Abstract
Metabolic mapping with fluorodeoxyglucose (FDG), a radiolabeled glucose analog, was used to assess regional activity changes in the mouse brain that result from extinction of a conditioned emotional response (CER). In the extinction group, Pavlovian tone–foot shock conditioning, followed by repeated tone-alone presentations, resulted in the reduction of the CER (freezing behavior). A second group underwent CER acquisition alone (nonextinction group), and a third group showed no CER after pseudorandom training. Then mice were injected with FDG, and tone-evoked brain activity was mapped. In the auditory system, increased activity resulted from the associative effects of acquisition training. Effects common to extinction and nonextinction groups, presumably reflecting the tone–foot shock association independently of CER expression, were found in the medial geniculate, hippocampus, and subiculum. In the extinction group, a major finding was the elevated activity in prefrontal cortex regions. In addition, brain–behavior correlations between FDG uptake and freezing behavior confirmed that subjects with higher prefrontal activity were more successful at inhibiting the CER. Interregional activity correlations showed extensive functional coupling across large-scale networks in the extinction group. The increased activity of the prefrontal cortex and its negative interactions with other regions within the extinction group suggest a functional network inhibiting the CER composed of prefrontal cortex, medial thalamus, auditory, and hippocampal regions. This is the first time that such a functional network resulting from Pavlovian extinction has been demonstrated, and it supports Pavlov's original hypothesis of extinction as the formation of cortical inhibitory circuits, rather than unlearning or reversal of the acquisition process.
Keywords: metabolic mapping, extinction, prefrontal cortex, Pavlovian conditioning, fluorodeoxyglucose, learning and memory
Introduction
The behavioral and neural literature on extinction has been reviewed recently (Myers and Davis, 2002). Pavlov (1927) explained extinction as the formation of inhibitory circuits that reduce the conditioned response (CR) by counteracting the previously acquired excitatory associations between the conditioned stimulus (CS) and the unconditioned stimulus (US). After observing the phenomenon of spontaneous recovery, in which a previously extinguished CR reoccurs after an interval of time, Pavlov concluded that the original CS–US association is not destroyed and that extinction entailed new learning that inhibits the CR. Although behavioral phenomena such as spontaneous recovery, rapid reacquisition, renewal, and reinstatement (Rescorla, 1997; Falls, 1998) suggest that CS–US associative effects are not completely erased after extinction, other conceptualizations of extinction have described it in terms of the weakening of the CS–US association (Rescorla and Wagner, 1972) or the reversal of the acquisition process (Richards et al., 1984). The present study was meant to determine, in part, which of these viewpoints is closer to the neural effects of extinction.
As a natural defensive response when a predator is detected, a rodent will stop moving or “freeze” to decrease the likelihood that a predator will notice it (Fanselow, 1989). This freezing behavior can be conditioned as a form of conditioned emotional response (CER) in anticipation of a predatory strike or foot shock. Morgan et al. (1993) and Quirk et al. (2000) showed that rats with lesions of the ventromedial prefrontal cortex have across-day retention deficits during extinction of conditioned freezing. However, Gewirtz et al. (1997) found that lesions of the rat ventromedial prefrontal cortex failed to affect CER acquisition and extinction across days. Although it was reported long ago that amygdala lesions disrupt CER acquisition (for review, see Goddard, 1964), recent findings suggest that the retention of CER extinction is linked to prefrontal cortex (Milad and Quirk, 2002).
To explore this topic in intact animals, brain changes caused by a tone after extinction of a tone-conditioned CER were assessed with uptake of fluorodeoxyglucose (FDG), a radiolabeled glucose analog. Neural activity can be mapped with FDG because brain cells use glucose and its analogs for energy metabolism (Sokoloff, 1992). Different brain metabolic effects of the same tone in groups of control and conditioned mice exposed to the same CS and US served to identify which regional activity changes were caused by the tone after CER extinction. We inferred which neural mechanisms might be unique to Pavlovian extinction by comparing extinction effects with other FDG studies of CR inhibition, such as conditioned inhibition (McIntosh and Gonzalez-Lima, 1993, 1994), instrumental response extinction (Nair et al., 1999, 2001a,b), blocking (Jones and Gonzalez-Lima, 2001a), and differential inhibition (Jones and Gonzalez-Lima, 2001b).
We hypothesized that the largest increase in metabolic activity evoked by the tone after extinction would be in the prefrontal cortex. We also hoped to find changes in other regions, particularly in auditory and limbic networks, resulting from the savings of CS–US associative effects. The results supported this hypothesis and contradicted the simpler notions of extinction as unlearning or reversal of acquisition.
Materials and Methods
Subjects were 48 male CBA/J mice, 5 weeks of age when delivered from the supplier (Jackson Laboratory, Bar Harbor, ME). An initial pilot study with 16 mice was conducted to determine the parameters for a training paradigm that would result in the extinction of freezing behavior.
For the subsequent FDG study, 32 naive subjects were divided into three groups, with n = 11 in the extinction group, n = 11 in the nonextinction group, and n = 10 in the pseudorandom group. Subjects were housed under standard laboratory conditions, two to a cage, with a 12 hr light/dark cycle and ad libitum access to food and water. Subjects were handled every day for 7 d before the start of training. All animal experimentation was approved by the University of Texas Institutional Animal Care and Use Committee and complied with all applicable federal and NIH guidelines.
Apparatus
Phase I (the acquisition phase) of the experiment occurred in context A. The training apparatus for the acquisition phase consisted of a conditioning chamber (22 × 14 × 22 cm) (MED Associates, St. Albans, VT) enclosed in a sound-attenuated box illuminated by a red light. Two sides of the chamber were aluminum, with clear Plexiglas for the front, back, and top. Tones were generated by two Wavetek Sweep/Modulation generators (Wavetek, San Diego, CA) and presented through speakers mounted in the top of each chamber. The acoustic CS was a frequency-modulated tone of 1–2 kHz, two sweeps per second, 15 sec in duration, with an intensity of 65 dB, measured at the center of the floor of the chamber. The US was a foot shock of 0.5 mA, 0.75 sec in duration, delivered through metal bars separated by 0.6 cm forming the floor of the chamber, which was wired to a Lafayette Instruments Master Shocker (Lafayette Instrument Co., Lafayette, IN). Presentations of stimuli were controlled by computer programs, created using the MED-PC for Windows programming language (MED Associates). Between sessions the operant chambers were washed with soap.
Phase II (the extinction training) and the FDG uptake period of the experiment used a different context (context B): a clear plastic cage (19 × 25 × 15 cm), with a speaker mounted in the lid, placed in an illuminated testing room. Between sessions, each extinction box was washed and swabbed with iodine to provide a distinctive olfactory environment.
Behavioral training
Conditioned behavior. The CER that was measured was freezing behavior, operationally defined as the mouse having all four feet on the floor, with minimal head movements and shallow, rapid breathing for at least 3 sec. The CS was conditioned to elicit a freezing response through pairing with the US. Each 15 sec tone CS was divided into five 3 sec bins, with the subject's behavior scored for each of the five bins. Behavior was recorded for the 15 sec before the onset of the tone, as well as the subsequent 15 sec during presentation of the tone CS, to provide a comparison between activity with and without the CS. An experimenter unaware of the subject's group did the behavioral recordings.
Experimental design. Before training, subjects were randomly assigned to one of three groups: extinction, nonextinction, and pseudorandom (Table 1). The first two groups underwent tone-shock pairing, but one group was trained to extinguish the CER and the other was not. The pseudorandom group underwent no repeated tone-shock pairings and developed no CER. This experimental design permitted dissociating among (1) the brain effects of CER extinction (extinction group vs nonextinction and pseudorandom), (2) the effects of CER expression (nonextinction group vs extinction and pseudorandom), and (3) the effects of tone-shock pairing (extinction and nonextinction groups vs pseudorandom).
Table 1.
Experimental design
|
Groups |
Phase I (days 3-4) |
Probe CER (day 5) |
Phase II (days 5-6) |
Probe CER (day 7) |
FDG test (day 7) |
|---|---|---|---|---|---|
| Extinction (extinction after acquisition) | T→S | CER | T | No CER | T |
| Non-extinction (no extinction after acquisition) | T→S | CER | No T | CER | T |
| Pseudorandom (no extinction, no acquisition)
|
T,S
|
No CER
|
T/No T
|
No CER
|
T
|
T, 1-2 kHz FM tone sweep (15 sec); S, 0.5 mA foot shock (0.75 sec); →, temporally contiguous tone/shock; comma, pseudorandomly timed discontiguous tone and shock; CER, freezing behavior scored during 15 sec tone.
Phase I. Days 1–2 of training consisted of habituation to context A for all subjects. During this period, each subject explored the chamber for 1 hr, with no tones or shocks. Days 3–4 of training were again conducted in context A and consisted of acquisition training for two groups (extinction and nonextinction groups) and pseudorandom tone and shock presentations for the pseudorandom group. Daily acquisition training consisted of four tone-shock presentations over 15 min, with intertrial intervals ranging from 2, 2.5, 3, 3.5, to 4 min, randomly shuffled by the MED-PC program. During each trial of acquisition training, the 15 sec tone and 0.75 sec foot shock coterminated. Daily pseudorandom training consisted of alternating presentations of four tones and four shocks over 15 min, with intervals ranging from 1, 1.5, to 2 min, randomly shuffled by the MED-PC program. The pseudorandom training also included exactly one paired presentation of CS–US over the 2 d to prevent the tone from being conditioned as a safety signal.
Phase II. Because our goal was to examine the neural effects evoked by the tone rather than by context A, all subsequent steps (probe trials, extinction training, and FDG uptake) were conducted in context B to minimize the effects of excitatory conditioning to context A. At the start of day 5, each subject was placed in context B for 15 min and given probe trials consisting of four presentations of the CS. Behavior was scored as described above. Days 5–6 of training involved 1 hr sessions in context B and consisted of tone alone presentations in the extinction group and no tones in the nonextinction (acquisition alone) group. For the pseudorandom group, mice received either tone alone or no tone. No behavioral or brain differences were found in the pseudorandom subgroups, so these mice were all treated as one group for the statistical analysis. Tone alone consisted of 60 presentations of the 15 sec tone CS in 1 hr, with 45 sec between each CS presentation. No tone consisted of 1 hr in context B with no presentations of CS or US.
FDG test. Day 7 consisted of probe trials, FDG administration, and exposure to the CS. Probe trials were conducted as described above and compared with the day 5 probe trial results to verify that the CER was still present in the nonextinction group and extinguished in the extinction group. This was followed by injection of FDG. Subjects received an intraperitoneal injection of 18 μCi/100 gm body weight of 14C(U)-FDG (specific activity, 300 mCi/mmol; American Radiolabeled Chemicals) in 0.1 ml of sterile saline. Subjects weighed a mean of 25 gm at the time of FDG administration. Subjects were immediately placed in context B (the extinction context) and exposed to the tone in a 5 sec on, 1 sec off cycle for 45 min, a period chosen from our pilot study to preserve the CER and optimize FDG uptake to the tone. Because most of the FDG uptake is trapped in the first 10 min after injection, most of the FDG label reflects the subject's initial response to the CS, consisting of freezing in the nonextinction group and no behavioral differences in the extinction and pseudorandom groups. Then subjects were removed from the room and quickly decapitated with a guillotine. Each brain was removed rapidly and frozen in –40°C isopentane for ∼3 min.
FDG autoradiography
The standard FDG autoradiographic procedure (for review, see Gonzalez-Lima, 1992) was chosen for metabolic mapping because it has several advantages over 2-deoxy-glucose (2-DG) and because FDG has been used in all of our previous conditioning studies. Briefly, FDG is structurally more similar to glucose than 2-DG and is thus a better glucose analog; the blood–brain barrier is significantly more permeable to FDG than to 2-DG; FDG phosphorylation in the brain is significantly greater than that of 2-DG, and thus FDG is trapped more readily by the brain than 2-DG. Because all six carbons of FDG are radiolabeled, it also has a greater specific activity as a tracer than 2-DG with one radiolabeled carbon.
Sections of the brain were cut at 40 μm at –20°C on a Reichert-Jung 2800 Frigocut E cryostat. Sections used for FDG autoradiography were picked up on slides and immediately dried on a hot plate at 60°C. Slides were affixed to poster board with double-sided tape, along with plastic standards of known 14C concentration (Amersham Biosciences, Arlington Heights, IL) that were used to calibrate the imaging system and report 14C concentrations. In a darkroom, the slides were closely apposed to Kodak EB-1 film and tightly packed inside Kodak X-O-Matic cassettes (Eastman Kodak, Rochester, NY) for 2 weeks. Films were developed in Kodak D-19 for 2 min and rinsed in stop bath for 1 min and fixer for 8 min. After development, films were hung to dry, labeled, and stored in protective covers.
Image analysis
FDG uptake was quantified using JAVA image analysis software (version 1.4, Jandel Scientific, San Rafael, CA). The film was placed on a light box and artifact-free images were captured through a black-and-white video camera (Javelin model JE2362, Meyers Instruments, Houston, TX). Images were digitized and corrected for film background and optical distortions from the camera through subtraction of the background. The absolute gray levels of the 14C standards on each film were used to create a calibration curve unique to each individual film, which allowed all optical density measurements taken from brain regions to be automatically expressed in terms of isotope incorporation per gram of brain tissue (nanocuries per gram). The mouse brain atlases by Paxinos and Franklin (2001) and Slotnick and Leonard (1975) were used to locate each region that was measured. FDG incorporation was measured from 17 auditory and 64 extra-auditory brain regions (see Fig. 3).
Figure 3.
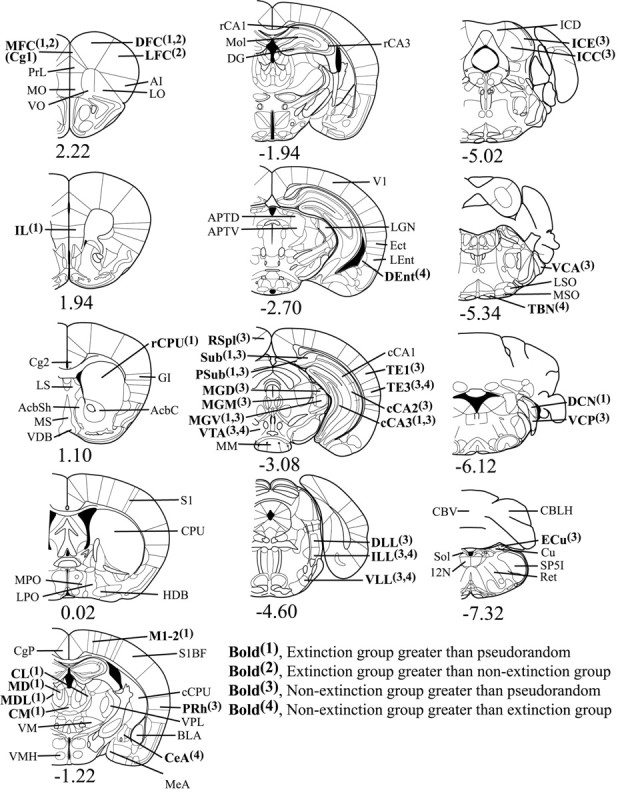
Coronal brain diagrams of locations of regions of interest by bregma level. The significant mean activity differences (p < 0.01) observed in each region are indicated in boldface. Anterior–posterior bregma coordinates are indicated below each diagram. [Section diagrams were reproduced with permission from Paxinos and Franklin (2001)]. MFC, Medial frontal cortex [Cg1 in Paxinos and Franklin (2001)]; PrL, prelimbic frontal cortex; MO, medial orbital cortex; VO, ventral orbital cortex; DFC, dorsal frontal cortex; LFC, lateral frontal cortex; AI, agranular insular cortex; LO, lateral orbital cortex; IL, infralimbic cortex; Cg2, anterior cingulate; LS, lateral septal nucleus; MS, medial septal nucleus; AcbSh, accumbens shell; AcbC, accumbens core; VDB, ventral diagonal band nucleus; rCPU, caudate–putamen rostral; GI, granular insular cortex; MPO, medial preoptic area; LPO, lateral preoptic area; HDB, horizontal limb of diagonal band posterior; S1, parietal cortex anterior; CPU, caudate–putamen middle; CgP, posterior cingulate; CL, central lateral thalamic nucleus; MD, medial dorsal thalamic nucleus; MDL, medial dorsal lateral thalamic nucleus; CM, centromedial thalamic nucleus; VM, ventromedial thalamic nucleus; VMH, ventromedial hypothalamus; M1–2, parietal cortex medial; S1BF, parietal cortex lateral; cCPU, caudate–putamen caudal; PRh, perirhinal cortex anterior; VPL, ventral posterior lateral thalamic nucleus; BLA, basolateral amygdala; CeA, central amygdala; MeA, medial amygdala; rCA1, anterior hippocampus CA1; rCA3, anterior hippocampus CA3; DG, dentate gyrus; Mol, hippocampal molecular layers; APTD, anterior pretectal area dorsal; APTV, anterior pretectal area ventral; V1, visual cortex; LGN, lateral geniculate nucleus; Ect, ectorhinal cortex posterior; LEnt, lateral entorhinal cortex; DEnt, deep entorhinal cortex; RSpl, retrosplenial cortex; Sub, subiculum; Psub, presubiculum; MGD, medial geniculate nucleus dorsal; MGM, medial geniculate nucleus medial; MGV, medial geniculate nucleus ventral; VTA, ventral tegmental area; MM, mammillary bodies; cCA1, posterior hippocampus CA1; cCA2, posterior hippocampus CA2; cCA3, posterior hippocampus CA3; TE1, auditory cortex dorsal; TE3, auditory cortex ventral; DLL, lateral lemniscus nucleus dorsal; ILL, lateral lemniscus nucleus intermediate; VLL, lateral lemniscus nucleus ventral; ICD, inferior colliculus nucleus dorsal; ICE, inferior colliculus nucleus external; ICC, inferior colliculus nucleus, central; VCA, ventral cochlear nucleus anterior; LSO, lateral superior olivary nucleus; MSO, medial superior olivary nucleus; TBN, trapezoid body nucleus; DCN, dorsal cochlear nucleus; VCP, ventral cochlear nucleus posterior; CBV, cerebellum vermis; CBLH, cerebellum lateral hemisphere; ECu, external cuneate nucleus; Cu, cuneate nucleus; SP5I, spinal trigeminal nucleus; Ret, medullary reticular formation; Sol, solitary tract nucleus; 12N, hypoglossal nucleus.
Three adjacent sections at 80 μm intervals were measured for each region of interest (ROI), with four readings taken in each section. The size of the measurement window was adjusted to allow four nonoverlapping measurements covering the entire region. In addition to measuring activity in the regions of interest, readings from white matter (the optic tract) were taken to serve as covariates.
Statistical analyses
Behavior. Changes in behavior across training days and differences in behavior across groups were evaluated on the basis of probe trial data from days 5 and 7. Evidence of extinction was evaluated by comparing the behavior of the extinction group between days 5 and 7 (between which the subjects undergo extinction training) using an ANOVA with tests for simple effects where appropriate; differences between groups were analyzed with ANOVA as well.
Mean brain activity. Group means of FDG uptake were analyzed as in our recent study of blocking of tone conditioning (Jones and Gonzalez-Lima, 2001a). To reduce variability resulting from individual differences in FDG uptake unrelated to the experimental paradigm, white matter readings were used as covariates in an analysis of covariance (ANCOVA) of brain activity measurements. Because there was no statistically significant difference in the white matter readings between groups, the ANCOVA can use the covariate to compensate for small variations in isotope incorporation across individuals. The significance level was set at 99% confidence (p < 0.01). Mean activity readings were then expressed in nanocuries per gram of tissue for each ROI, adjusted by the covariate white matter readings, with 99% confidence intervals for each group. If the measurements for two groups were both outside the other's 99% confidence interval, the effect was considered significant. Each ROI was treated as independent of the others, and one simply accepted the possibility that 1 of 100 comparisons (for p = 0.01) may have been type 1 errors. This is a standard procedure in neuroimaging studies that cannot apply any correction for multiple comparisons because of the large number of ROIs being sampled (Nobrega, 1992). Optical density measurements of the cochlear nuclei in one pseudorandom group subject were not available, and a linear interpolation was used to create two data points for the ventral and dorsal cochlear nuclei in this control subject. This did not change any pattern of significance in the pseudorandom group correlations among the cochlear nuclei but served to provide a uniform comparison for the other two groups.
Interregional correlations. Because extinction of conditioned behavior is manifested as neural changes in interactivity, the functional relationships among the regional brain activity data were analyzed in terms of pair-wise correlations within each group, as in our FDG study of extinction of instrumental behavior (Nair and Gonzalez-Lima, 1999). For the interregional correlation analysis, Pearson product–moment correlations were computed, including pair-wise comparisons of each region that showed a mean difference between groups as revealed by the ANCOVA means analysis. Optical density measurements of the optic tract were again used as a covariate to control for individual differences in film processing. To ensure the reliability of correlations, a jackknife procedure was performed in which each individual subject was dropped from a group, and then correlations were calculated again without that subject's data. This procedure was iterated until each subject had been sequentially dropped and the analysis performed again. Correlations were considered to be “reliably” significant only if they remained significantly (p < 0.01) different from zero throughout all iterations. This is a conservative method sensitive to outliers that avoids inflated type 1 errors caused by the large number of interregional correlations computed relative to the sample sizes.
Brain–behavior correlations. Correlations between brain activity and behavioral measurements of freezing during probe trials were also calculated for regions showing major extinction effects, using an extinction retention index defined as the freezing behavior ratio between post-acquisition and post-extinction probe trials (phase I freezing/phase II freezing). A high index reflects a greater reduction of freezing behavior and therefore more behavioral extinction. This value was calculated for both the extinction and nonextinction groups and correlated with the brain activity of these two groups. (The pseudorandom group was not included because they did not develop a CER.) Positive brain–behavior correlations reflect a linear relationship between increased regional brain activity and reduction of the CER.
Results
Behavioral results
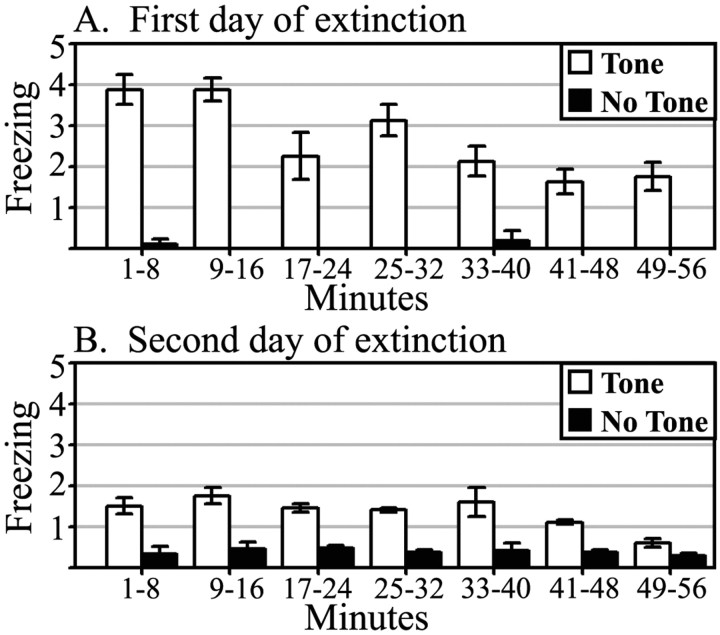
The CER demonstrated by the CBA/J mice was quite resilient; two 1 hr extinction sessions were required to extinguish the freezing behavior. Figure 1 shows freezing scores during tone and no tone presentations for the first and second extinction sessions, which demonstrate that a specific CER to the tone is present during the 45 min period selected for the FDG session.
Figure 1.
Freezing behavior during first (A) and second (B) day of extinction sessions. The CER was scored during tone-alone presentations and averaged across 8 min bins. White bars represent counts of freezing during tone CS; black bars represent counts of freezing with no tone CS, as measured before tone onset.
Average freezing counts during tone-alone probe trials after both phase I acquisition and phase II extinction training are summarized in Figure 2. Analysis of the probe trial behavioral data with ANOVA confirmed the significant increases in freezing behavior in the extinction (F(1,19) = 41.94; p < 0.01) and nonextinction (F(1,19) = 40.41; p < 0.01) groups after phase I associative tone-shock training, compared with pseudorandom. After phase II training, the extinction group showed a significant (F(1,20) = 123.02; p < 0.01) decrease in freezing behavior relative to the nonextinction group. The nonextinction group continued to respond with significantly more freezing behavior than the pseudorandom group (F(1,19) = 27.95; p < 0.01). There was no significant difference in freezing behavior between the extinction and pseudorandom groups after extinction training. Finally, there was no freezing behavior to the context during the tone-off periods preceding each trial (pre-CS), demonstrating that contextual excitatory effects were not transferred from context A to B and that the CER observed was evoked by the tone.
Figure 2.
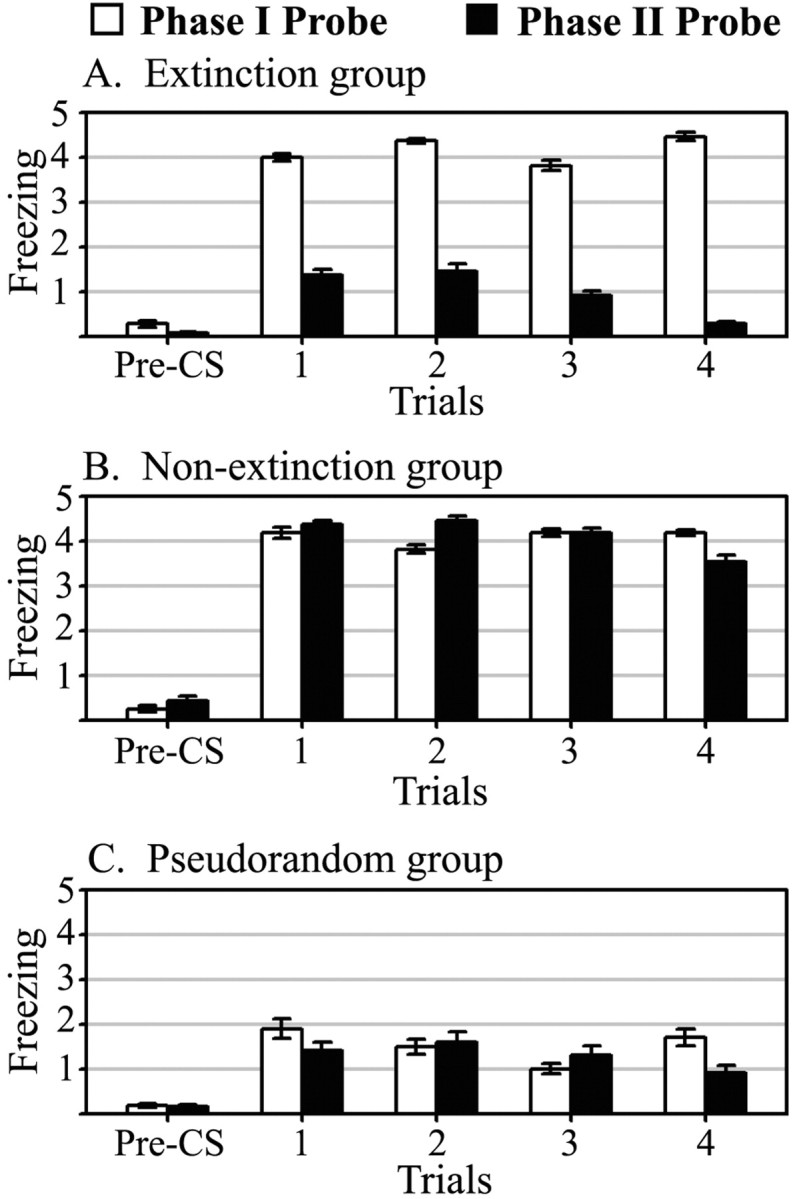
Probe trial freezing behavior. Data for each of four tone CS presentations during probe sessions I and II are shown. White bars represent counts of freezing during probe I (post-acquisition); black bars represent counts of freezing during probe II (post-extinction). Pre-CS freezing was measured during the 15 sec before tone onset and averaged across trials.
Mean brain activity results
Of 81 total regions of interest measured, 34 showed significant effects (p < 0.01) from the ANCOVA. In all, 14 of 17 auditory regions showed a significant activity increase resulting from acquisition training, and 20 of 64 extra-auditory regions showed significant effects caused by acquisition or extinction, or both. In general, three classes of effects were observed: (1) elevated activity in the extinction group; (2) elevated activity in the nonextinction group; and (3) extinction and nonextinction groups greater than pseudorandom group. A summary of these effects is shown in Table 2, and the regions are illustrated in Figure 3.
Table 2.
Significant group effects in mean activity values
|
|
Extinction |
Nonextinction |
Pseudorandom |
|---|---|---|---|
| Elevated activity in the extinction group | |||
| Extinction group greater than both nonextinction and pseudorandom | |||
| Medial frontal cortex (Cg1) | 481±18 | 409±17 | 408±16 |
| Dorsal frontal cortex (DFC) | 478±21 | 414±19 | 411±18 |
| Extinction group greater than pseudorandom | |||
| Infralimbic cortex (IL) | 364±19 | 323±17 | 295±16 |
| Medial dorsal thalamus (MD) | 459±14 | 443±14 | 409±15 |
| Medial dorsal lateral thalamus (MDL) | 508±13 | 481±13 | 450±14 |
| Centrolateral thalamus (CL) | 519±13 | 493±13 | 472±13 |
| Centromedial thalamus (CM) | 457±12 | 436±12 | 415±13 |
| Rostral caudate-putamen (rCPU) | 520±28 | 463±25 | 435±24 |
| Medial parietal cortex (M1-2) | 461±11 | 445±11 | 426±12 |
| Dorsal cochlear nucleus (DCN) | 563±19 | 561±19 | 508±26 |
| Extinction group greater than nonextinction | |||
| Lateral frontal cortex (LFC) | 443±21 | 380±19 | 383±18 |
| Elevated activity in the nonextinction group | |||
| Nonextinction group greater than both extinction and pseudorandom | |||
| Auditory cortex, ventral (TE3) | 366±19 | 411±20 | 355±19 |
| Lateral lemniscal nucleus, intermediate (ILL) | 411±24 | 489±32 | 377±29 |
| Lateral lemniscal nucleus, ventral (VLL) | 415±21 | 476±28 | 380±25 |
| Ventral tegmental area (VTA) | 269±12 | 327±11 | 287±10 |
| Nonextinction group greater than pseudorandom group | |||
| Auditory cortex, dorsal (TE1) | 419±19 | 429±20 | 385±19 |
| Medial geniculate, dorsal (MGD) | 359±14 | 383±14 | 333±16 |
| Medial geniculate, medial (MGM) | 389±13 | 410±13 | 362±15 |
| Inferior colliculus, external (ICE) | 473±23 | 509±23 | 428±25 |
| Inferior colliculus, central (ICC) | 749±30 | 765±30 | 689±33 |
| Lateral lemniscal nucleus, dorsal (DLL) | 357±20 | 373±27 | 314±25 |
| Ventral cochlear nucleus, anterior (VCA) | 489±17 | 515±17 | 449±22 |
| Ventral cochlear nucleus, posterior (VCP) | 498±22 | 520±22 | 436±29 |
| Perirhinal cortex (PRh) | 315±9 | 333±9 | 292±9 |
| Retrosplenial cortex (RSpl) | 384±16 | 406±17 | 355±18 |
| Posterior hippocampus, CA2 (cCA2) | 322±12 | 342±13 | 299±13 |
| External cuneate nucleus (ECu) | 578±22 | 605±22 | 538±22 |
| Nonextinction group greater than extinction group | |||
| Trapezoid body nucleus (TBN) | 339±15 | 373±15 | 342±21 |
| Central amygdala (CeA) | 175±15 | 226±13 | 202±13 |
| Deep entorhinal cortex (DEnt) | 226±12 | 279±11 | 246±10 |
| Extinction and nonextinction groups greater than pseudorandom group | |||
| Posterior hippocampus, CA3 (cCA3) | 292±11 | 309±12 | 263±12 |
| Subiculum (Sub) | 409±14 | 414±15 | 370±15 |
| Presubiculum (PSub) | 400±13 | 414±14 | 365±15 |
| Medial geniculate nucleus, ventral (MGV)
|
423±14
|
427±14
|
383±16
|
The indicated group differences are significant at p < 0.01 after ANCOVA. Regional FDG uptake means±SEs are expressed as nanocuries of FDG incorporation per gram tissue wet weight. The mean activity values for all regions sampled can be found in supplementary Tables A and B (available at www.jneurosci.org).
Elevated activity in the extinction group
Extinction group greater than both nonextinction and pseudorandom groups
The most prominent effect revealed by the mean activity analysis was the significantly elevated metabolism of prefrontal regions in the extinction group. There was a trend for elevated FDG uptake throughout prefrontal cortex, with medial prefrontal and dorsal frontal regions showing significant increases (15–18%) relative to both the pseudorandom and nonextinction groups.
Extinction group greater than pseudorandom group
Other regions showed greater activity in the extinction group as compared with the pseudorandom but not the nonextinction group. The infralimbic cortex showed the largest increase, with 23% greater FDG uptake than the corresponding value in the pseudorandom group. Neural activity in the medial thalamus increased in a widespread manner (10–13%), particularly for the medial dorsal thalamic nuclei, which are reciprocally connected with the frontal regions. Also affected were the centromedial and central lateral nuclei, which make widespread, diffuse modulatory connections throughout the cortex. Increased metabolism in the extinction group was also seen in the rostral caudate–putamen (19%) and medial parietal cortex (8%), which serve as a part of the mouse's sensorimotor US representation, and in the dorsal cochlear nucleus (DCN) (11%), which is part of the auditory CS representation.
Extinction group greater than nonextinction group
Although the lateral prefrontal cortex only approached statistical significance relative to pseudorandom, it was significantly higher (17%) than the nonextinction group, which was similar in activity to pseudorandom. However, other prefrontal regions such as prelimbic and orbital regions showed no significant effects.
Elevated activity in the nonextinction group
Nonextinction group greater than both extinction and pseudorandom groups
Parts of the auditory system, including auditory cortex (TE3) and both the intermediate and ventral nuclei of the lateral lemniscus, showed elevated activity in the nonextinction group, relative to both extinction (12–19%) and pseudorandom (16–30%) groups. Another region showing this excitatory effect (14–22%) was the ventral tegmental area (VTA), which may be involved in the expression of the CER. These nonextinction group increases may reflect tone-evoked CER excitatory components greater than any CS–US associative savings common to extinction and nonextinction groups.
Nonextinction group greater than pseudorandom group
The most consistent examples of this effect were seen in the auditory system, at virtually every level, from the primary auditory cortex (TE1) (11%) through medial geniculate nuclei (13–15%), the inferior colliculus (11–19%), dorsal lateral lemniscus nucleus (19%), to ventral cochlear nuclei (15–19%). For the nonextinction group, the tone CS acquired an excitatory salience not evident in the pseudorandom group, and as such, the auditory system showed increased neuronal metabolism at all levels of processing.
Similar excitatory effects (14%) were found in perirhinal and retrosplenial cortices and the CA2 region of the hippocampus. The external cuneate nucleus of the brainstem, which relays somatosensory information, also showed a 13% increase in metabolism in the nonextinction group.
Nonextinction group greater than extinction group
The nucleus of the trapezoid body (TBN) in the nonextinction group showed 10% higher activity than in the extinction group, which displayed activity similar to the pseudorandom group, and the difference between the nonextinction and pseudorandom groups approached statistical significance. Given the consistent excitatory effects for the other auditory system regions, and the similar pattern exhibited in the TBN, this region may be showing a weaker excitatory effect.
The central amygdala and deep entorhinal cortex also showed significant increases (23–29%) in the nonextinction group relative to the extinction group, but neither group was significantly different from the nonassociative pseudorandom group. Relative to the pseudorandom group, the trends were for lower activity in the extinction group and higher activity in the nonextinction group, which may reflect nonassociative influences on the CER.
Extinction and nonextinction groups both greater than pseudorandom
Despite the different CER expressed in the nonextinction and extinction groups, their common acquisition training resulted in similar tone-evoked effects in the CA3 hippocampus, subiculum, and presubiculum, seen as 10–18% increases relative to the pseudorandom group. These effects cannot be attributed to CER expression at the time of FDG uptake, because although the nonextinction group demonstrated freezing behavior, the extinction group did not. The simplest explanation is that acquisition training resulted in these changes because of the original tone-shock association. The medial geniculate, which also showed significant increases (10–12%) in both conditioned groups, might have entered into this association, contributing the auditory component of the CS–US savings.
Interregional within-group correlations
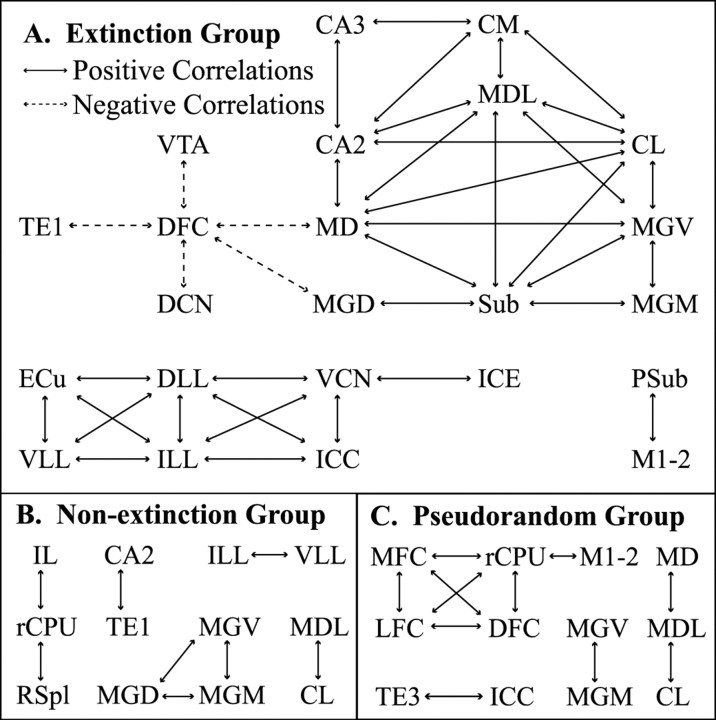
A striking finding about the interregional correlations of FDG uptake activity was the large number of reliably significant correlations among regions within the extinction group, far more than were found in either the nonextinction or pseudorandom groups. Of 50 pair-wise correlations that were found to be reliably significant at p < 0.01 after the conservative jackknife procedure, 37 of these were found in the extinction group, with 5 of those as 0.99 negative correlations between dorsal frontal cortex and other structures. These patterns of interactivity are illustrated in Figure 4.
Figure 4.
Pair-wise interregional activity correlations by group. Solid arrows indicate significant positive correlations in metabolic activity between two regions; dashed arrows indicate significant negative correlations (p < 0.01). The extensive functional coupling in the extinction group implies the existence of a network of thalamic, hippocampal, and auditory regions, with frontal cortex showing negative correlations with other regions. The significant correlation values by group can be found in supplementary Table C (available at www.jneurosci.org).
In the extinction group, dorsal frontal cortex (DFC) activity was inversely correlated with the VTA, medial dorsal thalamus (MD), and auditory regions [DCN, medial geniculate nucleus dorsal (MGD), TE1]. These strong negative correlations provided compelling evidence for a negative relationship between the prefrontal cortex and circuits linked to tone-evoked excitation of the conditioned response. The prefrontal cortex showed high colinearity among some of its regions, indicating a unified general pattern of activation and influences similar to other regions. Although a similar pattern of interactivity was found for DFC–lateral frontal cortex (LFC)–medial frontal cortex (MFC), only the stronger negative correlations between DFC and other regions reached significance in the extinction group. Two other functional circuits were formed in the extinction group, a higher-level circuit between medial thalamic and hippocampal regions and a lower-level circuit between auditory and somatosensory brainstem nuclei. In the higher circuit, the MD and MGD were functionally connected to a highly interactive thalamic–hippocampal network combining medial thalamic nuclei (medial dorsal lateral thalamic nucleus, centromedial thalamic nucleus, central lateral thalamic nucleus, medial geniculate nucleus ventral, medial geniculate nucleus medial) and hippocampal regions (CA2, CA3, subiculum). A brainstem circuit was also formed between CS and US relay pathways, comprising the lower auditory nuclei (ventral cochlear nucleus posterior, lateral lemniscus nucleus dorsal, lateral lemniscus nucleus intermediate, lateral lemniscus nucleus ventral, inferior colliculus nucleus central, inferior colliculus nucleus external) and a somatosensory relay nucleus (external cuneate nucleus).
In the nonextinction group, a smaller number of reliably significant correlations were found between infralimbic cortex, rostral caudate–putamen, and retrosplenial cortex, as well as auditory thalamocortical system/hippocampal correlations. The pseudorandom group showed significant positive relationships among the frontal regions and more coupling between frontal regions and corticostriatal motor regions (caudate–putamen rostral, parietal cortex medial). In both the nonextinction and pseudorandom groups, the rostral caudate–putamen showed significant correlations with other regions. These correlations were not observed in the extinction group.
As a result of the jackknife procedure, some high correlations were not reliably significant at p < 0.01. For example, the value of the correlation between dorsal frontal and lateral frontal cortex was 0.95–0.96 for all three groups, but only the pseudorandom group remained significant throughout all the jackknife iterations. Dropping subject 12 from the extinction group resulted in p = 0.039, whereas dropping subject 16 from the nonextinction group gave p = 0.011. Although many more high correlation coefficients could reach a less conservative probability level, the same within-group patterns of interactivity were found. Rather than focusing on particular correlation coefficients, the interregional covariance approach emphasized how the pattern of relationships among many regions and systems was manifested in each group.
Brain–behavior correlations
Correlations between brain activity and extinction behavior for the five regions showing significant (p < 0.05) correlations are presented in Table 3. This analysis confirmed that each of the more activated regions in the extinction group showed positive correlations between regional activation and extinction of the CER. The MFC in particular showed a 0.99 correlation between the extinction retention index and cortical activity (p < 0.01). The subjects with higher medial frontal cortex activity were more successful at inhibiting the CER.
Table 3.
Significant brain–behavior correlations between FDG activity and extinction index
|
Region |
Correlation (r value) |
|---|---|
| Medial frontal cortex | 0.99** |
| Dorsal frontal cortex | 0.61* |
| Lateral frontal cortex | 0.82** |
| Infralimbic cortex | 0.64** |
| Rostral caudate—putamen
|
0.48*
|
Significant at p < 0.01; *significant at p < 0.05.
Discussion
In an intact mammalian brain, prefrontal cortex activation and its negative interactions with extensive networks of medial thalamic, auditory, and hippocampal regions underlie the retention of extinction. Behavioral phenomena such as spontaneous recovery suggest that CS–US associative effects are not eliminated after extinction; however, many neural studies assume that extinction is simply the reversal of acquisition. For example, in the mollusk Hermissenda, Richards et al. (1984) concluded that extinction results from a reversal of the acquisition process, in terms of behavior and electrophysiology. Although this may be the extinction mechanism in simple organisms like invertebrates, animals with complex brains have more complex mechanisms of extinction that cannot be reduced to a simple cellular event.
Neural activity differences between extinction, nonextinction, and pseudorandom groups of mice might indicate the neural mechanisms involved in inhibition of the conditioned response. Because the extinction group as well as the pseudorandom group did not show a CER during FDG uptake sampling of brain activity, neural effects unique to the extinction group cannot be discounted simply as reflecting differences in CER performance. The lack of tone-evoked conditioned effects in the basal and lateral amygdala has been a consistent negative finding in every FDG study of conditioning for the past 20 years. Some regions might be involved at the beginning of conditioning but not after several days of training, when we tested the tone-evoked effects.
A strong argument can be made for a crucial role of the prefrontal cortex in the retention of CER inhibition after extinction. The lesion literature, however, is not conclusive. First, lesions are often made before acquisition training, which would interfere with normal brain interactions in acquisition and extinction. For example, the frontal–auditory correlation (DFC–DCN) was modified from positive to negative in the acquisition and extinction groups (0.52 to –0.99). Frontal cortex lesions would compromise this interaction during both acquisition and extinction. Second, lesions assume that the mechanism of extinction is localized to one brain region or pathway. This is not the case, as shown by the extensive network of interactions between brain regions in the extinction group (Fig. 4). Lesions of the rat ventromedial prefrontal cortex resulted in across-days extinction deficits in studies by Morgan et al. (1993) and Quirk et al. (2000) but not in Gewirtz et al. (1997). Morgan and LeDoux (1995) performed electrolytic lesions of the rat dorsomedial prefrontal cortex before Pavlovian conditioning and found that rats increased their freezing during both acquisition and extinction. Vouimba et al. (2000) performed electrolytic lesions of the mouse dorsomedial prefrontal cortex after acquisition of Pavlovian conditioning and found no effect on extinction. The assumption that there is one region or pathway responsible for extinction is too simplistic considering that in intact brains the extinguished tone produced activational effects in many regions (Fig. 3) and that there were >30 different interregional interactions in the extinction group that were not observed in the acquisition and pseudorandom groups (Fig. 4).
Milad and Quirk (2002) provided electrophysiological evidence for the involvement of the rat infralimbic cortex in the retention of extinction. Single unit recordings showed enhanced firing rates in infralimbic cortex but not in medial orbital or prelimbic cortex. Unit responses to the tone were stronger in rats showing more extinction of freezing. Furthermore, electrical stimulation of the infralimbic cortex led to less freezing during extinction. The electrical stimulation of projections from MD to prefrontal cortex can also modify extinction of conditioned freezing (Herry et al., 1999; Herry and Garcia, 2002). These findings are consistent with our FDG results. The mouse infralimbic cortex showed the largest increase in FDG uptake (23%) among the regions with significantly elevated activity in the extinction group; however, orbital and prelimbic cortex showed no significant group differences. Infralimbic activity was also correlated with our behavioral extinction index. Extinction effects, however, were not limited to the infralimbic cortex.
We found that medial, dorsal, and lateral regions of the prefrontal cortex, located anterior and dorsal to infralimbic cortex, showed higher correlations between their activity and the extinction index (Table 3), and they satisfied our criterion of showing differences as compared with both nonextinction and pseudorandom controls. The medial, dorsal, and lateral frontal regions are labeled as Cg1, M2, and M1, respectively, in the Paxinos and Franklin (2001) atlas. Our medial frontal cortex region (Fig. 3) is a neocortical region with six layers that needs to be distinguished from the histologically different cingulate cortex located more posterior, also labeled Cg1 by Paxinos and Franklin (2001). DFC and LFC are labeled as M2 and M1, respectively, in the Paxinos and Franklin (2001) atlas at this level (2.22 mm anterior to bregma) and at much more posterior levels (1.22 mm posterior to bregma). We cannot use the M1–M2 labels at all of these levels because although there is evidence that posterior M1–M2 are motor regions, there is no such purely motor evidence for our much more anterior dorsolateral frontal regions. M1–M2 showed no differences between extinction and nonextinction groups, and our results show that DFC and LFC are linked to extinction rather than to purely motor effects.
There were also large-scale networks of interactions between dorsal frontal, medial thalamic, hippocampal, and auditory regions in the extinction group, which may reflect an inhibitory relationship between frontal cortex and auditory and limbic networks with CS–US associative effects. This interpretation is consistent with human neuroimaging studies of tone conditioning. For example, Molchan et al. (1994) found an increase in frontal cortex blood flow with extinction, whereas auditory and medial temporal regions showed increases during acquisition. Schreurs et al. (2001) examined the interactions of prefrontal cortex during acquisition and extinction of tone-conditioned eye blink in young and old people using blood flow data. Consistent with our FDG findings in mice, humans showed greater activity in the prefrontal cortex during extinction retention. Moreover, after extinction the prefrontal cortex interacted extensively with other regions that were activated during acquisition, including negative correlations with auditory regions (superior temporal areas 42, 22) and limbic regions (hippocampus, perihippocampal area). Older subjects with impaired tone conditioning did not show these interactions. Admittedly, homologies between human and rodent brains are difficult to make, and the spatial resolution in the human studies was very limited as compared with our FDG mapping. Nevertheless the general pattern of brain effects produced by an extinguished tone is essentially the same in mice and men.
The prefrontal cortex is also involved in other behavioral inhibitory phenomena, such as the blocking of tone conditioning (Jones and Gonzalez-Lima, 2001a) and the partial reinforcement extinction effect (Nair et al., 2001a,b). Our FDG study of the extinction of an instrumental response in infant rats found significant interactions between medial prefrontal, orbitofrontal, and anterior cingulate cortices, but only in the older pups, which extinguished faster than the younger pups. Extensive functional coupling was found in the older group during extinction but not in handled controls, suggesting that the functional network involving frontal cortex was present as a result of their extinction training. McIntosh et al. (1999) described a positron emission tomography study in which human subjects showed progressively greater prefrontal activity to a tone CS– than another tone CS+. The better they were at inhibiting their response to the CS–, the more blood was routed to their prefrontal cortex. This form of behavioral inhibition is similar to the extinction paradigm, in which a tone-evoked CER is being suppressed.
Our findings also provided evidence that some auditory system changes produced by acquisition are present after extinction. Most auditory system activation is linked to tone-signaled CERs, as in our previous FDG mapping studies comparing tone-conditioned CER excitation and inhibition (McIntosh and Gonzalez-Lima, 1994). Associative effects in the auditory system are not observed in the pseudorandom group, in which the tone was not associated with the US. Enhanced activity in the ventral medial geniculate nucleus in nonextinction and extinction groups suggests that extinction does not involve unlearning of excitatory CS–US neural associations in some auditory structures.
The activational effects in the hippocampus, across both extinction and nonextinction groups, is further evidence that the previously acquired tone-shock association is still present in the extinction group, although the CER is extinguished. Interestingly, this lingering excitatory CS–US effect was not found in another form of CER inhibition called differential inhibition, in which the tone is never paired with the foot shock and no excitatory tone-shock association can be formed. In the case of differential inhibition, hippocampal and septal areas exhibited decreased FDG uptake to the tone inhibitor as compared with a pseudorandom group (Jones and Gonzalez-Lima, 2001b). Therefore, excitatory CS–US associative effects in certain auditory and hippocampal regions are not destroyed with extinction, and the brain effects of an extinguished tone are not the same as those of a tone inhibitor.
In conclusion, these findings suggest that prefrontal activation inhibits the associative components of the tone-evoked conditioned response via its negative interactions with auditory and hippocampal networks. They also support Pavlov's (1927) ideas of extinction, namely that the original CS–US associative effects remain partially intact and that inhibitory cortical circuits are formed to reduce the CS-evoked conditioned response.
Footnotes
This work was supported by National Institutes of Health Grant R01 NS37755 to F.G.-L. This work was submitted by D.B. in partial fulfillment of the requirements for a PhD degree at the University of Texas. We thank J. D. Berndt, A. Kalia, and M. Robinson for technical assistance.
Correspondence should be addressed to Dr. F. Gonzalez-Lima, University of Texas at Austin, Department of Psychology, 1 University Station A8000, Austin, TX 78712-0187. E-mail: gonzalez-lima@psy.utexas.edu.
D. Jones's present address: Department of Psychology, Trinity University, San Antonio, TX 78212.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235740-10$15.00/0
References
- Falls WA ( 1998) Extinction: a review of theory and the evidence suggesting that memories are not erased with nonreinforcement. In: Learning and behavior therapy (O'Donohue W, ed), pp 205–229. Boston: Allyn and Bacon.
- Fanselow MS ( 1989) The adaptive function of conditioned defensive behavior: an ecological approach to Pavlovian stimulus-substitution theory. In: Ethoexperimental approaches to the study of behavior (Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, eds), pp 151–166. Boston: Kluwer.
- Gewirtz JC, Falls WA, Davis M ( 1997) Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medial prefrontal cortex in rats. Behav Neurosci 111: 712–726. [DOI] [PubMed] [Google Scholar]
- Goddard GV ( 1964) Functions of the amygdala. Psychol Bull 62: 89–109. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F ( 1992) Brain imaging of auditory learning functions in rats: studies with fluorodeoxyglucose autoradiography and cytochrome oxidase histochemistry. In: Advances in metabolic mapping techniques for brain imaging of behavioral and learning functions (Gonzalez-Lima F, Finkenstädt T, Scheich H, eds), pp 39–109. Boston: Kluwer.
- Herry C, Garcia R ( 2002) Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci 22: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Vouimba RM, Garcia R ( 1999) Plasticity in the mediodorsal thalamo-prefrontal cortical transmission in behaving mice. J Neurophysiol 82: 2827–2832. [DOI] [PubMed] [Google Scholar]
- Jones DA, Gonzalez-Lima F ( 2001a) Mapping Pavlovian conditioning effects on the brain: blocking, contiguity and excitatory effects. J Neurophysiol 86: 809–823. [DOI] [PubMed] [Google Scholar]
- Jones DA, Gonzalez-Lima F ( 2001b) Associative effects of Pavlovian differential inhibition of behaviour. Eur J Neurosci 14: 1915–1927. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F ( 1993) Network analysis of functional auditory pathways mapped with fluorodeoxyglucose: associative effects of a tone conditioned as a Pavlovian excitor or inhibitor. Brain Res 627: 129–140. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F ( 1994) Network interactions among limbic cortices, basal forebrain, and cerebellum differentiate a tone conditioned as a Pavlovian excitor or inhibitor: fluorodeoxyglucose mapping and covariance structural modeling. J Neurophysiol 72: 1717–1733. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Rajah MN, Lobaugh NJ ( 1999) Interactions of prefrontal cortex in relation to awareness in sensory learning. Science 284: 1531–1533. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ ( 2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–74. [DOI] [PubMed] [Google Scholar]
- Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG ( 1994) A functional anatomical study of associative learning in humans. Proc Natl Acad Sci USA 91: 8122–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE ( 1995) Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109: 681–688. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE ( 1993) Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 163: 109–113. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M ( 2002) Behavioral and neural analysis of extinction. Neuron 36: 567–584. [DOI] [PubMed] [Google Scholar]
- Nair HP, Gonzalez-Lima F ( 1999) Extinction of behavior in infant rats: development of functional coupling between septal, hippocampal, and ventral tegmental regions. J Neurosci 19: 8646–8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair HP, Berndt JD, Barrett D, Gonzalez-Lima F ( 2001a) Metabolic mapping of brain regions associated with behavioral extinction in preweanling rats. Brain Res 903: 141–153. [DOI] [PubMed] [Google Scholar]
- Nair HP, Berndt JD, Barrett D, Gonzalez-Lima F ( 2001b) Maturation of extinction behavior in infant rats: large-scale regional interactions with medial prefrontal cortex, orbitofrontal cortex, and anterior cingulate cortex. J Neurosci 21: 4400–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega JN ( 1992) Brain metabolic mapping and behavior: assessing the effects of early developmental experiences in adult animals. In: Advances in metabolic mapping techniques for brain imaging of behavioral and learning functions (Gonzalez-Lima F, Finkenstädt T, Scheich H, eds), pp 125–149. Boston: Kluwer.
- Pavlov IP ( 1927) Conditioned reflexes. London: Oxford UP.
- Paxinos G, Franklin K ( 2001) The mouse brain in stereotaxic coordinates, Ed 2. San Diego: Academic.
- Quirk GJ, Russo GK, Barron JL, Lebron K ( 2000) The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20: 6225–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA ( 1997) Spontaneous recovery after Pavlovian conditioning with multiple outcomes. Anim Learn Behav 25: 99–107. [Google Scholar]
- Rescorla RA, Wagner AR ( 1972) A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Classical conditioning II (Black AH, Prokasy WF, eds), pp 64–99. New York: Appleton-Century-Crofts.
- Richards WG, Farley J, Alkon DL ( 1984) Extinction of associative learning in Hermissenda: behavior and neural correlates. Behav Brain Res 14: 161–170. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Bahro M, Molchan SE, Sunderland T, McIntosh AR ( 2001) Interactions of prefrontal cortex during eyeblink conditioning as a function of age. Neurobiol Aging 22: 237–246. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Leonard CM ( 1975) A stereotaxic atlas of the albino mouse forebrain. Rockville, MD: Department of Health, Education and Welfare.
- Sokoloff L ( 1992) Imaging techniques in studies of neural functions. In: Advances in metabolic mapping techniques for brain imaging of behavioral and learning functions. (Gonzalez-Lima F, Finkenstädt T, Scheich H, eds), pp 1–37. Boston: Kluwer.
- Vouimba RM, Garcia R, Baudry M, Thompson RF ( 2000) Potentiation of conditioned freezing following dorsomedial prefrontal cortex lesions does not interfere with fear reduction in mice. Behav Neurosci 114: 720–724. [DOI] [PubMed] [Google Scholar]