Abstract
Gsα is a subunit of the heterotrimeric G-protein complex, expressed ubiquitously in all types of cells, including neurons. Drosophila larvae, which have mutations in the Gsα gene, are lethargic, suggesting an impairment of neuronal functions. In this study, we examined synaptic transmission at the neuromuscular synapse in Gsα-null (dgsR60) embryos shortly before they hatched. At low-frequency nerve stimulation, synaptic transmission in mutant embryos was not very different from that in controls. In contrast, facilitation during tetanic stimulation was minimal in dgsR60, and no post-tetanic potentiation was observed. Miniature synaptic currents (mSCs) were slightly smaller in amplitude and less frequent in dgsR60 embryos in normal-K+ saline. In high-K+ saline, mSCs with distinctly large amplitude occurred frequently in controls at late embryonic stages, whereas those mSCs were rarely observed in dgsR60 embryos, suggesting a developmental defect in the mutant. Using the Gal4-UAS expression system, we found that these phenotypes in dgsR60 were caused predominantly by lack of Gsα in presynaptic neurons and not in postsynaptic muscles. To test whether Gsα couples presynaptic modulator receptors to adenylyl cyclase (AC), we examined the responses of two known G-protein-coupled receptors in dgsR60 embryos. Both metabotropic glutamate and octopamine receptor responses were indistinguishable from those of controls, indicating that these receptors are not linked to AC by Gsα. We therefore suggest that synaptic transmission is compromised in dgsR60 embryos because of presynaptic defects in two distinct processes; one is uncoupling between the yet-to-be-known modulator receptor and AC activation, and the other is a defect in synapse formation.
Keywords: Gsα, Drosophila, synaptic transmission, metabotropic glutamate receptor, octopamine receptor, neuromuscular junction.
Introduction
In a variety of cells, extracellular signals induce cellular responses through a family of receptors with seven transmembrane domains (7TMR). Essential components of this cascade are intermediary heterotrimeric G-proteins composed of α, β, and γ subunits. These G-proteins couple the receptors to appropriate intracellular effectors (Morris and Malbon, 1999). This signaling pathway plays a key role in triggering physiological responses to a wide variety of hormones, neurotransmitters, and sensory stimuli. In this scheme, the α subunit is essential for coupling of receptors to appropriate effectors, and many 7TMRs may couple to the same α subunit to mediate cellular responses in different contexts.
The well studied example of this signal transduction pathway involves 7TMR coupling to G-protein complexes containing Gsα. When the receptor is activated, Gsα stimulates adenylyl cyclase (AC), resulting in elevation of cAMP. Because involvement of cAMP in learning and memory has been well documented in a variety of systems (Kandel and Abel, 1995), Gsα may play an important role in neural functions. To study Gsα function, Drosophila mutants were isolated (Wolfgang et al., 1990, 1991). A hypomorphic allele, dgsB19, is viable, and neuromuscular transmission has been studied in third instars. At low-frequency stimulation, synaptic transmission was normal. However, synaptic facilitation during tetanus and post-tetanic potentiation (PTP), i.e., short-term plasticity, were not observed (W. J. Wolfgang, C. Clay, J. Parker, R. Delgado, Y. Kidokoro, P. Labarca, and M. Forte, unpublished observation). Similar phenotypes are found in rutabaga1 (rut1), in which Ca2+–calmodulin-responsive AC is defective (Livingstone et al., 1984; Zhong and Wu, 1991). If modulator receptors, activated by tetanus, are coupled to AC through Gsα, we expect dgs-null mutants to manifest similar phenotypes as rut1.
At neuromuscular synapses of newly hatched Drosophila larvae, activation of metabotropic glutamate receptors (mGluRs) facilitates synaptic transmission, which is mediated by the cAMP–PKA cascade. Both a membrane-permeant cAMP analog and forskolin, an activator of AC, mimic the mGluR response, and the response is inhibited by a blocker of AC and greatly reduced in rut1. It appears that presynaptic mGluRs are positively coupled to AC, possibly through Gs (Zhang et al., 1999). Because there is only one gene for Gsα (Quan and Forte, 1990), Gsα-null mutation provides us an opportunity to test Gs involvement in mGluR responses. Another modulator at crustacean and insect synapses is octopamine (Breen and Atwood, 1983; Klaassen and Kammer, 1985; Hidoh and Fukami, 1987). Activation of octopamine receptors, cloned from Drosophila heads, elevates the cAMP level (Han et al., 1998).
dgs also appears to be involved in synapse formation. Boutons at neuromuscular synapses in Drosophila third instars are more numerous in a hyperexcitable double mutant, ether-á-go-go, Shaker, than in wild-type (Budnik et al., 1990; Zhong et al., 1992). This phenotype was suppressed by additional mutation in dgs, indicating that activity-induced synapse formation is dependent on Gsα activities (Wolfgang, Clay, Parker, Delgado, Kidokoro, Labarca, and Forte, unpublished observation).
We studied neuromuscular synaptic transmission in Gsα-null embryos (dgsR60) using the patch-clamp techniques and found that synaptic transmission is presynaptically impaired and neither mGluRs nor octopamine receptors are coupled through Gsα to AC.
Materials and Methods
Fly stocks. Primarily, embryos (19–21 hr after egg laying, AEL) of the strain dgsR60c/CyO-GFP were used for the experiment. Homozygotes, which are embryonic lethal, are called dgsR60 in the text. Heterozygotes are called dgsR60/+ and were used as a control. As an additional control, we used a rescued strain of dgsR60 with a Gs27 transgene, designated Gs27. This rescue construct contains the entire dgs gene, which is located on the X chromosome (Gs27; dgsR60c/dgsR60c) (Wolfgang et al., 2001). The following transgenic strains were also used to selectively express a transgene: GsW24, in neurons or in muscles; dgsR60c/CyO-GFP; UAS-GsW24; dgsR60c/CyO-GFP; elav-Gal4; dgsR60c/CyO-GFP; and MHC82-Gal4. To compare phenotypes, we also used rut1 (Livingstone et al., 1984). In the metabotropic glutamate receptor response and octopamine receptor response, the involvement of the cAMP–PKA cascade was confirmed by the use of a mutant, DC0, in which a major catalytic subunit of PKA is lacking (Lane and Kalderon, 1993). Because a noncontracting double mutant, DC0 Mhc1, was readily available, we used it for this purpose. Mhc1 by itself does not affect synaptic transmission (Yoshihara et al., 2000).
Electrophysiology. Electrophysiological procedures for voltage-clamping embryonic muscles have been published previously (Nishikawa and Kidokoro, 1995). The abdominal longitudinal muscle 6 was used for physiological recordings. The membrane potential was held at -65 mV except for measurement of glutamate-induced currents, in which case the holding potential was -35 mV to reduce the current amplitude.
Nerve stimulation. A tip of micropipette filled with 4 m potassium acetate and having a resistance of ∼10 MΩ was placed in the ventral nerve cord at the site from which motor nerves emerged. Rectangular pulses of ∼1 μA and 2 msec in duration were delivered to stimulate motor nerves. The amplitude of nerve-evoked synaptic currents was measured in external saline containing 0.5 mm Ca 2+. The initial 10 responses evoked at 0.3 Hz were averaged to assess the mean amplitude. To estimate the failure rate, 40 pulses were delivered at 0.3 Hz in the external solution containing 0.2 mm Ca 2+. Subsequently, tetanic stimulation was given at 10 Hz for 10 sec. Finally, the stimulus frequency was returned to 0.3 Hz, and 40 stimuli were delivered to assess PTP.
Recording of miniature synaptic currents in high-K+saline. In normal saline with 0.2 mm Ca 2+, the frequency of miniature synaptic currents was low, and it was difficult to determine the mean amplitude accurately. To increase the miniature synaptic current (mSC) frequency, high-K + saline was used (see below for its ionic composition). The frequency was higher in this solution: ∼300 events for each cell were collected within a few minutes for measurement of their amplitudes, and an amplitude histogram was constructed.
The amplitude histograms of mSCs were not normally distributed. Some of them were skewed to larger amplitudes. To quantify the extent of skewness, we calculated the following statistical parameters for each histogram:
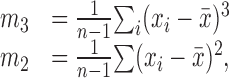 |
where xi is the amplitude of the ith mSC, x̄ is the mean, and n is the total number of mSCs. Here, m3 and m2 are the third and second moments about the mean. Using these parameters, the skewness is defined as follows:
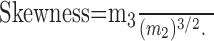 |
The value of skewness is zero when the amplitude histogram is normally distributed, positive when the distribution is skewed toward larger values (as in Fig. 4 Ea), and negative when it is skewed toward smaller values.
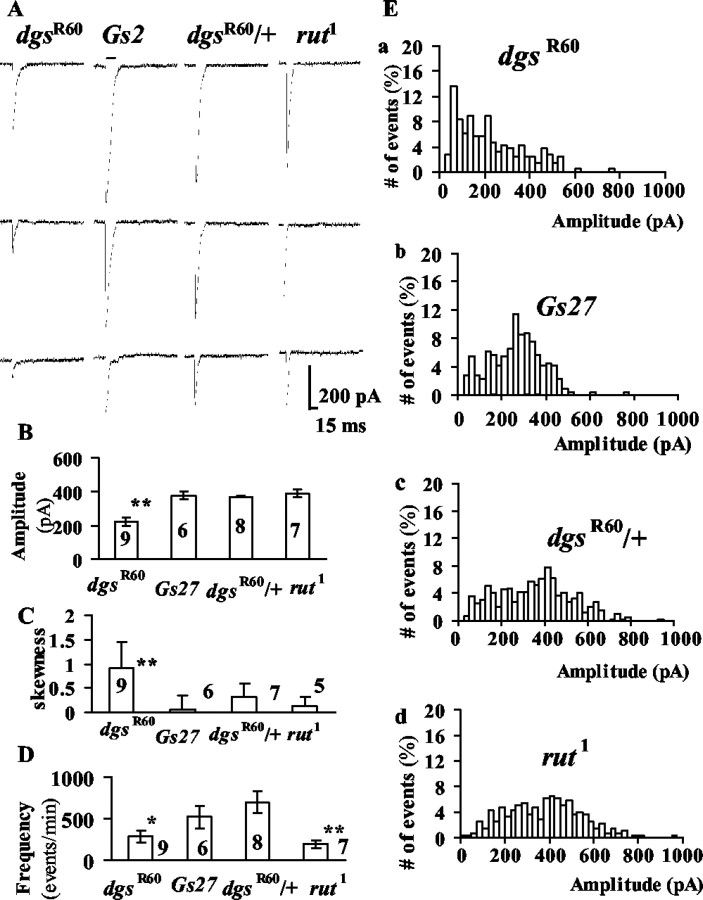
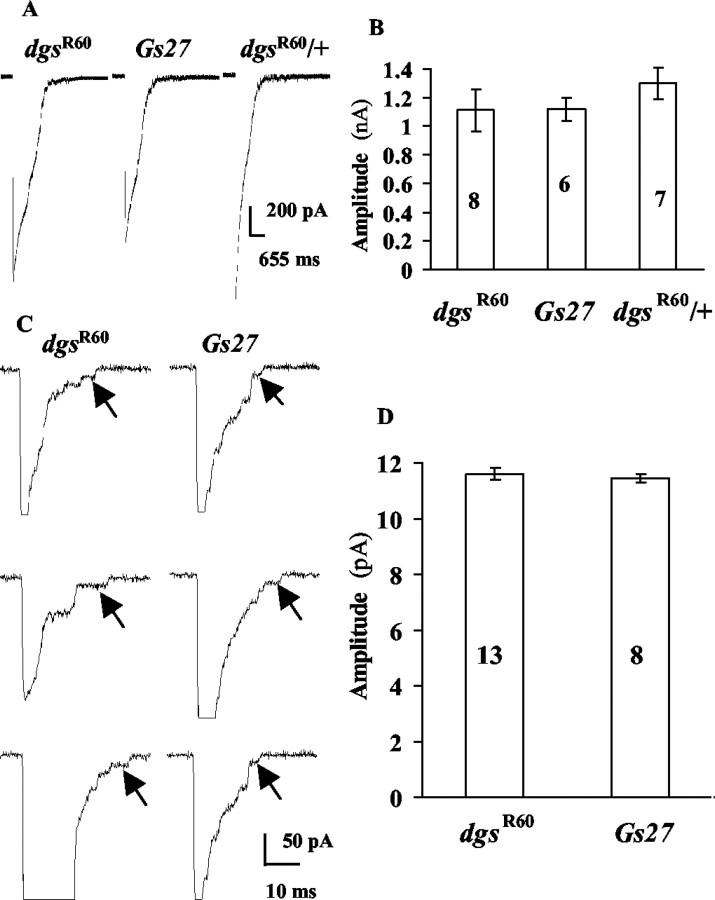
Figure 4.
mSCs in high-K + saline with 0.1 mm Ca 2+. A, Sample current traces. Three samples are shown for each strain. B, The mean amplitude for each strain. The bar at the top of each column is the SEM. Double asterisks indicate a statistical difference at p = 0.01 from Gs27, dgsR60/+ and rut1. The number in each column is the number of cells examined. C, The skewness of amplitude histogram. The skewness is defined in Materials and Methods. The bar at the top of each column is the SEM. The number is the number of cells examined. Double asterisks indicate a statistical difference at p = 0.01 from Gs27, dgsR60/+, and rut1. D, The frequency of mSCs. The bar at the top of each column is the SEM. The number is the number of cells examined. A single asterisk indicates a statistical difference at p = 0.05; double asterisks indicate a statistical difference at p = 0.01 from Gs27 and dgsR60/+. E, Amplitude histograms from a cell in each strain.
Application of glutamate to activate ionotropic glutamate receptors. To estimate the total amount of glutamate receptors in the postsynaptic membrane, glutamate-induced currents were measured at muscle 6 in Ca 2+-free saline. Glutamate was dissolved in a Ca 2+-free bath solution at 1mm and included in a glass pipette that had a tip diameter of ∼2 μm. Glutamate was delivered at the synapse by a pulse (100 msec) of gas pressure of 0.5 kg/cm 2. To prevent leaking of glutamate from the puff pipette, a latex bead with a diameter of 2.5 μm (Polysciences, Warrington, PA) was attached at the tip with steady negative pressure inside the puff pipette. This bead readily flew away with an application of positive pressure for glutamate delivery. A typical glutamate-induced inward current had a rise time (time to rise from 10 to 90% of amplitude) of ∼30 msec and started to decline during the puff pulse of 100 msec. The amplitude of glutamate-induced currents was often large, >2 nA, at a holding potential of -65 mV, which caused a problem of voltage clamping with a series resistance. To avoid this problem, the holding potential was reduced to -35 mV. Desensitization of glutamate receptors was severe. The second pulse evoked only 50–75% of the first glutamate-induced inward current amplitude after a 3 min resting period.
It should be noted that this method for glutamate application, although aimed at the synaptic area, also activates extrasynaptic receptors. The precise contribution of extrasynaptic glutamate receptor channels to the glutamate-induced currents is not known but is probably small, because receptors are highly localized at the postsynaptic area (Saitoe et al., 2001).
Application of agonists to activate metabotropic glutamate receptors or octopamine receptors. Glutamate (100 μm) or an agonist of metabotropic glutamate receptor, (S)4C3HPG (100 μm) or octopamine (10 μm), was puff-applied for 40 sec in high-K + saline containing 0.05 mm Ca 2+ and 3 μm tetrodotoxin (TTX). The frequency of mSCs was counted every 10 sec. The starting point of the 10-sec period was aligned at the onset of the puff pulse for comparison among records from different cells.
Solutions. Ca 2+-free saline had the following ionic composition (in mm): 140 NaCl, 2 KCl, 6 MgCl2, and 5 HEPES-NaOH, at a pH of 7.1. To evoke synaptic currents by nerve stimulation, 0.2 or 0.5 mm CaCl2 was added, and the equivalent amount of MgCl2 was reduced. The ionic composition of high-K + saline with 0.1 mm Ca 2+ was as follows (in mm): 78 NaCl, 62 KCl, 5.9 MgCl2, 0.1 CaCl2, and 5 HEPES-NaOH, at a pH of 7.1. Activation of metabotropic glutamate receptors and octopamine receptors was performed in the following high-K + saline (in mm): 78 NaCl, 62 KCl, 5.95 MgCl2, 0.05 CaCl2, and 5 HEPES-NaOH, at a pH of 7.1.
Biochemicals. (S)4C3HPG and MCCG-I were purchased from Tocris (Essex, UK), and TTX, octopamine, and glutamate were purchased from Sigma (St Louis, MO).
Statistics. First, parameters were tested for the normal distribution using the Kolmogorov–Smirnov test at a value of p = 0.05. When they were found to be normally distributed, we used Student's t test to compare two groups, and to compare more than two groups, we used ANOVA with Scheffé's criteria. When they were not normally distributed, we used the Mann–Whitney test.
Results
Synaptic transmission is subtly impaired in dgs-null mutant embryos
Although the dgs-null mutations are lethal, morphologically the neuromuscular synapse forms normally in embryos (Wolfgang et al., 2001). We examined synaptic transmission at the neuromuscular synapse in mutant embryos at late embryonic stages (19–21 hr AEL) using the patch-clamp technique in the whole-cell configuration.
Synaptic transmission in external saline containing 0.5 mm Ca2+
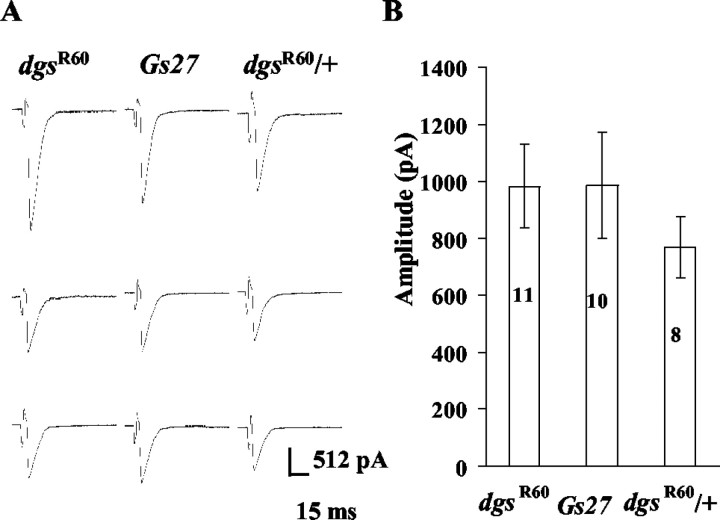
In the external solution containing 0.5 mm Ca2+, nerve stimulation at 0.3 Hz evoked robust synaptic currents in dgsR60 embryos (dgsR60/dgsR60). The amplitude of synaptic currents varied widely within one postsynaptic muscle cell and among different cells, and nerve stimulation rarely failed to evoke synaptic currents (Fig. 1A, left three traces). As a control, we used embryos of a strain in which a transgene, Gs27, was introduced into the background of dgsR60 (Gs27; dgsR60c/dgsR60c) (Wolfgang et al., 2001) (hereafter, this strain will be called Gs27). In these embryos, nerve stimulation also induced robust synaptic currents, rarely failing to evoke them, and the amplitude varied widely (Fig. 1A, middle three traces). Similar synaptic transmission was also observed in heterozygotes (dgsR60/+) (Fig. 1A, right three traces). The mean amplitude, including failures, was calculated by averaging the amplitudes of >10 synaptic currents in each cell of dgsR60 embryos and was not significantly different from that in Gs27 or heterozygous embryos (dgsR60/+) (Fig. 1B). These results are in accord with those in third instar larvae of a hypomorphic allele of dgs, dgsB19 (Wolfgang, Clay, Parker, Delgado, Kidokoro, Labarca, and Forte, unpublished observation).
Figure 1.
Nerve-evoked synaptic currents in dgsR60, Gs27, and dgsR60/+. External saline contained 0.5 mm Ca 2+. A, Three sample traces are shown for each strain. The amplitude varied in a large range. B, The mean amplitudes are not significantly different among these strains. The bar at the top of each column indicates the SEM,and numbers in columns are the number of cells examined.
Synaptic transmission in external saline containing 0.2 mm Ca2+
The large variation of evoked synaptic current amplitudes within a cell and among different cells may prevent detection of subtle impairment of synaptic transmission in dgsR60 embryos. A part of the variation of synaptic current amplitudes in embryos originates from a large variation of quantal synaptic current amplitudes in embryos (mSCs) (Kidokoro and Nishikawa, 1994). To circumvent this problem, we next measured the quantal content of synaptic currents by the failure method in a lower-external-Ca2+ solution, assuming the Poisson statistics for quantal release (Katz, 1969).
In the external solution containing 0.2 mm Ca2+, nerve stimulation at 0.3 Hz often failed to evoke synaptic currents in dgsR60 embryos (failure rate, 0.88 ± 0.11, mean ± SD, n = 16) (Fig. 2A1, left open column). This failure rate was not different from that in Gs27 embryos (0.92 ± 0.05, n = 8) (Fig. 2B1, left open column) but was significantly higher than that in heterozygous embryos, dgsR60/+ (0.76 ± 0.05, n = 8; p < 0.05) (Fig. 2C1, left open column), suggesting that synaptic transmission is slightly impaired in dgsR60 and Gs27 embryos.
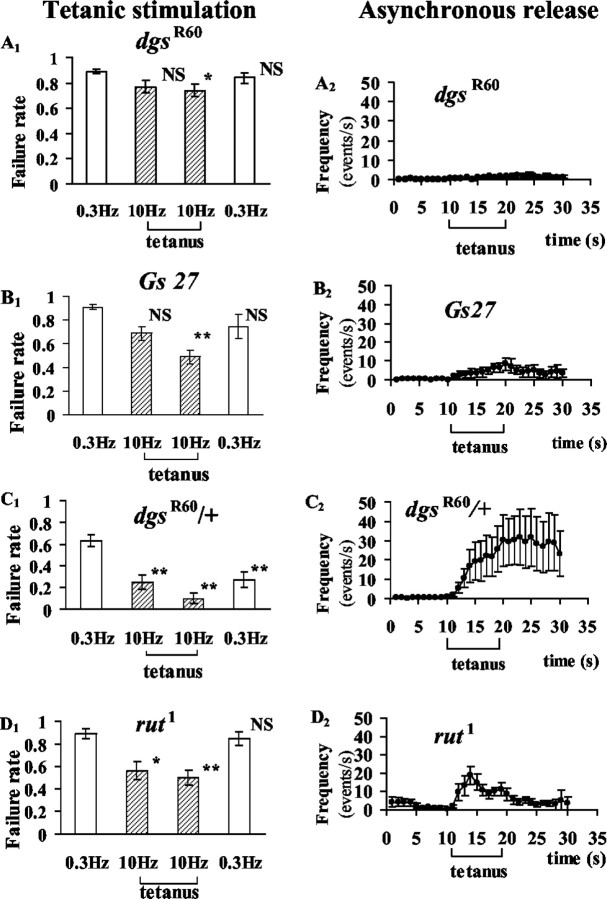
Figure 2.
Facilitation during tetanic stimulation and PTP in various strains (tetanic stimulation; A1–D1) and asynchronous release of quantal events (asynchronous release; A2–D2). At first, the nerve was stimulated 40 times at 0.3 Hz,and the failure rate was determined (left open columns in A1–D1). Then stimulation was switched to 10 Hz for 10 sec. The failure rates for the first 50 stimuli (left shaded columns) and those for the last 50 stimuli (right shaded columns) were depicted separately. Finally, the stimulation was switched back to 0.3 Hz (40 stimuli) to assess PTP (right open columns). A, dgsR60; n = 12, where n = number of cells examined. B, Gs27; n = 7. C, dgsR60/+; n = 7. D, rut1; n = 6. Bars at the top of each column in A1–D1 and at each data point in A2–D2 are the SEM. A single asterisks indicates a statistical difference from the pretetanic failure rate at p = 0.05; double asterisks indicate statistical significance at p = 0.01. NS, Nosignificance. This series of experiments was performed in normal saline with 0.2 mm Ca2+.
We further examined the synaptic facilitation during tetanus and PTP. When the nerve was stimulated at 10 Hz for 10 sec, facilitation during tetanus in dgsR60 embryos was not as prominent as in Gs27 and far less than in dgsR60/+ (Fig. 2A1–C1, middle two shaded columns). When the failure rate during the last 50 stimuli (right shaded column) was compared with that before tetanus (left open column), it was slightly lower in dgsR60 embryos, whereas it was much lower in Gs27 or in dgsR60/+ embryos (Fig. 2A1–C1). After tetanus, synaptic transmission was potentiated, and consequently the failure rate was reduced for a prolonged period of time in dgsR60/+ (Fig. 2C1, right open column) but not in dgsR60 embryos or in Gs27 embryos (Fig. 2A1,B1, right open columns). Thus, synaptic transmission in dgsR60 and Gs27 embryos appears to be mildly impaired presynaptically. Because the Gs27 transgene includes the entire Gsα gene, it is surprising that synaptic transmission in Gs27 embryos was not as robust as in dgsR60/+. This could be a positional effect of the Gs27 insertion site on the X chromosome. In addition, although it contains all Gsα sequences encoding Gsα protein and flanking 5′ and 3′ genomic regions, it may be that the Gs27 transgene does not contain all sequences required to precisely mimic the regulation of the endogenous Gsα gene in vivo.
In a mutant, rut1, in which AC is defective (Livingstone et al., 1984), PTP is abolished in third instar larvae (Zhong and Wu, 1991). If Gsα were positively coupling a synaptic modulator receptor with AC, we would expect the phenotype in dgsR60 embryos to be similar to rut1. We thus examined rut1 embryos in the same protocol and found that facilitation during tetanus was clearly observed but PTP was not (Fig. 2D1). Thus, these phenotypes in rut1 embryos are qualitatively similar to those in dgsR60, although quantitatively, facilitation during tetanus was more pronounced in rut1 (Fig. 2D1). Furthermore, the reduced facilitation during tetanus and lack of PTP were also found in third instar dgsB19 larvae (Wolfgang, Clay, Parker, Delgado, Kidokoro, Labarca, and Forte, unpublished observation) as reported previously in rut1 third instars (Zhong and Wu, 1991).
During and after tetanus, asynchronous transmitter release was clearly enhanced in the controls (in Gs27 and dgsR60/+) (Fig. 2B2,C2), whereas that in dgsR60 embryos was minimal (Fig. 2A2). In rut1, an enhancement of asynchronous release was clearly observed during tetanus but was much less after tetanus (Fig. 2D2) compared with that in dgsR60/+ (Fig. 2C2). Thus, facilitation of nerve-evoked synaptic transmission and asynchronous release during and after tetanus changed in parallel among different strains, suggesting that the same mechanism, such as an elevation of [Ca2+]i and/or cAMP in the terminal, underlies both of these phenomena.
Miniature synaptic currents were infrequent and smaller in Gsα-null embryos
In saline with a normal Ca2+ concentration (1.5 mm), muscles occasionally contracted and stretched presynaptic nerves, which increased the frequency of mSCs. Thus, it was difficult to accurately estimate the resting mSC frequency. To avoid this problem, mSCs were examined in 0.2 mm Ca2+ saline with 3 μm TTX during a 10 min recording period (Fig. 3A). The frequency was significantly lower in dgsR60 than in Gs27 or in dgsR60/+ embryos (Fig. 3B). This result again suggests that synaptic transmission in dgsR60 embryos is presynaptically compromised. In rut1, the mean frequency was not different in controls (Fig. 3B).
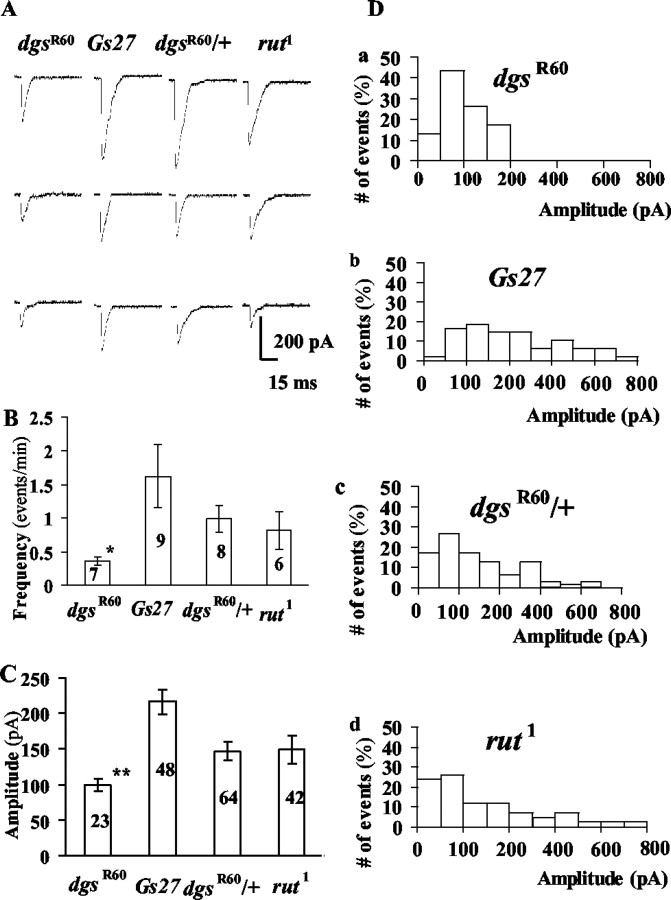
Figure 3.
Miniature synaptic currents (mSCs) in normal saline with 0.2 mm Ca 2+. A, Sample current traces. Three traces are shown for each strain. B, The mean frequency of mSCs in each strain. The number in each column is the number of cells examined. The bar at the top of each column is the SEM. Double asterisks indicate a statistical difference at p = 0.01 from Gs27 and dgsR60/+. C, The mean amplitude of mSCs in each strain. The number in each column is the number of events that were pooled among cells examined. Double asterisks indicate a statistical difference from Gs27, dgsR60/+,andrut1.D, Amplitude histograms for each strain. Events from a number of cells recorded in each strain were pooled. The number of events for each strain is the same as shown in C.
We also noticed that amplitudes of mSCs in dgsR60 embryos were somewhat smaller compared with those in Gs27 or in dgsR60/+ embryos (Fig. 3C). Because the amplitude varied widely in all strains and there were not enough events in each cell during the 10-min observation period, all synaptic events were pooled, and the mean amplitudes were compared. The mean amplitude in dgsR60 was significantly smaller than that in Gs27 or in dgsR60/+ embryos (Fig. 3C). This result suggests that the quantal size of synaptic currents is smaller in dgsR60 embryos than that in Gs27 or in dgsR60/+. However, because the numbers of events were small and the amplitudes varied widely (Fig. 3D), this conclusion should be considered tentative. In rut1, the mean amplitude was not different in dgsR60/+ or in Gs27.
To confirm the smaller amplitude of mSCs in dgsR60 embryos, we next measured mSCs in 62 mm K+ saline with 0.1 mm Ca2+, in which the mSC frequency was higher and sufficient numbers of events (>300) were collected within 2 min in each cell (Fig. 4). The amplitudes of mSCs were larger in high-K+ saline in all strains compared with those in normal-K+ saline (Fig. 4A,B). This is, at least in part, a result of the higher conductivity of K+ compared with Na+ through the Drosophila glutamate receptor channel: at the -65 mV membrane potential, K+ ions carry ∼27% more inward currents through glutamate receptor channels than Na+ ions do when K+ totally substitutes Na+ in the external solution (Chang et al., 1994). Under the conditions of the present experiments, we would expect ∼12% larger inward currents. Because in normal saline, the mean mSC amplitude in wild-type embryos is ∼190 pA at the holding potential of -65 mV (Kidokoro and Nishikawa, 1994; Deitcher et al., 1998), this difference in the channel conductance for K+ and Na+ does not fully account for the large mSCs (380–400 pA on average) (Fig. 4B) in control strains. An inspection of the amplitude histograms in Figure 4Eb,c immediately revealed that in high-K+ saline, there were distinctly more mSCs with large amplitudes, whereas in normal saline the mSC distribution was skewed. Thus, in high-K+ saline, mSCs with large amplitudes occurred frequently, resulting in larger mean amplitudes and less skewed amplitude distributions in controls. These large mSCs are not likely to be a result of coincidental superimposition of multiple mSCs, because the rise times in large mSCs were as short as those in small mSCs (Kidokoro and Nishikawa, 1994). But other explanations for large mSCs are possible (Llano et al., 2000). In contrast, the amplitude distribution of mSCs in dgsR60 was skewed toward larger amplitudes, and the majority of them were small (Fig. 4Ea). Consequently, the skewness (see Materials and Methods for definition) was significantly larger in dgsR60 than in controls (Fig. 4C). We will later discuss a possible mechanism for this change in the amplitude distribution in high-K+ saline in controls and the significance of this phenotype in dgsR60. In rut1, the amplitude distribution was similar to that in controls (Fig. 4Ed).
The frequency of mSCs in high-K+ saline was lower in dgsR60 and rut1 embryos than in controls (Fig. 4D). It should be noted that the mSC frequency in normal saline was not low in rut1 (Fig. 3B). Because the frequency of mSCs was high in high-K+ saline, the lower mSC frequency in rut1 is probably reflecting the smaller size of the exo/endo cycling pool (readily releasable pool) (Kuromi and Kidokoro, 2000).
Properties of postsynaptic glutamate receptor channels are not different in dgsR60 embryos from those in controls
The smaller amplitude of mSCs in dgsR60 embryos could be a result of either presynaptic factors, such as smaller amount of glutamate in vesicles, or postsynaptic factors, such as lower densities of glutamate receptors or smaller conductance of synaptic glutamate receptor channels. To distinguish these possibilities, we next examined the properties of postsynaptic glutamate receptor channels.
Glutamate-induced currents
To assess the total number of glutamate receptors in the postsynaptic membrane, 1 mm glutamate was puff-applied at the neuromuscular synapse in abdominal longitudinal muscle 6. The mean amplitude of glutamate-induced inward currents was not different in dgsR60 embryos from that in controls (Fig. 5A,B), suggesting that the total number of postsynaptic receptors is not the cause for smaller amplitudes of mSCs in dgsR60 embryos.
Figure 5.
Glutamate-induced currents and single glutamate receptor channels currents. A, Glutamate-induced currents. Glutamate (1 mm) was puffed for 100 msec to the synaptic area in Ca 2+-free saline. The holding potential was -35 mV. B, The mean amplitude of each strain. The bar at the top of each column is the SEM, and the number is the number of cells examined. The holding potential was -35 mV. C, Single glutamate receptor channel currents. Three sample synaptic currents are shown in each strain. The peak of synaptic currents is saturated. Spontaneous synaptic currents were recorded in high-K + saline with 0.05 mm Ca 2+. On the falling phase of synaptic currents, there are distinct steps (arrows) that are most likely because of opening of a single channel in the postsynaptic membrane (Nishikawa and Kidokoro, 1995). The amplitude of those steps was measured in >10 synaptic currents for each cell. D, The average of single-channel currents in each strain. The bar at the top of each column is the SEM, and the number is the number of cells examined.
Single-channel current amplitudes
In the falling phase, the synaptic current often changes in steps, revealing underlying single-channel currents. The smallest step amplitude probably corresponds to unitary channel current amplitude of the synaptic glutamate receptor channel (Nishikawa and Kidokoro, 1995). To assess the properties of synaptic glutamate receptor channels, we measured the amplitude of each step in dgsR60 embryos. The mean amplitude of smallest step amplitudes in dgsR60 embryos (11.6 ± 0.8 pA; n = 13) was not different from that in Gs27 (11.4 ± 0.4 pA; n = 8) in high-K+ saline (62 mm K+) (Fig. 5C,D). These values are ∼10% larger than those observed in normal saline at the same holding potential (10.5 pA for wild-type embryos) (Nishikawa and Kidokoro, 1995), which is most likely to be caused by the higher conductivity of K+ through the Drosophila glutamate receptor channels than Na+ (it is expected to be ∼12% higher in this ionic condition) (Chang et al., 1994). Thus, the unitary glutamate receptor channel current is not different in dgsR60 embryos than in Gs27 or wild-type embryos.
Metabotropic glutamate receptor responses in dgsR60 were indistinguishable from those in controls
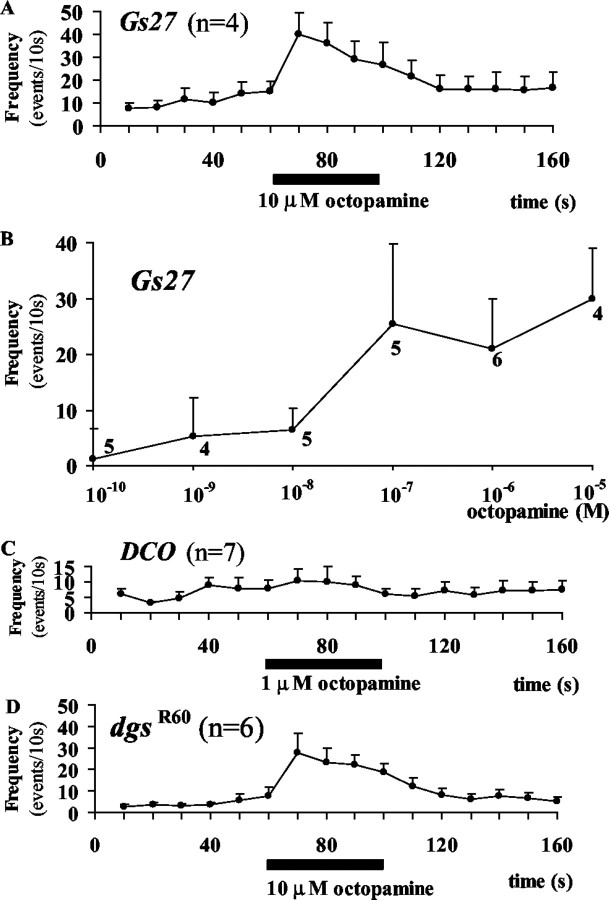
Activation of metabotropic glutamate receptors (mGluRs) in the presynaptic terminal clearly increases the frequency of mSCs in Ca2+-free saline and enhances synaptic transmission at the neuromuscular synapse of first instar larvae (Zhang et al., 1999). However, in embryos, the effect of an mGluR agonist, (S)4C3HPG, on the mSC frequency was somewhat capricious in Ca2+-free saline, and in some cells the effect was not observed. We found that the increase of mSC frequency by glutamate (Fig. 6A) or by (S)4C3HPG (Fig. 6C) was more consistently observed in high-K+ saline with low Ca2+ (0.05 mm)in Gs27 embryos and was blocked by an antagonist, MCCG-I, indicating that this response is the result of activation of mGluRs (Fig. 6B). The response to (S)4C3HPG was not observed in DC0 (Fig. 6D), in which a major subunit of PKA is missing (Lane and Kalderon, 1993), suggesting strongly that the cAMP–PKA cascade is involved in this response in accord with previous results in first instars (Zhang et al., 1999).
Figure 6.

mGluR responses inGs27(A–C), in DC0(D),and in dgsR60 embryos(E). A, Glutamate at 100 μm was puff-applied for 40 sec in high-K+ saline with 0.05 mm Ca2+, and quantal synaptic events were counted individually every 10 sec. The means of four cells are plotted. Bars attached to each point are the SEM. B, A specific mGluR antagonist, MCCG-I, at 200 μm was puff-applied together with 100 μm glutamate. The response was abolished. C, A specific mGluR agonist, (S)4C3HPG, 100 μm,was puff-applied for 40 sec in high-K+ saline with 0.05 mm Ca2+. The number of cells examined is seven. D, (S)4C3HPG at 100 μm was applied in DC0 embryos. No response was observed. The number of cells examined is four. E, The mGluR response evoked with 100μm (S)4C3HPG in dgsR60 embryos. The number of cells examined is nine.
In dgsR60 embryos, puff application of 100 μm (S)4C3HPG clearly increased the mSC frequency (Fig. 6E). The mGluR response in dgsR60 embryos was not different from that in Gs27 embryos (Fig. 6C), indicating that the mGluR response at the embryonic neuromuscular synapse is not mediated by Gsα.
Octopamine receptor responses in dgsR60 were indistinguishable from those in controls
The effect of octopamine on synaptic transmission was also examined in high-K+ saline with low Ca2+ (0.05 mm). Puff application of 10 μm octopamine for 40 sec increased the mSC frequency in G27 embryos (Fig. 7A). The dose–response curve is shown in Figure 7B, indicating that an apparent Kd is ∼10 nm. This value is smaller than that reported for the cloned octopamine receptor (190 nm) (Han et al., 1998), but it is larger than that reported for octopamine response in the crayfish (<1nm) (Breen and Atwood, 1983). The octopamine response is likely to be mediated by the cAMP–PKA cascade, because the response was not observed in DC0 (Fig. 7C).
Figure 7.
Octopamine receptor responses in Gs27 (A, B), in DC0 (C), and in dgsR60 embryos (D). A, Octopamine at 10μm was puff-applied in high-K + saline with 0.05 mm Ca 2+ for 40 sec, and quantal synaptic events were counted every 10 sec. The means of four cells are plotted, and the bars attached to each point are the SEM. B, The dose–response curve for octopamine. Neighboring two data points were connected by a straight line, and apparent Kd was estimated as a octopamine dose that produces the half-maximal response. Bars attached to each point are the SEM, and numbers are the number of cells examined. C, Lack of the octopamine receptor responses in DC0. The means of seven cells are plotted, and the bars attached to each point are the SEM. D, The octopamine receptor response in dgsR60 embryos. The means of six cells are plotted, and the bars attached to each point are the SEM.
In dgsR60 embryos, responses similar to those in Gs27 were observed (Fig. 7D), indicating that the octopamine response is not mediated by Gsα.
Expression of a Gsα transgene in neurons rescued the synaptic impairment in dgsR60, whereas its expression in postsynaptic muscles did not
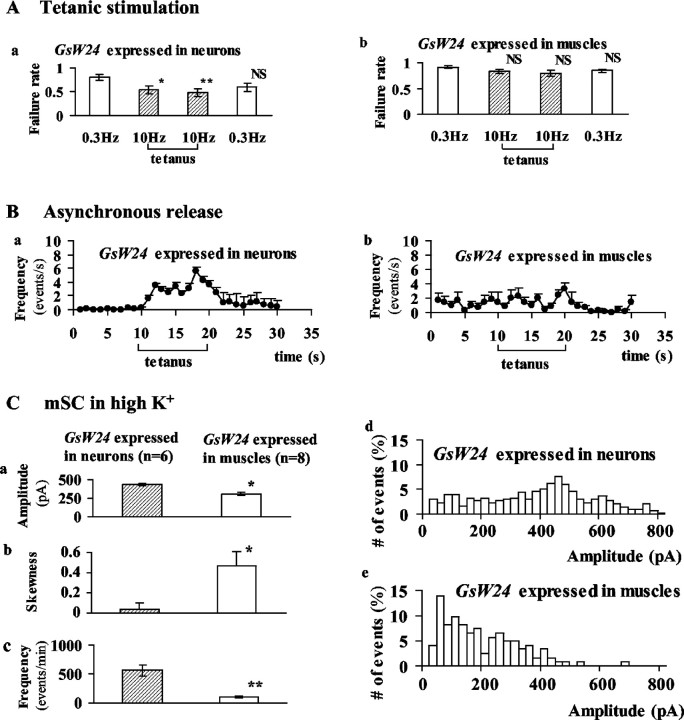
In wild-type embryos, Gsα is expressed not only in the presynaptic nerve terminals but also in postsynaptic muscles (Wolfgang et al., 2001). Therefore, the defects in synaptic transmission in dgsR60 embryos could be a result of the lack of Gsα in either the presynaptic or postsynaptic cells. To distinguish these two possibilities, we used the Gal4-UAS expression system and selectively expressed a Gsα transgene, GsW24, in neurons or in muscles in the dgsR60 background (Wolfgang et al., 2001). In the transgenic embryos, in which GsW24 was expressed in neurons, the failure rate of evoked synaptic currents in the external solution containing 0.2 mm Ca2+ (Fig. 8Aa, left open column) was not significantly different from that in dgsR60 (Figs. 2A1,8Aa), but synaptic facilitation during tetanus was more prominent (Figs. 2A1, 8A, shaded columns). Asynchronous release was observed more frequently in GsW24-expressing transgenic embryos than in dgsR60 (Figs. 2A2, 8Ba). These phenotypes are similar to those in Gs27 (Fig. 2B1,B2). Furthermore, the amplitude of mSCs in high-K+ saline was significantly larger in GsW24-expressing embryos than in dgsR60 and not different from controls (Figs. 4B, 8Ca). The amplitude histograms were widely spread in GsW24-expressing embryos (Fig. 8Cd), and the skewness was small (Fig. 8Cb), as it was in controls (Fig. 4C). The frequency of mSCs was as high as in controls (Fig. 8Cc). Altogether, the properties of mSCs in embryos expressing GsW24 in neurons were similar to those in controls, except that PTP was not as strong as that observed in heterozygous embryos (Fig. 2C). In wild-type, Gsα is abundantly expressed in muscles (Wolfgang, Clay, Parker, Delgado, Kidokoro, Labarca, and Forte, unpublished observation). Thus, it is possible that expression of Gsα in muscles is also required for full recovery of functions at the wild-type level. In addition, the positional effect of the GsW24 insertion site in the chromosome might also contribute to the less complete rescue by GsW24.
Figure 8.
Expression of a Gsα transgene, GsW24, in neurons rescued the synaptic impairment in dgsR60, whereas its expression in postsynaptic muscles did not. A wild-type transgene, GsW24, was expressed with a driver, elav-Gal4, in neurons and with another driver, MHC82-Gal4, in muscles in the dgsR60 background. A, Facilitation during tetanic stimulation and PTP. At first, the nerve was stimulated 40 times at 0.3 Hz, and the failure rate was determined (left open columns in Aa, Ab). Then, the stimulation was switched to 10 Hz for 10 sec. The failure rates for the first 50 stimuli (left shaded columns) and that for the last 50 stimuli (right shaded columns) were depicted separately. Finally, the stimulation was switched back to 0.3 Hz (40 stimuli) to assess PTP (right open columns). When GsW24 was expressed in neurons (Aa), facilitation during tetanus was observed, but when GsW24 was expressed in muscles (Ab), it was not. Bars at the top of each column are the SEM. A single asterisk indicates a statistical difference from the pretetanic failure rate at p = 0.05; double asterisks indicate a statistical difference at p = 0.01. NS, No significance. The number of cells examined is 10 for Aa and 7 for Ab. B, Asynchronous release of quantal events occurred during this series of stimulations, which increased during and after tetanus as shown in Ba, in which GsW24 was expressed in neurons, but not in Bb,in which GsW24 was expressed in muscles. This series of experiments was performed in normal saline with 0.2 mm Ca 2+.The number of cells examined is 10 for Ba and 7 for Bb. C, mSCs in high-K + saline with 0.1 mm Ca 2+. Ca, The amplitude. Left shaded columns are for a strain in which GsW24 was expressed in neurons (n = 6), and right open columns are for a transgenic strain in which GsW24 was expressed in muscles (n = 8). Cb, The skewness of amplitude histogram. Cc, The frequency of mSCs. The bar at the top of each column is the SEM. The number is the number of cells examined. A single asterisk indicates a statistical difference at p = 0.05; double asterisks indicate a statistical difference at p = 0.01. Cd, Amplitude histogram from a cell in which GsW24 was expressed in neurons. Similar histograms were observed in six cells in which GsW24 was expressed in neurons. Ce, Amplitude histogram from a cell in which GsW24 was expressed in muscles. Similar histograms were observed in eight cells in which GsW24 was expressed in muscles.
In contrast, when the transgene, GsW24, was expressed in muscles in the dgsR60 background, none of above-mentioned phenotypes were rescued (Fig. 8Ab,Bb,Ca–Cc,Ce).
Together, we conclude that the synaptic impairment in dgsR60 embryos is the result of lack of Gsα primarily in presynaptic neurons rather than in muscles.
Discussion
Presynaptic defects of synaptic transmission in dgsR60 embryos
Two distinct sets of phenotypes in synaptic transmission at the neuromuscular synapse in dgsR60 embryos were revealed, as follows. (1) Slightly smaller quantal content: The failure rate of stimuli to evoke synaptic currents in dgsR60 embryos was slightly greater in saline containing 0.2 mm Ca2+ compared with heterozygotes (Fig. 2), suggesting smaller quantal contents of evoked synaptic currents. This subtle impairment in nerve-evoked synaptic transmission probably correlates with lower frequencies of mSCs (Figs. 3B, 4D). Synaptic impairment was more clearly demonstrated on stimulation at 10 Hz. In dgsR60 embryos, we found minimal synaptic facilitation during tetanus and no PTP. Furthermore, asynchronous release of quanta during and after tetanus was much less than in controls. The lack of PTP found in dgsR60 embryos was similar to that in rut1 (Fig. 2D1). At the light-microscopic level, the morphology of neuromuscular synapses in dgsR60 embryos is not different from that of controls (Wolfgang et al., 2001). Then the defects in dgsR60 embryos could be in a lower release probability, in a smaller number of release sites, or in a smaller number of release-ready vesicles. (2) Smaller quantal size: Amplitudes of mSCs in dgsR60 embryos were slightly smaller in normal-K+ saline than in controls. The difference in mean mSC amplitude was more clearly demonstrated in high-K+ saline (Fig. 4), in which large mSCs occurred more frequently in controls than in dgsR60 embryos. Consequently, the amplitude histogram was broadly distributed in controls, whereas it was skewed toward large amplitudes in dgsR60 (Fig. 4E). The frequent occurrence of large mSCs in high-K+ saline in controls may reflect a developmental process in synapse maturation, which might be defective in dgsR60 embryos.
These two distinct sets of phenotypes in synaptic transmission in dgsR60 embryos are both a result of presynaptic defects.
Some phenotypes in dgsR60 embryos are similar to those in rut1, but others are distinctly different
In rut1, Ca2+–calmodulin-responsive AC is defective (Livingstone et al., 1984), and mGluR response are markedly reduced. AC coded by rut therefore appears to at least partly mediate mGluR responses (Zhang et al., 1999). If Gsα couples a modulator receptor to AC in nerve terminals, we would expect similar phenotypes in dgsR60 and in rut1.
During tetanic stimulation, synaptic transmission was slightly facilitated in rut1 embryos and in dgsR60, but PTP was absent in both mutants (Fig. 2). In third instars of a dgs hypomorph, dgsB19, both facilitation during tetanus and PTP were absent (Wolfgang, Clay, Parker, Delgado, Kidokoro, Labarca, and Forte, unpublished observation), and in third instars of rut1, there was slight facilitation during tetanus but no PTP (Zhong and Wu, 1991). These phenotypes are similar between rut1 and dgs. However, the mean amplitude of mSCs in high-K+ saline was smaller in dgsR60 embryos than in rut1 (Fig. 4B). The amplitude histogram was skewed in dgsR60 embryos (Fig. 4Ea), whereas in rut1 it was more widely distributed (Fig. 4Ed) and indistinguishable from controls (Fig. 4Eb,Ec). Thus, between the two distinct sets of phenotypes in dgsR60 embryos, the slightly smaller quantal content is shared with rut1, but the smaller quantal size is not. It seems unlikely that the phenotypes in dgsR60 embryos result entirely from a mechanism similar to that in rut1, in which a low level of cAMP production during tetanus is probably underlying the lack of PTP (Zhong and Wu, 1991).
Neither mGluRs nor octopamine receptors are coupled to a G-protein containing Gsα
Both mGluR and octopamine receptor responses in dgsR60 embryos were indistinguishable from those in Gs27 (Figs. 6, 7), indicating that Gsα does not constitute a G-protein that couples these receptors to AC activation in the presynaptic terminal. At the neuromuscular synapse of first instar larvae, activation of mGluRs with agonists increased the mSC frequency, which was blocked by a specific mGluR antagonist. The effect of mGluR activation was mimicked by forskolin and a membrane-permeant analog of cAMP. Furthermore, an adenylyl cyclase inhibitor blocked the mGluR agonist-induced effects, and in rut, the effects were greatly reduced (Zhang et al., 1999). These observations strongly suggest that mGluRs at the presynaptic terminal are coupled to AC, possibly through the Gs-protein. In this study, however, we showed that Gsα is not involved in the mGluR response. Because there is only one gene for Gsα (Quan and Forte, 1990), the coupling between mGluR and AC must be indirect. For example, mGluRs might be coupled to the phospholipase C cascade, activation of which leads to an increase of internal Ca2+ and activates Ca2+–calmodulin-responsive AC.
Because there are many G-protein-coupled receptors, our negative findings with two synaptic modulator receptors are not surprising. We found that of two distinct sets of phenotypes in dgsR60 embryos, one set, slightly smaller quantal content, is similar to that in rut1. Therefore, it is still possible that an unknown modulator receptor in the presynaptic terminal is coupled to AC through a G-protein containing Gsα.
Frequent occurrence of large mSCs in high-K+ saline in control embryos
Unexpectedly, we observed in high-K+ saline many distinctly large mSCs in Gs27 and dgsR60/+ embryos at the late embryonic stage. The mean amplitude was ∼80% larger than in normal saline (compare Figs. 3C and 4B). The major factor contributing to these large mean amplitudes is frequent occurrence of large synaptic currents (Fig. 4E). Large mSCs do occur in normal-K+ saline, but their frequency is low. In high-K+ saline, their frequency was elevated disproportionately, resulting in the amplitude histograms with a broader and less-skewed distribution (Fig. 4C).
Two peaks in the mSC amplitude distribution have been reported in Xenopus nerve–muscle cultures. In younger cultures, the mean amplitude of mSCs is smaller and the amplitude distribution is skewed toward larger amplitudes. The second symmetrical peak in the large-amplitude range appears in older cultures. This change in the amplitude distribution is considered to be a developmental process (Kidokoro, 1984). In Drosophila, a similar transition of amplitude histogram from a skewed distribution with a single peak to a distribution with two peaks during development has not been demonstrated (Kidokoro and Nishikawa, 1994). In this study, we observed broader amplitude distributions and sometimes two peaks in control strains in high-K+ saline. This could be a change in Drosophila embryos corresponding to that observed in Xenopus nerve–muscle cultures.
Why do large mSCs occur frequently in high-K+ saline? Among other possibilities, we favor the following scenario. In rapidly developing embryos, some release sites may be more mature than others. In high-K+ saline with Ca2+, in which Ca2+ levels in the presynaptic nerve terminal are elevated, fusion of vesicles may occur more frequently at those mature release sites than at immature sites. These mature release sites probably face a postsynaptic membrane with a higher receptor density. In dgsR60 embryos, however, fewer release sites may be mature and face a postsynaptic membrane with a high receptor density. In addition, those release sites may not be responding to an elevated Ca2+ in high-K+ saline to produce large mSCs, resulting in the smaller mean amplitude with a skewed distribution. In normal saline, these mature release sites may be regulated not to initiate excessive vesicle fusion. Because the mean amplitude of glutamate-induced currents reflecting the total number of receptors was not different between dgsR60 embryos and controls (Fig. 5A,B), these mature release sites with high receptor densities could not be more numerous in controls but must be releasing vesicles more frequently in high-K+ saline.
In this study, we found the skewed amplitude distributions of mSCs in dgsR60 embryos in high-K+ saline, whereas in controls, amplitude distributions were broader. Because a transition from a skewed amplitude distribution to a broader one has been observed during synapse formation (Kidokoro, 1984), the skewed amplitude distributions in dgsR60 embryos could be an indication of immature synapses, suggesting the involvement of Gsα in synapse formation. An observation pointing to the involvement of Gsα in synapse formation was also made in third instar larvae of a hypomorphic mutant, dgsB19. The numbers of boutons and branches of presynaptic terminals at the neuromuscular synapse were smaller in dgsB19 third instars than in controls. These phenotypes were not observed in second instars of dgsB19. This finding suggests that during the period of rapid muscle expansion and synapse formation in third instars, activation of Gsα is required (Wolfgang, Clay, Parker, Delgado, Kidokoro, Labarca, and Forte, unpublished observation). The presynaptic defects in dgsR60 embryos may be related to a similar process during early synapse formation.
The effects of the null-mutation in dgs on synaptic transmission were observed in two aspects. One could be because of uncoupling between the as-yet-unknown modulator receptor and AC activation. This phenotype is similar to that in rut1. The other is probably a defect in synapse formation. Mature release sites with high receptor densities may not be well developed in dgsR60 embryos. To pinpoint the process in which Gsα is involved, it is necessary to further examine synaptic transmission at early stages of development.
Footnotes
This work was supported by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan to Y.K. and by National Institutes of Health Grant R01-NS42841 to M.F. D.H. is supported by a scholarship from the Japanese Government.
Correspondence should be addressed to Dr. Yoshi Kidokoro, Gunma University School of Medicine, 3-39-22 Showa-machi, Maebashi 371-8511, Japan. E-mail: kidokoro@med.gunma-u.ac.jp.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235897-09$15.00/0
References
- Breen CA, Atwood HL ( 1983) Octopamine: a neurohormone with presynaptic activity-dependent effects at crayfish neuromuscular junctions. Nature 303: 716-718. [DOI] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu C-F ( 1990) Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci 10: 3754-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Ciani S, Kidokoro Y ( 1994) Ion permeation properties of the glutamate receptor channel in cultured embryonic Drosophila myotubes. J Physiol (Lond) 476: 1-16. [PMC free article] [PubMed] [Google Scholar]
- Deitcher DL, Ueda A, Stewart BA, Burgess RW, Kidokoro Y, Schwarz TL ( 1998) Distinct requirements for evoked and spontaneous release of neurotransmitter are revealed by mutations in the Drosophila gene neuronal-synaptobrevin. J Neurosci 18: 2028-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K-A, Millar HS, Davis RL ( 1998) A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci 18: 3650-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidoh O, Fukami J ( 1987) Presynaptic modulation by octopamine at a single neuromuscular junction in the mealworm (Tenebrio molitor). J Neurobiol 18: 315-326. [DOI] [PubMed] [Google Scholar]
- Kandel E, Abel T ( 1995) Neuropeptides, adenylyl cyclase, and memory storage. Science 268: 825-826. [DOI] [PubMed] [Google Scholar]
- Katz B ( 1969) The release of neural transmitter substances. Springfield: Thomas.
- Kidokoro Y ( 1984) Two types of miniature endplate potentials in Xenopus nerve-muscle cultures. Neurosci Res 1: 157-170. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y, Nishikawa K ( 1994) Miniature endplate currents at the newly formed neuromuscular junction in Drosophila embryos and larvae. Neurosci Res 19: 143-154. [DOI] [PubMed] [Google Scholar]
- Klaassen LW, Kammer AE ( 1985) Octopamine enhances neuromuscular transmission in developing and adult moths, Manduca sexta J Neurobiol 16: 227-243. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y ( 2000) Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron 27: 133-143. [DOI] [PubMed] [Google Scholar]
- Lane ME, Kalderon D ( 1993) Genetic investigation of cAMP-dependent protein kinase function in Drosophila development. Genes Dev 7: 1229-1243. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Sziber PP, Quinne WG ( 1984) Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga: a Drosophila learning mutant. Cell 37: 205-215. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A ( 2000) Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci 3: 1256-1265. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Malbon CC ( 1999) Physiological regulation of G protein-linked signaling. Physiol Rev 79: 1373-1430. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Kidokoro Y ( 1995) Junctional and extrajunctional glutamate receptor channels in Drosophila embryos and larvae. J Neurosci 15: 7905-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan F, Forte M ( 1990) Two forms of Drosophila melanogaster Gs alpha are produced by alternate splicing involving an unusual splice site. Mol Cell Biol 10: 910-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoe M, Schwarz TL, Umbach JA, Gundersen CB, Kidokoro Y ( 2001) Absence of junctional receptor clusters in Drosophila mutants lacking spontaneous transmitter release. Science 293: 514-517. [DOI] [PubMed] [Google Scholar]
- Wolfgang WJ, Quan F, Goldsmith P, Unson C, Spiegel A, Forte M ( 1990) Immunolocalization of G protein α-subunits in the Drosophila CNS. J Neurosci 10: 1014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang WJ, Quan R, Thambi N, Forte M ( 1991) Restricted spatial and temporal expression of G-protein α-subunits during Drosophila embryogenesis. Development 113: 527-538. [DOI] [PubMed] [Google Scholar]
- Wolfgang WJ, Roberts I, Hoskote A, Jackson S, Forte M ( 2001) Genetic analysis of the Drosophila Gsα gene. Genetics 158: 1189-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Suzuki K, Kidokoro Y ( 2000) Two independent pathways mediated by cAMP and protein kinase A enhance spontaneous transmitter release at Drosophila neuromuscular junctions. J Neurosci 20: 8315-8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kuromi H, Kidokoro Y ( 1999) Activation of metabotropic glutamate receptors enhances synaptic transmission at the Drosophila neuromuscular junction. Neuropharmacology 38: 645-657. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu C-F ( 1991) Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science 252: 198-201. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Budnik V, Wu C-F ( 1992) Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J Neurosci 12: 644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]