Abstract
OBJECTIVES
To determine the efficacy and safety of statins for primary prevention of atherosclerotic cardiovascular disease (ASCVD) events in older adults, especially those aged 80 and older and with multimorbidity.
METHODS
The National Institute on Aging and the National Heart, Lung and Blood Institute convened A multidisciplinary expert panel from July 31 to August 1, 2017, to review existing evidence, identify knowledge gaps, and consider whether statin safety and efficacy data in persons aged 75 and older without ASCVD are sufficient; whether existing data can inform the feasibility, design, and implementation of future statin trials in older adults; and clinical trial options and designs to address knowledge gaps. This article summarizes the presentations and discussions at that workshop.
RESULTS
There is insufficient evidence regarding the benefits and harms of statins in older adults, especially those with concomitant frailty, polypharmacy, comorbidities, and cognitive impairment; a lack of tools to assess ASCVD risk in those aged 80 and older; and a paucity of evidence of the effect of statins on outcomes of importance to older adults, such as statin-associated muscle symptoms, cognitive function, and incident diabetes mellitus. Prospective, traditional, placebo-controlled, randomized clinical trials (RCTs) and pragmatic RCTs seem to be suitable options to address these critical knowledge gaps. Future trials have to consider greater representation of very old adults, women, underrepresented minorities, and individuals of differing health, cognitive, socioeconomic, and educational backgrounds. Feasibility analyses from existing large healthcare networks confirm appropriate power for death and cardiovascular outcomes for future RCTs in this area.
CONCLUSION
Existing data cannot address uncertainties about the benefits and harms of statins for primary ASCVD prevention in adults aged 75 and older, especially those with comorbidities, frailty, and cognitive impairment. Evidence from 1 or more RCTs could address these important knowledge gaps to inform person-centered decision-making.
Keywords: statins, primary prevention, older adults, randomized clinical trial, benefits, risks
Cardiovascular events are the leading cause of morbidity and mortality in adults aged 75 and older, with coronary heart disease (CHD) and stroke accounting for 60% of deaths in those aged 85 and older.1 Randomized controlled trial (RCT) evidence supporting the benefit of statin therapy for primary prevention of fatal and nonfatal atherosclerotic cardiovascular disease (ASCVD) events in adults younger than 75 is substantial and consistent. A meta-analysis of 27 RCTs comparing statin therapy with a control or usual care demonstrated a statistically significant reduction in the relative risk of major ASCVD events of 37% for those in the lowest ASCVD risk category (<5.0%) and 49% for those in the moderate ASCVD risk category (5.0–9.9%)2,3 (Table 1). Although statin therapy reduce low-density lipoprotein cholesterol (LDL-C) levels irrespective of age, evidence supporting reduction of ASCVD events with statin therapy in adults aged 75 and older is limited.2–6 Assuming efficacy similar to that in RCTs of younger individuals, primary prevention with statins in all adults aged 75 to 94 could prevent 105,000 myocardial infarctions (MIs) and 68,000 CHD-related deaths at an incremental cost of approximately $25,000 per disability-adjusted life year over 10-years7; however, although an estimated 10% to 29% increase in the relative risk of functional limitation or mild cognitive impairment would offset these benefits,8 highlighting the need to assess the overall health benefit:risk of initiating statins in older adults.
Table 1.
Meta-Analyses of Randomized Trials of Statins for Primary Prevention Not Limited to Older Adults
| Study | Studies, n | Participants, n | Follow-Up, Years | Age Criteria | Age | CVD Outcome | Non-CVD outcome |
|---|---|---|---|---|---|---|---|
| Cholesterol Treatment Trialists collaborators 3 | 22 statin v placebo 5 high- vs low-dose statin | 134,537 39,612 | 4.8 5.1 | No restriction | 59 ± 8 61 ± 9 | -For low ASCVD risk (<5.0%), fatal and nonfatal ASCVD event, RR=0.57, 99% CI=0.36–0.89 -For medium ASCVD risk (5.0–9.9%), fatal and nonfatal ASCVD event, RR=0.61,99% CI=0.5 to −0.74 | Annual excess risk of hemorrhagic stroke per 1.0 mmol/L low-density lipoprotein cholesterol reduction was 0.5/1,000 people over 5 years; Absolute excess risk of DM was 0.1% per year |
| U.S. Preventive Services Task Force 4–6 | 19 RCTs; statin vs placebo | 71 344 | 0.5–6 | No restriction | 51–66 | All-cause death, RR=0.86, 95% CI=0.80–0.93; cardiovascular mortality, RR=0.69, 95% CI=0.54–0.88; stroke, RR=0.71, 95% CI=0.62–0.82; myocardial infarction, RR=0.64, 95% CI=0.57–0.71; composite cardiovascular outcomes, RR=0.70, 95% CI=0.63–0.78 | Statins vs placebo: No significantly greater risk of myalgia (RR=0.96, 95% CI=0.79–1.16), liver-related harm (RR=1.10, 95% CI=0.90–1.35), or DM (RR=1.05, 95% CI=0.91–1.20). One RCT found that high-intensity statins were associated with greater risk of DM (RR=1.25, 95% CI=1.05–1.49). |
CVD=cardiovascular disease; ASCVD=atherosclerotic CVD; RR=relative risk; CI=confidence interval; DM=diabetes mellitus; RCT=randomized controlled trial.
The lack of clear evidence-based guidance for initiating statins in individuals aged 75 and older is emphasized in the 2013 American College of Cardiology (ACC)/American Heart Association (AHA) Cholesterol Lowering Guideline.2 Two important aspects of this guideline complicate decision-making in initiating statins for primary ASCVD prevention in older adults: the recommended use of the pooled cohort equations (PCE) to estimate 10-year ASCVD risk in white and black men and women9 and the stipulation to have a discussion to evaluate the individualized ASCVD benefit and risk of statin therapy, taking into account the adverse effects, drug–drug interactions, and patient preferences when deciding to initiate, continue, or intensify statin therapy in individuals aged 75 and older. Although it is stated that the current ACC/AHA calculator can estimate ASCVD risk accurately up to age 79, age drives the calculated risk score such that this instrument always calculates persons aged 75 and older to be at high ASCVD risk (>7.5% 10 year predicted risk), even without other known risk factors,9 but the guidelines do not indicate that simply achieving 7.5% risk should trigger a statin prescription in those aged 75 and older. This should be decided after discussion of additional factors (ASCVD risk-reduction benefits, adverse effects, drug–drug interactions, and personal preference) between the clinician and the individual. 2 The guideline’s recommendation for primary ASCVD prevention in those aged 75 and older is that, “Statin therapy may be considered in selected individuals” (Class IIb; Evidence Level C), but consideration of comorbidities, safety, and focusing on an individual’s priorities of care before initiating therapy are underscored. These cautions underpin the concluding guideline statement that use of statins for primary ASCVD prevention in adults aged 75 and older is a “high priority area of research.” Furthermore, because the PCE 10-year ASCVD risk calculator has not been validated for those aged 80 and older, tools or methods to accurately estimate ASCVD risk in representative populations are needed.
From July 31 to August 1, 2017, the National Institute on Aging (NIA) and the National Heart, Lung, and Blood Institute (NHLBI) convened experts from diverse disciplines (cardiology, geriatrics and gerontology, general internal medicine, endocrinology, clinical pharmacology, biostatistics, clinical investigation, neuropsychology) for a workshop entitled, “Opportunities for Trials on Effects of Statins in Primary Prevention in Older Adults,” to review existing evidence, identify knowledge gaps, and consider whether there is sufficient evidence of statin safety and efficacy in persons aged 75 and older without ASCVD; consider whether existing data can inform the feasibility, design, and implementation of future statin trials in “real-world” older adults without ASCVD; and consider ASCVD clinical trial options and designs to address knowledge gaps. The presentations and discussions at that workshop stimulated and informed this article.
Existing Evidence and Remaining Gaps on Statins for Primary ASCVD Prevention in Older Adults
No RCTs designed and powered a priori to assess whether statins should be initiated for primary prevention in older adults have been completed. Available evidence is derived from observational studies, subgroup and post hoc analyses, and meta-analyses of RCTs (Table 2). In a subgroup analysis from the Heart Protection Study (total n=20, 536 adults), the proportional reduction in the rate of first major vascular events among 28 % of adults ≥ 70 years (23.6% vs 28.7% for simvastatin vs placebo) was similar across age categories (< 65 and ≥ 65 < 70). However, the majority of participants in the study had history of coronary heart disease (65%), stroke, peripheral arterial disease or diabetes.10 Similarly, pravastatin was not associated with a significant reduction in a composite endpoint of coronary death, nonfatal MI, and fatal or nonfatal stroke over 3.2 years in the 56% of the 5,804 subjects aged 70 to 82 without existing ASCVD in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER).11
Table 2.
Meta-Analyses of Randomized Trials of Statins for Primary Prevention in Older Adults
| Study | Studies, n | Participants | Follow-Up, Years | Age Criterion, Years | Mean Age, Years | CVD Outcome | Non-CVD outcome |
|---|---|---|---|---|---|---|---|
| Savarese et al.13 | 7 RCTs, placebo vs statin, 1 open label | N=24,674 without atherosclerotic CVD; 43% female | 3.5 | ≥65 | 73 | - 1.2% ARR in MI (NNT=84) - 0.7% ARR in stroke (NNT=143) - No significantly lower CVD mortality |
No significant difference in all-cause mortality |
| Teng et al.14 | 8 RCTs, statin vs placebo or usual care’ | N=25,952 (n=12,974 statin, 12,978 placebo) | 3.5 | >65 | 72.7 | - Major adverse cardiovascular events
(RR=0.82) -MI (RR=0.74) -Non-fatal MI (RR=0.75) -No significant difference in fatal MI or stroke |
No significant difference in all-cause mortality or significant adverse events, including myalgia, transaminitis, and new-onset DM |
| Ridker et al.15 | 2 RCTs, rosuvastatin vs placebo | N=8,781 | Median: JUPITER, 1.8 HOPE-3, 5.6 | ≥70 | JUPITER, median 66 HOPE-3, mean 65.7 | -Nonfatal MI, nonfatal ischemic stroke and CV death, HR=0.74, 95% CI=0.61–0.91 | JUPITER: DM: rosuvastatin, n=270, 3%; placebo, n=216, 2.4%; HR=1.25, 95% CI=1.05–1.49, P=.01. In those with DM risk factors who were taking rosuvastatin, 134 vascular events or deaths were prevented at the cost of 54 new cases of DM. Progression to DM in those taking rosuvastatin occurred ≈ 5.4 weeks earlier than with placebo. HOPE-3: muscle pain or weakness: rosuvastatin, n=367, 5.8%; placebo, n=296, 4.7%, P=.005; no significant difference in withdrawals because of muscle symptoms (rosuvastatin, n=83, 1.3%; placebo, n=76, 1.2%, P=.63); rhabdomyolysis or myopathy (rosuvastatin, n=2; placebo, n=1), cancer (rosuvastatin, n=267; placebo, n=286); cataract surgery (rosuvastatin, n=241, 3.8%; placebo, n=194, 3.1%, P=.02)17 |
RCT=randomized controlled trial; CVD = cardiovascular disease; ARR=absolute risk reduction; NNT=number needed to treat; MI=myocardial infarction; RR=relative risk; HR=hazard ratio; CI=confidence interval; DM=diabetes mellitus.
A post hoc analysis of the Lipid-Lowering Trial (LLT) component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) compared outcomes associated with pravastatin (40 mg/d) in those aged 75 and older with outcomes in those aged 65 to 74.12 In 2,867 participants without ASCVD (mean age 71), there was no significant difference in risk of CHD events or all-cause death between pravastatin 40 mg/d and usual care. A trend toward a higher rate of the primary outcome of all-cause mortality was seen in those aged 75 and older receiving pravastatin (hazard ratio (HR)=1.34, 95% confidence interval (CI)=0.98–1.84). Limitations of ALLHAT-LLT study include use of a low-potency statin; use of usual care rather than placebo control; and conduct of the study 15 to 21 years ago, when usual care standards differed from contemporary practice. In addition, 29% of usual care participants were taking a statin (including one of the newer statins entering the market, which were more potent than pravastatin), and 22% of those in the pravastatin arm were not taking a statin by the end of the trial, which may have diluted the LDL-C-lowering difference between the intervention and comparator arms.
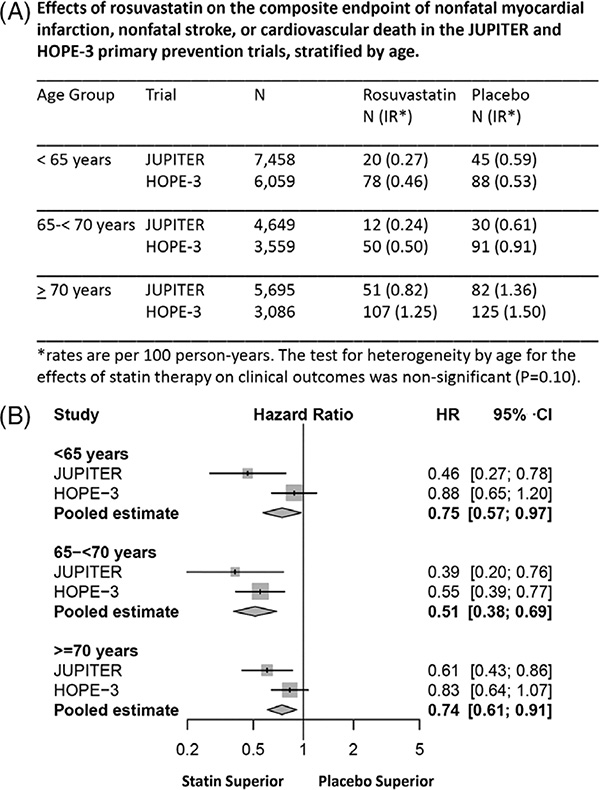
Several meta-analyses of RCTs of statins for primary prevention in adults aged 65 and older have been reported, but persons aged 80 and older, especially those with geriatric syndromes (e.g., frailty, sarcopenia, functional, cognitive decline) and multiple chronic conditions, are underrepresented in the contributing studies.3–6,13–15 (Tables 1 and 2) A meta-analysis of data from two placebo-controlled RCTs,15 Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) (mean age 66),16 in which rosuvastatin (20 mg) was used, and Heart Outcomes Prevention Evaluation (HOPE-3) (mean age 66),17, in which low-dose rosuvastatin (10 mg)) was used, reported a 26% reduction with rosuvastatin in risk of the composite endpoint of nonfatal MI, nonfatal ischemic stroke, and cardiovascular death in participants aged 70 and older (mean age 74), but the magnitude of the effect was statistically significant only in JUPITER (Figure 1).15
Figure 1.
Meta-analyses of Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin trial (JUPITER) and Heart Outcomes Prevention Evaluation-3 (HOPE-3) for efficacy of rosuvastatin for primary atherosclerotic cardiovascular disease (ASCVD) prevention in older adults. (A) Effects of rosuvastatin on the composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death in the JUPITER and HOPE-3 primary prevention trials. *Numbers of individuals at risk, incidence rates, and hazard ratios for nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death in the JUPITER and HOPE-3 primary prevention trials, stratified according to age. (B) Meta-analysis within age subgroups of the JUPITER and HOPE-3 primary prevention trials evaluating the effects of rosuvastatin on the composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. Reused with permission from Ridker PM et al. Primary Prevention With Statin Therapy in the Elderly. New Meta-Analyses From the Contemporary JUPITER and HOPE-3 Randomized Trials. Circulation 2017;135:1979–1981. Available at https://www.ahajournals.org/journal/circ
Noncardiovascular Outcomes and Potential Harms of Statins in Older Adults
Most primary prevention RCTs have evaluated the effect of statins on ASCVD outcomes. These studies have rarely evaluated non-ASCVD outcomes and were underpowered to detect harms. Measures of overall universal health outcomes meaningful to older adults, such as dementia- and disability-free survival, physical and cognitive function, independence, and health-related quality of life (QOL), are crucial to convey individualized risks and benefits of statins needed to provide care concordant with people’s priorities.18,19 Age-associated changes may increase vulnerability to harmful outcomes, such as cognitive decline, muscle symptoms, and fatigue. (Table 3, Supplementary Table S1). Thus, more robust and consistent measurement of global health outcomes that older adults value is needed in future trials of statins for primary prevention in older adults.18,19.
Table 3.
Evidence Gaps Regarding Statins for Primary Prevention in Adults Aged 75 and Older Without Atherosclerotic Cardiovascular Disease
| Characteristics | Area Requiring New Clinical Trial Evidence |
|---|---|
| Patient groups | • Larger numbers of older adults,
especially aged ≥80, with characteristics representative of the
target U.S. population (e.g., multimorbidity, at risk for functional or
cognitive decline, polypharmacy) • Data in older women • Data in underrepresented subpopulations such as minorities |
| Inclusion of measures important to older adults | • Universal health
outcomes • Measures of cognition, function, heath-related quality of life, frailty, multiple chronic condition burden, disability, incident dementia, and atherosclerotic cardiovascular disease |
| Time to benefit | • Data on optimal time to start or stop statin therapy |
| Specific statin | • Dose and type of statin based on individualized risk assessment |
Reports on a possible relationship between statins and cognitive decline are conflicting. A Cochrane review of 2 large placebo-controlled RCTs of statins to prevent dementia in 26,340 participants (aged 40 to 82, n=11,610 aged ≥ 70) reported no statin-related reduction in incident dementia.20 There were limitations of the included RCTs involving the cognitive assessments used, and the studies included participants at moderate to high vascular risk only. In contrast, several observational studies reported a protective effect of statin therapy against incident dementia (HR=0.80, 95% CI=0.68–0.95).21 A longitudinal study of older adults with baseline normal cognition noted a slower rate of annual worsening of cognitive performance in statin users than in nonusers (n=2,363),22 and statin users (n=1224, mean age 72.8 ± 8.17) out-performed nonusers in measures of attention. Older adults at highest risk of cognitive decline, including very old individuals and persons with low education levels, are underrepresented in most trials. Assessment of the potential protective effect of statins against incident and worsening dementia in RCTs is limited because measures of cognition and dementia are rarely a prespecified outcome and are assessed using various methods at various time points, including passive collection of adverse events in some studies. Thus, existing data highlight the need for validated measures of cognition to assess populations with differing baseline cognitive function and dementia risk in adequately powered RCTs of statins.
In contrast to the purported protective effect of statins against dementia, several postmarketing reports of cognitive impairment associated with statins (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) weeks to years after statin use have prompted a labeling change by the U.S. Food and Drug Administration,23 although a meta-analysis of placebo-controlled trials failed to show significant adverse effects of statins on cognition in cognitively normal subjects (n=45,564 participants from 18 RCTs, 9 of which included participants aged ≥65) or those with Alzheimer’s disease (n=1,153 participants from four RCTs; mean age > 68).24 Future studies should evaluate whether statins have a deleterious effect on cognition in oldest adults.
Data are also needed on statin-associated muscle symptoms (SAMS) in older adults to clarify their incidence, pathophysiology, and possible effect on muscle strength, fatigue, frailty, physical function, and risk of falls.25 A Dutch observational study of adults aged 75 and older compared 1,261 statin users with 3,094 nonusers and found no difference in prevalence of muscle complaints (3.3% vs 2.5%, P = .18).26 Recent genome-wide association studies have shown that common variants in SLCO1B1*5 are strongly associated with greater risk of simvastatin-induced myopathy27 and other mild statin-induced side effects.28 The clinical significance and underlying mechanism of SAMS could be elucidated by including prospective definitions of SAMS29 and objective measurement of strength and activity and by using well-established diagnostic biomarkers such as creatinine kinase levels in future studies. Methods to integrate statin withdrawal and rechallenge and to assess muscle structure and function would clarify the clinical importance of SAMS and help distinguish it from common musculoskeletal conditions in older adults, including arthritis, sarcopenia, frailty, and deconditioning. It is important to determine whether age-related alterations in skeletal muscle structure and function increase vulnerability to SAMS, as well as the potential influence of comorbidities, polypharmacy, pharmacokinetics, pharmacodynamics, and frailty on the risk of SAMS.
The greater risk of incident diabetes mellitus (DM) associated with statin use is of special concern for older adults, especially older women, because it is likely that age-related changes in body composition and metabolism increase vulnerability to DM.30 A meta-analysis of 17 trials reported greater risk of incident DM (odds ratio=1.09, 95% CI=1.02–1.17),31 with a higher risk reported with higher-intensity than moderate-intensity statins.32 A RCT showed that only participants with 1 or more risk factors for DM (e.g., high body mass index, glycosylated hemoglobin, high fasting blood sugar, metabolic syndrome) progressed to DM after statin initiation.33
Can Current Data Be Extrapolated to Older Adults?
Given the limited numbers of older adults included in RCTs of statins for primary prevention, is it reasonable to extrapolate trial data from persons younger than 75 to this population? Age-related concerns that limit the generalizability of existing data from younger to older adults are shown in Supplementary Table S1.
The strength of the association between high serum LDL cholesterol and an increase in the risk of ASCVD events becomes weaker with advancing age, in part because of survival bias, despite the increasing absolute risk of ASCVD in older adults.34,35(Figure 1A) Thus, the efficacy of statins in mitigating the risk of incident ASCVD events by lowering LDL-C is less clear in those aged 75 and older, particularly as they age further. Age-associated changes in pharmacodynamics and pharmacokinetics, declining kidney function, other comorbid conditions, and polypharmacy may influence the benefit-to-harm ratio of statins in older adults. Moreover, better understanding of how age-related alterations in body composition (adipose, free water), drug metabolism, drug-protein binding, and reduced renal clearance affect the potency and effect of specific statins on clinical outcomes may identify ways to lessen their harms.
It is likely that the differences in time to benefit (the time for a population to realize the intended benefits of a medication) of statins to reduce risk of CHD, ischemic stroke, or non-ASCVD outcomes, versus time to harm, especially in the setting of advancing age with limited life-span, influence decision-making on if and when statins are recommended for preventive therapy. Older adults with limited life expectancy may be exposed to the immediate risks of statins adversely affecting QOL, with little likelihood of surviving long enough to reap their benefits. The timing of initiation and duration of statin therapy were identified as crucial evidence gaps that necessitate careful planning of enrollment criteria for future trials. Whether future RCTs enroll statin-naïve participants, those who are already taking statins, or both may depend on the feasibility of enrollment. Feasibility challenges may also determine whether future RCTs could be placebo controlled. Evidence to support guidance on when to stop statin therapy in older adults with a limited lifespan is also needed.
The Statin Therapy for Reducing Events in the Elderly Trial
Launched in 2015, the Statin Therapy for Reducing Events in the Elderly (STAREE) trial is comparing the effect of atorvastatin 40 mg/d (starting at 20 mg/d and titrated to 40 mg/d) with that of placebo on the composite co-primary outcomes of time from randomization to death or development of dementia or significant disability and to a major fatal or nonfatal cardiovascular event in 18,000 adults aged 70 and older () (www.staree.org/au). Secondary outcomes include the individual composite endpoint components, new-onset DM, incident cancer, cognitive decline or incident dementia, frailty or disability, need for permanent residential care, and QOL. Eligible participants must be independent living (without assistance), and exclusion criteria include known ASCVD, DM, dementia or a Modified Mini-Mental State Examination score less than 78 at entry, total cholesterol greater than 290 mg/dL, an estimated glomerular filtration rate less than 45 mL/min per 1.73 m2, chronic liver disease, serious intercurrent illness likely to cause death within the next 5 years, unwillingness to stop current statin or other lipid-lowering therapy, absolute contraindication to statin therapy, or long-term use of selected cytochrome P450 3A4 inhibitors.
Although STAREE is expected to bolster the evidence base of the efficacy and safety of statin therapy for primary prevention in older adults, several aspects of the design are worth highlighting. First, it is likely that enrolling participants aged 70 and older will lead to having approximately half of enrollees being aged 70 to 75, which directly overlaps with data available from JUPITER and HOPE-3 and limits much-needed data on those aged 80 and older.15 Second, exclusion of individuals with common comorbidities (e.g., DM, kidney disease) will lower cardiovascular event rates and reduce generalizability to the growing population of older adults with these comorbidities. Third, inclusion of development of dementia (even if mild) as part of the co-primary endpoint may preclude the ability of the trial to address disability, functional status, and QOL outcomes in individuals with established dementia. Finally, because the trial’s main sites are in Australia, limited racial and ethnic diversity and differences in background medical care may affect generalizability of the findings to the increasingly diverse U.S. population of older adults.
Potential Trial Options to Guide Decision-Making on Statins for Primary Prevention in Octogenarians and Beyond
One or more prospective, placebo-controlled RCTs could address the important knowledge gaps identified regarding the efficacy and safety of statins in persons aged 75 and older without ASCVD, especially those aged 80 and older and with multiple chronic conditions. Future clinical trial design considerations include a traditional efficacy RCT versus a large simple pragmatic RCT embedded in healthcare networks and implemented in “real-world” settings; inclusion and exclusion of participants with specific comorbidities, cardiovascular risk factors, frailty, and functional or cognitive impairment; the optimal lower age cut-off of participants for inclusion in the study (≥70, ≥ 75, or ≥ 80) to address existing knowledge gaps; choice and daily dosage of statin therapy; and trial duration. Examples of 2 potential trial designs are summarized in Table 4.
Table 4.
Summary of Possible Randomized Trial Options of Statins for Primary Prevention in Older Adults without Atherosclerotic Cardiovascular Disease (ASCVD)
| Design Features | Pragmatic Clinical Trial | Traditional Clinical Trial |
|---|---|---|
| Randomization | Individual-level randomization, at level of healthcare system, or according to site or provider within a healthcare system | Individual-level randomization |
| Age criteria | ≥75, with significant number ≥80 | ≥75, with significant number ≥80 |
| Target population | No known clinical ASCVD and not currently taking statins. Cohort will include significant number of women, minorities, participants with frailty, mobility limitations, cognitive impairment, targeted comorbidities. | No known clinical ASCVD and not currently taking statins. Cohort will include significant number of women, minorities, participants with frailty, mobility limitations, cognitive impairment, targeted comorbidities. |
| Intervention | Statin vs placebo. High-dose atorvastatin (80 mg/d) should be avoided. If lower dose initiated, plan for up-titration could be considered, but goal serum LDL-C is unclear in older adults. | Statin vs placebo. High-dose atorvastatin (80 mg/d) should be avoided. If lower dose initiated, plan for up-titration could be considered, but goal serum LDL-C is unclear in older adults. |
| Variables to be considered for inclusion | Measures of cognitive function, physical function, SAMS, health-related quality of life, disability, comorbidities, medications, symptom burden | Measures of cognitive function, physical function, SAMS, health-related quality of life, disability, comorbidities, medications, symptom burden |
| Treatment duration | Average 4–5 years | Average 4 years |
| Outcomes | Two outcomes considered as primary and secondary endpoints vs co-primary endpoints: - Survival free of dementia or persistent physical disability - Fatal and nonfatal CVD events | Two outcomes considered as primary and secondary endpoints vs co-primary endpoints: - Survival free of dementia or persistent physical disability - Fatal and nonfatal CVD events |
| Sample size | 15,000–17,000 | 13,000 |
| Power | 90% at 2-sided alpha of 0.05 to detect 13% reduction in incidence of death, dementia, and persistent physical disability and 18% reduction in incidence of composite CVD outcome | 90% at 2-sided alpha of 0.025 to detect 15% reduction in incidence of death, dementia, and persistent physical disability and 18% reduction in incidence of composite CVD outcome |
LDL-C=low-density lipoprotein cholesterol, SAMS=statin induced muscle symptoms, CVD=cardiovascular disease.
Several outcomes were discussed including one representing global health, such as freedom from dementia and disability and an ASCVD efficacy outcome, such as fatal and nonfatal ASCVD events. These outcomes could be considered as primary and secondary endpoints or as co-primary endpoints. A pragmatic RCT could ideally leverage existing networks of healthcare systems (e.g., Health Care Systems Research Network,36 Cardiovascular Research Network,37 NIH Collaboratory,38 Patient-Centered Outcomes Research Network 39. Randomization could occur at the level of the individual, the site, or the healthcare system.
Many design elements, including variables and outcomes of importance to older adults, could be incorporated into either type of trial design. A recruitment strategy that ensures adequate enrollment of individuals in their 80s and 90s; women; underrepresented minorities; and clinical, functional, and social characteristics consistent with the general U.S. population of older adults without clinical ASCVD would best help to fill knowledge gaps. Additionally, incorporation of validated measures of cognition, physical function, disability, frailty, SAMS, heath-related QOL, and burden of multimorbidity are needed in future clinical trials. The lack of evidence supports the need for future placebo-controlled trials in “real-world” older adults who are truly representative of this population. Other considerations for future trials in older adults include specific statin selection and dosage, potential drug–drug interactions, specific statin properties affecting potency (protein-binding, metabolism and clearance), and drug safety considerations. Existing data support the need for future RCTs that exceed 4 years of follow-up to evaluate efficacy for the main endpoints, which could be challenging if recruiting a high proportion of the oldest adults (e.g., aged ≥ 85).
Feasibility Analyses of the Eligible Population Within Kaiser Permanente Northern California and Kaiser Permanente Northwest
Retrospective cohort analyses in 2 large integrated healthcare delivery systems providing comprehensive care for approximately 4.8 million people in Northern California, Oregon, and Washington (~0.6 million) presented at the workshop evaluated the feasibility of a primary prevention statin trial in older adults. Adults aged 75 and older with at least 12 months of continuous enrollment before entry and no known ASCVD were identified between October 1, 2010, and September 30, 2015. Of the 269,155 eligible persons aged 75 and older, 41% were receiving a statin at entry. Those with DM (65%) and chronic kidney disease (51%) had the highest rates of statin use. Of those not taking statins at entry, 30% initiated a statin, mainly atorvastatin or simvastatin. For statin users, the annual incidence of death was 6.5% and of the composite of MI or ischemic stroke was 0.3%.
For successful implementation of a primary prevention RCT, the question of equipoise between providers and participants must be assessed. Given that 41% of older adults without ASCVD take statins, further feasibility studies may be necessary to assess whether providers and patients in healthcare systems are willing to be randomly assigned to statin or placebo or to stop a statin that is being taken. Although these preliminary data support the feasibility of an adequately powered RCT, they also highlight a likely limited window of opportunity to conduct such a trial before the use of statin therapy increases further in older adults.
Conclusion
There is a critical evidence gap relating to the benefits and risks of initiating statins for primary prevention in older adults, especially those aged 80 and older with multiple chronic conditions, which existing data or extrapolating evidence from trials in adults younger than 75 cannot address. Elucidation of how age-associated changes in physiology, body composition, and pharmacokinetics may increase vulnerability to statin-related harms and incorporation of validated methods to identify the risk of primary ASCVD events in adults aged 75 and older are warranted to fill these gaps. One or more prospective, placebo-controlled RCTs that include specific design elements pertinent to age-related changes could provide evidence to support person-centered decision-making regarding whether to initiate statins for primary prevention in adults aged 75 and older without ASCVD. Inclusion of global health outcomes important to older adults, including survival free of dementia and disability, independence, QOL, cognitive function, SAMS, and DM, in future trials would further contribute to informed decision-making. Possible trial designs include traditional RCTs and pragmatic RCTs, which are relevant to the majority of older adults currently seen in clinical practice. Feasibility data from 2 large healthcare delivery systems suggest that there is sufficient power for ASCVD events, but given that more than 40% of adults aged 75 and older without ASCVD are taking statins for primary prevention without supporting evidence of benefit, the timeliness of future trials in this setting is important, as is the willingness of providers and participants to be randomized.
Supplementary Material
Supplemental Table S1. Issues to Consider Regarding Statins for Primary Prevention in Older Adults
ACKNOWLEDGMENTS
Financial Disclosure: The National Institute on Aging (NIA) funded the “Opportunities for Trials on Effects of Statins in Primary Prevention in Older Adults” workshop, which was held in Bethesda, Maryland, July 31-August 1, 2017, with support and participation from representatives of the National Heart, Lung, and Blood Institute (NHLBI). Funding for this work was also supported by R24AG045050 from the NIA (JG, SF, AG).
The study authors acknowledge the contributions of Daniel E. Forman, MD, to the present manuscript.
The authors thank the following NIA and NHLBI staff for coordinating and participating in the workshop: Lawrence C. Fine, MD, NHLBI; Evan Hadley, MD, Sergei V. Romashkan MD, PhD, and Marcel Salive, MD, NIA.
We thank the following individuals whose presentations at the workshop, in addition to those of the coauthors, stimulated the development of this article:
Lesley Curtis, PhD, Duke Clinical Research Institute
Michelle Oden, PhD, MS, Oregon State University
Eric D. Peterson, MD, Duke Clinical Research Institute
Paul M. Ridker, MD, Brigham and Women’s Hospital, Harvard School of Medicine
Janice B. Schwartz MD, University of California San Francisco
Mary Sano, PhD, Icahn School of Medicine
Paul D. Thompson, MD, Hartford HealthCare Heart & Vascular Institute
The content of this manuscript reflects the views of the authors and does not necessarily reflect the official views or policies of the NIA, NHLBI, the National Institutes of Health, or the Department of Health and Human Services.
Conflict of Interest. Dr. Singh reports giving expert testimony for plaintiffs’ attorneys in litigation involving a statin. Dr. Go reports receiving a research grant through his institution from Sanofi. Dr. Wenger reports research grants, contracts, trial steering committee, trial data safety and monitoring board involvement with Gilead Sciences, NHLBI, Pfizer, and Society for Women’s Health Research. She also reports consultancies with Amgen, AstraZeneca, Gilead Sciences, Janssen Pharmaceuticals, and Merck. Dr. Zoungas reports past participation in advisory boards, educational meetings or research on behalf of Monash University (for work unrelated to this paper) with AstraZeneca Pty Ltd, Eli Lilly Australia Pty Ltd, Merck Sharp & Dohme (Australia) Pty Ltd, and Novo Nordisk Pty Ltd. Dr. Gurwitz serves as a member of the UnitedHealthcare Pharmacy and Therapeutics Committee.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Akushevich I, Kravchenko J, Ukraintseva S, Arbeev K, Yashin AI. Age patterns of incidence of geriatric disease in the U.S. elderly population: Medicare-based analysis. J Am Geriatr Soc 2012;60:323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–2934. [DOI] [PubMed] [Google Scholar]
- 3.Mihaylova B, Emberson J, Blackwell L et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012; 380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibbins-Domingo K, Grossman DC, Curry SJ et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016;316:1997–2007. [DOI] [PubMed] [Google Scholar]
- 5.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: Evidence report and systematic review for the us preventive services task force. JAMA 2016;316:2008–2024. [DOI] [PubMed] [Google Scholar]
- 6.Chou R, Dana T, Blazina I et al. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Statin Use for the Prevention of Cardiovascular Disease in Adults: A Systematic Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. [PubMed] [Google Scholar]
- 7.Odden MC, Pletcher MJ, Coxson PG et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muldoon MF, Ryan CM, Sereika SM, Flory JD, Manuck SB. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med 2004;117:823–829. [DOI] [PubMed] [Google Scholar]
- 9.Goff DC, Lloyd-Jones DM, Bennett G et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Journal of the American College of Cardiology 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002;360:7–22. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J, Blauw GJ, Murphy MB et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002;360:1623–30. [DOI] [PubMed] [Google Scholar]
- 12.Han BH, Sutin D, Williamson JD et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: The ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017;177: 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savarese G, Gotto AM Jr., Paolillo S, D’Amore C, Losco T, Musella F, et al. Benefits of statins in elderly subjects without established cardiovascular disease: A meta-analysis. J Am Coll Cardiol 2013;62:2090–2099. [DOI] [PubMed] [Google Scholar]
- 14.Teng M, Lin L, Zhao YJ et al. Statins for primary prevention of cardiovascular disease in elderly patients: Systematic review and meta-analysis. Drugs Aging 2015;32:649–661. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Lonn E, Paynter NP, Glynn R, Yusuf S. Primary prevention with statin therapy in the elderly: New meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation 2017;135: 1979–1981. [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Danielson E, Fonseca FA et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S, Bosch J, Dagenais G et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31. [DOI] [PubMed] [Google Scholar]
- 18.Tinetti ME, McAvay GJ, Chang SS et al. Contribution of multiple chronic conditions to universal health outcomes. J Am Geriatr Soc 2011;59: 1686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Case SM, O’Leary J, Kim N, Tinetti ME, Fried TR. Older Adults’ Recognition of Trade-Offs in Healthcare Decision-Making. J Am Geriatr Soc 2015; 63:1658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database System Rev 2016;1:CD003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: A cohort study validated by comparison with randomized trials. Br J Clin Pharmacol 2009;67:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steenland K, Zhao L, Goldstein FC, Levey AI. Statins and cognitive decline in older adults with normal cognition or mild cognitive impairment. J Am Geriatr Soc 2013;61:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.FDA Drug Safety Communication: Important Safety Label Changes to Cholesterol-Lowering Statin Drugs (online). Available at https://www.fda.gov/Drugs/DrugSafety/ucm293101.htm Accessed December 6.
- 24.Ott BR, Daiello LA, Dahabreh IJ et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med 2015;30:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenson RS, Baker S, Banach M et al. Optimizing cholesterol treatment in patients with muscle complaints. J Am Coll Cardiol 2017;70:1290–1301. [DOI] [PubMed] [Google Scholar]
- 26.van der Ploeg MA, Poortvliet RK, van Blijswijk SC et al. Statin use and self-reported hindering muscle complaints in older persons: A population based study. PloS One 2016;11:e0166857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Link E, Parish S, Armitage J et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008;359:789–799. [DOI] [PubMed] [Google Scholar]
- 28.Voora D, Shah SH, Spasojevic I et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J Am Coll Cardiol 2009;54: 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker BA, Capizzi JA, Grimaldi AS et al. Effect of statins on skeletal muscle function. Circulation 2013;127:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Culver AL, Ockene IS, Balasubramanian R et al. Statin use and risk of diabetes mellitus in postmenopausal women in the women’s health initiative. Arch Intern Med 2012;172:144–152. [DOI] [PubMed] [Google Scholar]
- 31.Mills EJ, O’Regan C, Eyawo O et al. Intensive statin therapy compared with moderate dosing for prevention of cardiovascular events: A meta-analysis of > 40 000 patients. Eur Heart J 2011;32:1409–1415. [DOI] [PubMed] [Google Scholar]
- 32.Preiss D, Seshasai SR, Welsh P et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: A meta-analysis. JAMA 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012;380:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Psaty BM, Anderson M, Kronmal RA et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: The Cardiovascular Health Study. J Am Geriatr Soc 2004;52: 1639–1647. [DOI] [PubMed] [Google Scholar]
- 35.Orkaby AR, Gaziano JM, Djousse L, Driver JA. Statins for primary prevention of cardiovascular events and mortality in older men. J Am Geriatr Soc 2017;65:2362–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Health Care Systems Research Network (online). Available at http://www.hcsrn.org/en/ Accessed January 22, 2018.
- 37.Cardiovascular Research Network (online). Available at http://cvrn.org/Pages/default.aspx Accessed January 22, 2018.
- 38.NIH Collaboratory (online). Available at http://www.rethinkingclinicaltrials.org Accessed January 22, 2018.
- 39.PCORnet The National Patient-Centered Clinical Research Network. Available at http://www.pcornet.org Accessed January 22, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Issues to Consider Regarding Statins for Primary Prevention in Older Adults