Abstract
Epidemiology, in vitro, and in vivo studies strongly implicate a role for cholesterol in the pathogenesis of Alzheimer's disease (AD). We have examined the impact of aberrant intracellular cholesterol transport on the processing of the amyloid precursor protein (APP) in a mouse model of Niemann-Pick type C (NPC) disease. In the NPC mouse brain, cholesterol accumulates in late endosomes/lysosomes. This was associated with the accumulation of β-C-terminal fragments (CTFs) of APP, but the level of β-secretase and its activity were not affected. α-Secretase activity and secreted APPα generation were also not affected, suggesting CTFs increased because of decreased clearance. The level of presenilin-1 (PS-1) was unchanged, but γ-secretase activity was greatly enhanced, which correlated with an increase in Aβ40 and Aβ42 levels. These events were associated with abnormal distribution of PS-1 in the endosomal system. Our results show that aberrant cholesterol trafficking is associated with the potentiation of APP processing components in vivo, leading to an overall increase in Aβ levels.
Keywords: amyloid, cholesterol, Niemann-Pick, NPC, β-CTF, γ-secretase, presenilin, Rab 5, endosome
Introduction
Recent epidemiological studies suggest that cholesterol plays a significant role in the development of Alzheimer's disease (AD), because patients taking cholesterol synthesis inhibitors (statins) have reduced incidence of the disease (Jick et al., 2000; Wolozin et al., 2000). In vivo studies have shown that a transgenic mouse model of AD fed a high fat/high cholesterol diet had exacerbated features of the disease (Refolo et al., 2000), and the converse was seen after treatment with cholesterol synthesis inhibitors (Refolo et al., 2001; Petanceska et al., 2002).
These findings are supported by several biochemical studies that show that the processing of the amyloid precursor protein (APP) is directly affected by cellular cholesterol content (Bodovitz and Klein, 1996; Simons et al., 1998; Wahrle et al., 2002). APP is a transmembrane protein containing a short C-terminal cytoplasmic domain and a large N-terminal ectodomain. It undergoes a series of proteolytic cleavages that result in the production of either the nonamyloidogenic C-terminal fragment (CTF) P3, or a CTF that includes the amyloidogenic Aβ domain. It is the Aβ fragment that accumulates in the distinctive amyloid plaques that is a hallmark of AD. The generation of Aβ peptides relies on the activity of two proteases, β-secretase and γ-secretase, thought to be β-amyloid cleaving enzyme (BACE) and presenilin-1 (PS-1), respectively, which cleave APP in sequence. Cleavage within the Aβ region by α-secretase results in secreted APPα (sAPPα) and P3 production, thus precluding Aβ generation (for review, see De Strooper, 2000; Vassar, 2001).
The Niemann-Pick type C1 (NPC1) protein is an intracellular chaperone involved in the transport of cholesterol from late endosomes/lysosomes to the endoplasmic reticulum (ER) (Neufeld et al., 1996). NPC1 shares sequence homology with the cholesterol-sensing domains of several other proteins, including 3-hydroxy-methylglutaryl-CoA (HMG-CoA) and SCAP, both of which are implicated in cholesterol homeostatic mechanisms. NPC disease is an autosomal recessive disorder caused by a mutation in the NPC1 gene. It is characterized by a fatal build up of unesterified cholesterol and sphingolipids in late endocytic organelles, leading to demyelination and progressive neurodegeneration (Carstea et al., 1997; Patterson et al., 2000). The BALB/c npcnih mouse (Loftus et al., 1997) synthesizes abnormal NPC1 protein because of an insertion in the NPC1 gene. It develops progressive neurodegeneration and dies at 8–10 weeks of age.
In the present study, we use the mutant NPC mouse to examine the effects of aberrant cholesterol transport on Aβ synthesis and APP processing in the brain. We provide evidence that intracellular cholesterol is intrinsically linked to APP processing and that disruption of normal cholesterol trafficking enhances amyloidogenesis via redistribution of PS-1, enhancement of γ-secretase activity, and accumulation of β-CTFs.
Materials and Methods
Animals. Mice used in this study were homozygous mutant BALB/c npc nih mice (Loftus et al., 1997) from Jackson Laboratories, and their wild-type (wt) littermates. All animals were maintained and euthanized according to National Institutes of Health guidelines. Homozygous NPC mice developed neurological abnormalities at 6–8 weeks of age and died within 10 weeks of age. Eight-week-old animals were anesthetized and perfused through the left cardiac ventricle with 10 ml of cold 10 mm PBS, pH 7.4. After perfusion, the brain was removed quickly, dissected into hemibrains, the cerebellum and brain stem were removed, and the remaining region (mainly cortex/hippocampus) was either snap frozen on dry ice or postfixed for 18 hr with 4% paraformaldehyde at 4°C. Unless otherwise specified, eight NPC mice and eight wt littermate mice were examined at each stage.
Aβ ELISA preparation and assay. Two homogenization procedures were used for this series of experiments. The first method used the organic solvent diethyl acetate (DEA) to extract soluble Aβ (Savage et al., 1998). Briefly, hemibrains were homogenized in 20 mm Tris buffer containing 1 mm EDTA, 1 mm EGTA, 250 mm sucrose, and protease inhibitors, pH 7.4. The lysate was further homogenized with 0.4% DEA in 100 mm NaCl and centrifuged at 135,000 × g for 60 min. The supernatant was neutralized by adding 0.5 m Tris-HCl, pH 6.8. The second method involves sequential TBS, Triton X-100, SDS, and 70% formic acid (FA) extraction (Kawarabayashi et al., 2001). Brain samples were homogenized in 1.5 ml of 20 mm TBS with 1 mm EDTA and protease inhibitor mixture. Samples were centrifuged at 130,000 × g for 45 min in a TLA 100.3 rotor (Beckman). The supernatant was removed and stored (TBS extract). The pellet was resuspended in 1% Triton X-100 in TBS with EDTA and protease inhibitor mixture by sonicating for 15 sec. The centrifugation step was repeated, and the supernatant was stored (Triton X-100 extract). The pellet was resuspended in 2% SDS in TBS containing EDTA and protease inhibitor mixture. Samples were centrifuged, and the supernatant was stored (SDS extract). The final resuspension was in 70% FA in water, sonicated for 60 sec. The sample was centrifuged again. No pellet was seen after the final spin (FA extract). The Aβ ELISA assay was performed as described previously (Refolo et al., 2001). Briefly, Nunc-immuno plates (Maxisorp; Nunc A/S, Roskilde, Denmark) were coated with 10 μg/ml JRF/cAβ40/10 or JRF/cAβ42/26. Mouse-specific antibody JRF/Aβ1–15/2-HRPO was used to detect the presence of Aβ peptides.
Endosomal fractionation. Endosome fractions were prepared on a sucrose gradient as described previously (van der Goot, 1997). Briefly, brain regions from NPC and wt mice (n = 3 for each) were homogenized in 1.5 ml of 2 m sucrose in HEPES-EDTA buffer containing protease inhibitor mixture. A postnuclear supernatant (PNS) was prepared by centrifugation at 1,000 × g for 10 min, followed by 3,000 × g for 10 min, at 4°C. PNS (0.5 ml) was diluted 1:1 with sucrose and imidazole solution in HEPES-EDTA buffer to make a final sucrose concentration of 40% and imidazole concentration of 3 mm. This was overlaid with 1.5 ml of 35% sucrose with 3 mm imidazole, 1 ml of 25% sucrose with 3 mm imidazole, and 8% sucrose with 3 mm imidazole. The sucrose gradient was centrifuged in a SW55 rotor (Beckman) at 150,000 × g for 90 min. Interfaces between sucrose concentrations were clearly visible, and 100 μl aliquots of each interface were extracted from the top of the centrifuge tube.
Immunoblot analysis. Equal amounts of protein from Triton X-100 extracted samples were loaded onto 10–16% tricine gels. Ten microliters of the endosomal fraction were mixed with 10 μl of sample buffer and loaded. Blots were exposed to the following antibodies: Rab 5a and Rab 7 (Santa Cruz Biotechnology, San Diego, CA), apolipoprotein E (apoE) (Calbiochem, San Diego, CA), β-COP (Golgi) and calnexin (ER) (Sigma-Aldrich, St. Louis, MO), BACE (Affinity Bioreagents Inc, Golden, Co), glial fibrillary acidic protein (GFAP) (against reactive astrocytes; Sigma, St. Louis, MO), antibody 14 (PS-1), antibody 22C11 (Chemicon, Temecula, CA), and antibody 369 (APP). For β-CTF identification, 500 μg of protein were immunoprecipitated with antibody 369, specific for the C-terminal of APP, and then immunolabeled with JRF/Aβ1–15/2, which is specific for murine β-CTF and Aβ, but not α-CTF. For sAPPα identification, brain TBS homogenates were centrifuged at 100,000 × g for 1 hr. Two hundred micrograms of protein were immunoprecipitated with JRF/Aβ1–15/2, specific for sAPPα but not sAPPβ. Precipitate was then probed with 22C11.
Secretase activity assay. α-Secretase, β-secretase, and γ-secretase activity was measured using commercially available kits (R & D Systems, Minneapolis, MN). Briefly, brain samples were homogenized in supplied buffers. The homogenate was then added to a secretase-specific APP peptide conjugated to the reporter molecules EDANS and DABCYL. In the uncleaved form, the fluorescent emissions from EDANS are quenched by the physical proximity of the DABCYL moiety, which exhibits maximal absorption at the same wavelength (495 nm). Cleavage of the peptide by the secretase physically separates the EDANS and DABCYL, allowing for the release of a fluorescent signal. The level of secretase enzymatic activity in the homogenate is proportional to the fluorometric reaction (R & D Systems).
Confocal microscopy. Thirty-micrometer brain sections were dual labeled for PS-1 (antibody 14) and either late endosomes (Rab 7) or early endosomes (Rab 5). Sections were examined under a confocal microscope (Leica DM 1 RBE; Leica Camera AG, Solms, Germany). Images were acquired with an UV 100× 1.4 NA oil PL AP01 objective with pinhole settings at ∼1 airy unit.
Statistical analysis. Statistical analysis was performed using SPSS version 11.0. All ELISA data were analyzed using a two-tailed t test, with results deemed significant when p < 0.05. Immunoblots were quantified, and data from NPC mice were expressed as percentage of wt control. Results were deemed significant when p < 0.05 using a nonparametric Mann–Whitney U test.
Results
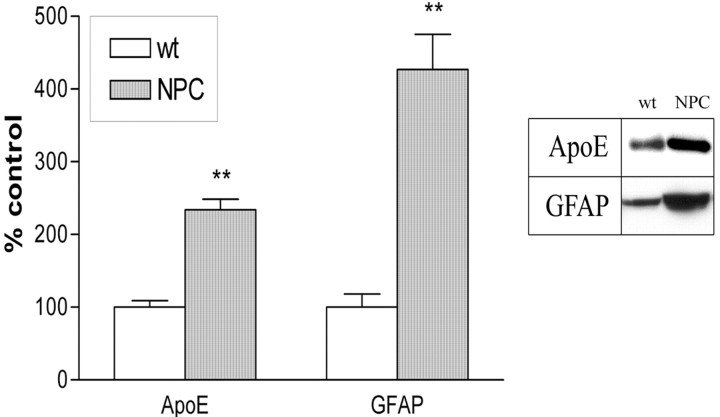
NPC mice show profound astrocytosis and upregulate apoE production
Alterations in CNS cholesterol are associated with modulated apoE levels, and we observed a large increase in apoE production in NPC mice (p < 0.01) (Fig. 1). However, apoE is synthesized in both oligodendrocytes and astrocytes (Boyles et al., 1985; Stoll et al., 1989; Krul and Tang, 1992) and apoE mRNA is increased in response to neuronal damage, to allow for cholesterol and lipid redistribution to the affected areas (Poirier et al., 1991). NPC mice undergo degeneration and reactive astrocytosis and gliosis; thus, increased apoE levels in the NPC mice may reflect enhanced astrocyte activity and not altered response to cholesterol metabolism. To test whether astrocyte markers in general were altered, we examined the level of the GFAP and found it to be significantly elevated in NPC mice (p < 0.01) (Fig. 1). With astrocytes being a major source of apoE in the CNS, it is not unreasonable to assume that apoE is upregulated in response to the neurodegenerative affects of the NPC mutation and probably not due to fluctuations in cholesterol homeostatic mechanisms.
Figure 1.
ApoE levels are enhanced in NPC mice. There was a significant increase in apoE levels in NPC mice that was associated with marked elevation of GFAP, a marker of activated astrocytes. Graphs show means ± SEM for n = 8. Mann–Whitney U nonparametric test: *p < 0.05; **p < 0.01 versus wt control.
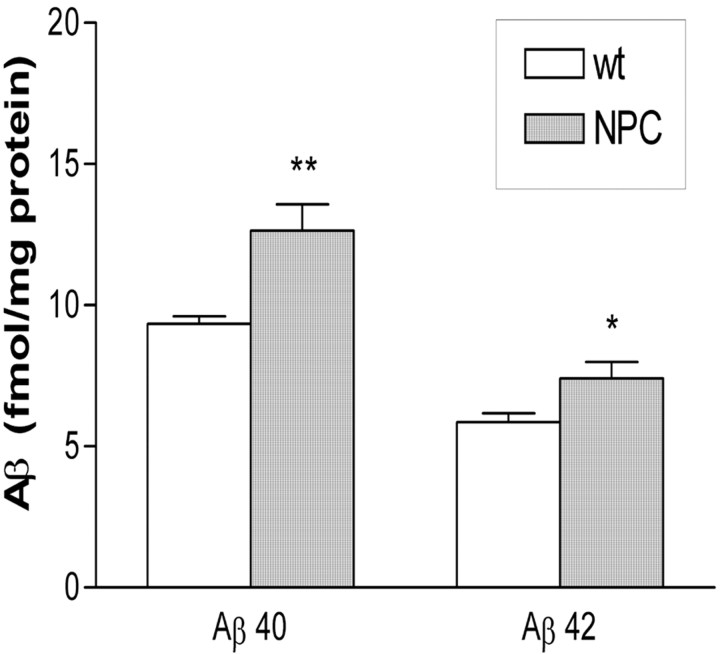
Aβ production is increased in NPC mice
We examined the effect of altered cholesterol trafficking in NPC mice on both soluble and insoluble Aβ production. DEA causes partial solubilization of the cell membrane and allows examination of soluble extracellular and intracellular compartments, but not membrane-bound proteins (Savage et al., 1998) or insoluble fibrillar proteins. We did not find any alteration in Aβ production in DEA extracted fractions (results not shown). When Aβ was extracted using a four-step extraction that extracts soluble, membrane-bound, and insoluble Aβ, we found an increase in both Aβ40 (p < 0.01) and Aβ42 (p < 0.05) in the Triton X-100 extract (Fig. 2). Our ELISA assay was inhibited by the presence of 2% SDS, and attempts to dilute the SDS to suitable levels led to undetectable levels of Aβ. We were, therefore, unable to assay Aβ levels in the SDS fraction. Compartments known as lipid rafts are one of the purported sites of Aβ production (Lee et al., 1998; Tun et al., 2002; Wahrle et al., 2002). These compartments are detergent insoluble and are positive for the marker protein flotillin. Flotillin immunolabeling of SDS and Triton-X fractions was the same between NPC and wt mice, with the majority of the flotillin being identified in the SDS fraction in both mouse groups (data not shown). Insoluble (FA extractable) Aβ was not identified in brain samples from any mice, suggesting that, although elevated, Aβ levels did not reach sufficiently high enough levels for amyloid aggregation to occur. Immunohistochemistry using anti-Aβ antibodies confirmed this finding (data not shown).
Figure 2.
Aβ40 and Aβ42 levels are increased in NPC mice. ELISA performed on homogenates extracted in Triton-X 100 showed increased Aβ40 and Aβ42 in NPC mice. Graph show means ± SEM; n = 8 for each group. Two-tailed t test: *p < 0.05; **p < 0.01 versus wt control.
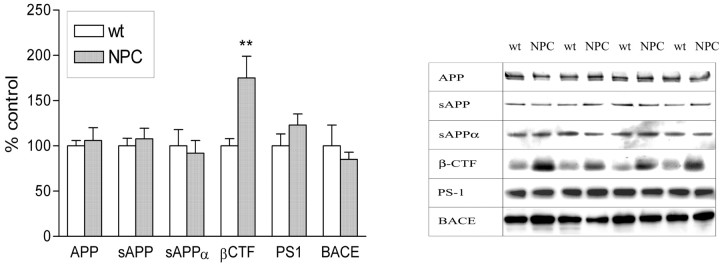
Alteration in APP processing components
There was no change in APP holoprotein levels, suggesting that APP is not upregulated in NPC mouse brain. There was also no alteration in sAPPtotal or sAPPα protein levels, suggesting (by extrapolation) that sAPPβ was also unaltered in NPC mice. To assess whether proteases involved in sAPPα and sAPPβ production were affected by aberrant intracellular cholesterol trafficking in NPC mouse brain, we measured the levels of β-secretase (BACE) and assayed for the activity of α-secretase and β-secretase in brain homogenates. Neither the level of BACE (Fig. 3) nor the activity of either α-secretase or β-secretase (Fig. 4b) were altered in NPC mice compared with wild type. Despite these findings, β-CTF levels were increased by ∼75% in NPC mouse brain (p < 0.01). These data suggest that production of β-CTF was not increased, but degradation and clearance were decreased. PS-1 has been proposed to be the γ-secretase that is responsible for cleavage of the C-terminal of Aβ (Wolfe et al., 1999). PS-1 levels were unchanged in NPC mice (Fig. 3), however, the activity of γ-secretase was increased by over 50% when compared with wt littermate controls (Fig. 4b).
Figure 3.
NPC mice have altered APP processing. APP, sAPP, sAPPα, PS-1, and BACE protein levels were all unchanged in NPC compared with wt mouse brain. However, a large increase in β-CTF levels was noted. Mann–Whitney U nonparametric test: **p < 0.01 versus wt control.
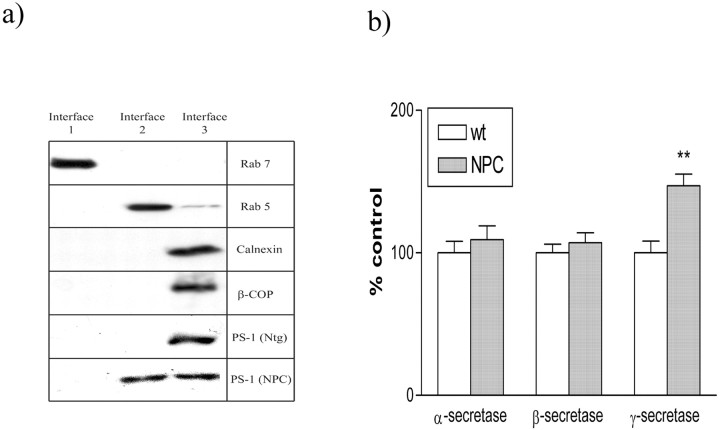
Figure 4.
PS-1 is redistributed to early endosomes in NPC mice. a, Interface 1 contains late endosomes, as detected by Rab 7. Interface 2 contains early endosomes, as detected by Rab 5. Interface 3 contains ER and Golgi components, as detected by calnexin and β-COP, respectively. PS-1 is localized to ER in wt mice, but is present in both early endosomes and ER in NPC mutant mice. b, Bar graph shows α-secretase, β-secretase, and γ-secretase activity in cortical homogenates. Two tailed t test: **p < 0.01 versus wt control.
PS-1 accumulates in early endosomes from NPC mice
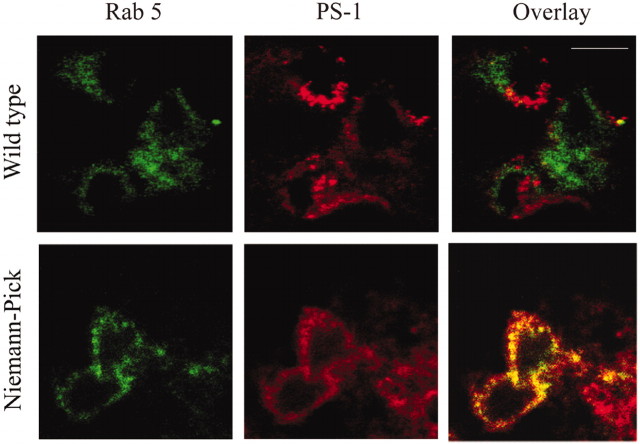
Although the increase in γ-secretase activity may partially explain the increase in Aβ production, it does not account for discrepancies between the large accumulation of β-CTF, yet relatively small increase in Aβ. We, therefore, examined the distribution of PS-1 in NPC mice (Figs. 4a, 5). Crude separations showed an accumulation of PS-1 from NPC mice, but not wt controls, in a compartment that had similar buoyancy to early endocytic (Rab 5 positive) organelles. No accumulation was seen in late endocytic (Rab 7 positive) compartments (Fig. 4a). These endosomal fractions were negative for markers of ER (calnexin) and Golgi bodies (β-COP), suggesting there was no cross-contamination of fractions and that NPC mice had a relatively normal distribution of these organelles. Confocal microscopy confirmed the distribution of PS-1 in NPC brain tissue. Dual labeling with PS-1 and Rab 5 showed no colocalization of these proteins in wt mice. However, PS-1 was present in Rab 5-positive organelles in NPC mice (Fig. 5).
Figure 5.
Confocal microscopy shows PS-1 accumulating in early endosomes in NPC mutant mice. Rab 5 (green) and PS-1 (red) do not converge in wt animals. However, in NPC mice there was a significant, but not complete, colocalization (yellow).
Discussion
Cholesterol homeostatic pathways are complex in the NPC mouse brain. Intracellularly, the defective NPC protein causes an accumulation of cholesterol in late endosomes/lysosomes (Xie et al., 2000), which in most organs is associated with decreased rates of esterification (Xie et al., 1999a,b). One important study has shown that the critical factor influencing Aβ production may not just be total cholesterol levels, but rather the ratio of free cholesterol to cholesterol ester, or the compartmentalization of cholesterol within the cell (Puglielli et al., 2001). Thus, the NPC mouse may have an alteration in the free cholesterol-to-cholesterol ester ratio, which may be critical to Aβ accumulation.
This study shows that aberrant intracellular cholesterol transport in NPC mice is associated with a profound alteration in β-CTF levels, γ-secretase activity, and subcellular distribution of PS-1 and is associated with increased formation of both Aβ40 and Aβ42 peptides. These findings agree with a previous in vitro study that showed an accumulation of Aβ in cultured NPC mutant cells, and also with a small study on NPC mouse brain showing increased Aβ production (Yamazaki et al., 2001). Our data suggest that abnormal cholesterol transport results in a shift in the distribution of PS-1 to early endosomal compartments, which is associated with increased γ-secretase activity and greater production of Aβ.
Previous reports have shown a correlation between cholesterol and α-secretase (Bodovitz and Klein, 1996), β-secretase (Simons et al., 1998), or γ-secretase activity (Wahrle et al., 2002). We have shown that full-length APP holoprotein levels were unaffected in NPC mice. α-Secretase activity was unaltered, and the level of sAPPα was correspondingly unchanged. The level of β-secretase and its activity was also unaltered, and the levels of sAPPβ were unchanged. Thus, the generation of sAPP fragments and the reciprocal production of CTFs was unaltered. The level of β-CTF was, however, significantly increased, suggesting that the clearance and degradation of β-CTF was dramatically reduced in NPC mice. Interestingly, there was a 50% increase in γ-secretase activity in NPC brain that would be expected to have reduced the levels of β-CTF, generating increased Aβ. We did observe significantly elevated Aβ levels in the NPC mice, but it would appear that despite this increase in γ-secretase-mediated clearance, the levels of β-CTF remained above those seen in wt mice. It is possible that the observed increase in γ-secretase activity was not sufficient to clear all of the β-CTF, the secretase and its substrate were not temporally or spatially fully colocalized to some degree, or other CTF clearance mechanisms (such as those mediated by the proteosome) are defective in NPC mice. Runz et al. (2002) studied the effect of a NPC mimicking compound, U18666A, on APP processing and Aβ production in vitro. In their system, cholesterol transport inhibition led to inhibited β-secretase activity and a reduction in Aβ (Runz et al., 2002). However, they showed an increase in γ-secretase activity when SP-C99-transfected cells that express truncated APP lacking the BACE cleavage site were treated with the same compound. Because our NPC mice undergo progressive degeneration that is not replicated in vitro, the sequence of events (such as temporal redistribution of PS-1, or the impact of other degenerating cellular pathways) may not be equivalent in the two systems. It is also possible that β-secretase activity was affected regionally or in some cellular compartments, but the effects were masked in our whole-lysate assay.
Aβ production occurs by sequential cleavage of APP by β-secretase and γ-secretase. One site that has been suggested as a site for Aβ production is the cholesterol-rich lipid raft because APP, β-secretase, γ-secretase, and Aβ all localize to these transmembrane domains (Lee et al., 1998; Tun et al., 2002; Wahrle et al., 2002). Triton X-100 disrupts cellular membranes and it is in this fraction that we found altered Aβ levels. Because lipid rafts are Triton insoluble and the majority of the raft-marker flotillin was found in the SDS fraction as expected, it is unlikely that the site of aberrant production of Aβ in these mice is in lipid rafts.
Cholesterol is known to accumulate in late endosome/lysosome-like compartments. Indeed, both Yamazaki et al. (2001) and Runz et al. (2002) implicate late endosomes as the site for Aβ and PS-1 accumulation in NPC cells. We, therefore, used a sucrose gradient to separate late and early endosomes. In NPC mice, PS-1 was present in the ER as expected, but it also accumulated in organelles with similar buoyancy to early endosomal fractions (Rab 5 positive). No PS-1 was detected in late endosomal (Rab 7 positive) fractions. When brain tissue was examined using confocal microscopy, PS-1 and Rab 5 immunoreactivity overlapped in NPC, but not in wt mice. Although there is some discrepancy between in vitro and in vivo data, our data showing the accumulation of β-CTFs and the accumulation of PS-1 in early endosomes in the NPC mouse brain are in agreement with recent data from human NPC postmortem brains (Jin et al., 2002; Shie et al., 2002).
In conclusion, our study shows that the intracellular accumulation of cholesterol has important consequences for the compartmentalization of PS-1, the activity of γ-secretase, and the subsequent production of Aβ. We found that the accumulation of cholesterol in these mice decreases β-CTF clearance and increases Aβ production. These changes are accompanied with, or caused by a concurrent accumulation of, PS-1 in the endosomal pathway and increased γ-secretase activity. These findings join a growing consensus between both preclinical and clinical studies, indicating a role for cholesterol in amyloidogenesis. This study also adds to the characterization of the NPC mouse model, which may aid in understanding the mechanisms of this disease and may help identify common pathways leading to pathological features common to both human NPC and AD, such as tauopathy.
Footnotes
This work was supported by grants from the National Institutes of Health (to K.D.). We thank Dr. Sam Gandy for antibodies 14 and 369 and Johnson and Johnson Pharmaceuticals for anti-Aβ antibodies.
Correspondence should be addressed to Dr. Karen Duff, Center for Dementia Research, Nathan S. Kline Institute, 140 Old Orangeburg Road, Orangeburg, NY 10962. E-mail: duff@nki.rfmh.org.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235645-05$15.00/0
References
- Bodovitz S, Klein WL ( 1996) Cholesterol modulates α-secretase cleavage of amyloid precursor protein. J Biol Chem 271: 4436–4440. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM ( 1985) Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest 76: 1501–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Tagle DA ( 1997) Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 277: 228–231. [DOI] [PubMed] [Google Scholar]
- De Strooper B ( 2000) Alzheimer's disease. Closing in on γ-secretase. Nature 405: 627–629. [DOI] [PubMed] [Google Scholar]
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA ( 2000) Statins and the risk of dementia. Lancet 356: 1627–1631. [DOI] [PubMed] [Google Scholar]
- Jin L-W, Shie FS, Nochlin D, Vincent I ( 2002) Interneuronal accumulation of Aβ and APP-CTF in Niemann-Pick type C disease. Neurobiol Lipids 5: 1. [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG ( 2001) Age-dependent changes in brain, CSF, and plasma amyloid (β) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci 21: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krul ES, Tang J ( 1992) Secretion of apolipoprotein E by an astrocytoma cell line. J Neurosci Res 32: 227–238. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Liyanage U, Bickel PE, Xia W, Lansbury Jr PT, Kosik KS ( 1998) A detergent-insoluble membrane compartment contains A β in vivo Nat Med 4: 730–734. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ ( 1997) Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science 277: 232–235. [DOI] [PubMed] [Google Scholar]
- Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ ( 1996) Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem 271: 21604–21613. [DOI] [PubMed] [Google Scholar]
- Patterson MC, Vanier M, Suzuki K, Morris JA, Carstea ED, Neufeld EB, Blanchette-Mackie EJ, Pentchev PG ( 2000) Niemann-Pick disease, type C: a lipid trafficking disorder. In: Metabolic and molecular bases of inherited disease (Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler K, Vogelstein B, eds), pp 3611–3634. New York: McGraw Hill.
- Petanceska SS, DeRosa S, Olm V, Diaz N, Sharma A, Thomas-Bryant T, Duff K, Pappolla M, Refolo LM ( 2002) Statin therapy for Alzheimer's disease: will it work? J Mol Neurosci 19: 155–161. [DOI] [PubMed] [Google Scholar]
- Poirier J, Hess M, May PC, Finch CE ( 1991) Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res Mol Brain Res 11: 97–106. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Konopka G, Pack-Chung E, Ingano LA, Berezovska O, Hyman BT, Chang TY, Tanzi RE, Kovacs DM ( 2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat Cell Biol 3: 905–912. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA ( 2000) Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol Dis 7: 321–331. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE ( 2001) A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis 8: 890–899. [DOI] [PubMed] [Google Scholar]
- Runz H, Rietdorf J, Tomic I, de Bernard M, Beyreuther K, Pepperkok R, Hartmann T ( 2002) Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J Neurosci 22: 1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage MJ, Trusko SP, Howland DS, Pinsker LR, Mistretta S, Reaume AG, Greenberg BD, Siman R, Scott RW ( 1998) Turnover of amyloid β-protein in mouse brain and acute reduction of its level by phorbol ester. J Neurosci 18: 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shie F-S, Maezawa I, Vincent I, Jin L-W ( 2002) Accumulation of Aβ in neurons with Niemann-Pick type C defects. Neurobiol Lipids 5: 1. [Google Scholar]
- Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K ( 1998) Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc Natl Acad Sci USA 95: 6460–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Meuller HW, Trapp BD, Griffin JW ( 1989) Oligodendrocytes but not astrocytes express apolipoprotein E after injury of rat optic nerve. Glia 2: 170–176. [DOI] [PubMed] [Google Scholar]
- Tun H, Marlow L, Pinnix I, Kinsey R, Sambamurti K ( 2002) Lipid rafts play an important role in A β biogenesis by regulating the beta-secretase pathway. J Mol Neurosci 19: 31–35. [DOI] [PubMed] [Google Scholar]
- van der Goot FG ( 1997) Separation of early steps in endocytic membrane transport. Electrophoresis 18: 2689–2693. [DOI] [PubMed] [Google Scholar]
- Vassar R ( 2001) The β-secretase, BACE: a prime drug target for Alzheimer's disease. J Mol Neurosci 17: 157–170. [DOI] [PubMed] [Google Scholar]
- Wahrle S, Das P, Nyborg AC, McLendon C, Shoji M, Kawarabayashi T, Younkin LH, Younkin SG, Golde TE ( 2002) Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis 9: 11–23. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ ( 1999) Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 398: 513–517. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G ( 2000) Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol 57: 1439–1443. [DOI] [PubMed] [Google Scholar]
- Xie C, Turley SD, Dietschy JM ( 1999a) Cholesterol accumulation in tissues of the Niemann-pick type C mouse is determined by the rate of lipoprotein-cholesterol uptake through the coated-pit pathway in each organ. Proc Natl Acad Sci USA 96: 11992–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Turley SD, Pentchev PG, Dietschy JM ( 1999b) Cholesterol balance and metabolism in mice with loss of function of Niemann-Pick C protein. Am J Physiol 276: E336–E344. [DOI] [PubMed] [Google Scholar]
- Xie C, Burns DK, Turley SD, Dietschy JM ( 2000) Cholesterol is sequestered in the brains of mice with Niemann-Pick type C disease but turnover is increased. J Neuropathol Exp Neurol 59: 1106–1117. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Chang TY, Haass C, Ihara Y ( 2001) Accumulation and aggregation of amyloid beta-protein in late endosomes of Niemann-pick type C cells. J Biol Chem 276: 4454–4460. [DOI] [PubMed] [Google Scholar]