Abstract
Patient: Female, 22
Final Diagnosis: Nephrogenic cystinosis
Symptoms: Bilateral eye pain
Medication: —
Clinical Procedure: —
Specialty: Ophthalmology
Objective:
Rare co-existance of disease or pathology
Background:
Infantile nephropathic cystinosis is the most common and severe variant of cystinosis, which is a rare autosomal recessive condition related to a defect in the transportation of the protein cystine resulting in its deposition in various organs. Due to the rarity of this condition, only 1 case with extensive ocular involvement has been found in the English-language literature. Here, we report a second such case to highlight the significance of early diagnosis in avoiding devastating but preventable vision loss.
Case Report:
We describe the extensive asymmetrical ocular involvement in a 22-year-old woman who had nephropathic cystinosis since childhood. Despite frequent follow up and systemic and topical cysteamine therapy, she developed ocular complications, including increased intraocular pressure, uveitis, and retinal changes with complete loss of vision in her left eye. In addition, her general condition requires a renal transplant in the near future.
Conclusions:
Ophthalmologists should be aware of cystinosis and the sequalae of ocular involvement in this disease, despite its rarity. Identification of the earliest corneal deposits should not be overlooked, especially in the context of other systemic manifestations that are indicative of the nephropathic variant of cystinosis.
MeSH Keywords: Corneal Diseases, Cysteamine, Cystinosis, Intraocular Pressure, Saudi Arabia, Uveitis
Background
Cystinosis is a rare autosomal recessive disorder which occurs in all ethnic groups, with a prevalence of 1 in every 100 000 to 200 000 live births. It is caused by mutations in the CTNS gene encoding a protein responsible for the transportation of cystine. Affected individuals show 1 of 3 variants of the disease: (1) nephrogenic cystinosis (also known as infantile or classical cystinosis), (2) intermediate cystinosis (also known as late-onset nephropathic cystinosis), or (3) non-nephropathic cystinosis (also known as adult-onset or ocular cystinosis) [1]. The nephrogenic variant is the most common and severe form of cystinosis, affecting approximately 95% of these patients [1,2]. In this article, we report the case of a 22-year-old woman with nephropathic cystinosis complicated by a wide variety of ocular manifestations, asymmetrically affecting both eyes, with severe manifestations in her left eye.
Case Report
A 22-year-old Saudi woman presented to the Department of Ophthalmology with a diagnosis of nephrogenic cystinosis, complicated by Fanconi syndrome, end-stage renal disease (for 13 years), and hypothyroidism. She has been born via full-term spontaneous vaginal delivery with no complications to second-degree consanguineous parents. Her birth weight was 2 kg. As a neonate, she had a failure to thrive due to oral feeding intolerance, for which a gastrostomy tube was inserted. She had global speech and motor developmental delay. She has been on levothyroxine daily, systemic cysteamine tablets daily, and dialysis 3 times per week.
Her family history was positive of chronic renal failure of unknown etiology affecting her paternal uncle with onset in his 30s. She had 2 siblings who suffered from ocular cystinosis variant, which started at around 10–15 years of age and did not require any treatment. The other 3 siblings were healthy. Her surgical history included her disease-related gastrostomy tube insertion and AV fistula for hemodialysis.
This patient was referred, as a case of nephrogenic cystinosis confirmed by renal biopsy and white blood cell cystine levels, to the Ophthalmology Department at King Faisal Specialist Hospital and Research Center (KFSH&RC) in Saudi Arabia. On her first ocular examination in 2006, her visual acuity (VA) was 20/70 in her right eye and 20/100 in her left eye. Intraocular pressure (IOP) was in the high 20s in both eyes. Slit-lamp examination of both eyes showed cystine crystal deposition in both corneas (Figure 1A, 1B). On fundoscopy, hyperpigmentation of the retinal pigmented layer (RPE) of the macular area and the peripheral retina was noted. She was started on cysteamine eyedrops 0.5% every 2 h in both eyes and was scheduled for follow-up at the clinic. Her corneal deposits and fundoscopy changes progressed slowly over the following years (Figure 2A–2D).
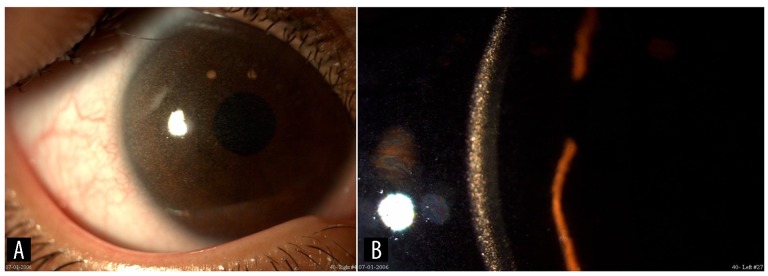
Figure 1.
(A) The clinical appearance of the patient’s right cornea at the initial presentation, showing slight corneal haze. (B) The slit-lamp appearance of the left cornea clearly demonstrating the deposition of cystine crystals within the cornea.
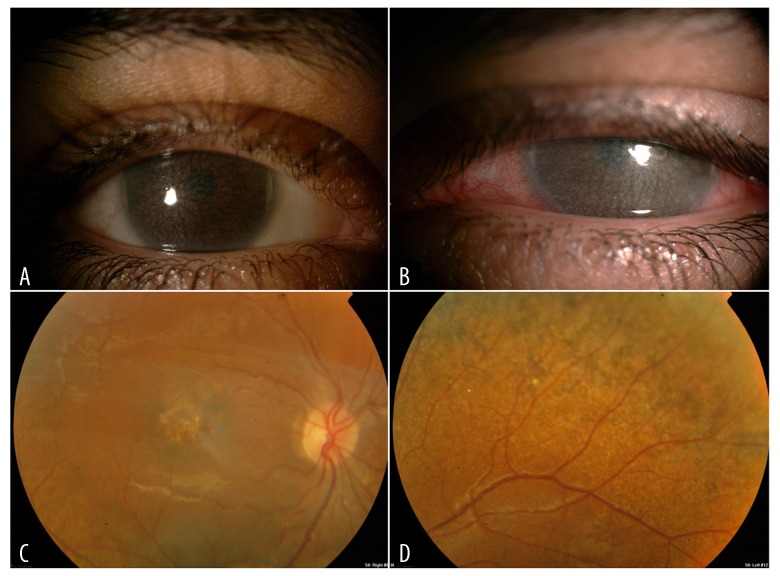
Figure 2.
(A) Follow up clinical photo in the same case with increasing corneal deposits and haze in the right eye. (B) Follow up clinical photos in the same case with increasing corneal deposits and haze in the left eye. (C) Fundoscopy image showing the right eye macular changes. (D) Fundoscopy image showing the left eye peripheral pigmentary changes.
In 2017, she presented to the Emergency Department with severe left eye pain that was associated with redness, tearing, blurred vision, nausea, and headache. On ocular examination, her VA was 20/100 in her right eye, while it dropped to counting fingers (CF) at 50 cm in her left eye. IOP was normal in her right eye and measured 32 mmHg in the left. Slit-lamp examination of the right eye showed diffuse cysteine crystals with a hazy view of the fundus. The left eye showed ciliary injection, diffuse cystine crystals involving the full thickness cornea, and total obscuration of the anterior chamber, lens, and fundus. Corneal pachymeter showed thick corneas with irregular curvature maps. The corneal thicknesses were 800–900 microns and 900–1000 microns in the right and left eye, respectively.
The patient was diagnosed with acute angle-closure glaucoma and was started on topical anti-glaucoma medications: brimonidine drops 0.15% in both eyes 3 times daily, latanoprost drops 0.005% in both eyes once daily, and analgesics in the form of acetaminophen as needed. Three days later, her pain and systemic symptoms had improved, with mild residual left eye injection and IOP of 23 mmHg.
Two weeks later, she presented again to the Emergency Department with recurrent episodes of bilateral eye pain and redness and was found to be non-compliant to her medications. She was advised to continue on the same previously prescribed treatment and was discharged home.
On follow up 3 months later, she presented with significant worsening of the pain and redness. On ocular examination, her visual acuity remained the same in the right eye and had worsened further to hand motion in the left. IOP was controlled in both eyes. Slit-lamp examination showed left eye ciliary injection, marked posterior synechiae, and anterior chamber reaction with +1 flare and +2 inflammatory cells in both eyes. Fundus examination was not performed; therefore, she underwent ultrasound imaging, which showed vitreous hemorrhage and crystal deposits in her corneas as well as the wall of the globes posteriorly at all levels (Figure 3A). Accordingly, she was diagnosed with uveitis, and prescribed prednisolone drops 1% in the left eye twice a day for therapy. During the last few visits, she was found to have recurrent corneal abrasions due to her corneal edema, which was believed to contribute to her frequent pain.
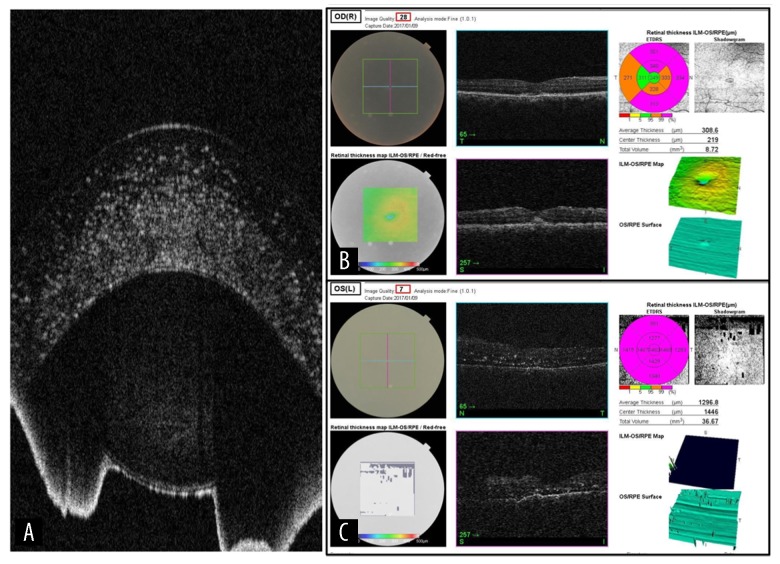
Figure 3.
(A) The ultrasound image showing the diffuse extensive corneal deposits. (B) The OCT image with crystal deposits in the right macular area. (C) The OCT image with crystal deposits in the left retina.
On her latest follow up in December 2018, her visual acuity was stable in her right eye. However, she lost her vision in the left eye with no light perception. Her OCT images clearly showed the crystal deposits in her cornea and also showed the corresponding retinal changes and deposits in the macular area and retinal periphery (Figure 3B, 3C). She was maintained on the same topical palliative ophthalmic drops in addition to cyclopentolate eye drops every 2 h for recurrent pain, which we believe was caused by a multitude of factors. The steroids were kept to be used as needed when her pain worsened. She was offered left eye enucleation, but the parents refused. The appearance of both corneas is demonstrated in Figure 4, which clearly shows the progression of her disease. She is currently awaiting a kidney transplant for her end-stage renal disease.
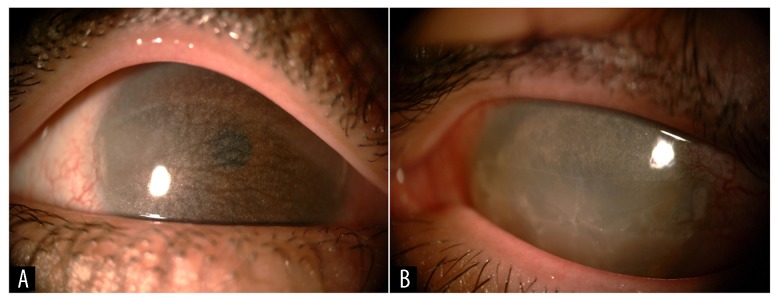
Figure 4.
(A) The slit-lamp appearance of her right cornea with marked progression compared to the image shown in Figure 1A above. (B) The slit-lamp appearance of the cornea in the left non-seeing eye.
Discussion
Cystinosis is caused by a pathogenic variant of the CTNS gene which normally encodes the protein cystinosin, which is responsible for transporting the disulfide amino acid cystine out of the lysosome and into the cytoplasm. The mutated CTNS genes produce abnormal cystinosin, leading to the accumulation of cystine crystal in different organs of the body [1]. There are over 100 reported mutations in the gene, including deletions, insertions, duplications, missense, nonsense, and splice site mutations [3,4]. The infantile cystinosis form is known to be caused by severe truncating mutations, while the milder variants of the disease are shown to be caused by milder mutations in at least 1 allele [5].
Signs and symptoms of the most common nephrogenic variant appear during the first year of life, with the most common early feature of morning vomiting, poor appetite, and feeding difficulties that lead to failure to thrive. By 1 year of age, they are typically at the third percentile for height and weight, with normal head circumference [1]. By 6 months of age, they develop Fanconi syndrome, which presents as severe polyuria, polydipsia, dehydration, and hypochloremic metabolic acidosis. They may also develop hypophosphatemic or hypocalcemic rickets due to the high excretion of phosphate and calcium, leading to bone deformities, seizures, and tetany due to vitamin D and calcium deficiency [1]. At the end of the first decade of life, patients typically develop hypothyroidism and possibly primary hypogonadism in affected males [6]. Cystine may accumulate in many other organs, manifesting as delayed eruption and development of teeth, mildly altered craniofacial morphology, and decreased airway size [7]. They ultimately develop renal failure at around 10 years of age due to a gradual deterioration in glomerular function. Patients typically reach puberty 1–2 years late, with impaired visual and spatial cognition, but their language and intellectual functions are preserved [6,8].
The ocular manifestations of the same variant begin with the common complaint of photophobia at the end of the first decade of life due to the deposition of cystine crystals in all the corneal layers, which diffracts and scatters the incoming light [1,9]. The density of the stromal crystal deposition is directly related to the severity of photophobia. Later in the course of the disease, the cystine crystals may deposit in other structures of the eye, resulting in anterior segment problems including band keratopathy, filamentary keratitis, recurrent epithelial erosions, peripheral corneal neovascularization, and posterior synechiae [1,10–12]. The cystine crystals may also accumulate irreversibly in the retinal layers, leading to pigmentary retinopathy with degeneration of the photoreceptors, which shows a mottled pattern on fundus examination. Such patients present with decreased color and night vision, as well as visual field loss [1,10,13].
The diagnosis of cystinosis is often delayed owing to the rarity of the disease. It is diagnosed based on 1 of the following: (1) slit-lamp identification of cystine crystals in the cornea after 12 months of age, which is always present after 16 months of age, (2) an increase in cystine concentration in polymorpho-nuclear leukocytes, (3) an increase in cystine content in cultured fibroblasts or in the placenta at birth, or (4) a positive molecular genetic test result for a bi-allelic pathogenic variant of the CTNS gene [1].
Oral cysteamine drugs are usually prescribed at diagnosis to slow progression of the renal and retinal manifestations of the disease. The treatment of cystinosis is highly dependent on the cysteamine drug formulations, which include oral administration, topical drops, and topical lubricants [1,14–17]. However, since the cornea is avascular, the oral formulation does not seem to have a significant effect. Several previous reports have shown that corneal crystals can effectively be dissolved and reversed by the topical form, in turn improving the photophobia and ocular pain [1,18–20]. The main challenge with topical cysteamine drops is compliance, as it requires administration every 1–2 h and cold storage [21]. Strict adherence to the treatment is required to prolong the lifespan of cystinosis and decrease the rate of its complications [2,19,21,22]. The current lifespan of patients with nephropathic cystinosis has increased from less than 10 years of age to at least the mid-forties or fifties, with interventions including renal transplants and cystine depletion therapy [1,23].
Our thorough search of the English-language literature found only 1 case of infantile nephropathic cystinosis, with a presentation similar to ours, reported by Tsilou et al. in 2007. Their 31-year-old male patient had a similar clinical picture, but the cystine deposits were extensive bilaterally. Their patient underwent therapeutic keratoplasty to improve vision in his right eye [12].
Conclusions
Our case is a unique presentation of nephrogenic cystinosis with an unfortunate outcome despite topical and systemic therapy. Ophthalmologists and family physicians should be aware of the early manifestation of this rare disease to ensure prompt treatment and follow-up with better visual and systemic outcomes. Further biochemical studies of the pathogenesis of the disease and evolution of the therapeutic agents’ pharmacodynamics may help achieve more success in arresting the progression of disease.
Acknowledgments
Dr. Doaa Alshammari, Ophthalmic Photographer, Ophthalmology Department, King Faisal Specialist Hospital and Research Center.
Footnotes
Department and Institution where work was done
Department of Ophthalmology, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
Conflicts of interest
None.
References:
- 1.Nesterova G, Gahl WA. Cystinosis. 2001 Mar 22 [Updated 2017 Dec 7] In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet] Seattle (WA): University of Washington; 1993–2018. Seattle; Available from: https://www.ncbi.nlm.nih.gov/books/NBK1400/ [Google Scholar]
- 2.Emma F, Nesterova G, Langman C, et al. Nephropathic cystinosis: An international consensus document. Nephrol Dial Transplant. 2014;29(Suppl. 4):iv87–94. doi: 10.1093/ndt/gfu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Town M, Jean G, Cherqui S, et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18(4):319–24. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 4.Kalatzis V, Nevo N, Cherqui S, et al. Molecular pathogenesis of cystinosis: Effect of CTNS mutations on the transport activity and subcellular localization of cystinosin. Hum Mol Genet. 2004;13(13):1361–71. doi: 10.1093/hmg/ddh152. [DOI] [PubMed] [Google Scholar]
- 5.Levtchenko E, Van den Heuvel L, Emma F, Antignac C. Clinical utility gene card for: Cystinosis. Eur J Hum Genet. 2014;22(5) doi: 10.1038/ejhg.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chik CL, Friedman A, Merriam GR, Gahl WA. Pituitary-testicular function in nephropathic cystinosis. Ann Intern Med. 1993;119(7 Pt 1):568–75. doi: 10.7326/0003-4819-119-7_part_1-199310010-00004. [DOI] [PubMed] [Google Scholar]
- 7.Bassim CW, Gautam P, Domingo DL, et al. Craniofacial and dental findings in cystinosis. Oral Dis. 2010;16(5):488–95. doi: 10.1111/j.1601-0825.2010.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spilkin AM, Ballantyne AO, Babchuck LR, Trauner DA. Non-verbal deficits in young children with a genetic metabolic disorder: WPPSI-III performance in cystinosis. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):444–47. doi: 10.1002/ajmg.b.30448. [DOI] [PubMed] [Google Scholar]
- 9.Labbé A, Niaudet P, Loirat C, et al. In vivo confocal microscopy and anterior segment optical coherence tomography analysis of the cornea in nephropathic cystinosis. Ophthalmology. 2009;116(5):870–76. doi: 10.1016/j.ophtha.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Tsilou ET, Rubin BI, Reed GF, et al. Age-related prevalence of anterior segment complications in patients with infantile nephropathic cystinosis. Cornea. 2002;21(2):173–76. doi: 10.1097/00003226-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser-Kupfer MI, Caruso RC, Minkler DS, Gahl WA. Long-term ocular manifestations in nephropathic cystinosis. Arch Ophthalmol. 1986;104(5):706–11. doi: 10.1001/archopht.1986.01050170096030. [DOI] [PubMed] [Google Scholar]
- 12.Tsilou E, Zhou M, Gahl W, et al. Ophthalmic manifestations and histopathology of infantile nephropathic cystinosis: Report of a case and review of the literature. Surv Ophthalmol. 2007;52(1):97–105. doi: 10.1016/j.survophthal.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsilou ET, Rubin BI, Reed G, et al. Nephropathic cystinosis: Posterior segment manifestations and effects of cysteamine therapy. Ophthalmology. 2006;113(6):1002–9. doi: 10.1016/j.ophtha.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Labbé A, Baudouin C, Deschênes G, et al. A new gel formulation of topical cysteamine for the treatment of corneal cystine crystals in cystinosis: The Cystadrops OCT-1 study. Mol Genet Metab. 2014;111(3):314–20. doi: 10.1016/j.ymgme.2013.12.298. [DOI] [PubMed] [Google Scholar]
- 15.Makuloluwa AK, Shams F. Cysteamine hydrochloride eye drop solution for the treatment of corneal cystine crystal deposits in patients with cystinosis: An evidence-based review. Clin Ophthalmol. 2018;12:227–36. doi: 10.2147/OPTH.S133516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gahl WA, Balog JZ, Kleta R. Nephropathic cystinosis in adults: Natural history and effects of oral cysteamine therapy. Ann Intern Med. 2007;147(4):242–50. doi: 10.7326/0003-4819-147-4-200708210-00006. [DOI] [PubMed] [Google Scholar]
- 17.Liang H, Labbé A, Le Mouhaër J, et al. A new viscous cysteamine eye drops treatment for ophthalmic cystinosis: An Open-Label Randomized Comparative Phase III Pivotal Study. Invest Ophthalmol Vis Sci. 2017;58(4):2275–83. doi: 10.1167/iovs.16-21080. [DOI] [PubMed] [Google Scholar]
- 18.Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347(2):111–21. doi: 10.1056/NEJMra020552. [DOI] [PubMed] [Google Scholar]
- 19.Gahl WA, Kuehl EM, Iwata F, et al. Corneal crystals in nephropathic cystinosis: Natural history and treatment with cysteamine eyedrops. Mol Genet Metab. 2000;71(1–2):100–20. doi: 10.1006/mgme.2000.3062. [DOI] [PubMed] [Google Scholar]
- 20.Elmonem MA, Veys KR, Soliman NA, et al. Cystinosis: A review. Orphanet J Rare Dis. 2016;11:47. doi: 10.1186/s13023-016-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shams F, Livingstone I, Oladiwura D, Ramaesh K. Treatment of corneal cystine crystal accumulation in patients with cystinosis. Clin Ophthalmol. 2014;8:2077–84. doi: 10.2147/OPTH.S36626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodin-sartorius A, Tête MJ, Niaudet P, et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81(2):179–89. doi: 10.1038/ki.2011.277. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser-Kupfer MI, Gazzo MA, Datiles MB, et al. A randomized placebo-controlled trial of cysteamine eye drops in nephropathic cystinosis. Arch Ophthalmol. 1990;108(5):689–93. doi: 10.1001/archopht.1990.01070070075038. [DOI] [PubMed] [Google Scholar]