Abstract
Myelin oligodendrocyte glycoprotein (MOG) is, quantitatively, a relatively minor component of the myelin membrane. Nevertheless, peritoneal administration of MOG evokes potent cellular and humoral immunoreactivity, resulting in an experimental allergic encephalitis with immunopathology similar to multiple sclerosis. Moreover, antibodies against MOG cause myelin destruction in situ. Therefore, it appears that MOG-related demyelination is dependent on anti-MOG antibody, but the mechanism(s) by which it occurs is unclear. Of potential significance are observations that some proteins are selectively partitioned into specialized plasma membrane microdomains rich in glycosphingolipids and cholesterol (“lipid rafts”). In particular, during ligand or antibody cross-linking, various plasma membrane receptors undergo enhanced partitioning into rafts as an obligatory first step toward participation in early signal transduction events. In contrast to mature myelin, in oligodendrocytes (OLs) in culture MOG is not raft associated [Triton X-100 (TX-100) soluble, 4°C]. However, in this study we show that antibody cross-linking (anti-MOG plus secondary antibody) of MOG on the surface of OLs results in the repartitioning of ∼95% of MOG into the TX-100-insoluble fraction. This repartitioning of MOG is rapid (≤1 min), antibody dose dependent, requires an intact cytoskeleton, leads to phosphorylation or dephosphorylation of tyrosine, serine, and threonine residues in specific proteins (e.g., β-tubulin, Gβ1–2), and invokes a rapid retraction of OL processes. After removal of the cross-linking antibodies, these events are reversed. We hypothesize that antibody-mediated repartitioning of MOG into TX-100-insoluble glycosphingolipid–cholesterol-rich microdomains initiates specific cellular signaling that could be related to initial steps of MOG-mediated demyelination.
Keywords: myelin oligodendrocyte glycoprotein, oligodendrocytes, multiple sclerosis, lipid rafts, cytoskeleton, signaling
Introduction
Myelin is a unique, lipid-rich biological membrane produced in the CNS by oligodendrocytes (OLs) (Pfeiffer et al., 1993; Madison et al., 1999). Loss or damage of this functionally active membrane results in neurological deficits such as occur in multiple sclerosis (MS). Myelin oligodendrocyte glycoprotein (MOG) is an integral myelin membrane protein present primarily in the outer lamella of the myelin sheath. Despite its relatively low abundance (0.01–0.05% of the total myelin protein), it has been implicated as a potentially major player in demyelinating diseases (Linington et al., 1984).
Besides inflammatory mediators derived from T cells, there is increasing evidence that anti-MOG effector mechanisms play an important role in the pathogenesis of demyelination (Reindl et al., 1999; Iglesias et al., 2001; Von Büdingen et al., 2001). Anti-MOG antibodies are found in the CSF and in disintegrating myelin around axons in lesions of acute MS patients (Linington and Lassmann, 1987; Xiao et al., 1991; Genain et al., 1995; Reindl et al., 1999). Presentation of purified MOG induces severe demyelinating experimental allergic encephalomyelitis (EAE) in both rodents and primates (Johns and Bernard, 1999; Iglesias et al., 2001). When a monoclonal antibody (mAb) against MOG is injected into rodents, there is extensive demyelination (Schluesener et al., 1987; Linington et al., 1988). In addition, demyelination was produced in aggregating brain cell cultures by anti-MOG, whereas antibodies against other myelin proteins had no effect (Kerlero de Kosbo et al., 1990). Despite the growing conviction that MOG/anti-MOG interactions are mediators of demyelination in rat EAE and MS, a mechanism has not been established. The demyelinating capacity of the MOG/anti-MOG complex may be related to its ability to activate complement (Piddlesden et al., 1993); however, MOG-related demyelination also occurs independently of complement (Dyer and Matthieu, 1994; Menon et al., 1997).
Glycosphingolipids and cholesterol form lateral assemblies (“rafts”) in the plasma membrane of cells, into which certain proteins can partition whereas others are excluded (Simons and Ikonen, 1997; Brown and London, 1998; Friedrichson and Kurzchalia, 1998; Varma and Mayor, 1998). Lipid rafts have an important role as platforms for the initiation of signal transduction by favoring specific protein–protein interactions necessary for signal transduction. Ligand or antibody cross-linking of some proteins results in their enhanced partitioning into rafts and their participation in early signal transduction events (Simons and Toomre, 2000). We have proposed that the high content of glycosphingolipids and cholesterol in myelin sheaths may contribute functionally to OL–myelin physiology (Bansal and Pfeiffer, 1989; Pfeiffer et al., 1993; Kim et al., 1995; Bansal et al., 1999). Consistent with this hypothesis, myelin proteins are differentially partitioned into rafts, including the glycosylphosphatidylinositol-anchored proteins NCAM (neural cell adhesion molecule)-120 and contactin, doubly acylated proteins Fyn and Lyn kinases, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) and MOG (Kim et al., 1995; Kramer et al., 1997, 1999; Kim and Pfeiffer, 1999; Simons et al., 2000; Taylor et al., 2002).
In this study we show that antibody-induced cross-linking of MOG in OLs in a complement-independent manner (1) results in the partitioning of MOG into the detergent-insoluble fraction produced by Triton X-100 (TX-100) extraction at 4°C, (2) concomitantly activates dephosphorylation of β-tubulin and the Gβ subunit of the trimeric G-protein complex, as well as the phosphorylation of other yet unidentified proteins, and (3) triggers OL process retraction. We propose that similar events may contribute to a molecular mechanism leading to the initial steps of MOG-related demyelination.
Materials and Methods
Materials. Antibodies were obtained from the following sources: monoclonal anti-MOG antibody (8–18C5) (Dr. C. Linington, Max-Planck Institute, Germany); anti-proteolipid protein (PLP) antibody (AA3) (Dr. M. Lees, Shriver Center, Waltham, MA); anti-CNP (Bansal and Pfeiffer, 1985); anti-phosphoserine (Calbiochem, San Diego, CA); anti-phosphothreonine (Zymed, San Francisco, CA); anti-phosphotyrosine (4G10), polyclonal anti-caveolin antibody, and goat anti-mouse IgG (Transduction Laboratories, San Diego, CA); anti-Gβ1–4 (Santa Cruz Biotechnology, Santa Cruz, CA); horse radish peroxidase-conjugated goat anti-mouse IgG (Chemicon, Temecula, CA); and anti-β-actin and anti-β-tubulin antibodies (Sigma, St. Louis, MO). Saponin, methyl-β-cyclodextrin (MCD), filipin complex, peroxidase-conjugated cholera toxin B subunit, cytochalasin D, nocodazol, okadaic acid, vanadate, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), and Hoechst 33342 were obtained from Sigma. The general tyrosine kinase inhibitor PD166285 was a gift from Parke Davis (Ann Arbor, MI). All solutions were prepared with MilliQ H2O. Protein concentrations were determined by DC protein assay kit (Bio-Rad, Richmond, CA).
Cell culture. Mixed primary cultures and highly enriched populations of maturing OLs were prepared and maintained as described previously (Pfeiffer et al., 1993; Bansal et al., 1996). OL populations were grown in defined medium [modified N2 (mN2)] (Bottenstein and Sato, 1979; Gard and Pfeiffer, 1989) for 7 d to obtain MOG-expressing OLs.
Immunofluorescence microscopy. Cells were incubated with HEPES-buffered Earle's balanced salt solution (EBSS-HEPES) containing 3% normal goat serum (NGS) (also used for diluting antibodies) to block nonspecific absorption, and live cells were stained (20 min, 4°C) with O4 mAb (1:25), in some cases double-immunolabeled for the surface antigens galactocerebroside (GalC) (O1 mAb, 1:25) (Sommer and Schachner, 1981; Bansal et al., 1989) or MOG (8–18C5 mAb) (1:100). Cells were then incubated with the appropriate secondary antibodies for 20 min: FITC-conjugated goat anti-mouse IgM (1:50; μ-chain specific, for O1 or O4; Chemicon) plus Cy3-conjugated goat anti-mouse IgG (1:500; γ-chain specific, for MOG; Jackson ImmunoResearch, West Grove, PA). Cells were mounted in 50% glycerol, pH 8.6, and 2.5% diazobicyclo-(2,2,2) octane to suppress fading and examined by epifluorescence microscopy. Total cell number was determined by counting cells labeled with a nuclear counterstain (1 μg/ml Hoechst dye 33342) included with the secondary antibodies. Washing between steps was performed with three 5 min changes of 1% NGS and EBSS-HEPES. Data of cell counting from at least 10 fields were used for each preparation. To assess the integrity of microtubules and microfilaments after nocodazol (10 μm; 0, 30, 60, 90, and 120 min) or cytochalasin D (20 μm; 0, 30, 60, 90, and 120 min) treatments, insoluble tubulin and actin, respectively, were stained as described by Pigino et al. (2001). Briefly, cells were fixed with 4% paraformaldehyde (15 min, 4°C), washed with 0.13 m HEPES, pH 6.9, 2 mm MgCl2, 10 mm EGTA, and extracted in the same buffer plus 0.2% TX-100 for 5 min at 37°C before tubulin staining, or with 0.02% saponin for 2 min at 37°C before actin staining.
Estimation of OL morphology after MOG cross-linking. OLs grown in mN2 medium were washed with 1% bovine serum albumin (BSA) in DMEM and incubated at 37°C with monoclonal anti-MOG antibody [8–18C5; 1:100 (156 μg/ml IgG diluted in freshly prepared mN2; antibody concentration was determined by QuantiType Radial Immunodiffusion kit, QED Bioscience, San Diego, CA)] for various time intervals (5–15 min). Antibody was washed out by two changes of DMEM. Goat anti-mouse IgG (1:500, diluted in DMEM) was added for 5–15 min at 37°C. In some experiments, plates were incubated with 0.1 μm PD166285 (3 hr, 37°C) or 10 nm okadaic acid (3 hr, 37°C) before MOG cross-linking. In some other experiments, the antibody-containing media was removed, and the cells were grown further in fresh medium for 2, 4, or 14 hr. Controls were subjected to the same schedule of washes and incubations. Plates were put on an ice tray, and antibody was washed out by two changes of EBSS-HEPES. Live cells were stained (20 min, 4°C) with O4 mAb (1:25) as described before and analyzed by epifluorescence microscopy. The areas occupied by mature OLs in randomly selected fields were compared, using Adobe Photoshop 5.0 (number of pixels per cell). Averages and SEM were calculated (n = 20–35), and evaluation of statistics significance between conditions was made using Student's two-tail t test.
Antibody perturbation and preparation of cell lysate. OLs grown in mN2 medium were washed with 1% BSA in DMEM and incubated at 37°C with monoclonal anti-MOG antibody [8–18C5; 1:25–1:500 (624–31 μg/ml IgG, diluted in fresh mN2)] for various time intervals (1–60 min). Antibody was washed out by two changes of DMEM. Goat anti-mouse IgG (1:25–1:10,000, diluted in DMEM) was added for 1–30 min at 37°C to cross-link MOG/anti-MOG complexes. Controls, including no additions or treatment with only primary or secondary antibody, were subjected to the same schedule of washes and incubations. Some plates were treated with monoclonal antibody O10 (recognizing an extracellular epitope of PLP, diluted 1:10) (Jung et al., 1996) and secondary antibody in the same way as anti-MOG treatment. In some experiments, plates were incubated with 5 mm MCD (15 min, 37°C; see below), 10 μm nocodazol (2 hr, 37°C), 20 μm cytochalasin D (2 hr, 37°C), 10 nm okadaic acid (3 hr, 37°C), or 0.1 μm PD166285 (3 hr, 37°C) before MOG cross-linking. In some cases, the control and antibody-containing media were removed, and the cells were grown further in fresh medium for 15 or 30 min. After cross-linking, plates were put on an ice-tray and washed twice with ice-cold PBS or with mN2 and then incubated at 37°C for 15 or 30 min. Cells were scraped into 0.5 ml of 150 mm NaCl, 5 mm EDTA, 25 mm Tris-Cl buffer, pH 7.5, containing 1 mm PMSF, 10 μg/ml leupeptin–aprotinin, 50 mm NaF, 10 mm NaP2O7, and 1 mm Na o-vanadate (scraping buffer) and passed 10 times (on ice) through a 30 ga needle.
Detergent extraction and sucrose gradient ultracentrifugation. Triton X-100 (1% final concentration) was added to cell lysates and incubated for 30 min at either 4 or 37°C (Kim and Pfeiffer, 1999; Taylor et al., 2002) (see Results). The TX-100 extracts were centrifuged (13,000 × g, 4°C, 10 min) to separate them into detergent-insoluble pellet and detergent-soluble supernatant fractions.
The supernatant fraction was precipitated with 2 vol of ethanol at –20°C overnight and centrifuged (13,000 × g, 4°C, 10 min), and the new supernatant was discarded. Finally, both the original detergent-insoluble pellet and the ethanol-precipitated soluble fraction pellets were solubilized in equal volumes (to allow comparison of the relative amount of each protein in the two fractions) of sample buffer for analysis by SDS-PAGE.
For sucrose gradient ultracentrifugation, TX-100-insoluble pellet fractions were resuspended in 0.5 ml of scraping buffer plus 1% TX-100, mixed with 2 m sucrose (1 ml), overlaid with 1 m (2 ml) and 0.2 m (1.5 ml) sucrose, and centrifuged for 16 hr at 45,000 rpm (SW 55 Ti, ∼200,000 × g; Beckman) at 4°C. After centrifugation, 0.5 ml fractions were collected at 4°C from the top to the bottom of the gradient.
Cholesterol extraction. To disrupt cholesterol in OL membranes before TX-100 extraction, cell lysates were treated with saponin (final 0.2%) on ice for 30 min and centrifuged (13,000 × g, 10 min) (Kim and Pfeiffer, 1999). The supernatant (S1) was collected. The pellet was extracted with 0.5 ml of 1% TX-100 in scraping buffer for 30 min (see above) and centrifuged (13,000 × g, 10 min), and the TX-100-soluble fraction (S2) and pellet were separated. Supernatants S1 and S2 were precipitated with ethanol at –20°C overnight and centrifuged (13,000 × g, 10 min). Pellets and ethanol-precipitated supernatant fractions were resuspended in equal volumes (to allow a comparison of the relative amount of each protein in the two fractions) of sample buffer for analysis by SDS-PAGE. To remove cholesterol from live cells, OLs in culture were treated with 5 mm MCD for 15 min at 37°C (Ledesma et al., 1998), before MOG cross-linking. Cholesterol levels were evaluated by filipin staining (50 μg/ml, 30 min, 4°C).
SDS-PAGE and Western blotting analysis. Equal volumes of the soluble and insoluble fractions (see above) of the various TX-100 extracts were solubilized in sample buffer [50 mm Tris-HCl, pH 6.8, 2.5% glycerol, 5% SDS, 4 m urea, 0.01% bromophenol blue, with or without 10 mm DTT (see Results)], loaded onto acrylamide gels (Protean II mini-cell apparatus, Bio-Rad), and run at constant voltage (120 V, 1–2 hr). The proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Hybond-P, Amersham Biosciences, Piscataway, NJ) at 4°C with a constant voltage (100 V, 1 hr). The blots were blocked with 5% nonfat milk or 5% BSA (Sigma) depending on the antibody (1 hr, room temperature) before immunostaining and detection [enhanced chemiluminescence (ECL Plus), Amersham Biosciences].
Two-dimensional PAGE. Mature OL cells were scraped into 25 mm Tris-Cl buffer, pH 7.5, containing 2% CHAPS, 1 mm PMSF, 10 μg/ml leupeptin–aprotinin, 50 mm NaF, 10 mm NaP2O7, and 1 mm Na o-vanadate. Proteins were precipitated overnight with 2 vol of ethanol at –20°C. Cell extracts (300 μg of protein) were solubilized in rehydration buffer: 7 m urea, 2 m thiourea, 2% CHAPS, 0.5% immobilized pH gradient (IPG) buffer 4–7 (Amersham Biosciences), 100 mm DTT, 0.001% bromophenol blue. All samples were left in rehydration buffer for 1 hr at room temperature with occasional mixing before centrifugation (10,000 × g, 10 min) to clear particulate matter. Sample supernatant was added to IPGphor coffins (Amersham Biosciences), and an Immobiline Dry Strip, pH 4–7, isoelectric focusing gel (Amersham Biosciences) was placed over the solution. The IPG strips were allowed to rehydrate overnight. Proteins were separated in the first dimension (200 V, 1 hr; 500 V, 1 hr; 1000 V, 1 hr; ramped to 8000 V, 30 min; held at 8000 V for 30,000 Vh) at 20°C using an IPGphor electrophoresis unit (Amersham Biosciences). After isoelectric focusing, the gel was equilibrated first for 15 min with 130 mm DTT in an equilibration buffer containing 6 m urea, 50 mm Tris, pH 6.8, 30% glycerol, 2% SDS, and second for 15 min with 135 mm iodoacetamide in the same equilibration buffer. SDS-PAGE was performed in 10% acrylamide running gels at a constant current (15 mA, 14 hr), using a Hoefer DALT vertical system (Amersham Biosciences). The proteins were transferred to PVDF membranes (Hybond-P, Amersham Biosciences) at constant current (400 mA, 14 hr). In some cases, gels were stained with ammoniacal silver nitrate or Colloidal Blue (Invitrogen, Carlsbad, CA).
Results
Antibody-induced cross-linking of MOG on the surface of differentiated OLs in culture induces its partitioning into a detergent insoluble fraction
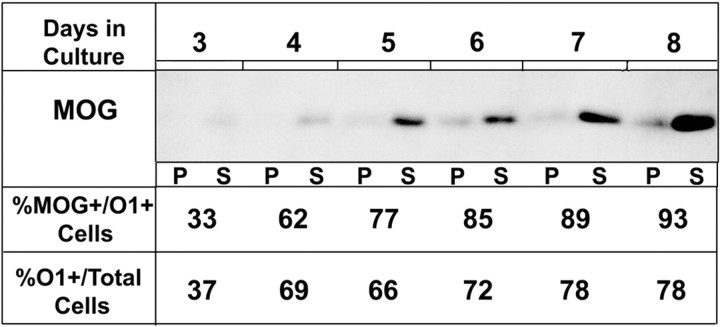
Glycosphingolipid–cholesterol microdomains are often studied biochemically by taking advantage of their insolubility in nonionic detergents. We have shown previously that in myelin membrane ∼40% of MOG is present in TX-100 (4°C)-insoluble, low-density, glycosphingolipid–cholesterol-rich microdomains (lipid rafts) (Kim and Pfeiffer, 1999; Taylor et al., 2002). In contrast, when cultures enriched for OLs were extracted with TX-100 at 4°C, nearly all of MOG was detected in the soluble fraction at all ages studied (Fig. 1). We conclude that the association of MOG in the relatively immature, myelin-like membranes of OLs in culture (Singh and Pfeiffer, 1985) differs in this important characteristic from that in mature myelin in association with axons.
Figure 1.
Developmental association of MOG with detergent-insoluble microdomains in OLs. Western blot analysis of MOG in pellet (P, insoluble) and supernatant (S, soluble) fractions during TX-100 extraction at 4°C from OLs (3–8 d in culture). Cell lysate (20 μg of protein) was extracted, and the entire yield in each fraction was loaded in the gels for each condition. The percentage of O1 + /total cells and MOG +/O1+ cells in each preparation is indicated (estimated by immunofluorescence microscopy as described in Materials and Methods). This figure represents a typical result of three independent experiments.
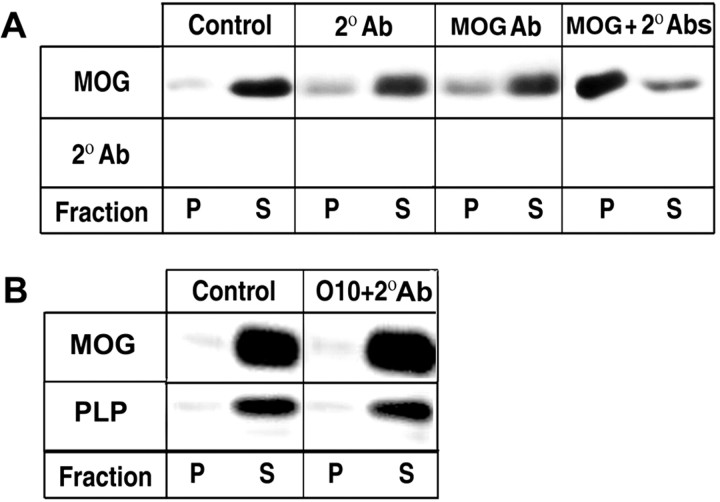
Noting that cross-linking of a number of membrane receptors with antibodies leads to their repartitioning into lipid rafts (Simons and Toomre, 2000), we treated MOG-positive OLs at 37°C with anti-MOG antibody (1:100) for 15 min before detergent extraction; again, nearly all of the MOG was found in the soluble fraction (Fig. 2A). However, when OLs were first treated with anti-MOG (primary antibody) and then were further treated with a cross-linking secondary antibody (1:100; anti-IgG) for an additional 15 min at 37°C, MOG was recovered nearly entirely in the detergent-insoluble fraction (Fig. 2A). Several control studies were performed (Fig. 2): (1) secondary antibody alone had no effect on MOG partitioning; (2) immunoblot analysis confirmed that the signal observed was not attributable to the detection of 25 kDa anti-MOG antibody light chains (which, were they present, could potentially be confused with 28 kDa MOG/anti-MOG signals in reducing gel Western blots); (3) the detergent solubilities of two other major myelin proteins, CNP and PLP (both mostly soluble during extraction of OLs in culture with TX-100 at 4°C), were unaltered by the anti-MOG/anti-IgG cross-linking (data not shown); (4) similar cross-linking of PLP with a monoclonal antibody against an extracellular domain (010, Jung et al., 1996) did not alter the detergent solubility of either PLP or MOG (Fig. 2B).
Figure 2.
MOG repartitioning after antibody-induced cross-linking. A, B, Western blot analyses of MOG (A, B), anti-IgG (to control for residual antibody light-chain reaction in the ∼ 25 kDa region of blots; 2° Ab) (A) and PLP (B) in pellet (P, insoluble) and supernatant (S, soluble) fractions during TX-100 extraction at 4°C of mature OLs. Cells were incubated for 15 min with anti-MOG mAb 8–18C5 (1:100, MOG Ab), anti-mouse IgG (1:100, 2° Ab), subsequently with MOG Ab and 2° Ab (A), subsequently with O10 (1:10) and anti-mouse IgM (1:100) (B) or media alone (control, no Ab). After scraping, cell lysates (20μg of protein) were extracted with TX-100 at 4°C and separated into pellets and supernatants by centrifugation, and the entire yield in each fraction was loaded in the gels for each condition. The figure represents a typical result of three independent experiments.
We conclude that cross-linking MOG on the surface of differentiated OLs in culture results in a major change in its detergent solubility properties. We therefore initiated a series of experiments to assess the time course, dose–response, biochemical characteristics, and potential functional significance of the MOG cross-linking-mediated detergent insolubility of MOG.
MOG partitioning occurs during treatment with low doses and short durations of anti-MOG
The detergent insolubility of MOG in response to antibody cross-linking was studied as a function of dose and duration of treatment of both the primary and secondary antibodies.
At a constant dilution of secondary antibody (1:100), the partitioning of MOG into the detergent-insoluble fraction was essentially complete at dilutions of primary antibody ranging from 1:25 (624 μg/ml IgG) to 1:500 (31 μg/ml IgG) (Fig. 3A). Treatment of OLs with anti-MOG (1:100) followed by 15 min of cross-linking with secondary antibody (1:100, 23 μg/ml) as a function of time (Fig. 3B) resulted in the partitioning into the insoluble fraction of ∼80% of MOG within 1 min and nearly all MOG within 5 min [with longer exposures (e.g., 1 hr), a minor amount of MOG reappeared in the soluble fraction in some experiments].
Figure 3.
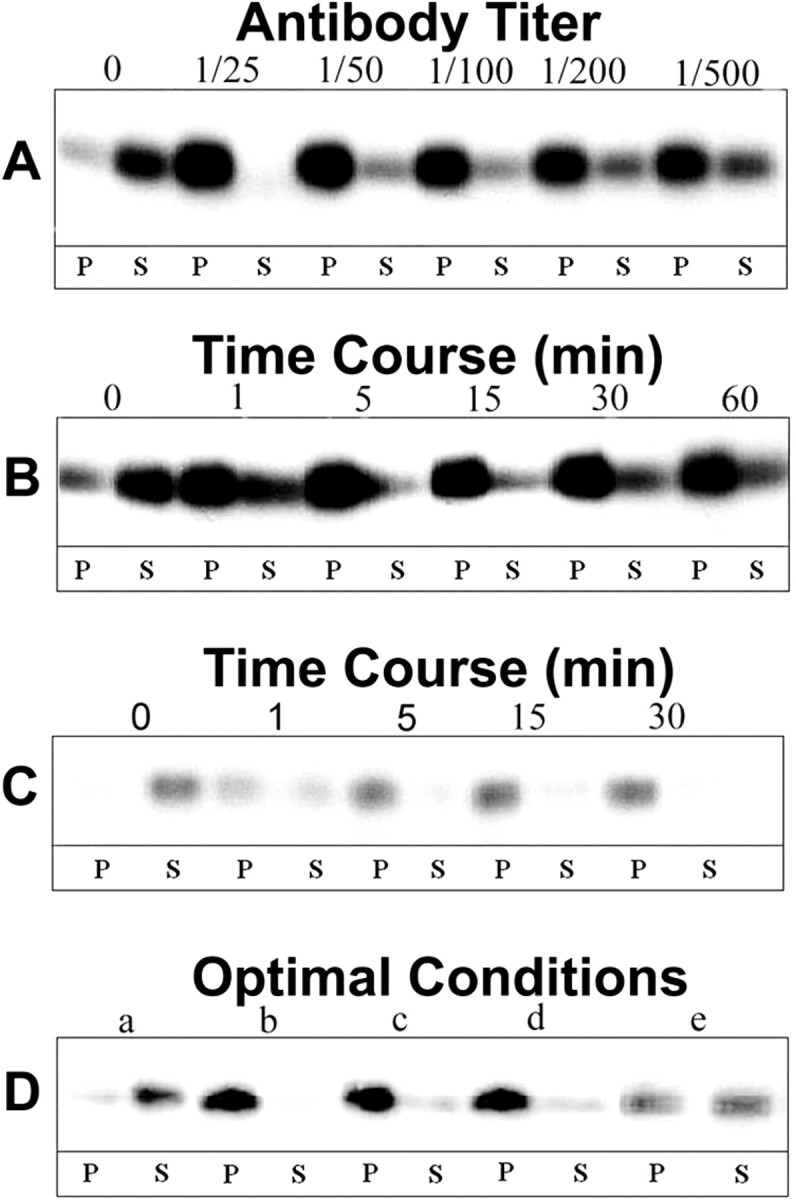
MOG repartitioning as a function of antibody dose and incubation time. Western blot analysis of MOG in the insoluble pellet (P) and soluble (S) fractions during TX-100 extraction at 4°C of mature OLs after various treatments. A, 1° Ab dose–response: cells were incubated for 15 min with varying concentrations of anti-MOG mAb 8–18C5 (1:25–1:500), and then for 15 min with anti-mouse IgG (1:100); B, 1° Ab time course: cells were incubated for 1–60 min with anti-MOG mAb 8–18C5 (1:100), and then for 15 min with anti-mouse IgG (1:100); C, 2° Ab time course: cells were incubated for 15 min with anti-MOG mAb 8–18C5 (1:100), and then for 1–30 min with anti-mouse IgG (1:100); D, OLs incubated under the following conditions for 1° and 2° Ab doses and incubation times: control, no Ab (a); 1° Ab 1:100 15 min, 2° Ab 1:100 15 min (b); 1° Ab 1:100 5 min, 2° Ab 1:100 5 min (c); 1° Ab 1:100 5 min, 2° Ab 1:500 5 min; (d) 1° Ab 1:100 1 min, 2° Ab 1:500 1 min (e). Controls were similarly incubated with media alone. After scraping, cell lysates (20μg of protein) were extracted with TX-100 at 4°C and separated into pellets and supernatants by centrifugation, and the entire yield in each fraction was loaded in the gels for each condition. The figure represents a typical result of three independent experiments.
With primary antibody at a constant dilution (1:100) and duration of treatment (15 min), >90% of MOG partitioned into the detergent-insoluble fraction during cross-linking with secondary antibody dilutions up to 1:500 (data not shown). Under these conditions, significant partitioning was observed within 1 min and was nearly complete within 5 min (Fig. 3C).
On the basis of these results, the following conditions were chosen for subsequent analyses: anti-MOG, 1:100 for 5 min; cross-linking secondary antibody, 1:500 for 5 min. Nevertheless, at these antibody concentrations (anti-MOG 1:100; secondary antibody 1:500) even 1 min incubations with each antibody resulted in ∼50% partitioning of MOG (Fig. 3D, e). We conclude that the cross-linking-induced partitioning of MOG into a detergent-insoluble fraction occurs rapidly and at low concentrations of antibody.
Detergent-insoluble MOG from differentiated OLs in culture has both low- and high-density components
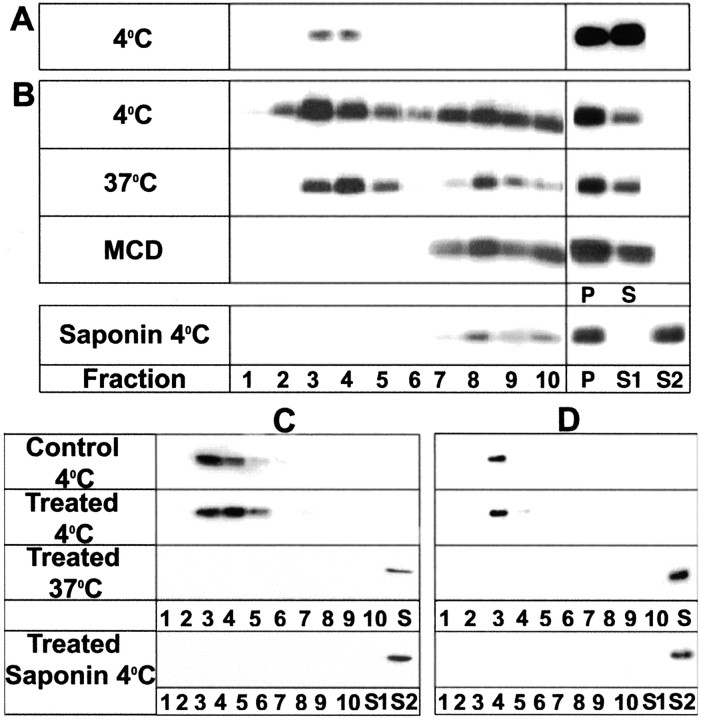
Insolubility of a protein in TX-100 at 4°C by itself is not sufficient to conclude that these proteins are associated with lipid rafts; insolubility can also be derived from protein–protein interactions with, for example, cytoskeletal elements (Pfeiffer et al., 1993; De Angelis and Braun, 1996; Kim and Pfeiffer, 1999; Taylor et al., 2002). We therefore applied three additional, well established biochemical criteria. During TX-100 extraction at 4°C, raft-associated proteins float to a characteristic, low-bouyant density in density (e.g., sucrose) gradients (Brown and Rose, 1992; Simons and Ikonen, 1997); raft-associated proteins insoluble in TX-100 at 4°C generally become solubilized when the extraction is performed either at 37°C (Brown and Rose, 1992) or at 4°C after previous treatment with the cholesterol-binding agent saponin (Rothberg et al., 1990; Cerneus et al., 1993; Hanada et al., 1995; Stulnig et al., 1997; Ledesma et al., 1998). These three criteria are fulfilled by MOG present in the detergent-insoluble fraction obtained during treatment of purified myelin with TX-100 at 4°C (Kim and Pfeiffer, 1999; Taylor et al., 2002) (Fig. 4A).
Figure 4.
Further analyses of the TX-100-insoluble fractions. Western blot analyses of MOG were performed on either purified myelin (extraction at 4°C) (A) or treated OLs (B) [anti-MOG mouse mAb 18–18C5 (1:100, 5 min) followed by anti-mouse IgG (1:500, 5 min)]. OLs were extracted with TX-100 at 4°C, 37°C, and 4°C after pretreatment of the cell lysate with saponin (Saponin 4°C) or MCD 4°C after pretreatment of the cells in culture with 5 mm methyl β-cyclodextrin for 15 min at 37°C. The insoluble fractions (myelin, 50 μg; OLs, 300 μg of protein) were then further fractionated by centrifugation on sucrose gradients as described in Materials and Methods (fraction 1, top of gradient; i.e., lowest density). C, D, Western blot analysis of GM1 detected by reaction with cholera toxin B subunit (C) or caveolin after various treatments (D) as described above in B. OLs were untreated (Control 4°C) or first antibody-treated (above) and extracted with TX-100 at 4°C (Treated 4°C) or 37°C (Treated 37°C) or at 4°C after pretreatment with saponin (Treated Saponin 4°C). P and S are the insoluble and soluble fractions, respectively, after extraction with TX-100 at the indicated temperatures. S1, Soluble fraction after pretreatment of the cell lysate with saponin; S2, soluble fraction after pretreatment with saponin followed by extraction with TX-100 at 4°C. Cell lysate (5 μg of protein in A; 20 μg of protein in B) was extracted, and the entire yield in each fraction was loaded in the gels for each condition (A, B, right panel). Typical results of four independent experiments are shown.
In contrast to myelin, the detergent-insoluble MOG from antibody cross-linked OLs (Fig. 2A) was distributed in both light and heavy fractions during floating on sucrose gradients, both of which were poorly solubilized by extraction with TX-100 at 37°C (Fig. 4B). On the other hand, pretreatment of the cell lysate with saponin, or depletion of cholesterol by previous treatment of the OLs with methyl β-cyclodextrin (see Fig. 6A,B), did result in efficient solubilization of the light fractions (but much less efficiently for the heavy fractions) during extraction with TX-100 at 4°C (Fig. 4B). The small amount of detergent-insoluble material obtained from untreated control cells had similar characteristics as that from cross-linked cells (data not shown). The light fraction was further analyzed for the presence of GM1 ganglioside (a widely used marker for lipid rafts) and caveolin (a marker of caveolae, a subgroup of lipid rafts) (Abrami et al., 2001) (Fig. 4C,D). Both of these markers were enriched in the light fractions during TX-100 extraction at 4°C, were completely solubilized during extraction at 37°C or pretreatment with saponin, and were similarly distributed in control and MOG cross-linked cells. We conclude that during antibody cross-linking, MOG becomes repartitioned into a preexisting TX-100-insoluble (4°C) fraction with key characteristics of lipid rafts (low density, solubilization by saponin pretreatment, GM1, caveolin) and a higher density fraction that is likely to be based on protein–protein interactions.
Figure 6.
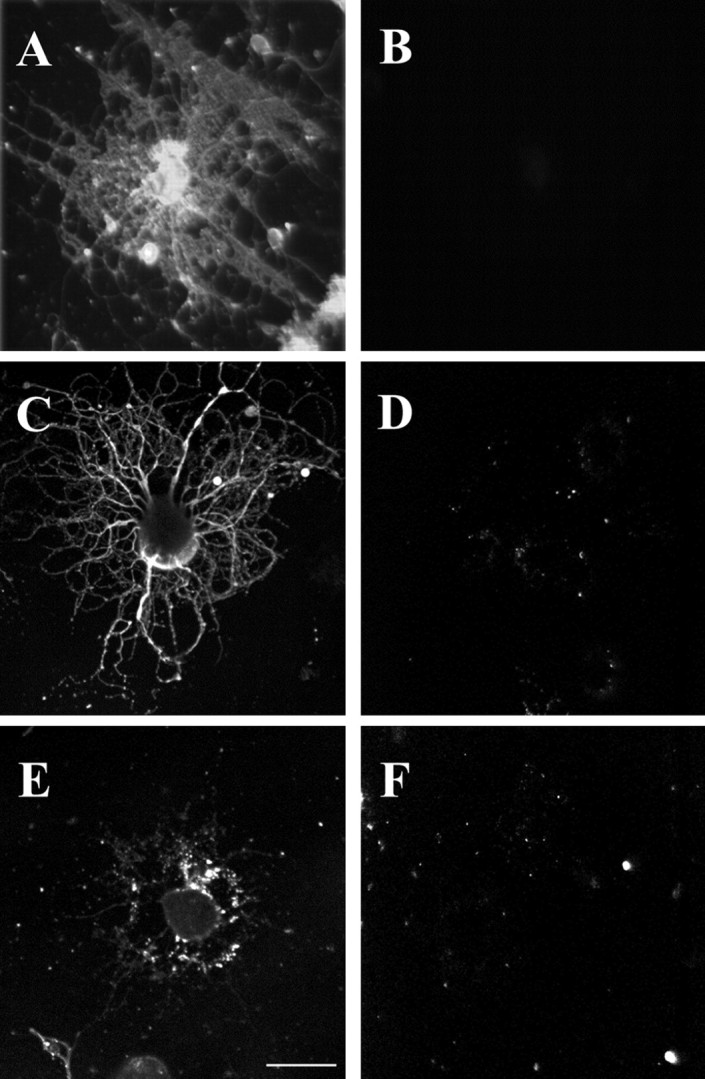
Removal of cholesterol, or depolymerization of microfilaments or microtubules, in OLs in culture. Treatment with MCD, nocodazol, or cytochalasin D resulted in the removal of nearly all of the cholesterol (A, B) or depolymerization of microtubules (C, D) or actin microfilaments (E, F), respectively. A, B, Mature OLs in culture were either not treated (A) or treated with 5 mm MCD for 15 min (B) before staining with filipin to assess the efficiency of removal of cholesterol by the drug.C, D, Mature OLs in culture were either not treated (C) or treated with 10 μm nocodazol for 2 hr (D) to assess the efficiency of depolymerization of microtubules by the drug, as analyzed by immunostaining with anti-tubulin antibodies. E, F, Mature OLs in culture were either not treated (E) or treated with 20 μm cytochalasin D for 2 hr (F) to assess the efficiency of depolymerization of actin microfilaments by the drug, as analyzed by immunostaining with anti-actin antibodies. See Materials and Methods for details. Scale bar, 5 μ.
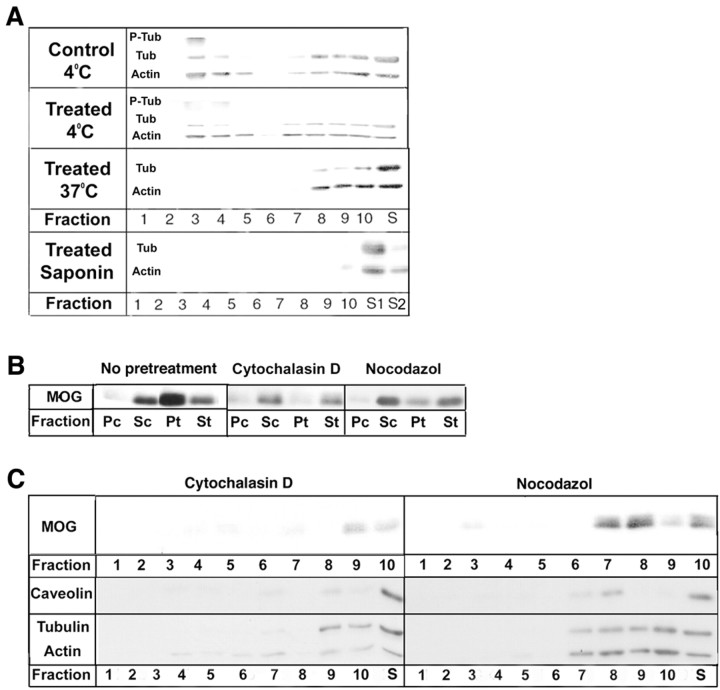
Role of the cytoskeleton in MOG cross-linking
Dyer and Matthieu (1994) reported that MOG becomes associated with microtubules after long exposures of OLs to high doses of anti-MOG antibodies. Because cytoskeletal elements can be insoluble in TX-100 at 4°C (Pereyra et al., 1988), the distributions on sucrose gradients of tubulin and actin in the TX-100-insoluble material (4°C) were analyzed (Fig. 5A). As for MOG, both proteins were present in both the light and heavy fractions. However, in contrast to MOG, both tubulin and actin in the light fractions were solubilized during TX-100 extraction at 37°C, as well as at 4°C after saponin pretreatment (Fig. 5A). When OLs were first treated with either nocodazol or cytochalasin D to depolymerize microtubules or microfilaments, respectively (Fig. 6C–F), the redistribution of MOG into the insoluble fraction (TX-100, 4°C) was nearly entirely eliminated, and the small amount of remaining insoluble material was present in the heavy fractions (Fig. 5B,C). However, further analysis on sucrose gradients showed that under these conditions caveolin, tubulin, and actin were also present in the heavy fractions (Fig. 5C), suggesting that cytoskeletal disruption affected raft integrity in general rather than the redistribution of MOG in particular.
Figure 5.
Role of cytoskeletal proteins in lipid raft formation and MOG repartitioning. Control and antibody-treated [anti-MOG mAb 8–15C5 (1:100, 5 min), anti-mouse IgG (1:500, 5 min)] OLs in culture were detergent extracted. The soluble (S) and insoluble pellet (P) fractions during TX-100 extraction (4°C or 37°C, or pretreatment with saponin followed by TX-100 extraction at 4°C) were analyzed by Western blot. A typical result from three independent experiments is shown. A, Analysis of phospho-β-tubulin (P-Tub), β-tubulin (Tub), and actin (Actin). Insoluble fractions (300 μg of protein) were loaded on sucrose gradients, and fractions were collected from the top (1) to the bottom (10) of the gradient. S, Soluble fraction after TX-100 extraction at 4 or 37°C; S1, soluble fraction after pretreatment with saponin; S2, soluble fraction after pretreatment with saponin followed by extraction with TX-100 at 4°C. Note that essentially all of the tubulin becomes dephosphorylated during cross-linking of MOG; tubulin and actin that are present in the low-density fractions (3–5) when cells are extracted at 4°C become soluble when extractions are performed at 37 or 4°C after pretreatment with saponin. B, Western blot analysis of MOG after a 2 hr pretreatment with either 20 μm cytochalasin D or 10 μm nocodazol. Soluble and insoluble fractions from control and antibody-treated cells after extraction with TX-100 at 4°C are Sc, Pc, St, and Pt, respectively. Cell lysate (20 μg of protein) was extracted, and the entire yield in each fraction was loaded in the gels for each condition. Note that the repartitioning of MOG into lipid rafts during cross-linking is virtually eliminated during dissociation of the actin (cytochalasin D) or tubulin (nocodazol) cytoskeleton. C, The TX-100 (4°C)-insoluble fractions from drug-and antibody-treated cells (B, Pt) were analyzed on sucrose gradients (300 μg of protein). Note that not only MOG but also caveolin, tubulin, and actin are absent from the low-density DIG fractions (3–5) during pretreatment of the cells with cytoskeleton-disrupting agents.
Specific proteins are phosphorylated in response to MOG antibody cross-linking
In a number of systems, ligand- or antibody-mediated partitioning of proteins into lipid rafts results in the initiation of signal transduction cascades (Simons and Toomre, 2000). Because phosphorylation of proteins is an integral part of signal transduction mechanisms, we examined by Western blotting the phosphorylation state of tyrosine, serine, and threonine residues in proteins from untreated and cross-linked OLs.
After cross-linking, two proteins present in the detergent-insoluble material became either tyrosine phosphorylated (∼160 kDa) (Fig. 7A) or dephosphorylated (50 kDa) [(Fig. 7A) nonreducing gel]; these changes in phosphorylation were not found in the detergent-soluble material (data not shown). The ∼160 kDa phosphotyrosine band was present in both the light and heavy fractions during floating on sucrose gradients; in contrast, the ∼50 kDa dephosphorylated protein was exclusively in the light fraction (Fig. 5A) (and data not shown). These changes in phosphorylation were not found when cells were treated with anti-MOG without secondary Ab (data not shown), and they were prevented if cells were pretreated with either a general tyrosine kinase (PD166285) (Fig. 7A) or phosphatase (vanadate) inhibitor (data not shown).
Figure 7.
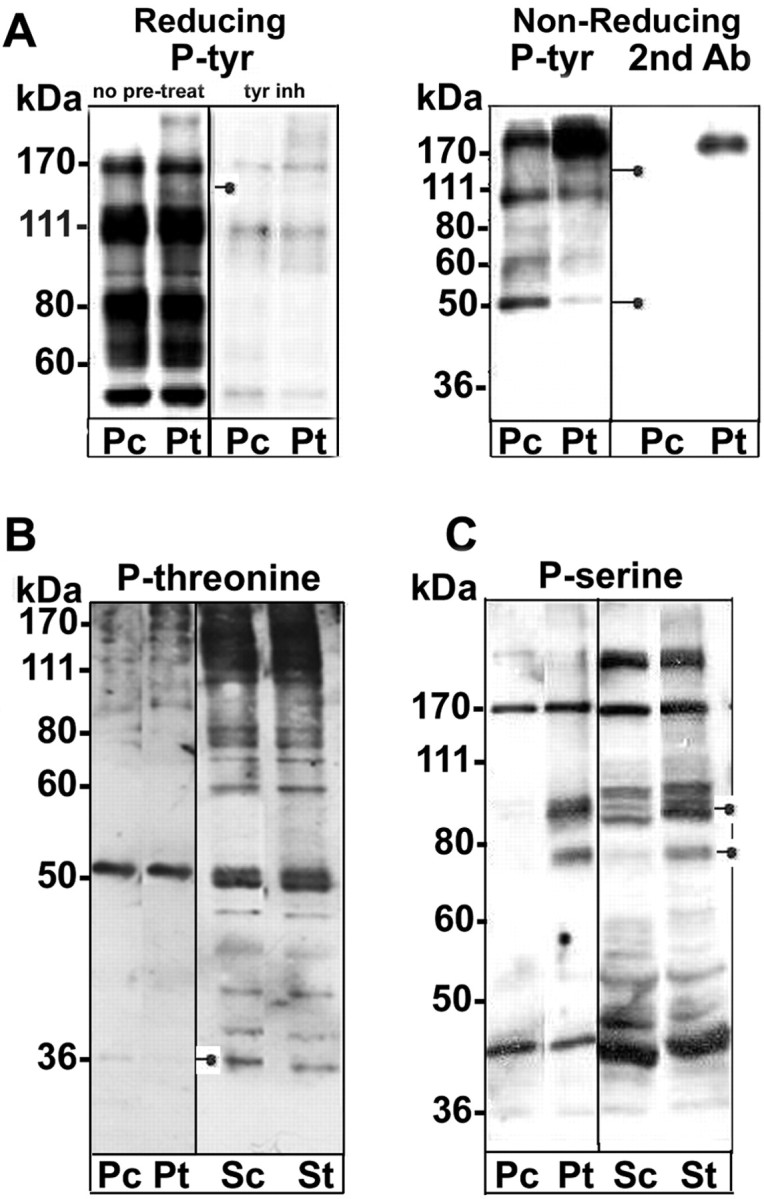
Altered protein phosphorylation mediated by MOG cross-linking. The soluble and insoluble fractions from TX-100 extractions (4°C; 20 μg of total cell protein was extracted and the entire protein yield in each fraction was loaded) of untreated control and antibody-treated [anti-MOG mAb 8–15C5 (1:100, 5 min), anti-mouse IgG (1:500, 5 min)] OLs in culture were analyzed by Western blot for protein phosphorylation. P, Insoluble pellet fraction; S, soluble fraction; c, control; t, treated to achieve cross-linking. The positions on the gels of proteins for which the phosphorylation pattern was altered by cross-linking are indicated by closed circles attached to short lines. Typical results from three independent experiments are shown. A, Western blots for proteins containing phosphotyrosine were analyzed by either reducing or nonreducing gel electrophoresis. The nonreducing gels were performed to demonstrate that the ∼ 50 kDa protein (weakly present in reducing gels, but not shown in A) was not residual antibody heavy chain. In some cases the cells were pretreated for 3 hr with 0.1 μm PD166285, a general tyrosine kinase inhibitor (A). A protein of ∼ 160 kDa was detected that became phosphorylated during MOG cross-linking. A second phosphoprotein of ∼ 50 kDa was identified that became dephosphorylated during cross-linking. B, Western blot for proteins containing phosphothreonine. A protein of ∼ 35 kDa was detected that became partially dephosphorylated during MOG cross-linking. C, Western blot for proteins containing phosphoserine. Two proteins (∼ 70 and 100 kDa) that became phosphorylated during MOG cross-linking were detected. The phosphorylation of the ∼ 70 kDa protein(s) was strongly upregulated in both the soluble and insoluble fractions. The phosphorylation of the ∼ 100 kDa protein(s) was strongly upregulated in the insoluble fraction and significantly so in the soluble fraction. Examples of potentially altered phosphorylation states in other proteins were tentatively identified but are not indicated.
Analyses with anti-phosphothreonine antibody identified a protein that in the insoluble fraction (TX-100, 4°C) became dephosphorylated during cross-linking (∼35 kDa). This protein has characteristics of a raft-associated protein (insoluble in TX-100 at 4°C, recovered at low density in sucrose gradients, soluble in TX-100 at 37°Corat4°C after cholesterol extraction) (Fig. 7B) (and data not shown). Analyses with anti-phosphoserine in Western blots revealed the appearance during cross-linking of at least two phosphoserine proteins (∼70 and ∼100 kDa) (Fig. 7C).
Levels of phospho-fyn, FAK (focal adhesion kinase), and MAPK (mitogen-activated protein kinase) were not changed after MOG cross-linking (data not shown).
We next attempted to identify proteins that change their phosphorylation status during MOG cross-linking by two-dimensional PAGE, analyzed by silver staining and Western blot with anti-phosphotyrosine, anti-phosphoserine, or anti-phosphothreonine antibodies. A similar pattern of proteins (silver-stained gels) was observed in control and cross-linked cells, indicating that the overall protein content and profile were not changed during MOG cross-linking (Fig. 8A). Phosphotyrosine Western blots of MOG cross-linked samples again detected the newly phosphorylated ∼160 kDa and the dephosphorylated ∼50 kDa proteins (Fig. 8B). The ∼50 kDa tyrosine dephosphorylated protein was identified by mass spectrometry and confirmed by Western blot analysis to be β-tubulin (Fig. 8C). The relatively low abundance of the ∼160 kDa protein precluded its identification by mass spectrometry. Phosphoserine Western blots of MOG cross-linked samples resolved the newly phosphorylated (∼70 kDa) protein observed on one-dimensional PAGE gels (Fig. 7C) into three proteins with different isoelectric points (Fig. 8D). Phosphothreonine Western blot analysis (Fig. 8E,F) detected the newly dephosphorylated ∼35 kDa protein, identified by mass spectrometry and confirmed by Western blot analysis to be the β(1–2) subunit of the heterotrimeric G-protein complex.
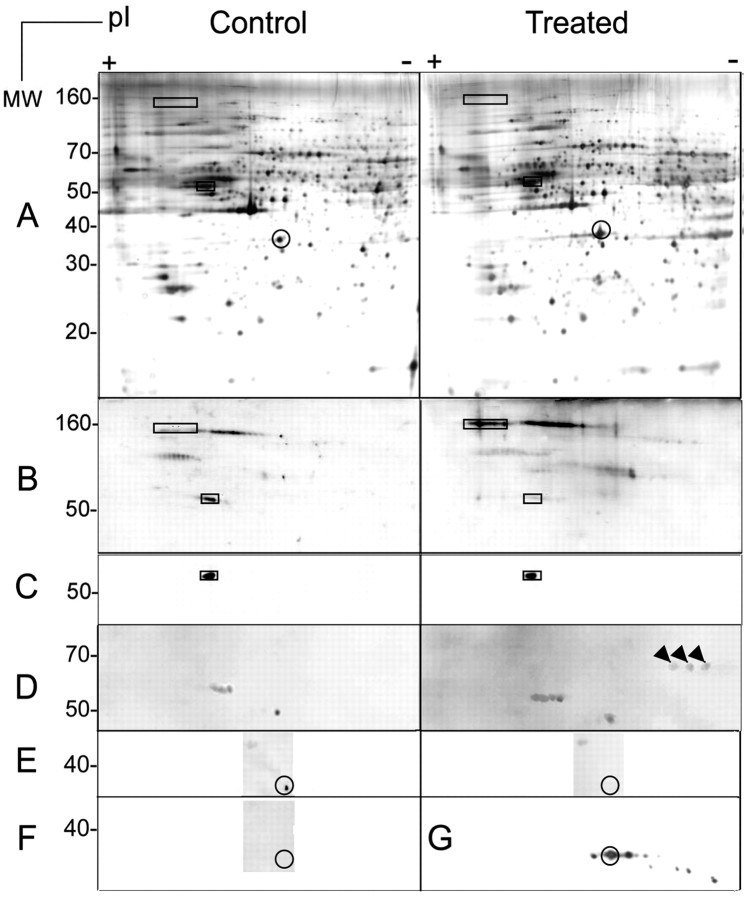
Figure 8.
Two-dimensional PAGE profiles of OL proteins before and after MOG cross-linking. Cell lysates (300 μg of total cell protein) from untreated and antibody-treated [anti-MOG mAb 8–15C5 (1:100, 5 min), anti-mouse IgG (1:500, 5 min)] OLs in culture were analyzed by two-dimensional electrophoresis. After cross-linking, the cells were harvested by scraping into collection buffer (25 mm Tris-HCl, pH 7.5, 2% CHAPS, 1 mm PMSF, 10 μg/ml leupeptin–aprotinin, 50 mm NaF, 10 mm NaP2O7, and 1 mm Na o-vanadate). Proteins were then precipitated with 2 vol of ethanol at –20°C and resolubilized into rehydration buffer (see Materials and Methods). Typical results from three independent experiments are shown. A, Silver staining. No significant differences in the patterns of proteins from control and treated cells were noted. B, Anti-phosphotyrosine. Two proteins of ∼ 160 kDa/pI ∼ 4.5 and ∼ 70 kDa/pI ∼ 6 were identified that became tyrosine phosphorylated, and one phosphotyrosine protein of ∼ 50 kDa/pI ∼ 5 was identified that became dephosphorylated, during MOG cross-linking. C, Anti-β-tubulin. The ∼ 50 kDa/pI ∼ 5 phosphotyrosine protein that became dephosphorylated during MOG cross-linking (A, B) is identified as β-tubulin (confirmed by mass spectroscopy). D, Anti-phosphoserine. A triplet of proteins of ∼ 70 kDa/pI ∼ 6.5 became further serine phosphorylated; their identity is currently unresolved. E, Anti-phosphothreonine. A phosphothreonine protein of ∼ 35 kDa/pI ∼ 6 becomes dephosphorylated during MOG cross-linking. F, Anti-phosphothreonine pretreated with a 50-fold excess of phosphothreonine to control for the specificity of the antibody. G, Anti-Gβ subunit of the trimeric G-protein. The ∼ 35 kDa/pI ∼ 6 phosphothreonine protein that becomes dephosphorylated during MOG cross-linking (E) is identified as the β-subunit of the heterotrimeric G-protein complex (initially identified by mass spectrometry). Squares indicate proteins for which the tyrosine phosphorylation pattern is altered during MOG cross-linking (A–C). Arrowheads indicate the triplet of serine-phosphorylated proteins (D), and circles indicate threonine-dephosphorylated Gβ subunit (A, E, F, G) after MOG cross-linking.
We conclude that cross-linking MOG on the surface of differentiated OLs growing in culture leads to the phosphorylation–dephosphorylation of tyrosine, serine, or threonine residues in specific proteins.
MOG cross-linking in the presence of tyrosine kinase or serine–threonine phosphatase inhibitors
The redistribution of MOG into the pellet (TX-100, 4°C) after MOG cross-linking was not affected by pretreatment of OLs with the general tyrosine kinase inhibitor PD166285, the serine–threonine phosphatase inhibitor okadaic acid (Fig. 9A), or the general phosphatase inhibitor vanadate (data not shown). This suggests that the observed phosphorylation changes elicited by these activities are a consequence, rather than the cause, of MOG redistribution.
Figure 9.
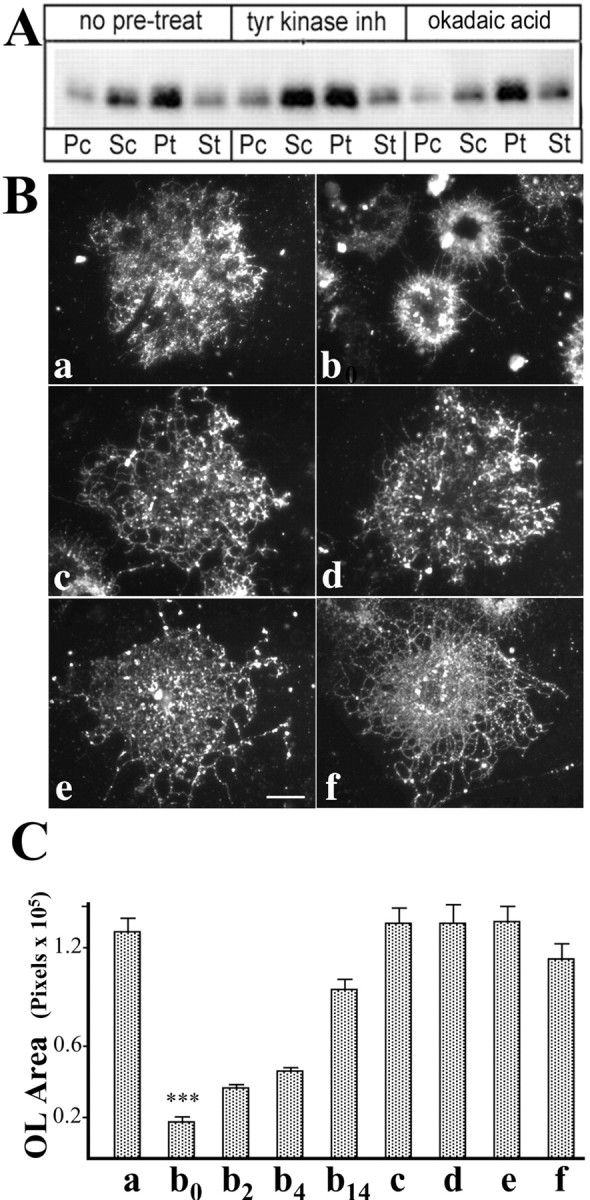
Changes in OL morphology during cross-linking of MOG. OLs in culture were either left untreated as controls (see Materials and Methods) or antibody treated [anti-MOG mAb 8–15C5 (1:100, 5 min), anti-mouse IgG (1:500, 5 min)]. A, Cell lysates were extracted with TX-100 (4°C; 20 μg total cellular protein) and separated into insoluble (P) and soluble (S) fractions by centrifugation, and the entire protein yield of the fractions was analyzed for MOG by Western blotting. c, Control; t, treated to achieve cross-linking. Pretreatment with either a tyrosine kinase (PD166285) or a serine–threonine phosphatase (okadaic acid) inhibitor did not affect the repartitioning of MOG into the insoluble fraction. B, Cells were immunostained with O4 antibody for epifluorescence microscopy. Scale bar, 5 μ. C, The areas occupied by randomly chosen cells were calculated by measuring the number of pixels per cell using Adobe Photoshop 5.0. B, C, Lowercase letters (a, c, e): no anti-MOG cross-linking; (b, d, f): anti-MOG cross-linked; (a, b0): no further treatment; (b2, b4, b14): the antibody-containing medium was removed, and the cells were grown further in fresh medium for 2 (b2), 4 (b4), or 14 hr (b14). c, d, Pretreatment for 3 hr with 0.1 μm PD166285, a tyrosine kinase inhibitor; e, f, pretreatment for 3 hr with 10 nm okadaic acid, a serine–threonine phosphatase inhibitor. ***, a/b0, and b0/b14 indicate statistically significant differences, p ≤ 0.0001; the values of a, c–f, and b0, b2, and b4 are not significantly different (p > 0.5). Error bars represent SEM; n = 20–35. Note that inhibition of phosphorylation or dephosphorylation did not alter the antibody-mediated repartitioning of MOG but did block the changes in morphology observed during MOG cross-linking of uninhibited cells. The effect of MOG cross-linking on OL morphology was reversible.
Morphological alterations in OLs after MOG cross-linking
Tubulin dephosphorylation in neurons is associated with growth cone collapse (Atashi et al., 1992). Therefore, the effect of cross-linking MOG and the accompanying tubulin dephosphorylation on the extension and integrity of OL processes (identified by O4 antibody staining, a marker for OLs) was examined (Fig. 9B,C). After MOG cross-linking (anti-MOG mAb 1:100, 5 or 15 min, and then anti-mouse IgG 1:500, 5 or 15 min), mature OLs underwent dramatic retraction of cell processes and membrane sheets, with a consequent reduction in the area occupied by the OLs (Fig. 9, compare a, b0,) (p < 0.0001). These changes in morphology were not observed in OLs treated with anti-MOG antibody alone (no cross-linking with secondary antibody), and they were prevented entirely if OLs were treated with either a general tyrosine kinase inhibitor (PD166285) (Fig. 9c,d) or a serine–threonine phosphatase inhibitor (okadaic acid) (Fig. 9e,f) before and during MOG cross-linking. Essentially identical results were obtained when the immunocytochemical analyses were performed using antiserum against myelin basic protein (MBP), marker of mature OLs, to estimate the areas occupied by OLs (data not shown). We conclude that MOG cross-linking leads to a reversible retraction of myelin-like membranes and processes as the result of alterations in phosphorylation of signal transduction and cytoskeletal proteins. The potential significance of these changes for demyelinating disease is intriguing.
Reversibility of MOG cross-linking
To assess the reversibility of MOG cross-linking-mediated events, the antibody was removed, and the cells were refed with fresh medium and further incubated at 37°C. Within 15 min both the amount of MOG that was insoluble in TX-100 at 4°C and the level of phosphorylation of β-tubulin returned nearly to levels observed in untreated control cells (Fig. 10A,B) (after 30 min, only a very small amount of MOG was still present in the detergent-soluble fraction; data not shown). These results indicate that MOG repartitioning and β-tubulin dephosphorylation after MOG cross-linking in OLs is rapidly reversible and that β-tubulin dephosphorylation is observed only when a significant amount of MOG is present in the insoluble fraction. Similarly, reversibility occurred with regard to cross-linking-induced changes in morphology. When the antibody-containing medium was removed and the cells were further incubated with fresh culture medium at 37°C, within 2–4 hr the treated OLs began to re-extend processes and increase their occupied area and underwent substantial recovery of process extension (to >75% of control cultures) within 14 hr of incubation (Fig. 9C, b0, b2, b4, b14). Thus, morphological recovery, albeit substantial, is a slower event than the return to control levels of MOG partitioning and phosphorylation states.
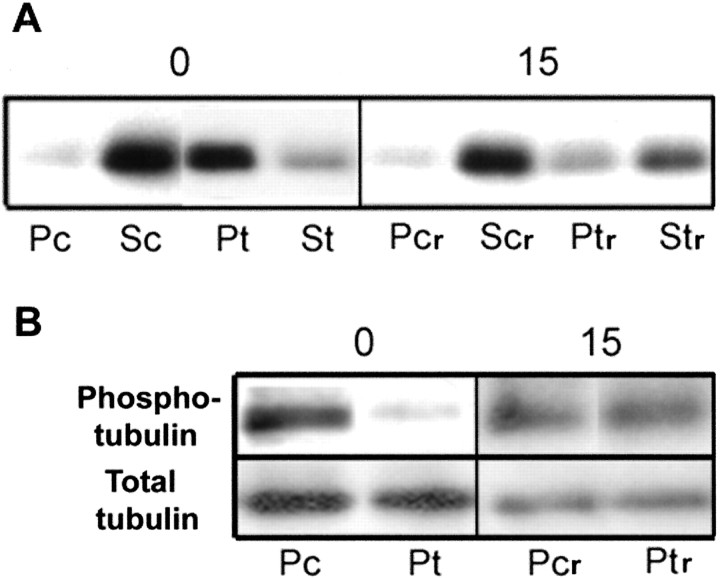
Figure 10.
Reversibility of MOG repartitioning and tubulin dephosphorylation. OLs in culture were first either left untreated as controls (Pc, Sc) or antibody-treated [Pt, St; anti-MOG mAb 8–15C5 (1:100, 5 min), anti-mouse IgG (1:500, 5 min)]. The control and antibody-containing media were then removed, and the cells were grown further in fresh medium (Pcr, Ptr) for 15 min. Cell lysate (20 μg of protein) was extracted with TX-100 (4°C) and separated into soluble (S) and insoluble (P) fractions by centrifugation, and the entire yield in each fraction was loaded in the gels for each condition and analyzed by Western blot. Typical results from three independent experiments are shown. A, Anti-MOG; B, anti-β-tubulin (phosphotubulin and total tubulin). Immediately after cross-linking followed by removal of the antibody (0; Pt, St), as expected (above), nearly all MOG is recovered in the insoluble (P) fraction (A), and nearly all β-tubulin is dephosphorylated (B). However, within 15 min after removal of the antibody (15; Ptr, Str), nearly all MOG is now recovered once again in the soluble fraction (Str), and β-tubulin has become rephosphorylated to a level nearly equal to that of untreated controls.
Discussion
Cross-linking of membrane proteins is a physiological phenomenon that can lead to the repartitioning of these proteins into lipid rafts, resulting in novel protein interactions and initiation of cell signaling (Simons and Toomre, 2000; Ikonen, 2001). Although occurring naturally via multivalent ligands, similar responses have been observed using antibodies (Simons and Toomre, 2000).
In this report we show that although MOG in OLs in culture is mostly detergent soluble (TX-100, 4°C), it becomes repartitioned into a detergent-insoluble fraction during antibody-induced cross-linking (anti-MOG + secondary antibody). Concomitantly, there is an upregulation of the phosphorylation or dephosphorylation of tyrosine, serine, or threonine residues in specific proteins, accompanied by a dramatic retraction of myelin-like membrane sheets and cellular processes. These observations are consistent with a model proposing that during MOG-cross-linking, specific signal transduction pathways are activated, resulting in alterations in the maintenance of OL cell processes and myelin-like membrane. This repartitioning of MOG is rapid (≤1 min) and reversible and occurs at concentrations of antibody substantially lower than previously used to perturb OLs in culture (Dyer and Matthieu, 1994). After the detergent-insoluble fraction is floated on sucrose gradients, MOG is distributed between light- and heavy-density fractions. The low-density fraction has characteristics of lipid rafts; these include, in addition to its low density, sensitivity to pretreatment with cholesterol perturbing agents before detergent extraction (TX-100, 4°C) that renders MOG soluble. The heavy-density fraction is enriched in the cytoskeletal proteins β-tubulin and β-actin, suggesting that protein–protein interactions are involved.
After MOG cross-linking there are specific changes in the phosphorylation of tyrosine, serine, and threonine residues of specific detergent-insoluble proteins that are associated with rafts, including β-tubulin and Gβ1–2. We propose that there is a causal relationship between partitioning of MOG into the detergent-insoluble fractions and the induced phosphorylation–dephosphorylation events. Although the partitioning of MOG is independent of cellular tyrosine kinase or serine–threonine phosphatase activities, the retraction of cell processes requires the changes in phosphorylation state. Therefore, we speculate a sequence of events in which MOG cross-linking induces repartitioning, implementing novel protein–protein interactions within lipid rafts, followed in turn by specific changes in protein phosphorylation state, resulting in cellular morphological alterations.
Moreover, this study, as well as others (Holowka et al., 2000; Fassett et al., 2001; Nebl et al., 2002), indicates that raft integrity depends on intact microtubules and microfilaments. Consistent with this, although the bulk of tubulin and actin were found in the heavy detergent-soluble fraction, significant levels of these proteins were also identified in the low-density lipid raft fraction, suggesting a close relationship between cytoskeletal elements and rafts (Holowka et al., 2000; Maekawa et al., 2001; this study). This is strongly supported by our observation that disruption of the cytoskeleton leads to a concomitant disruption of the OL lipid rafts, as indicated by the redistribution of raft markers (e.g., caveolin) from the detergent-insoluble light fraction to the heavy fraction.
The identification of proteins that change their phosphorylation status after MOG cross-linking is a key step toward identifying mechanisms leading to altered cell physiology. In particular, we identified the dephosphorylation of β-tubulin and G-protein β subunit. β-tubulin dephosphorylation affects microtubule polymerization (Atashi et al., 1992) and is likely to be a factor in the loss of OL membrane reported in this study (acute anti-MOG exposure) and by Dyer and Matthieu (1994) (chronic anti-MOG exposure). The G-protein βγ complex regulates the activity of a diverse set of effectors, including phospholipases, adenyl cyclases, and ion channels (Clapham and Neer, 1997). Phosphorylation of the β subunit activates the dissociated Gβγ complex (Sternweis, 1994; Nürnberg et al., 1996). The dephosphorylation of Gβγ complex could therefore affect a number of different downstream signaling pathways.
MOG is clearly implicated in demyelinating disease. Immunological studies in humans also identified MOG as a prevalent antigenic molecule among the myelin proteins (Von Büdingen et al., 2001). The encephalitogenic properties of MOG are mainly linked to the induction of antibody responses, which directly promote demyelination in CNS disorders such as MS. Although autoimmune responses directed against CNS antigens have generally been considered pathogenic, some reports show that both cellular and humoral immune responses can promote tissue repair after CNS injury and disease. In particular, the exciting observation that some polyreactive IgM autoantibodies reacting with OL surface antigens (not well characterized yet) promote myelin repair (Warrington et al., 2000; Bieber et al., 2001, 2002), as well as the previous finding that the IgM antibody O4 (against sulfogalactolipids) enhances OL differentiation (Bansal et al., 1988), suggests their possible application as therapeutic agents.
In rat and marmoset models, MOG-induced EAE demyelination is anti-MOG antibody dependent and reproduces the immunopathology seen in many cases of MS. The demyelinating activity of MOG-specific antibody has often been related to its ability to activate complement, which could compromise the metabolic integrity of the cell and/or directly kill OLs and thus disrupt myelin (Piddlesden et al., 1993; for review, see Iglesias et al., 2001; Von Büdingen et al., 2001). The finding that purified MOG binds C1q (Johns and Bernard, 1997) strengthens this hypothesis. However, MBP degradation and membrane loss leading to anti-MOG-induced demyelination was also found in models independent of complement action (Dyer and Matthieu, 1994; Menon et al., 1997; this report).
We propose a new, complement-independent model for MOG/anti-MOG-induced demyelination. According to this model, elevated titers of anti-MOG antibody lead to a sequence of events: (1) cross-linking of MOG results in the repartitioning of a large fraction of MOG into DIGs; (2) MOG forms complexes with specific protein partners (e.g., kinases and phosphatases) that are in DIGs; (3) specific signaling pathways become activated; (4) alterations of OL physiology critical for membrane maintenance are elicited. We propose further that similar events may occur in MS. Several questions need to be addressed, including whether antibody cross-linking is mimicking or exacerbating, or both, the action of an endogenous ligand, in which case this signaling could be related not only to anti-MOG-induced demyelination but also to normal MOG function within the OLs.
Footnotes
This work was supported by National Institutes of Health Grants NS10861 and NS41078 and in part by a Post-doctoral Fellowship from the National Multiple Sclerosis Society. We thank Dr. Christopher Linington for providing anti-MOG antibody and for fruitful discussions. We are pleased to acknowledge the excellent administrative support of Wendy Wolcott, Janice Seagren, and Jennifer Gilman.
Correspondence should be addressed to Cecilia Marta, Department of Neuroscience, University of Connecticut Medical School, 263 Farmington Avenue, Farmington, CT 06030-3401. E-mail: Marta@up.uchc.edu.
Copyright © 2003 Society for Neuroscience 0270-6474/03/235461-11$15.00/0
References
- Abrami l, Fivaz M, Kobayashi T, Kinoshita T, Parton R, Van der Goot FG ( 2001) Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J Biol Chem 276: 30729–30736. [DOI] [PubMed] [Google Scholar]
- Atashi JR, Klinz SG, Ingraham CA, Matten WT, Schachner M, Maness S ( 1992) Neural cell adhesion molecules modulate tyrosine phosphorylation of tubulin in nerve growth cone membranes. Neuron 8: 831–842. [DOI] [PubMed] [Google Scholar]
- Bansal R, Pfeiffer SE ( 1985) Developmental expression of 2′,3′-cyclic nucleotide 3′-phosphohydrolase in dissociated fetal rat brain cultures and rat brain. J Neurosci Res 14: 21–34. [DOI] [PubMed] [Google Scholar]
- Bansal R, Pfeiffer SE ( 1989) Reversible inhibition of oligodendrocyte progenitor differentiation by a monoclonal antibody against surface galactolipids. Proc Natl Acad Sci USA 86: 6181–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Gard AL, Pfeiffer SE ( 1988) Stimulation of oligodendrocyte differentiation in culture by growth in the presence of a monoclonal antibody to sulfated glycolipid. J Neurosci Res 21: 260–267. [DOI] [PubMed] [Google Scholar]
- Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE ( 1989) Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res 24: 548–557. [DOI] [PubMed] [Google Scholar]
- Bansal R, Kumar M, Murray K, Morrison RS, Pfeiffer SE ( 1996) Regulation of FGF receptors in the oligodendrocyte lineage. Mol Cell Neurosci 7: 263–275. [DOI] [PubMed] [Google Scholar]
- Bansal R, Winkler S, Bheddah S ( 1999) Negative regulation of oligodendrocyte differentiation by galactolipids. J Neurosci 19: 7913–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieber AJ, Warrington A, Pease LR, Rodriguez M ( 2001) Humoral autoimmunity as a mediator of CNS repair. Trends Neurosci [Suppl] 24: S39–44. [DOI] [PubMed] [Google Scholar]
- Bieber AJ, Warrington A, Asakura K, Ciric B, Kaveri SV, Pease L, Rodriguez M ( 2002) Human antibodies accelerate the rate of remyelination following lysolecithin-induced demyelination in mice. Glia 37: 241–249. [DOI] [PubMed] [Google Scholar]
- Bottenstein J, Sato G ( 1979) Growth of a rat neuroblastoma line in serum-free supplemented medium. Proc Natl Acad Sci USA 76: 514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E ( 1998) Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164: 103–114. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK ( 1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68: 533–544. [DOI] [PubMed] [Google Scholar]
- Cerneus D, Ueffing E, Posthuma G, Strous G, Van der Ende A ( 1993) Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis. J Biol Chem 268: 3150–3155. [PubMed] [Google Scholar]
- Clapham DE, Neer EJ ( 1997) G protein beta gamma subunits. Annu Rev Pharmacol Toxicol 37: 167–203. [DOI] [PubMed] [Google Scholar]
- De Angelis DA, Braun PE ( 1996) 2′,3′-cyclic nucleotide 3′-phosphodiesterase binds to actin-based cytoskeletal elements in an isoprenylation-independent manner. J Neurochem 67: 943–951. [DOI] [PubMed] [Google Scholar]
- Dyer CA, Matthieu JM ( 1994) Antibodies to myelin/oligodendrocyte-specific protein and myelin/oligodendrocyte glycoprotein signal distinct changes in the organization of cultured oligodendroglial membrane sheets. J Neurochem 62: 777–787. [DOI] [PubMed] [Google Scholar]
- Fassett MS, Davis DM, Valter MM, Cohen GB, Strominger JL ( 2001) Signaling at the inhibitory natural killer cell immune synapse regulates lipid raft polarization but not class I MHC clustering. Proc Natl Acad Sci USA 98: 14547–14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichson T, Kurzchalia TV ( 1998) Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature 394: 802–805. [DOI] [PubMed] [Google Scholar]
- Gard AL, Pfeiffer SE ( 1989) Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development 106: 119–132. [DOI] [PubMed] [Google Scholar]
- Genain CP, Cannella B, Hauser SL, Raine CS ( 1995) Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 5: 170–175. [DOI] [PubMed] [Google Scholar]
- Hanada K, Nishijima M, Akamatsu Y, Pagano R ( 1995) Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein in mammalian membranes. J Biol Chem 270: 6254–6260. [DOI] [PubMed] [Google Scholar]
- Holowka D, Sheets ED, Baird B ( 2000) Interactions between Fc(epsilon)RI and lipid raft components are regulated by the actin cytoskeleton. J Cell Sci 113: 1009–1019. [DOI] [PubMed] [Google Scholar]
- Iglesias A, Bauer J, Litzenburger T, Schubart A, Linington C ( 2001) T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia 36: 220–234. [DOI] [PubMed] [Google Scholar]
- Ikonen E ( 2001) Roles of lipid rafts in membrane transport. Curr Opin Cell Biol 13: 470–477. [DOI] [PubMed] [Google Scholar]
- Johns TG, Bernard CA ( 1997) Binding of component C1q to myelin oligodendrocyte glycoprotein: a novel mechanism for regulating CNS inflammation. Mol Immunol 34: 33–38. [DOI] [PubMed] [Google Scholar]
- Johns TG, Bernard CA ( 1999) The structure and function of myelin oligodendrocyte glycoprotein. J Neurochem 72: 1–9. [DOI] [PubMed] [Google Scholar]
- Jung M, Sommer I, Schachner M, Nave KA ( 1996) Monoclonal antibody O10 defines a conformationally sensitive cell-surface epitope of proteolipid protein (PLP): evidence that PLP misfolding underlies dysmyelination in mutant mice. J Neurosci 16: 7920–7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerlero de Kosbo N, Honegger P, Lassmann H, Matthieu JM ( 1990) Demyelination induced in aggregating brain cell cultures by a monoclonal antibody against myelin/oligodendrocyte glycoprotein. J Neurochem 55: 583–587. [DOI] [PubMed] [Google Scholar]
- Kim T, Pfeiffer SE ( 1999) Myelin glycosphingolipid/cholesterol-enriched microdomains selectively sequester the non-compact myelin proteins CNP and MOG. J Neurocytol 28: 281–293. [DOI] [PubMed] [Google Scholar]
- Kim T, Fieldler K, Madison DL, Krueger WH, Pfeiffer SE ( 1995) Cloning and characterization of MVP-17: a developmentally regulated myelin protein in oligodendrocytes. J Neurosci Res 42: 413–422. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Koch T, Niehaus A, Trotter J ( 1997) Oligodendrocytes direct glycosyl phosphatidylinositol-anchored proteins to the myelin sheath in glycosphingolipid-rich complexes. J Biol Chem 272: 8937–8945. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Klein C, Koch T, Boytinck M, Trotter J ( 1999) Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem 274: 29042–29049. [DOI] [PubMed] [Google Scholar]
- Ledesma MD, Simons K, Dotti CG ( 1998) Neuronal polarity: essential role of protein-lipid complexes in axonal sorting. Proc Natl Acad Sci USA 95: 3966–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linington C, Lassmann H ( 1987) Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin/oligodendrocyte glycoprotein (MOG). J Neuroimmunol 17: 61–69. [DOI] [PubMed] [Google Scholar]
- Linington C, Webb M, Woodhams PL ( 1984) A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J Neuroimmunol 6: 387–396. [DOI] [PubMed] [Google Scholar]
- Linington C, Bradl M, Lassmann H, Brunner C, Vass K ( 1988) Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol 130: 443–454. [PMC free article] [PubMed] [Google Scholar]
- Madison DL, Krueger WH, Cheng D, Trapp BD, Pfeiffer SE ( 1999) SNARE complex proteins, including the cognate pair VAMP-2 and syntaxin-4, are expressed in cultured oligodendrocytes. J Neurochem 72: 988–998. [DOI] [PubMed] [Google Scholar]
- Maekawa S, Morii H, Kumanogoh H, Sano M, Naruse Y, Sokawa Y, Mori N ( 2001) Localization of neuronal growth-associated, microtubule-destabilizing factor SCG10 in brain-derived raft membrane microdomains. J Neurochem (Tokyo) 129: 691–697. [DOI] [PubMed] [Google Scholar]
- Menon K, Piddlesden SJ, Bernard CCA ( 1997) Demyelinating antibodies to myelin oligodendrocyte glycoprotein and galactocerebroside induce degradation of myelin basic protein in isolated human myelin. J Neurochem 69: 214–222. [DOI] [PubMed] [Google Scholar]
- Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ ( 2002) Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem 277: 43399–43409. [DOI] [PubMed] [Google Scholar]
- Nürnberg B, Harhammer R, Exner T, Schulze RA, Wieland T ( 1996) Species- and tissue-dependent diversity of G-protein β subunit phosphorylation: evidence for a cofactor. Biochem J 318: 717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra PM, Horvath E, Braun PE ( 1988) Triton X-100 extractions of central nervous system myelin indicate a possible role for the minor myelin proteins in the stability in lamellae. Neurochem Res 13: 583–595. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R ( 1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol 3: 191–197. [DOI] [PubMed] [Google Scholar]
- Piddlesden SJ, Lassmann H, Zimprich F, Morgan BP, Linington C ( 1993) The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol 143: 555–564. [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Pelsman A, Mori H, Busciglio J ( 2001) Presenilin-1 mutations reduce cytoskeletal association, deregulate neurite growth, and potentiate neuronal dystrophy and Tau phosphorylation. J Neurosci 21: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl M, Linington C, Brehm U, Egg R, Dilitz E, Deisenhammer F, Poewe V, Berger T ( 1999) Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain 122: 2047–2056. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Ying YS, Kamen BA, Anderson RGW ( 1990) Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol 111: 2931–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluesener HJ, Sobel RA, Linington C, Weiner HL ( 1987) A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol 139: 4016–4021. [PubMed] [Google Scholar]
- Simons K, Ikonen E ( 1997) Functional rafts in cell membranes. Nature 387: 569–572. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D ( 2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39. [DOI] [PubMed] [Google Scholar]
- Simons M, Kramer EM, Thiele C, Stoffel W, Trotter J ( 2000) Assembly of myelin by association of proteolipid protein with cholesterol- and galactosylceramide-rich membrane domains. J Cell Biol 151: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H, Pfeiffer SE ( 1985) Myelin-associated galactolipids in primary cultures from dissociated fetal rat brain: synthesis, accumulation, and cell surface expression. J Neurochem 45: 1371–1381. [DOI] [PubMed] [Google Scholar]
- Sommer I, Schachner M ( 1981) Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol 83: 311–327. [DOI] [PubMed] [Google Scholar]
- Sternweis PC ( 1994) The active role of beta gamma in signal transduction. Curr Opin Cell Biol 6: 198–203. [DOI] [PubMed] [Google Scholar]
- Stulnig TM, Berger M, Sigmund T, Stockinger H, Horejsi V, Waldhausl W ( 1997) Signal transduction via glycosyl phosphatidylinositol-anchored proteins in T cells is inhibited by lowering cellular cholesterol. J Biol Chem 272: 19242–19247. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Coetzee T, Pfeiffer SE ( 2002) Detergent-insoluble glycosphingolipid/cholesterol microdomains of the myelin membrane. J Neurochem 81: 993–1004. [DOI] [PubMed] [Google Scholar]
- Varma R, Mayor S ( 1998) GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394: 798–801. [DOI] [PubMed] [Google Scholar]
- Von Büdingen HC, Tanuma N, Villoslada P, Ouallet JC, Hauser S, Genain C ( 2001) Immune responses against the myelin/oligodendrocyte glycoprotein in experimental autoimmune demyelination. J Clin Immunol 21: 155–170. [DOI] [PubMed] [Google Scholar]
- Warrington A, Asakura K, Bieber AJ, Ciric B, Van Keulen V, Kaveri S, Kyle R, Pease L, Rodriguez M ( 2000) Proc Natl Acad Sci USA 97: 6820–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao BG, Linington C, Link H ( 1991) Antibodies to myelin-oligodendrocyte glycoprotein in cerebrospinal fluid from patients with multiple sclerosis and controls. J Neuroimmunol 31: 91–96. [DOI] [PubMed] [Google Scholar]