quinpirole (1 μm) activated an inwardly rectifying current ∼300% greater than the agonist-independent current (n = 8) (Fig. 2a, Table 1). Both the agonistindependent and quinpirole-induced inwardly rectifying currents were abolished with the potassium channel blockers cesium (1 mm) or barium (100 μm) (Table 1). Additional neurons were exposed to quinpirole during applied voltage steps and then examined with TH/DAB immunolabel. Fourteen of 15 TH-expressing wild-type neurons (11 derived from C57BL6 mice and 4 derived from wild-type mice from the same mixed 129Sr/C57BL6 genomic background as the D2–/– mutants) displayed inwardly rectifying currents in response to quinpirole.
Figure 2.
a, A voltage ramp (–20 to –120 mV) elicited an endogenous small inwardly rectifying current in a wild-type dopaminergic SN neuron (control). A prominent rectifying current was elicited in the presence of 1μm quinpirole. After removal of quinpirole, there was a recovery to nearly initial levels. The thin black lines superimposed on the current traces indicate the slopes, between –70 and –110 mV, reported in Table 1. b, In a dopaminergic SN neuron derived from a D2–/– mutant, little endogenous inwardly rectifying current was observed (control), and quinpirole did not activate any inwardly rectifying current. A second control voltage ramp after quinpirole again elicited no inwardly rectifying current (recovery); however, a subsequent application of 50 μm baclofen elicited an inwardly rectifying current.
Table 1.
Magnitude of the inwardly rectifying currents (determined by the slope between -70 and -110 mV) as indicated in Figure 2a
|
Wild-type neurons |
|
| Control | -143 ± 25 |
| Quinpirole | -396 ± 73* |
| Quinpirole/Cs/Ba | -16 ± 10* |
| D2 -/- neurons | |
| Control | -85 ± 28 |
| Quinpirole | -88 ± 20 |
| Baclofen | -481 ± 149* |
| Baclofen/Cs/Ba
|
-19 ± 19
|
Data indicate mean ± SEM values in picoamperes per second. In wild-type SN dopamine neurons, the inward currents were activated by 1 μM quinpirole (n = 8). The response to quinpirole was abolished in 1 mM cesium or 100 μM barium. In D2 -/- mutant SN dopamine neurons, the inwardly activating currents were not activated by quinpirole but were activated by 50 μM baclofen (n = 7). The baclofen-elicited currents were abolished in cesium or barium. Two additional D2 -/- mutant SN dopamine neurons did not respond to quinpirole but were not tested with baclofen, and four additional D2 -/- mutant SN dopamine neurons did not respond to either quinpirole or baclofen; these are not included in the table. *Different from control by p < 0.01 (ANOVA followed by Student-Newman-Keuls multiple comparison test).
To test whether expression of D3 autoreceptors was sufficient for the quinpirole-activated inwardly rectifying current, we examined SN dopamine neurons derived from D2–/– mice. Quinpirole did not potentiate the endogenous voltage-dependent inward rectifying current in any mutant neuron (n = 13) (Fig. 2b, Table 1). The fractions of either TH-positive wild-type or TH-positive D2–/– neurons that responded to quinpirole (14 of 15 and 0 of 13, respectively) strongly suggest that the expression of D2 receptors is required for quinpirole activation of SN GIRK currents (p < 0.0001; Fisher's exact test).
The absence of quinpirole activation of GIRK current in D2–/– neurons could be caused by either a lack of D2 receptors or an absence of functional GIRK channels in the mutant SN neurons. To test whether GIRK currents were still functional in D2–/– SN dopamine neurons, we applied the GABAB receptor agonist baclofen (50 μm), which activates GIRK channels in SN neurons (Slesinger et al., 1997; Uezono et al., 1998). For those D2–/– SN dopamine neurons exposed to quinpirole and baclofen in succession, baclofen induced an inwardly rectifying current in 7 of 11 neurons, whereas quinpirole produced an inwardly rectifying current in none (Fig. 2b, Table 1). We conclude that functional GIRK channels were present in SN dopaminergic neurons of D2–/– mutant mice.
Discussion
Although a physiological response to D3 dopamine autoreceptor activation has not been identified in neurons, D3 receptors activate GIRK currents in cell lines that coexpress D3 and GIRK2, (Kuzhikandathil et al., 1998; Kuzhikandathil and Oxford, 2000). To test a similar function for neuronal D3 autoreceptors directly, we elicited GIRK currents in acutely dissociated SN dopamine neurons derived from wild-type and D2–/– mice with quinpirole, a D2/D3 agonist. The quinpirole-induced currents were identified as GIRK channels because (1) they were abolished by the potassium channel blockers cesium and barium, (2) they displayed inward current below the activation range of most other potassium channels, (3) the voltage-ramp protocol used inactivates voltage-gated K channels before the onset of inwardly rectifying currents, and (4) the absence of sodium in the internal and external solutions, as well as the presence of tetrodotoxin, prevented activation of Ih currents.
We used acutely dissociated neurons from 3-week-old mice because this is the period of highest D3 autoreceptor expression in wild-type mice, as well as a period of higher D3 expression in D2–/– than in wild-type mice (Jung et al., 1999). We observed GIRK current responses to quinpirole application in 14 of 15 wild-type SN dopamine neurons recorded, but in none of 13 SN dopamine neurons derived from D2–/– mice. Because quinpirole is an agonist for both D2 and D3 receptors, this striking difference argues against coupling of D3 autoreceptors and GIRK currents in SN neurons. Because of the fractions of neurons with detectable D3 autoreceptors and GIRK subunit mRNAs (21 and 74%, respectively) and the small sample size of D2–/– SN DA neurons that we recorded (n = 13), we may have missed a subpopulation of SN DA neurons in which D3 activation elicits GIRK currents. If there is D3 activation of GIRK currents in SN neurons, however, it must be exceedingly rare in comparison with SN neurons that exhibit D2 activation of these currents.
A possible criticism of our approach is that the function of GIRK2 or D3 could be disrupted in acutely dissociated neurons. We tested this possibility in various control experiments. First, we showed that the lack of a response to quinpirole in D2–/– mice was not caused by a loss of GIRK channel expression, because GIRK-mediated currents were elicited in D2–/– SN dopamine neurons by a GABAB receptor agonist. Second, although we cannot prove directly that these GIRK currents are mediated by GIRK2 subunits by the voltage-clamp recordings, we confirmed high GIRK2 subunit expression in SN dopamine neurons, as shown in vivo (Inanobe et al., 1999; Liss et al., 1999). Third, although we do not have a means to prove that D3 autoreceptor is functional, we showed that its message is present under conditions in which the D2 autoreceptor is functional.
It should be noted that the fraction of neurons that express GIRK2 subunits and D3 autoreceptor as detected by single-cell RT-PCR is likely underestimated, because the GIRK subunit mRNA within the fraction of extracted cytosol may not be abundant enough to be detected in every neuron, and degradation may occur during cell harvesting. Although 26% of wild-type TH-expressing SN neurons (5 of 19) were devoid of GIRK subunit mRNA, 14 of 15 (93%) of wild-type dopamine SN neurons that we recorded exhibited quinpirole-induced GIRK currents. Similarly, although only 21% of acutely dissociated neurons displayed detectable D3 mRNA, immunocytochemical results indicate that all SN dopamine neurons express D3 autoreceptor protein (Diaz et al., 2000); however, the physiological function of the D3 autoreceptor remains to be elucidated.
Footnotes
This work was supported by National Science Foundation, National Institute of Mental Health (Z.Y.), National Heart, Lung, and Blood Institute (D.L.), National Institute on Drug Abuse, National Institute of Neurological Disorders and Stroke, National Alliance for Research on Schizophrenia on Depression, Parkinson's Disease Foundation, and Lowenstein Foundation (D.S.). We are grateful to Drs. James Surmeier and Yvonne Schmitz for critiques and comments and to Dr. Claudia Schmauss for providing mutant mice and comments.
Correspondence should be addressed to David Sulzer, 650 West 168th Street, New York, NY 10032. E-mail: ds43@columbia.edu.
V. Davila's current address: The Vollum Institute, Oregon Health and Science University, 3181 Sam Jackson Park Road, Portland OR 97201-3098.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235693-05$15.00/0
References
- Aretha CW, Galloway MP ( 1996) Dopamine autoreceptor reserve in vitro: possible role of dopamine D3 receptors. Eur J Pharmacol 305: 119–122. [DOI] [PubMed] [Google Scholar]
- Burke RE, Antonelli M, Sulzer D ( 1998) Glial cell line-derived neurotrophic growth factor inhibits apoptotic death of postnatal substantia nigra dopamine neurons in primary culture. J Neurochem 71: 517–525. [DOI] [PubMed] [Google Scholar]
- Diaz J, Pilon C, Le Foll B, Gros C, Triller A, Schwartz JC, Sokoloff P ( 2000) Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci 20: 8677–8684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, Zahniser NR ( 1999) Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem 72: 148–156. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Sotnikova TD, Grekhova TV, Rayevsky KS ( 1996) In vivo evidence for preferential role of dopamine D3 receptor in the presynaptic regulation of dopamine release but not synthesis. Eur J Pharmacol 308: 261–269. [DOI] [PubMed] [Google Scholar]
- Inanobe A, Yoshimoto Y, Horio Y, Morishige KI, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, Kurachi Y ( 1999) Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci 19: 1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, Robakis NK, Polites HG, Pintar JE, Schmauss C ( 1999) Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience 91: 911–924. [DOI] [PubMed] [Google Scholar]
- Koeltzow TE, Xu M, Cooper DC, Hu XT, Tonegawa S, Wolf ME, White FJ ( 1998) Alterations in dopamine release but not dopamine autoreceptor function in dopamine D3 receptor mutant mice. J Neurosci 18: 2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Oxford GS ( 2000) Dominant-negative mutants identify a role for GIRK channels in D3 dopamine receptor-mediated regulation of spontaneous secretory activity. J Gen Physiol 115: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Yu W, Oxford GS ( 1998) Human dopamine D3 and D2L receptors couple to inward rectifier potassium channels in mammalian cell lines. Mol Cell Neurosci 12: 390–402. [DOI] [PubMed] [Google Scholar]
- Lejeune F, Millan MJ ( 1995) Activation of dopamine D3 autoreceptors inhibits firing of ventral tegmental dopaminergic neurones in vivo. Eur J Pharmacol 275: R7–9. [DOI] [PubMed] [Google Scholar]
- L'Hirondel M, Cheramy A, Godeheu G, Artaud F, Saiardi A, Borrelli E, Glowinski J ( 1998) Lack of autoreceptor-mediated inhibitory control of dopamine release in striatal synaptosomes of D2 receptor-deficient mice. Brain Res 792: 253–262. [DOI] [PubMed] [Google Scholar]
- Liss B, Neu A, Roeper J ( 1999) The weaver mouse gain-of-function phenotype of dopaminergic midbrain neurons is determined by coactivation of wvGirk2 and K-ATP channels. J Neurosci 19: 8839–8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer G, Adelbrecht C, Lauritzen I, Lesage F, Lazdunski M, Agid Y, Raisman-Vozari R ( 1997) An immunocytochemical study on the distribution of two G-protein-gated inward rectifier potassium channels (GIRK2 and GIRK4) in the adult rat brain. Neuroscience 80: 345–357. [DOI] [PubMed] [Google Scholar]
- Slesinger PA, Stoffel M, Jan YN, Jan LY ( 1997) Defective gammaaminobutyric acid type B receptor-activated inwardly rectifying K+ currents in cerebellar granule cells isolated from weaver and Girk2 null mutant mice. Proc Natl Acad Sci USA 94: 12210–12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Yan Z, Song WJ ( 1998) Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. Adv Pharmacol 42: 1020–1023. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Sun BC, Martin LP, Creese I ( 1997) Functional roles of dopamine D2 and D3 autoreceptors on nigrostriatal neurons analyzed by antisense knockdown in vivo J Neurosci 17: 2519–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezono Y, Akihara M, Kaibara M, Kawano C, Shibuya I, Ueda Y, Yanagihara N, Toyohira Y, Yamashita H, Taniyama K, Izumi F ( 1998) Activation of inwardly rectifying K+ channels by GABA-B receptors expressed in Xenopus oocytes. NeuroReport 9: 583–587. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E ( 2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408: 199–203. [DOI] [PubMed] [Google Scholar]
- Yan Z, Song WJ, Surmeier DJ ( 1997) D2 dopamine receptor reduce N-type Ca 2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein kinase C-insensitive pathway. J Neurophysiol 77: 1003–1015. [DOI] [PubMed] [Google Scholar]
- Zapata A, Witkin JM, Shippenberg TS ( 2001) Selective D3 receptor agonist effects of (+)-PD 128907 on dialysate dopamine at low doses. Neuropharmacology 41: 351–359. [DOI] [PubMed] [Google Scholar]