Abstract
BACKGROUND
The benefits of early continuous neuromuscular blockade in patients with acute respiratory distress syndrome (ARDS) who are receiving mechanical ventilation remain unclear.
METHODS
We randomly assigned patients with moderate-to-severe ARDS (defined by a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen of <150 mm Hg with a positive end-expiratory pressure [PEEP] of ≥8 cm of water) to a 48-hour continuous infusion of cisatracurium with concomitant deep sedation (intervention group) or to a usual-care approach without routine neuromuscular blockade and with lighter sedation targets (control group). The same mechanical-ventilation strategies were used in both groups, including a strategy involving a high PEEP. The primary end point was in-hospital death from any cause at 90 days.
RESULTS
The trial was stopped at the second interim analysis for futility. We enrolled 1006 patients early after the onset of moderate-to-severe ARDS (median, 7.6 hours after onset). During the first 48 hours after randomization, 488 of the 501 patients (97.4%) in the intervention group started a continuous infusion of cisatracurium (median duration of infusion, 47.8 hours; median dose, 1807 mg), and 86 of the 505 patients (17.0%) in the control group received a neuromuscular blocking agent (median dose, 38 mg). At 90 days, 213 patients (42.5%) in the intervention group and 216 (42.8%) in the control group had died before hospital discharge (between-group difference, −0.3 percentage points; 95% confidence interval, −6.4 to 5.9; P = 0.93). While in the hospital, patients in the intervention group were less physically active and had more adverse cardiovascular events than patients in the control group. There were no consistent between-group differences in end points assessed at 3, 6, and 12 months.
CONCLUSIONS
Among patients with moderate-to-severe ARDS who were treated with a strategy involving a high PEEP, there was no significant difference in mortality at 90 days between patients who received an early and continuous cisatracurium infusion and those who were treated with a usual-care approach with lighter sedation targets. (Funded by the National Heart, Lung, and Blood Institute; ROSE ClinicalTrials.gov number, .)
THE ACUTE RESPIRATORY DISTRESS SYN-drome (ARDS) is an inflammatory form of lung injury that results in respiratory failure with hypoxemia, decreased lung compliance, and bilateral alveolar opacities on chest imaging.1 It is well established that the approaches used for the application of mechanical ventilation in patients with ARDS can affect survival and outcomes after discharge from the intensive care unit (ICU). For example, neuromuscular blockade reduces patient–ventilator dyssynchrony, the work of breathing, and the accumulation of alveolar fluid; patients with ARDS could benefit from these outcomes.2 However, prolonged administration of neuromuscular blocking agents is associated with subsequent neuromuscular weakness.3,4 The largest multicenter trial to date (the ARDS et Curarisation Systematique [ACURASYS] trial)5 was conducted a decade ago, and ICU practices have changed since then. The investigators of that trial reported that the early administration of a 48-hour infusion of neuromuscular blockade in patients with moderate-to-severe ARDS (defined by a ratio of the partial pressure of arterial oxygen [Pao2] to the fraction of inspired oxygen [Fio2] of <150 mm Hg with a positive end-expiratory pressure [PEEP] of ≥5 cm of water) resulted in lower mortality than a strategy of deep sedation without routine neuromuscular blockade.5 Despite these encouraging results, early neuromuscular blockade is not widely adopted and is only weakly recommended in current guidelines.6–9 Potential concerns include the lack of research comparing neuromuscular blockade and deep sedation with current practice (which promotes lighter sedation targets8,10–12) as well as limited data on the effect of neuromuscular blockade on neuromuscular function and other long-term outcomes.2,13 In addition, neuromuscular blockade requires deep sedation, which itself can result in negative outcomes.6,12,14
The Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network of the National Heart, Lung, and Blood Institute (NHLBI) conducted the Reevaluation of Systemic Early Neuromuscular Blockade (ROSE) trial — a multicenter, unblinded, randomized trial of patients with moderate-to-severe ARDS — to determine the efficacy and safety of early neuromuscular blockade with concomitant heavy sedation as compared with a strategy of usual care with lighter sedation targets. We hypothe sized that the use of early neuromuscular blockade would result in lower all-cause in-hospital mortality at 90 days than usual care.
METHODS
TRIAL DESIGN AND OVERSIGHT
We designed the ROSE trial to be consistent with certain elements of the ACURASYS trial.5,15 Similarities included the use of the same neuromuscular blocking agent (cisatracurium) with the same dosing regimen and duration of treatment. A key difference was our use of lighter sedation targets in the control group to be consistent with current practice recommendations.6,8,9 To minimize potentially confounding differences in the use of cointerventions, we specified the approach to mechanical ventilation in the protocol, including the use of a strategy involving a high PEEP, and we recommended the use of a conservative fluid strategy.16–18 To capture potential differences in late sequelae, assessors who were unaware of the group assignment interviewed surviving patients or their proxies at 3, 6, and 12 months after randomization. We published the protocol and submitted the statistical analysis plan (available with the full text of this article at NEJM.org) to the NHLBI before data analysis.15 A central institutional review board and a data and safety monitoring board appointed by the NHLBI provided oversight. Our coordinating center gathered and analyzed the data, and the protocol committee wrote the first draft of the manuscript. We vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. We obtained written informed consent from representatives of all patients.
PATIENTS
We enrolled patients who were undergoing mechanical ventilation through an endotracheal tube and had the following criteria present for less than 48 hours: Pao2:Fio2 of less than 150 mm Hg with a PEEP of 8 cm or more of water; bilateral pulmonary opacities on chest radiography or on computed tomography that could not be explained by effusions, pulmonary collapse, or nodules; and respiratory failure that could not be explained by cardiac failure or fluid overload. If results of arterial blood gas analysis were unavailable, the Pao2 was inferred from the oxygen saturation as measured by pulse oximetry (Spo2) and was used to estimate the Pao2: Fio2 at a PEEP of 8 cm or more of water.19,20 A full list of exclusion criteria is provided in the Supplementary Methods section in the Supplementary Appendix, available at NEJM.org.
RANDOMIZATION AND TREATMENTS
We randomly assigned patients in a 1:1 ratio to receive 48 hours of continuous neuromuscular blockade with concomitant deep sedation (intervention group) or to receive usual care without routine neuromuscular blockade and with lighter sedation targets (control group). Patients in the intervention group who were not under deep sedation at baseline were deeply sedated within 4 hours after randomization. Subsequently, patients in this group received an intravenous bolus of 15 mg of cisatracurium, followed by a continuous infusion of 37.5 mg per hour for 48 hours. Although treatment was not administered in a blinded manner, we chose not to adjust the dose of the neuromuscular blocking agent according to peripheral nerve stimulation both to replicate the dosing regimen used in the ACURASYS trial and to facilitate adherence to the trial protocol. Neuromuscular blockade could be stopped early if the patient met the criteria for freedom from mechanical ventilation (Fio2 ≤0.40 and PEEP ≤8 cm of water) for at least 12 hours. We recommended the use of light sedation in the control group. Light sedation was defined by a score on the Richmond Agitation–Sedation Scale of 0 or −1 (scores range from 4 [combative] to −5 [unresponsive], with a score of 0 indicating that the patient is alert and calm), a score on the Riker Sedation–Agitation Scale of 3 or 4 (scores range from 1 [unresponsive] to 7 [dangerous agitation], with a score of 4 indicating that the patient is calm and cooperative), or a score on the Ramsay Sedation Scale of 2 or 3 (scores range from 1 [anxious, restless] to 6 [unresponsive], with a score of 2 indicating that the patient is cooperative and oriented).21–23
COMMON TRIAL PROCEDURES
All patients were treated with a strategy of low tidal volume ventilation within 2 hours after randomization and a high PEEP strategy for up to 5 days after randomization.16,24,25 We allowed a lower PEEP if the clinician suspected that a higher PEEP worsened oxygenation, hypotension, high plateau pressures (>30 cm of water), or acidemia (pH <7.15) despite tidal-volume reductions, fluid boluses, or increases in respiratory rate. Lower PEEP was also permitted if a pneumothorax developed or if the patient was at high risk for barotrauma. The use of prone positioning was at the discretion of the clinician, though we recommended that clinicians wait at least 12 hours after the onset of ARDS, as suggested by current evidence,26 and avoid the automatic use of neuromuscular blockade. We allowed an open-label intravenous bolus injection of 20 mg of cisatracurium in both groups if patients met prespecified criteria (see the Additional Methods section in the Supplementary Appendix). After the 48-hour trial intervention period, decisions regarding further use of neuromuscular blockade, including the choice of agent, were left to the discretion of the treating clinician. To facilitate comparison, we report all neuromuscular blockade use as the equivalent cisatracurium dose.27
END POINTS
The primary end point was in-hospital death from any cause at 90 days (in-hospital was defined as the time in the trial hospital plus transfer to another hospital, including the time in long-term acute care facilities). Secondary end points were organ dysfunction (as assessed on the basis of the Sequential Organ Failure [SOFA] score28; scores range from 0 to 4 for each of six organ systems, with higher scores indicating more severe organ dysfunction), in-hospital death at day 28, days free of organ dysfunction, days not in the ICU, days free of mechanical ventilation, and days not in the hospital at day 28. End points assessed at 3, 6, and 12 months were survival, disability, health-related quality of life, patient-reported health, pain interference, symptoms resembling those of post-traumatic stress, cognitive function, and return to work.29–33 Safety end points included recall of paralysis (assessed with the modified Brice questionnaire), ICU-acquired weakness up to day 28 (assessed with the Medical Research Council scale, which includes scores for muscle strength in 6 muscle groups on each side of the body, for a total of 12 muscle groups; the score for each muscle group can range from 0 [no movement observed] to 5 [the muscle contracts normally against full resistance], with the overall score ranging from 0 to 60), limitations on physical activity (assessed with the ICU Mobility Scale; scores range from 0 [no movement] to 10 [walking without aid]), new-onset atrial fibrillation or supraventricular tachycardia, barotrauma, and investigator-reported adverse events.34–38 We could not ensure that the in-hospital assessors of end points were unaware of treatment group, but all postdischarge end points were assessed by trial personnel who were unaware of the group assignment.
STATISTICAL ANALYSIS
Under the assumption that 27% of patients in the intervention group and 35% in the control group would die, we calculated that 1408 patients would need to be enrolled to provide the trial with 90% power to reject the null hypothesis of no difference between the groups in treatment effect, at a two-sided alpha level of 0.05.5,25,39 The trial was designed to be stopped if superiority of either group was established using symmetric group sequential flexible stopping boundaries, with no stopping rule for futility.40 We compared the primary end point between groups with the use of a Wald test for the difference of two proportions. We performed pre-specified analyses according to severity of ARDS (Pao2: Fio2 <120 mm Hg or ≥120 mm Hg) and duration of ARDS (a duration less than or greater than the median time from meeting inclusion criteria to randomization) as well as for the potential effect of excluding patients who had previously received neuromuscular blockade (hospitals were divided into terciles on the basis of their exclusion rate of patients who had previously received neuromuscular blockade). We also tested for interactions between treatment group and sex, race, and ethnic group. All treatment-by-subgroup interactions were analyzed on the risk difference scale with the use of a generalized linear model with a binomial distribution function and an identity link function. Secondary end points are reported with observed differences and 95% confidence intervals. Adverse events were compared between groups, with the event the unit of analysis and with the use of weighted Poisson regression; nonserious events were weighted by 1 and serious events were weighted by 2. Mortality at 90 days and at 1 year was compared between the groups with the use of a z-test, which was based on the point estimates and standard errors of the within-group non-parametric interval-censored survival functions. All analyses were performed according to the intention-to-treat principle, without adjustment for multiple comparisons. Two-sided P values of less than 0.05 were considered to indicate statistical significance. Analyses were performed with SAS software, version 9.4 (SAS Institute).
RESULTS
PATIENTS
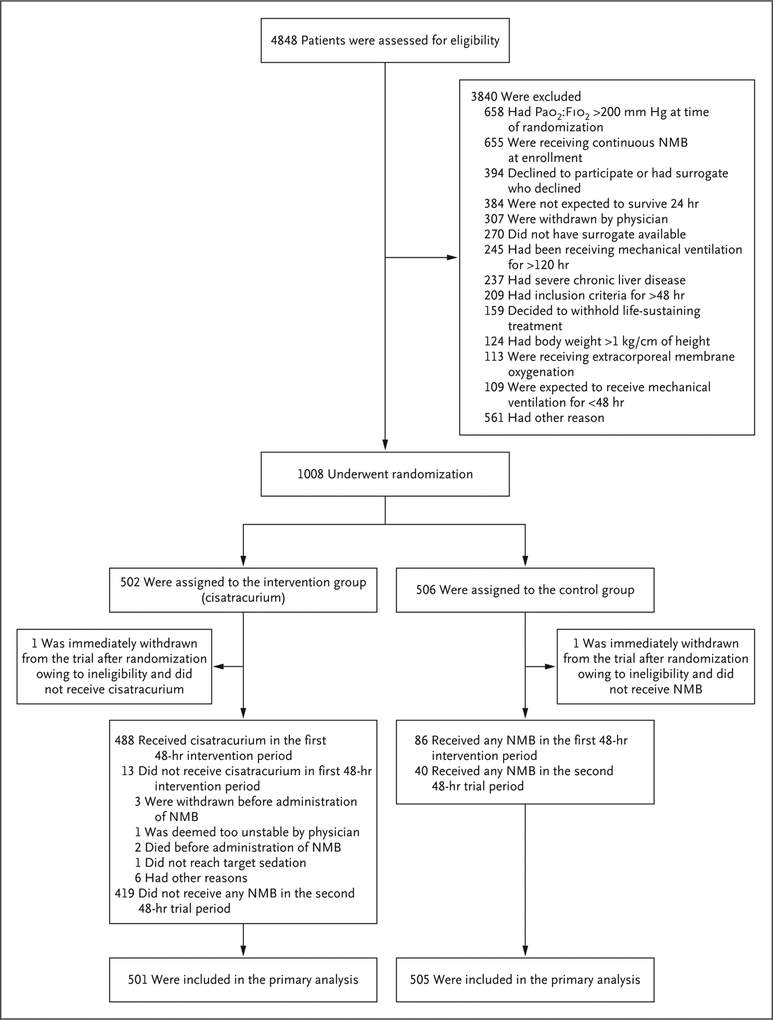
From January 2016 through April 2018, we screened 4848 patients at 48 hospitals across the United States, and 1006 patients were included in the primary analysis (Fig. 1). After the second interim analysis, the decision to stop the trial for futility was made independently by the data and safety monitoring board; the decision was endorsed by the NHLBI and accepted by the PETAL steering committee. The most common reason for exclusion was improvement in the Pao2: Fio2 before enrollment (658 patients). The most common reason for exclusion after screening was the previous receipt of neuromuscular blockade (655 patients). Of the patients who were enrolled, 501 were randomly assigned to the intervention group, and 505 to the control group. Baseline characteristics were similar in the two groups (Table 1, and Table S1 in the Supplementary Appendix). Patients were enrolled a median of 7.6 hours (interquartile range, 3.7 to 15.6) after diagnosis of moderate-to-severe ARDS; 9.3% of the patients (94 patients) were enrolled with a qualifying Spo2: Fio2 (Table S2 in the Supplementary Appendix).
Figure 1. Patient Screening, Enrollment, and Follow-up.
Patients may have had more than one reason for exclusion. Two patients were randomly assigned twice to the control group. No patients were lost to follow-up. NMB denotes neuromuscular blockade, and Pao2:Fio2 the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen.
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Intervention Group (N = 501) | Control Group (N = 505) |
|---|---|---|
| Age — yr | 56.6±14.7 | 55.1±15.9 |
| Female sex— no. (%)† | 210 (41.9) | 236 (46.7) |
| White race — no. (%)† | 361 (72.1) | 344 (68.1) |
| Shock at baseline — no. (%) | 276 (55.1) | 309 (61.2) |
| Median time from eligibility to randomization (IQR) — hr | 8.2 (4.0–16.4) | 6.8 (3.3–14.5) |
| Neuromuscular blockade use between meeting inclusion criteria and randomization — no./total no. (%) | 55/484 (11.4) | 50/484 (10.3) |
| Primary cause of lung injury— no. (%) | ||
| Pneumonia | 292 (58.3) | 301 (59.6) |
| Aspiration | 91 (18.2) | 75 (14.9) |
| Nonpulmonary sepsis | 68 (13.6) | 71 (14.1) |
| Other cause | 50 (10.0) | 58 (11.5) |
| Assessments and measurements | ||
| APACHE III score‡ | 103.9±30.1 | 104.9±30.1 |
| Total SOFA score§ | 8.7±3.6 | 8.8±3.6 |
| Tidal volume— ml/kg of predicted body weight¶ | 6.3±0.9 | 6.3±0.9 |
| Fio2∥ | 0.8±0.2 | 0.8±0.2 |
| Inspiratory plateau pressure — cm of water** | 25.5±6.0 | 25.7±6.1 |
| PEEP — cm of water†† | 12.6±3.6 | 12.5±3.6 |
| Pao2:Fio2 — mm Hg‡‡ | 98.7±27.9 | 99.5±27.9 |
| Imputed Pao2:Fio2 — mm Hg§§ | 94.8±26.7 | 93.2±28.9 |
Plus–minus values are means ±SD. There were no significant differences between the groups except for time from inclusion in the trial to randomization (P = 0.047) and shock at baseline (P = 0.05). Percentages may not total 100 because of rounding. IQR denotes interquartile range.
Sex and race were determined by the coordinators on the basis of hospital records or information from the next of kin.
Acute Physiology, Age, and Chronic Health Evaluation (APACHE III) scores range from 0 to 299, with higher scores indicating more severe illness.41 The APACHE III score was assessed in 455 patients in the intervention group and 459 in the control group.
Sequential Organ Failure Assessment (SOFA) scores were measured in 5 organ systems (respiratory, cardiovascular, hematologic, gastrointestinal, and renal; the neurologic system was not assessed), with each organ scored from 0 to 4, resulting in an aggregated score that ranges from 0 to 20, with higher scores indicating greater dysfunction.28 The SOFA score was not assessed in 1 patient in the control group.
The tidal volume was assessed in 445 patients in the intervention group and 443 in the control group.
The fraction of inspired oxygen (Fio2) was assessed in 469 patients in the intervention group and 474 in the control group.
The inspiratory plateau pressure was assessed in 274 patients in the intervention group and 266 in the control group.
The positive end-expiratory pressure (PEEP) was assessed in 492 patients in the intervention group and 495 in the control group.
The ratio of the partial pressure of arterial oxygen (Pao2) to Fio2 was assessed in 452 patients in the intervention group and 460 in the control group. The Fio2 value reflects the value that was recorded closest to the time of randomization within the 24 hours before randomization.
If an arterial blood gas analysis was not available at randomization, the Pao2: Fio2 could be inferred from the oxygen saturation as measured by pulse oximetry. The imputed Pao2: Fio2 was calculated in 49 patients in the intervention group and 45 patients in the control group.
NEUROMUSCULAR BLOCKADE, SEDATION, AND OTHER CARE PROCESSES
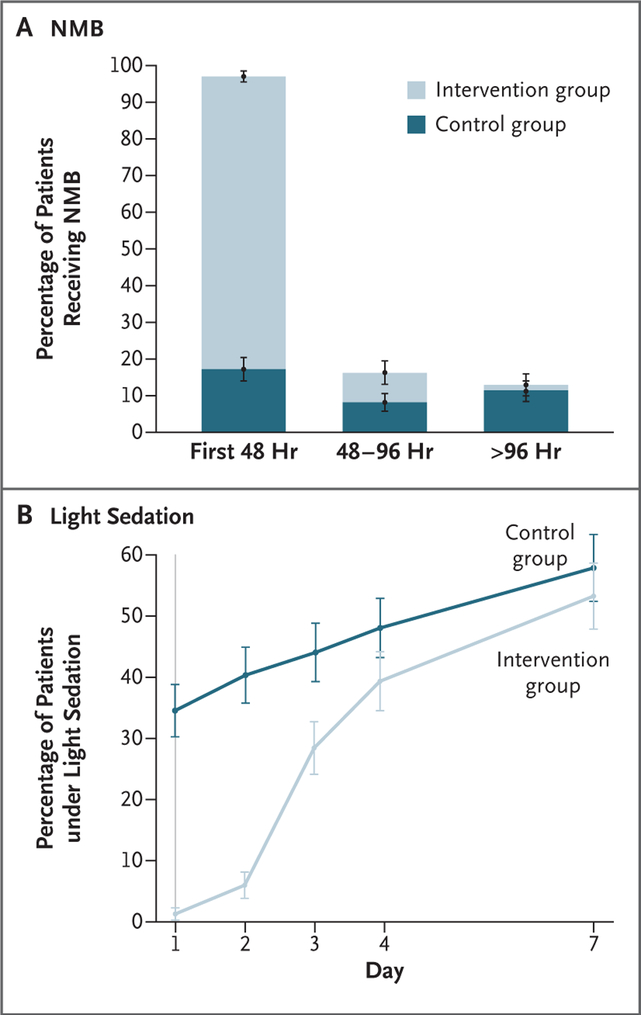
In the intervention group, 488 patients (97.4%) received a cisatracurium infusion, beginning a mean (±SD) of 1.9±1.4 hours after randomization. The median duration of cisatracurium administration over the 48-hour intervention period was 47.8 hours (interquartile range, 43.8 to 48.0), and the median cumulative dose was 1807 mg (interquartile range, 1706 to 1815). Overall, the cisatracurium infusion was stopped early in 74 patients (14.8%) because of clinical improvement. In the control group, 86 patients (17.0%) received a neuromuscular blocking agent during the first 48 hours at a median cisatracurium (or equivalent) dose of 38 mg (interquartile range, 14 to 200). Additional details on the dosing of neuromuscular blocking agents are provided in Table S3 in the Supplementary Appendix. Patients in the intervention group were under deeper sedation than patients in the control group both during the 48-hour intervention period and on the third trial day (Fig. 2). During the first 24 hours, patients in the intervention group had lower PEEP requirements than patients in the control group (between-group difference, −0.9 cm of water; 95% confidence interval [CI], −1.5 to −0.4). During the first and second 24-hour periods, patients in the intervention group also had lower minute ventilation (the between-group difference on day 1 was −0.7 liters per minute [95% CI, −1.1 to −0.2], and on day 2, −0.8 liters per minute [95% CI, −1.2 to −0.4]), lower Fio2 requirements (the between-group difference on both day 1 and day 2 was −0.04 [95% CI, −0.06 to −0.02]), and higher driving pressures (the between-group difference on day 1 was 0.7 cm of water [95% CI, 0.0 to 1.3], and on day 2, 0.8 cm of water [95% CI, 0.1 to −1.5]). However, there were no between-group differences in the Pao2: Fio2 from day 1 through day 7. Improvement in oxygenation was similar among patients who were enrolled early and those who were enrolled late after the onset of ARDS. From day 1 through day 7, there was good adherence to the protocol with respect to PEEP and Fio2 recommendations, and adherence to recommended ventilation guidelines ranged from 80.1 to 87.5% with respect to low tidal volume ventilation (≤6.5 ml per kilogram of predicted body weight) and 85.6 to 90.8% with respect to low plateau pressures (≤30 cm of water). The median daily fluid balance was 327 ml (interquartile range, −951 to 1456) on day 2 and −242 ml (interquartile range, −1432 to 728) on day 3, and there were no differences between trial groups. Additional details are provided in Figure S1 and Tables S4 through S8 in the Supplementary Appendix.
Figure 2. Neuromuscular Blockade and Sedation.
Panel A shows the mean percentage of patients who received continuous neuromuscular blockade, and Panel B shows the mean percentage of patients who were under light sedation during the first week of the trial. Light sedation was defined by a score of 0 or −1 on the Richmond Agitation–Sedation Scale (scores range from 4 [combative] to −5 [unresponsive], with a score of 0 indicating that the patient is alert and calm), a score of 3 or 4 on the Riker Sedation–Agitation Scale (scores range from 1 [unresponsive] to 7 [dangerous agitation], with a score of 4 indicating that the patient is calm and cooperative), or a score of 2 or 3 on the Ramsay Sedation Scale (scores range from 1 [anxious, restless] to 6 [unresponsive], with a score of 2 indicating that the patient is cooperative and oriented).21–23 More details are provided in Tables S3 and S4 in the Supplementary Appendix. I bars indicate standard errors.
PRIMARY END POINT
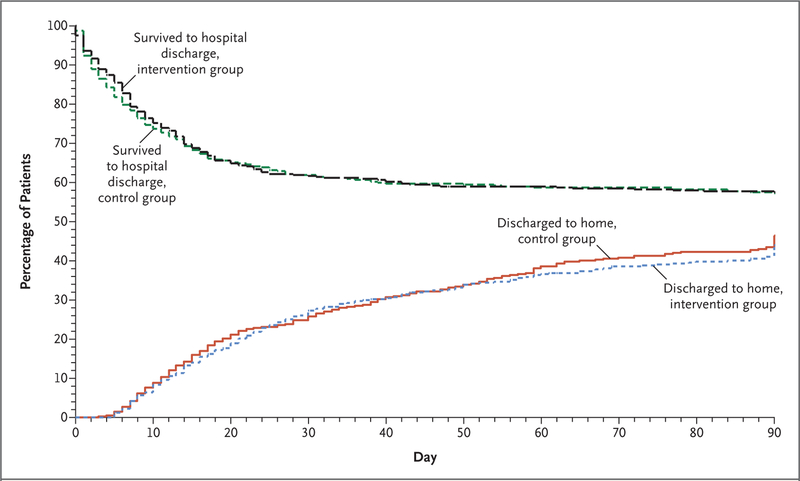
At 90 days, in-hospital death from any cause occurred in 213 patients (42.5%) in the intervention group and in 216 patients (42.8%) in the control group (between-group difference, −0.3 percentage points; 95% CI, −6.4 to 5.9; P = 0.93) (Fig. 3 and Table 2). Treatment-by-subgroup interactions were not significant with respect to ARDS severity, ARDS duration, or previous neuromuscular blockade use stratified according to hospital tercile. Other than the interaction of treatment assignment with ethnic group (P = 0.02 for interaction), no other interactions were significant (Fig. S2 and Tables S9 through S15 in the Supplementary Appendix).
Figure 3. Patients Who Survived to Hospital Discharge and Were Discharged Home during the First 90 Days after Randomization.
The period of hospitalization included transfer to other health care facilities.
Table 2.
End Points.*
| Variable | Intervention Group (N = 501) | Control Group (N = 505) | Between-Group Difference (95% CI) | P Value |
|---|---|---|---|---|
| percentage points | ||||
| Primary end point: in-hospital death by day 90 — no. (%)† | 213 (42.5±2.2) | 216 (42.8±2.2) | −0.3 (−6.4 to 5.9) | 0.93 |
| Secondary end points | ||||
| In-hospital death by day 28 — no. (%) | 184 (36.7) | 187 (37.0) | −0.3 (−6.3 to 5.7) | |
| Days free of ventilation at day 28‡ | 9.6±10.4 | 9.9±10.9 | −0.3 (−1.7 to 1.0) | |
| Days not in ICU at day 28 | 9.0±9.4 | 9.4±9.8 | −0.4 (−1.6 to 0.8) | |
| Days not in hospital at day 28‡ | 5.7±7.8 | 5.9±8.1 | −0.2 (−1.1 to 0.8) | |
| Safety end points | ||||
| In-hospital recall of paralysis | ||||
| Total no. of patients (%) | 9 (1.8) | 10 (2.0) | −0.2 (−1.9 to 1.5) | |
| Among patients who received neuromuscular blockade — no./total no. (%) | 9/487 (1.8) | 2/129 (1.6) | 0.3 (−2.1 to 2.7) | |
| MRC score§ | ||||
| Day 7 | 46.7±14.4 | 49.5±12.3 | −2.8 (−6.1 to 0.6)¶ | |
| Day 28 | 45.7±13.9 | 49.8±10.6 | −4.1 (−9.0 to 0.9)¶ | |
| ICU-acquired weakness — no./total no. (%)∥ | ||||
| Day 7 | 50/122 (41.0) | 41/131 (31.3) | −9.7 (−21.5 to 2.1) | |
| Day 28 | 22/47 (46.8) | 14/51 (27.5) | −19.4 (−38.2 to −0.6) | |
| Any time through day 28 | 107/226 (47.3) | 89/228 (39.0) | −7.3 (−15.7 to 1.1) | |
| Serious adverse events — no. of events** | 35 | 22 | 0.09 | |
| Serious cardiovascular adverse events— no. of events** | 14 | 4 | 0.02 | |
| Atrial fibrillation or SVT during ICU stay — no. (%) | 101 (20.2) | 99 (19.6) | 0.88 | |
| Barotrauma — no. (%) | 20 (4.0) | 32 (6.3) | 0.12 | |
| Pneumothorax on days 0 through 2 — no. (%) | 8 (1.6) | 10 (2.0) | 0.81 | |
| Pneumothorax on days 0 through 7 — no. (%) | 14 (2.8) | 25 (5.0) | 0.10 |
Unless otherwise indicated, plus–minus values are means ±SD. ICU denotes intensive care unit, and SVT supraventricular tachycardia.
Included are all deaths that occurred after randomization in any heath care facility before discharge home until day 90 of the trial. Patients in a health care facility at day 91 were considered to be alive. The plus–minus values in this category are standard errors.
If in-hospital death occurred before day 29, the days free of ventilation and the days not in the hospital at day 28 were considered to be zero.
The Medical Research Council (MRC) scale was used to assess muscle strength in 6 muscle groups on each side of the body, for a total of 12 muscle groups. The score for each muscle group can range from 0 (no movement observed) to 5 (muscle contracts normally against full resistance), with the overall score ranging from 0 to 60.37 The MRC score at day 7 was assessed in 122 patients in the intervention group and 131 in the control group; the score at day 28 was assessed in 47 patients in the intervention group and 51 in the control group.
The between-group difference is the difference in MRC score.
ICU-acquired weakness was defined as an MRC score of less than 48 if all 12 muscle groups were assessed, or a mean muscle-group score of less than 4 when at least 7 of the 12 muscle groups were assessed.
A list of all adverse events is provided in Table S24 in the Supplementary Appendix. Participants may have had more than 1 adverse event. Although mortality was high in both groups, only 1 death from complete heart block and refractory shock was considered possibly related to cisatracurium. No other deaths were reported by participating sites as possibly, probably, or definitely related to cisatracurium or any other procedure specified in the trial protocol.
SECONDARY END POINTS
At 28 days, there was no between-group difference in hospital mortality, days free of ventilation, days out of the ICU, or days out of the hospital (Table 2). Cardiovascular SOFA scores were higher in the intervention group than in the control group on day 1 (between-group difference, 0.2; 95% CI, 0.1 to 0.4) and day 2 (between-group difference, 0.3; 95% CI, 0.1 to 0.5). However, there were no differences thereafter, nor were there differences in total SOFA scores or other organ-specific SOFA scores. The use of adjunctive therapies appeared to be similar in the two groups during the 48-hour intervention period (between-group difference, 0.7 percentage points; 95% CI, −4.0 to 5.5) and through day 28 (between-group difference, 1.2 percentage points; 95% CI, −4.2 to 6.6). Overall, prone positioning was used in 15.8% of patients (159 patients), with similar use in the two groups (between-group difference, 1.9 percentage points; 95% CI, −2.6 to 6.4). Most (56% [42 patients]) of the 75 patients who underwent prone positioning in the control group did not receive concomitant neuromuscular blockade. Glucocorticoid use was also similar in the two groups. The mean (±SE) estimated mortality at 1 year was also not different between groups (51.1±2.2% in the intervention group and 51.1±2.2% in the control group). Patient-reported outcomes were similar between the groups at 3, 6, and 12 months, including health-related scores and health-related limitations with respect to disability, cognitive function, symptoms resembling those of post-traumatic stress, and pain. Additional information on secondary end points is provided in Tables S16 through S23 in the Supplementary Appendix.
SAFETY AND ADVERSE EVENTS
Safety and adverse events are summarized in Table 2 and in Tables S24 through S28 in the Supplementary Appendix. Although mortality was high in both groups, only one death was considered possibly related to cisatracurium, no deaths were considered probably or definitely related to cisatracurium, and there were no between-group differences in the percentage of patients who died during the 48-hour trial intervention period or up to 96 hours. Recall of paralysis was uncommon and did not differ between groups. Patients in the control group had higher mean levels of physical activity up to day 6. The rates of ICU-acquired weakness assessed were not different between groups, but many patients (range, 51.2 to 67.5%) could not complete the weekly in-hospital assessments of muscle strength. More serious cardiovascular events were reported in the intervention group than in the control group (14 vs. 4 events; P = 0.02), although the rates of new-onset atrial fibrillation and supraventricular tachycardia did not differ between groups. Rates of pneumothorax and overall barotrauma also did not differ between groups.
DISCUSSION
In a cohort of critically ill patients identified shortly after the diagnosis of moderate-to-severe ARDS, the addition of early continuous neuromuscular blockade with concomitant deep sedation did not result in lower mortality than a usual-care approach to mechanical ventilation that included lighter sedation targets. This trial had high adherence to the protocol, including minimal crossover use of neuromuscular blockade and high adherence to the recommended ventilation and fluid strategy. The results of prespecified subgroup analyses were consistent with those of the primary analysis across severity and duration of ARDS and across trial sites with different exclusion rates for previous neuromuscular blockade use.
Several factors may explain why our findings differed from those of ACURASYS, the previous multicenter trial that showed a benefit with early continuous neuromuscular blockade. First, we used a higher PEEP strategy in both groups to test our intervention in the context of best care and to reduce the likelihood of differential PEEP use across groups. Higher PEEP may itself reduce mortality among patients with moderate-to-severe ARDS, thereby blunting the potential treatment effect of early continuous neuromuscular blockade.16 Second, on the basis of current guideline recommendations and clinical studies,10–12,15 we designed this trial so that the sedation targets used in the control group were lighter than those used in the ACURASYS trial; deep sedation was used in both the intervention group and the control group in the ACURASYS trial. In our trial, the higher number of cardiovascular adverse events in the intervention group than in the control group could be the result of deep sedation in the intervention group, which could have induced hypotension, bradycardia, and other cardiovascular effects. Therefore, the use of the lighter sedation strategy in our control group may have decreased mortality in that group. Third, prone positioning reduces the risk of death in patients with ARDS when it is initiated during the first 12 to 24 hours after the onset of moderate-to-severe ARDS and is administered for at least 16 hours per day.26 The percentage of patients who underwent prone positioning in our trial was similar to that observed in a recent international epidemiologic study, but it was lower than in the ACURASYS trial.5,7 Whether early continuous neuromuscular blockade is more effective with prone positioning is unknown, but it is a possible explanation for the different results of our trial and the ACURASYS trial.
Patients in our trial were enrolled earlier after the onset of ARDS than those in the ACURASYS trial.42 Consequently, we may have included patients who might not have survived long enough to be included in the previous trial. Although we excluded patients whose Pao2: Fio2 improved to more than 200 mm Hg before randomization, we may also have recruited some patients with lung injury that was either rapidly improving or less established than that observed in the previous trial. However, analyses stratified according to the time from the onset of ARDS to enrollment did not suggest any between-group difference in the rate of improvement in oxygenation or treatment effect. The unexpected interaction between Hispanic ethnic group and treatment may be the result of random chance.
Our trial has limitations. The most common reason eligible patients were excluded was that they had previously received neuromuscular blockade. It is possible that treating physicians were identifying and treating a subset of patients who were more likely to benefit from neuromuscular blockade use. However, there was no evidence of benefit even when analyses were restricted to trial sites that rarely excluded those patients. We did not systematically measure the effect of neuromuscular blockade on ventilator dyssynchrony. However, in patients with ARDS or at risk for ARDS, neuromuscular blockade essentially eliminates ventilator dyssynchrony.43 Finally, nurses, physiotherapists, and other health care professionals were aware of the treatment assignments. This lack of blinding may have influenced short-term assessments of early neuromuscular function, the level of physical activity, and the reporting of adverse events. In conclusion, among patients with moderate-to-severe ARDS who were treated with a higher PEEP strategy, the administration of an early and continuous infusion of cisatracurium did not result in significantly lower mortality at 90 days than usual care with lighter sedation targets.
Supplementary Material
Acknowledgments
Supported by grants (U01HL123009, U01HL122998, U01HL123018, U01HL123023, U01HL123008, U01HL123031, U01HL123004, U01HL123027, U01HL123010, U01HL123033, U01HL122989, U01HL123022, and U01HL123020) from the National Heart, Lung, and Blood Institute.
Dr. Ferguson reports receiving lecture fees from Getinge and consulting fees from Sedana Medical and Baxter; Dr. Khan, receiving grant support from United Therapeutics, Actelion Pharmaceuticals/Johnson & Johnson, Lung, GlaxoSmithKline, Astra-Zeneca, and Reata Pharmaceuticals; Dr. Talmor, receiving lecture fees from Hamilton Medical and serving on an advisory board for Sedana Medical; Dr. Thompson, receiving consulting fees from Bayer, GlaxoSmithKline, and Boehringer Ingelheim; and Dr. Yealy, receiving fees for multiple tort actions for cases unrelated to paralysis in the intensive care unit. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and their families, the clinical and research staff at the participating sites, and the staff at the clinical coordinating center. We also thank Jennifer Vates for assistance with preparation of an earlier version of the manuscript and Travis Vermilye for figure development.
APPENDIX
The affiliations of the members of the writing committee are as follows: the Departments of Medicine (M.M.) and Emergency Medicine (A.A.G.), University of Colorado School of Medicine, Aurora; the Departments of Critical Care Medicine (D.T.H., D.C.A.) and Emergency Medicine (D.M.Y.), University of Pittsburgh School of Medicine, Pittsburgh; the Department of Medicine, Johns Hopkins University School of Medicine, Baltimore (R.G.B.); the Interdepartmental Division of Critical Care Medicine, Department of Medicine, University Health Network and Sinai Health System, University of Toronto, Toronto (N.D.F.); the Department of Medicine, Montefiore Hospital, New York (M.N.G.); the Department of Medicine, Intermountain Medical Center and the University of Utah, Salt Lake City (C.K.G.); the Department of Medicine, University of Washington, Seattle (S.G., C.L.H.); the Biostatistics Center (D.H.), the Department of Medicine (B.T.T.), and the PETAL Network Clinical Coordinating Center (C.A.U.), Massachusetts General Hospital, the Department of Emergency Medicine, Division of Emergency Critical Care Medicine, Brigham and Women’s Hospital (P.C.H.), and the Department of Anesthesia, Critical Care, and Pain Medicine, Beth Israel Deaconess Medical Center (D.T.) — all in Boston; the Department of Critical Care, Respiratory Institute, Cleveland Clinic, Cleveland (R.D.H.); the Department of Medicine, University of Michigan and Veterans Affairs Center for Clinical Research, Ann Arbor (T.J.I.); the Department of Medicine, Oregon Health and Science University, Portland (A.K.); and the Departments of Medicine and Anesthesia, University of California, San Francisco, San Francisco (K.D.L.).
The members of the writing committee (Marc Moss, M.D., David T. Huang, M.D., M.P.H., Roy G. Brower, M.D., Niall D. Ferguson, M.D., Adit A. Ginde, M.D., M.P.H., M.N. Gong, M.D., Colin K. Grissom, M.D., Stephanie Gundel, M.S., Douglas Hayden, Ph.D., R. Duncan Hite, M.D., Peter C. Hou, M.D., Catherine L. Hough, M.D., Theodore J. Iwashyna, M.D., Ph.D., Akram Khan, M.D., Kathleen D. Liu, M.D., Ph.D., Daniel Talmor, M.D., M.P.H., B. Taylor Thompson, M.D., Christine A. Ulysse, M.S., Donald M. Yealy, M.D., and Derek C. Angus, M.D., M.P.H.) assume responsibility for the overall content and integrity of this article. The affiliations of the members of the writing committee are listed in the Appendix.
Footnotes
This work does not necessarily represent the views of the Department of Veterans Affairs.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
A full list of the investigators in the Reevaluation of Systemic Early Neuromuscular Blockade (ROSE) trial and the Prevention and Early Treatment of Acute Lung Injury (PETAL) network is provided in the Supplementary Appendix, available at NEJM.org.
REFERENCES
- 1.The ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526–33. [DOI] [PubMed] [Google Scholar]
- 2.Slutsky AS. Neuromuscular blocking agents in ARDS. N Engl J Med 2010; 363: 1176–80. [DOI] [PubMed] [Google Scholar]
- 3.Price DR, Mikkelsen ME, Umscheid CA, Armstrong EJ. Neuromuscular blocking agents and neuromuscular dysfunction acquired in critical illness: a systematic review and meta-analysis. Crit Care Med 2016; 44: 2070–8. [DOI] [PubMed] [Google Scholar]
- 4.Puthucheary Z, Rawal J, Ratnayake G, Harridge S, Montgomery H, Hart N. Neuromuscular blockade and skeletal muscle weakness in critically ill patients: time to rethink the evidence? Am J Respir Crit Care Med 2012; 185: 911–7. [DOI] [PubMed] [Google Scholar]
- 5.Papazian L, Forel J-M, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363: 1107–16. [DOI] [PubMed] [Google Scholar]
- 6.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41: 263–306. [DOI] [PubMed] [Google Scholar]
- 7.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315: 788–800. [DOI] [PubMed] [Google Scholar]
- 8.Devlin JW, Skrobik Y, Gélinas C, et al. Executive summary: clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46: 1532–48. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2016. Crit Care Med 2017; 45: 486–552. [DOI] [PubMed] [Google Scholar]
- 10.Devlin JW, Pandharipande PP. Light sedation is the goal: making the evidence heavier. Crit Care Med 2018; 46: 1003–4. [DOI] [PubMed] [Google Scholar]
- 11.Shehabi Y, Bellomo R, Kadiman S, et al. Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality: a multinational prospective longitudinal cohort study. Crit Care Med 2018; 46: 850–9. [DOI] [PubMed] [Google Scholar]
- 12.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med 2012; 186: 724–31. [DOI] [PubMed] [Google Scholar]
- 13.Marini JJ. Early phase of lung-protective ventilation: a place for paralytics? Crit Care Med 2006; 34: 2851–3. [DOI] [PubMed] [Google Scholar]
- 14.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008; 371: 126–34. [DOI] [PubMed] [Google Scholar]
- 15.Huang DT, Angus DC, Moss M, et al. Design and rationale of the Reevaluation of Systemic Early Neuromuscular Blockade trial for acute respiratory distress syndrome. Ann Am Thorac Soc 2017; 14: 124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briel M, Meade M, Mercat A, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 2010; 303: 865–73. [DOI] [PubMed] [Google Scholar]
- 17.The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351: 327–36. [DOI] [PubMed] [Google Scholar]
- 18.The National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006; 354: 2564–75. [DOI] [PubMed] [Google Scholar]
- 19.Brown SM, Duggal A, Hou PC, et al. Nonlinear imputation of PaO2/FIO2 from SpO2/FIO2 among mechanically ventilated patients in the ICU: a prospective, observational study. Crit Care Med 2017; 45: 1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown SM, Grissom CK, Moss M, et al. Nonlinear imputation of Pao2/Fio2 from Spo2/Fio2 among patients with acute respiratory distress syndrome. Chest 2016; 150: 307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166: 1338–44. [DOI] [PubMed] [Google Scholar]
- 22.Riker RR, Fraser GL, Cox PM. Continuous infusion of haloperidol controls agitation in critically ill patients. Crit Care Med 1994; 22: 433–40. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974; 2: 656–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–8. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013; 368: 795–805. [DOI] [PubMed] [Google Scholar]
- 26.Guérin C, Reignier J, Richard J-C, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368: 2159–68. [DOI] [PubMed] [Google Scholar]
- 27.Hunter JM. New neuromuscular blocking drugs. N Engl J Med 1995; 332: 1691–9. [DOI] [PubMed] [Google Scholar]
- 28.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe orga ndys-function/failure: on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–10. [DOI] [PubMed] [Google Scholar]
- 29.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware J Jr, Kosinski M, Keller SDA. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–33. [DOI] [PubMed] [Google Scholar]
- 31.Twigg E, Humphris G, Jones C, Bram-well R, Griffiths RD. Use of a screening questionnaire for post-traumatic stress disorder (PTSD) on a sample of UK ICU patients. Acta Anaesthesiol Scand 2008; 52: 202–8. [DOI] [PubMed] [Google Scholar]
- 32.Hobson J The Montreal Cognitive Assessment (MoCA). Occup Med (Lond) 2015; 65: 764–5. [DOI] [PubMed] [Google Scholar]
- 33.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology 2005; 65: 559–64. [DOI] [PubMed] [Google Scholar]
- 34.Avidan MS, Palanca BJ, Glick D, et al. Protocol for the BAG-RECALL clinical trial: a prospective, multi-center, randomized, controlled trial to determine whether a bispectral index-guided protocol is superior to an anesthesia gas-guided protocol in reducing intraoperative awareness with explicit recall in high risk surgical patients. BMC Anesthesiol 2009;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002; 288: 2859–67. [DOI] [PubMed] [Google Scholar]
- 36.Hodgson C, Needham D, Haines K, et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung 2014; 43: 19–24. [DOI] [PubMed] [Google Scholar]
- 37.Kleyweg RP, van der Meché FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 1991; 14: 1103–9. [DOI] [PubMed] [Google Scholar]
- 38.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med 2014; 370: 1626–35. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38: 1573–82. [DOI] [PubMed] [Google Scholar]
- 40.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983; 70: 659–63. [Google Scholar]
- 41.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991; 100: 1619–36. [DOI] [PubMed] [Google Scholar]
- 42.Schenck EJ, Oromendia C, Torres LK, Berlin DA, Choi AMK, Siempos II. Rapidly improving ARDS in therapeutic randomized controlled trials. Chest 2019; 155: 474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sottile PD, Albers D, Higgins C, Mckeehan J, Moss M. The association between ventilator dyssynchrony, delivered tidal volume, and sedation using a novel automated ventilator dyssynchrony detection algorithm. Crit Care Med 2018; 46(2): e151–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.