Abstract
The physiological functions of astrocytes within neural circuits remain incompletely understood. There has been progress in this regard from recent work on striatal astrocytes, where detailed studies are emerging. In this review, findings on striatal astrocyte identity, form, and function, are summarized with a focus on how astrocytes regulate striatal neurons, circuits, and behavior. Specific features of striatal astrocytes are highlighted to illustrate how they may be specialized to regulate medium spiny neurons (MSNs) by responding to, and altering, excitation and inhibition. Further experiments should reveal additional mechanisms for astrocyte–neuron interactions in the striatum and potentially reveal insights into the functions of astrocytes in neural circuits more generally.
Astrocytes and Neural Circuits
Documented over 150 years ago [1], astrocytes are part of the larger population of central nervous system (CNS) cells called glia, which includes oligodendrocytes, microglia, and other specialized cells [2]. Although neural circuits are often thought of as comprising only neurons, an anatomically exact definition of a neural microcircuit is that ‘comprising neurons and associated cells such as glia, organized to carry out specific operations within a region of the nervous system’ [3]. Astrocytes do not operate in isolation and their functions relate to interactions with many types of brain cell. In these settings, established and proposed roles for astrocytes include interactions with the vasculature, the blood–brain barrier, regulation of synapse formation and loss, ion homeostasis, neurotransmitter clearance, metabolic support, regulation of action potential waveforms, and synaptic plasticity [4].
The proportion of astrocytes varies considerably between brain areas [5] and species. Current estimates suggest that astrocytes represent ~19–40% of brain cells [6]. Numbers aside, astrocytes represent an important type of cell necessary for neural circuits to develop and function [2]. Furthermore, astrocytes are widely implicated in brain disorders [7–9]. Broad advances in this field have renewed interest in the fundamental biology of astrocytes within fully developed brain areas. For instance, astrocyte regulation of neurons has been proposed to occur via: (i) vesicular gliotransmission; (ii) channel-mediated release of neuroactive substances; (iii) neurotransmitter homeostasis; (iv) ionic homeostasis; (v) movement of astrocyte processes towards or away from synapses; (vi) release of neurotoxic factors; (vii) regulation of extracellular diffusion; (viii) neurovascular coupling; (ix) metabolic coupling; and (x) astrocytes acting as intermediary mediators of neuromodulation [2,4,7,10–16]. However, there are few examples where astrocytes have been studied comprehensively in a single adult vertebrate brain area to uncover functions that may be tailored to that circuit (many of the aforementioned insights were from multiple brain areas). In this review, I summarize recent findings related to these topics in relation to astrocytes in the striatum, where detailed work is emerging. Other than two pioneering papers [17,18], little was known about striatal astrocytes until recently.
The Striatum: Exploring Astrocytes Systematically in a Defined Circuitry
The striatum is the largest input nucleus of the basal ganglia (BG), a group of interconnected subcortical nuclei [19]. In rodents, most (~95%) neurons within the striatum are γ-aminobutyric acid (GABA)ergic projection neurons called medium spiny neurons (MSNs) (see Glossary). The remainder are GABAergic and cholinergic interneurons. The striatum receives excitatory gluta-matergic input from the cortex and thalamus, which impinges on extensive dendritic spines of D1-dopamine receptor expressing and D2-dopamine receptor-expressing MSNs (Figure 1A). One essential function of the striatal circuit is to determine which subpopulations of MSNs fire during coordinated input, which alters the activity of downstream nuclei and, hence, BG-controlled behaviors [19–26]. In a simplified interpretation, activation of the D1 MSNs inhibits the firing of the BG output nuclei, the substantia nigra pars reticulata (SNr) and internal globus pallidus (GPi) via the ‘direct’ striatonigral projection, resulting in disinhibition of thalamocortical circuits. Conversely, activation of D2 MSNs enhances SNr activity via the striatopallidal (indirect pathway) circuit, with resultant inhibition of thalamocortical drive via activation of the striatopallidal circuit. While the relative activity level of each output pathway originating from the dorsolateral striatum was once thought of as having strictly opposing roles in movement facilitation and suppression (the rate model), recent work has indicated that it is a precise and concomitant pattern of complementary ensemble activity of these pathways, within spatially biased local clusters, that regulates successful and appropriate movement initiation, and that subsequently controls the smooth execution of action sequences or behavioral ‘syllables’ [27–29]. Thus, appropriate complementary activity patterns in D1 and D2 MSN pathways have important implications for pathological conditions that produce excessive repetitive behaviors, disrupted motor sequences, and/or excessive behavioral switching. An indication of the importance of the striatum derives from the fact that multiple neurological and psychiatric diseases involve striatal dysfunction, including Huntington’s disease (HD), Parkinson’s disease (PD), repetitive behaviors, attention-deficit hyperactivity disorders, obsessive-compulsive disorders, habits, rituals, and tics [20,22,30]. There are also peculiar pediatric throat infections that appear to alter striatal and BG function irreparably, leading to behavioral phenotypes such as obsessive-compulsive disorder (OCD) [31]. These involve astrocytes and other glia [32,33], which form part of the immune response of the brain. In HD, there is progressive striatal degeneration in proportion to the severity of motor, psychiatric, and cognitive deficits [30] as well as evidence to support a role for astrocytes [9]. Hence, the striatum provides an opportunity to study astrocytes in a nucleus with not only well-characterized inputs and outputs (Figure 1A), but also the potential to inform about behavior and disease (Figure 1B,C). Furthermore, MSNs are relatively homogenous and can be studied in tissue slices and in vivo, although the striatum itself comprises distinct anatomical units that mediate separable functions. As discussed later, multiple MSNs interact anatomically with single astrocytes, providing a basis to explore signaling between these cell types. Finally, the striatum is cytoarchitecturally distinct with a sizeable volume in mice (~23 mm3) and humans [34,35] that renders it amenable to a range of molecular, biochemical, and physiological approaches.
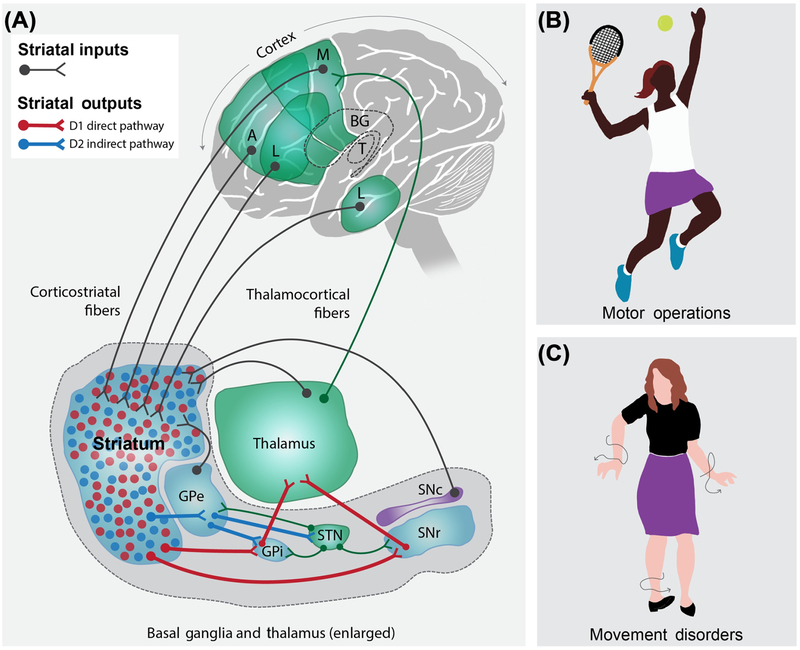
Figure 1. Simplified View of the Striatum in Relation to the Basal Ganglia Circuit and Its Known Roles in Behavior.
(A) Highly schematized cartoon illustrating the striatum in humans in relation to its synaptic inputs [thalamus (T), cortex – associative (A), motor (M), and limbic (L), substantia nigra pars compacta(SNc), and external segment of the globus pallidus(GPe)] and outputs via the basal ganglia (BG) circuitry [GPe, internal segment of the globus pallidus (GPi), and substantia nigra pars reticulate (SNr)]. The striatum receives glutamatergic excitation from multiple regions of the cortex, which impinges on D1 and D2 medium spiny neurons [MSNs (also called striatal projection neurons in some studies); colored red and blue, respectively]. These inputs project to downstream nuclei via the direct and indirect pathway formed by D1 and D2 MSNs, respectively. (B) Cartoon of a tennis player to illustrate that the striatum is intimately involved in movement, action selection, and motor operations. (C) Illustration of the chorea and abnormal movements observed in a patient with advanced-stage Huntington’s disease (HD), whereby movements are uncontrolled and abnormal. Other disorders thought to involve the BG and the striatum are mentioned in the main text. For simplicity, the striatum is shown as a single structure, although in humans it comprises the caudate nucleus, putamen, and nucleus accumbens. Abbreviation: STN, subthalamic nucleus.
Identity: Striatal Astrocyte Molecular Signatures
Transcriptomics
Since cell isolation and culture conditions alter astrocytes from their in vivo state [36], an alternative method to assess astrocyte transcriptomes in vivo was needed. For this purpose, RNA sequencing (RNA-Seq) of adult (P63) astrocytes using Aldh1/1-Cre/ERT2 × RiboTag mice was performed [37,38]. In these mice, the HA-tagged ribosomal subunit Rpl22HA [37] was expressed selectively in astrocytes [38]. Differential expression analysis revealed that 1180 genes were significantly enriched in striatal astrocytes relative to the hippocampus; that is, astrocytes were molecularly separable in the striatum relative to the hippocampus, a conclusion supported by other work [39–45].
The set of genes highly expressed and not differentially expressed between striatum and hippocampus includes ones involved in core astrocyte functions, for instance Slc1a2, Sparc, Kcnj10, and Slc6a11. There was significant expression of nine K+ channels and two auxiliary subunits in striatal astrocytes. Similarly, from known transmembrane Ca2+ flux pathways, significant gene expression for 23 proteins was found, consistent with the diversity of striatal astrocyte Ca2+ signals [46]. Furthermore, of the 249 known Ca2+ binding EF-hand containing proteins, 68 were expressed at appreciable levels [fragments per kilobase million (FPKM >10)] in striatal astrocytes, and 18 were differentially expressed in relation to the hippocampus. These data show that striatal astrocytes display richness in K+ channels, Ca2+ flux pathways, and proteins likely to buffer and respond to Ca2+. The RNA-Seq data are freely available (www.astrocyternaseq.org).
There were two surprises from RNA-Seq. The gene encoding glial fibrillary acid protein (Gfap) was not highly expressed in striatal astrocytes, but was highly expressed in hippocampal astrocytes. This is important, because it confirms that GFAP is not a robust marker for striatal astrocytes. This may have implications for the analysis of postmortem striatal tissue from neurodegenerative diseases, because such studies invariably use GFAP. Furthermore, the gene encoding μ-crystallin (Crym) was highly expressed in striatal, but not in hippocampal astrocytes. This suggests that Crym represents a striatum specific marker for astrocytes.
Proteomics
Proteomics was performed to validate RNA-Seq findings. The correlation between RNA and protein levels in striatum was strong, implying that mRNA levels reflected the abundance of most identified proteins. However, ~10% of the proteins showed high abundance, but low mRNA FPKM values (<10), which may indicate nonlinearity between protein lifetime and transcript levels. The top 20 proteins shared between hippocampus and striatum were: Actb, Gapdh, Hist1h2bf, Slc1a3, Hist1h4a, Slc1a2, Eno1, Atp1a2, Atp5b, Glud1, Ppia, Eno2, Atp5a1, Phgdh, Dpysl2, Tubb4b, Prdx1, Cfl1, Asrgl1, and Ndrg2 (listed by their gene symbols). Although some are well studied, little is known about the functions of others. Furthermore, statistical analyses revealed that, out of 143 proteins reproducibly detected, 18 were markedly enriched in striatal astrocytes. These were: Crym, Ppp1r1b, Tln1, Acaa2, Aldh5a1, Pdhb, Slc25a3, Epb4.1l2, Uqcrc2, Etfdh, Acadl, Acsf2, Acsbg1, Pcx, Aco2, Ldhb, Sept2, and Dld (listed with their gene symbols in order of enrichment). The functions of most of these in striatal physiology are unknown. Concordant with the transcriptomic profiles, the most differentially expressed striatal and hippocampal astrocyte proteins were μ-crystallin and GFAP, respectively. Furthermore, μ-crystallin expression was restricted to striatal astrocytes and was not detected in astrocytes from several brain areas, although it did occur in a small population of striatal neurons [47]. μ-Crystallin is thought to bind thyroid hormone and function as a ketimine reductase [48], but its function in the striatum remains unknown.
Marker Expression
Although GFAP was a poor marker of striatal astrocytes, antibodies against Aldh1l1, S100β, and GLT1 labeled most striatal astrocytes [38,46,49–53]. Kir4.1 expression identified most striatal astrocytes, but significant expression level differences existed between cells [49]. It is not clear whether this reflects true differences in Kir4.1 expression or exposure of the antibody epitope. By contrast, the astrocyte GABA transporter GAT-3 labeled ~30% of evenly distributed astrocytes [38,52]. Furthermore, μ-crystallin labeled ~85% of the astrocytes in the ventral striatum, but only ~30% in the dorsal regions, even though the density of astrocytes was equivalent (Figure 2). The significance of μ-crystallin expression within astrocytes along the dorsoventral axis may relate to the underlying neuronal circuity. It also suggests, but does not prove, local striatal astrocyte heterogeneity.
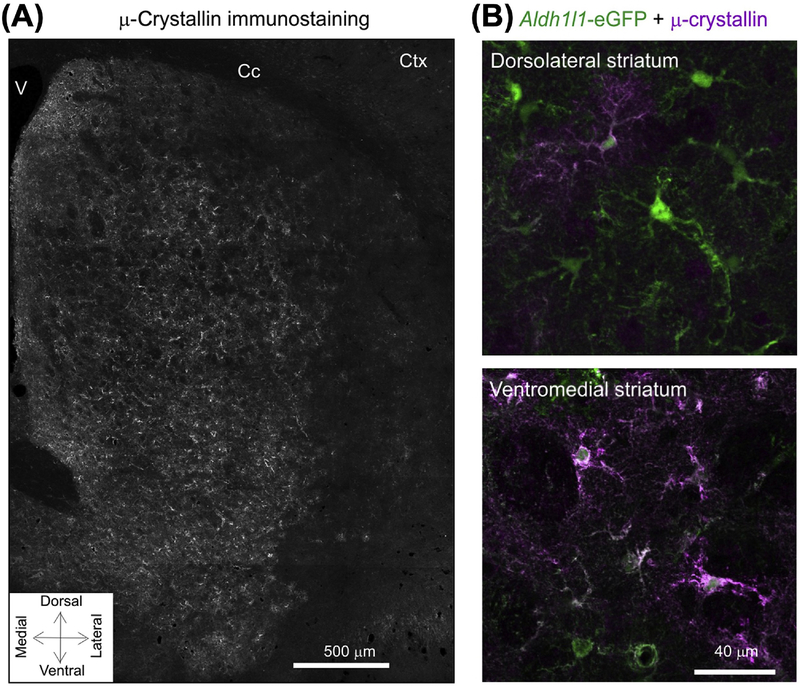
Figure 2. μ-Crystallin Displays a Gradient of Expression within Striatal Astrocytes.
(A) μ-crystallin immunostaining in striatum showing its spatial gradient. There are higher levels of expression in the ventral striatum compared with dorsal areas. (B) Representative images for μ-crystallin immunostaining in dorsolateral and ventromedial parts of the striatum in brain sections from Aldh1l1-eGFP mice. In the dorsolateral area, ~30% of astrocytes were μ-crystallin positive, whereas, in the ventromedial area, this was ~90%. Adapted from [50]. Abbreviations: Cc, corpus callosum; Ctx, cortex; V, ventricle.
Form: Striatal Astrocyte Morphology and Interactions with Neurons
Morphology
Striatal astrocytes are beautifully complex cells to observe under the microscope (Figure 3A), and comprise a cell body, six or so major primary branches that emanate from the soma, multiple secondary and tertiary branches, one or more thicker aquaporin-4-laden blood vessel-associated end-feet, and myriad finer branchlets and leaflets [4]. The finest leaflets are too small to be resolved with light microscopy and are irregular, thin, and sheet-like with dimensions on the tens of nanometer scale and with high surface area:volume ratios. They lack easily observed organelles, are devoid of GFAP, but show localized calcium signaling. Thus, up to 95% of an astrocyte comprises such leaflets [54], which are a defining feature of healthy astrocytes throughout the CNS. Together, these anatomical features give rise to the complex overall structure of astrocytes, which appear as ‘bushy’ cells with a clear soma when viewed with a cytosolic reporter, such as GFP, Lucifer yellow, or tdTomato [38,46,50,55] (Figure 3B). However, when viewed with a cell surface reporter, such as Lck-GFP, astrocytes appear as fluffy cloud-like cells [38], because the soma is not easily discernible.
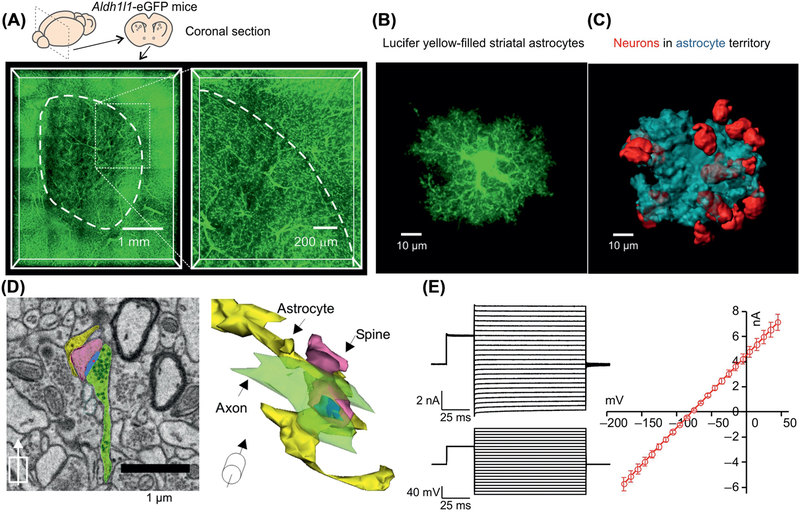
Figure 3. Properties of Striatal Astrocytes.
(A) Coronal sections of Aldh1l1-eGFP mouse brains cleared using the Sca/eS method and imaged using confocal microscopy to show abundant astrocytes within the striatum. The magnified section on the right shows the dorsolateral region of the striatum. Blood vessels are demarcated by their associations with GFP-expressing astrocyte end-feet. (B) Confocal volumes of a Lucifer yellow-filled striatal astrocyte. (C) 3D reconstructions of volumes enclosed by striatal astrocyte territories (blue) and NeuN (red). (D) Example of scanning electron microscopy (SEM) image from the striatum with corresponding 3D rendering displayed at an angle. The synaptic structures and closest astrocyte processes are colored as follows: yellow, astrocytes; blue, postsynaptic densities (PSDs); green, axons; and pink, spines. The center of the PSD is denoted by a red dot. (E) Representative current waveforms for a striatal astrocyte in response to stepwise changes membrane potential, along with an average current voltage relation from multiple cells. Reproduced from [50] (A–D); data in (E) from [51].
Although astrocytes are morphologically complex (Figure 3A), they do not appear to be strongly polarized [54]. The only polarized astrocyte compartment appears to be one or more end foot-bearing branches that demarcate the vasculature. Other astrocyte structures, such as the major branches, branchlets, and leaflets, do not have currently known markers that define them as molecularly separable. For example, Kir4.1 and GLT1 appear to be highly and evenly expressed within striatal astrocytes and not restricted to specific subcellular structures that can be detected with light microscopy. This means either that synapses exist throughout entire astrocytes or that Kir4.1 and GLT1 may perform functions broadly in the neuropil. Furthermore, striatal astrocyte leaflets at excitatory synapses do not appear to contain structures, such as vesicles, to support vesicular gliotransmission [50], although this requires further study.
Spatial Interactions with Neurons
Striatal astrocytes exist at a density of ~8 in the volume of a cube with 100-μm sides (~106 μm3) in Swiss-Webster mice [50]. A dye-filled striatal astrocyte has a volume of ~13 000 μm and the volume occupied by the smallest object around it (i.e., the volume that the astrocyte occupies) is ~59 000 μm3. These numbers are likely to be different for other mouse strains and vertebrate species, as is the volume of the striatum [35]. In a large area of dorsolateral striatum of several hundred square micrometers, there are about six times more neurons than astrocytes [50]. In accord, single fluorescently labeled striatal astrocytes contact multiple MSN somata (Figure 3C), with numbers that differ depending on whether MSN somata are counted only when they are entirely contained within an astrocyte territory (~11), or are also counted if they touch at the edges of the astrocyte (~20) [50,51]. The former represents a more conservative and consistent estimate, since the edge of an astrocyte is hard to define. Among the ~11 MSMs (on average) contacting each astrocyte, approximately six are D1 MSNs and five D2 MSNs [51]; this ratio is nearly identical to the relative densities of D1 and D2 MSN within the dorsolateral striatum [51]. Furthermore, the subpopulation of striatal astrocytes demarcated by Crym did not interact preferentially with D1 or D2 MSN somata [51]. The dendrites of a single MSN arborize locally to an area ~400 μm diameter in a plane [56], which would thus include ~170 astrocytes based on a flattened area of ~3000 μm2 per astrocyte [51]. The finding that striatal astrocytes are proximate to many MSN somata suggests that they regulate the extracellular milieu, as suggested by modeling in the lateral habenula [57] and by in-tissue K+ measurements in the striatum [49].
Spatial Interactions with Synapses
The density of excitatory synapses in the striatum [58] is ~0.9 per μm3, implying that single striatal astrocyte territories encompass ~50 700 excitatory synapses. The spatial interactions and approximate distances of astrocyte leaflets with such synapses has been assessed [51] with a fluorescence resonance energy transfer (FRET)-based method called the neuron astrocyte proximity assay (NAPA) and with serial block face scanning electron microscopy (SBF-SEM), although neither method accurately reflects the extracellular space. The proximity between striatal astrocyte leaflets and presynaptic terminals of asymmetric excitatory synapses that receive input from cortex and thalamus was evaluated [59]. The average distance from the pre- or postsynaptic membrane to the membrane of the nearest astrocyte leaflet was ~50 nm (Figure 3D). The distance between astrocyte leaflets and presynaptic terminals was variable: ~53% of the closest contacts were <10 nm away and likely measurable by NAPA. The other ~47% were between 10 nm and 400 nm away (i.e., at a distance longer than that measured with NAPA, but resolvable with colocalization).
The average distance from a tyrosine hydroxylase (TH)-identified putative dopaminergic pre- or postsynaptic membrane to the membrane of the nearest astrocyte leaflet was ~250 nm. Approximately 11% of the interactions were <10 nm away, but ~89% were between 10 nm and 1100 nm away (i.e., at a distance longer than that detected by NAPA). Taken together, these data show that excitatory and TH-positive synapses in the striatum are not ensheathed by astrocyte processes. Instead, astrocyte processes make variable finger-like associations with synapses [60,61]. NAPA was also used to assess where in an astrocyte different inputs occurred: the density of inputs tracked with the complexity of the astrocyte, implying that distinct inputs do not sample observable astrocyte subdomains, at least at the resolution discernible with light microscopy. Furthermore, stimulation of cortical inputs did not alter NAPA-based FRET between astrocyte leaflets and excitatory synapses, whereas strong ischemia in brain slices did [51]. Contact between astrocyte processes and excitatory synapses was altered in mouse models of HD [51]. The simplest explanation may be that the finest astrocyte processes withdraw from corticostriatal synapses in HD model mice at early stages of pathology.
Functional Properties
In rats, three electrophysiological signatures for striatal astrocytes have been reported, each with subtly different current-voltage relations and rectification [17,18]. However, in the dorsolateral region of the mouse striatum, the current-voltage relations were essentially linear [49–52] (Figure 3E). Striatal astrocytes displayed a negative resting membrane potential (Vm) of ~ −85 mV, a low membrane resistance of <10 MOhm and a passive Ohmic current-voltage relationship [46,49–52]. They expressed Kir4.1 channels, the blockade of which increased membrane resistance and depolarized the membrane by a few millivolts, implying that additional open K+ channels must draw the Vm towards the K+ equilibrium potential [49,50]. If ionic conductances with positive equilibrium potentials exist for Na+, Ca2+, or Cl−, then their contribution to Vm is dominated by K+ even when Kir4.1 is blocked. Thus, striatal astrocytes function like Nernstian K+ electrodes [49].
Gap-junctional coupling between astrocytes in the striatum is extensive: dialyzing a single astrocyte with dye led to spread to ~100 astrocytes within ~20 min [50], 98% of which were S100β-positive astrocytes [51]. With longer recording periods, this could be as high as 500 coupled cells [17,18]. The extent of gap-junctional coupling in vivo is unknown, but could alter how astrocytes regulate hundreds of MSNs within 3D volumes of coupled cells. The spread of dye between astrocytes was strongly inhibited by blocking gap junctions, and striatal astrocytes expressed significant mRNA for connexin 43 (encoded by Gja1) [50,51]. The PARIS method could be used to study regulation of gap junctional coupling between astrocytes in real time [62]. There are no available data on SWELL1 channel functions in striatal astrocytes [63], although they do occur there [50].
Function: Astrocyte–Neuron Interactions in Circuits and Behavior
After astrocyte Ca2+ signaling was reported to be regulated by neurotransmitters [64–66], Smith hypothesized that astrocyte Ca2+ signals may affect the function of neural circuits [67,68]. Testing such ideas has proven challenging. The exploration of astrocyte functions within the striatum is at a nascent stage, but progress has been made. Here, I summarize studies that have explored astrocytic regulation of MSNs with clear circuit, behavioral, or disease relevance. However, channelrhodopsin is not appropriate for stimulating striatal astrocytes, because it results in transient elevations in extracellular K+ [69]. Melanopsin may work, but has not been tried in striatum [70].
Striatal Astrocyte Ca2+ Signals
Striatal astrocytes display intracellular Ca2+ elevations, which are driven by Ca2+ entry across the plasma membrane and by Ca2+ release from intracellular stores [38,46,50,52,55]. At least three types of spontaneous Ca2+ signal are known. Global waves encompass the soma and large parts of astrocyte branches. Local waves are restricted and cover smaller parts of branches. Microdomains are frequent and cover only micrometer-scale areas of branches and somata. Fast signals lasting less than a few seconds in duration were not detected in striatal astrocytes [46]. Furthermore, striatal astrocytes responded poorly to corticostriatal axonal stimulation, but responded with intracellular Ca2+ elevations to applications of GABA, cannabinoids, ATP, phenylephrine, glutamate, and dopamine [38,46,50,52,53,55,71]. Mitochondrial Ca2+ signals were not evaluated, but have been reported elsewhere [72,73].
Circuit-Specific Signaling by Striatal Astrocytes
A recent study concluded that astrocytes in the dorsal striatum formed functional interactions with groups of D1 and D2 MSNs, suggesting that astrocyte subpopulations operate in a circuit-specific manner [71]. When the authors recorded from two D1 MSNs and strongly depolarized one to 0 mV for 5 s, they recorded the consequences of postsynaptic endocannabinoid release from the first MSN as an increase in astrocyte Ca2+ signaling and as a decrease in neurotransmitter release onto the first recorded neuron. The responses were mediated by astrocytic and presynaptic cannabinoid receptors (CB1Rs), respectively. However, in the second recorded D1 MSN, the authors measured an increase in glutamate release probability that was downstream of astrocyte CB1 receptor activation and the subsequent Ca2+-dependent release of astrocytic glutamate, which activated presynaptic metabotropic mGlur1/5 glutamate receptors. This only happened when they recorded from pairs of D1–D1 or D2–D2 MSNs (i.e., homotypic pairs) [71]. This is interesting, because the D1 and D2 MSNs are intermingled with no anatomical specificity in the dorsal striatum [74]. This led the authors to propose that astrocytes form subnetworks with specific populations of MSNs. This suggestion could have important implications in the striatum, but it should be interpreted with caution. At this point, there is no evidence to ascertain what the functional significance of such interactions could be for behavior or for in vivo circuit function [71]. The experiments were also reliant on 12–18-day-old mice (i.e., before gliogenesis is complete) [75]. Furthermore, striatal MSNs are well known to display prolonged postnatal development that extends to the end of Week 3 for the emergence of mature excitatory synapses and intrinsic membrane properties [76]. Although astrocytes were suggested to form subnetworks, intracellular dialysis results in coupling with many, perhaps all, astrocytes nearby [18,50]. In relation to mGlur1/5, in striatal astrocytes from adult mice, RNA-Seq and imaging instead show mGlur2/3 [38,46,50,77,78]. Astrocyte Ca2+-dependent glutamate gliotransmission occurs under some settings [71], but not in others [50], in the striatum and elsewhere [79,80].
Attenuation of Striatal Astrocyte Ca2+-Dependent Signaling
Intracellular Ca2+ signals have been explored as a possible basis for astrocyte–neuron interactions [81]. However, deletion of IP3 type 2 receptors produced few detectable behavioral effects [82–84] even though astrocyte Ca2+ signals were attenuated, including in the striatum [46]. To try to resolve this disconnect, another method to attenuate astrocyte Ca2+ signals was needed. A recent study used overexpression of a plasma membrane pump, called PMCA, and found evidence to support a role for astrocyte Ca2+ signaling in the regulation of neural circuit function and mouse behavior via a mechanism involving GAT-3. These findings are illustrated in Figure 4 with a current working model. Perhaps most unexpectedly, the attenuation of astrocyte Ca2+ signaling caused excessive self-grooming, which is related to OCD-like behaviors in humans [21]. The data showed that astrocytes regulate striatal MSNs and a specific behavior via ambient GABA-mediated neuromodulation of large volumes of brain tissue. However, further studies are needed, because the method used to attenuate astrocyte Ca2+ signaling lacks precise temporal resolution. Furthermore, the hPMCA2w/b approach worked well for striatum and hippocampus, but needs to be tested in other brain areas. Interpreted as a ‘loss-of-function’ experiment, these studies nonetheless provide evidence that astrocyte Ca2+ signaling is consequential for striatal function in adult mice [52].
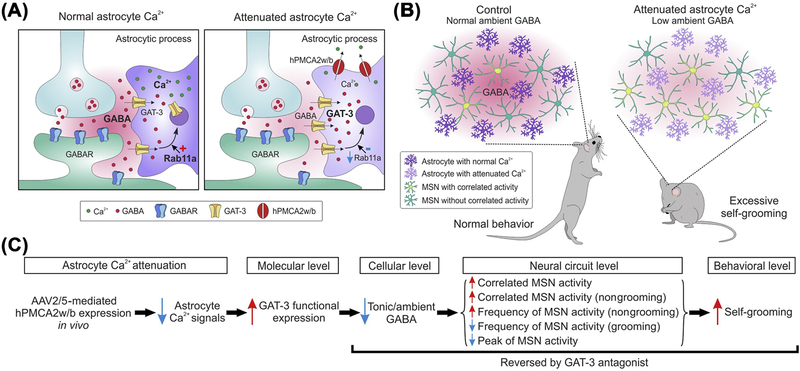
Figure 4. Working Model for How Attenuating Striatal Astrocyte Ca2+-Dependent Signaling In Vivo Altered Striatal Neural Circuit Function with Behavioral Consequences.
The cartoon summary is of the main findings at synaptic (A) and in vivo levels (B). (C) Description of the proposed sequence of events. In brief, attenuation of striatal astrocyte Ca2+ signals reduces Rab11a, which results in increased GAT-3 functional expression. This reduces ambient γ-aminobutyric acid (GABA) levels in the extracellular space and tonic inhibition. The data are consistent with a model in which reduced tonic inhibition alters medium spiny neurons (MSN) firing and downstream circuits to cause excessive self-grooming. In accord, tonic inhibition and self-grooming were rescued by a GAT-3 antagonist. Reproduced from [52].
Stimulation of Striatal Astrocyte–Neuron Interactions
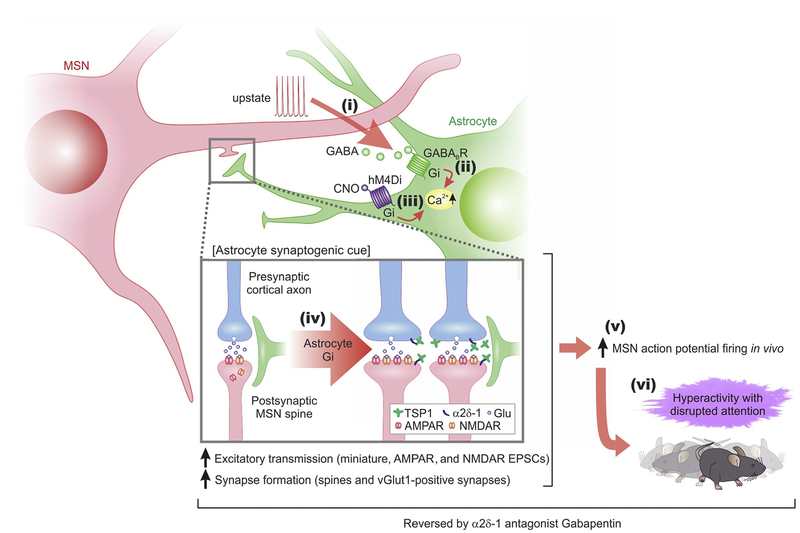
Striatal astrocytes responded to Gi-coupled G-protein-coupled receptor (GPCR) activation with elevations in intracellular Ca2+ levels [50]. This is consistent with data for astrocytes elsewhere in the brain [78,85,86] and likely occurred by activation of phospholipase C by Gβγ subunits and the subsequent generation of IP3. This type of response is not a peculiarity of astrocytes, and has been well documented [87]. However, the functional significance of Gi-mediated responses in the striatum were unknown. Recent progress and key findings are summarized in Figure 5. The working model suggests that MSNs communicate with astrocytes via the release of GABA during heightened activity, such as upstate transitions [53]. Such functional interactions are favored by the proximity of MSNs within single striatal astrocyte territories. The data are consistent with the hypothesis that GABA is released from MSN dendrites and activates Gi-coupled GABAB GPCRs, which are highly expressed in astrocytes [50,53]. GABAB receptor activation in turn results in the elevation of intracellular Ca2+ levels via release from stores. Selective activation of the Gi pathway in astrocytes using chemogenetics (hM4Di) resulted in elevated Thbsl expression, and the resultant TSP-1 actions (a known synaptogenic molecule) increased excitatory synapse formation, enhanced MSN firing in vivo, and were causal for the behavioral hyperactivity and disturbed attention phenotypes triggered by the striatal astrocyte Gi pathway. The data also show that physiological activity of neurons triggers astrocyte signaling, and that signaling from astrocytes to neurons is sufficient to alter circuits and behavior in adults. This could have relevance for brain plasticity and neural network dynamics on timescales beyond fast neuronal activity alone. More conceptually, therefore, the findings suggested that complex behavioral phenotypes that currently lack mechanistic understanding in adults have an astrocytic component. However, the findings should not be overinterpreted because the responses could only be assessed 30–120 min after chemogenetic activation of the astrocyte Gi pathway [53]. It remains to be determined whether additional responses occur on faster timescales of seconds or minutes. Furthermore, the available data only assessed the dorsal striatum, and the functional relevance for other parts of the striatum are unknown.
Figure 5. A Schematized Working Model for how Gi G-Protein-Coupled Receptor (GPCR)-Mediated Medium Spiny Neuron (MSN)–Astrocyte Bidirectional Interactions Affect Behavior.
When MSNs were depolarized to levels associated with upstates, they released γ-aminobutyric acid (GABA) (step i), which activated Gi-protein coupled GABAB G-protein-coupled receptors (GPCRs) on striatal astrocytes, leading to an increase in intracellular Ca2+ signals (step ii). Selectively stimulating the Gi pathway with hM4Di and clozapine-n-oxide (CNO) evoked Ca2+ signals in striatal astrocytes (step iii), upregulated the astrocyte synaptogenic molecule TSP-1, boosted excitatory synapse formation and fast excitatory synaptic transmission (step iv) and increased firing of MSNs (step v), which together resulted in hyperactivity with disrupted attention phenotypes in mice (step vi). The synaptic, circuit, and behavioral effects resulting from Gi pathway activation in vivo (steps iv–vi) were reduced or reversed by blocking TSP-1 actions on neuronal α2δ–1 receptors with gabapentin. Reproduced from [53]. Abbreviation: EPSC, excitatory postsynaptic current.
Striatal Astrocyte–Neuron Interactions in HD
HD is a progressive neurodegenerative disorder characterized by motor, cognitive, and psychiatric symptoms. It is caused by a single genetic defect, a polyglutamine expansion in Huntingtin (HTT) that results in the expression of a mutant protein (mHTT) [30]. Although HTT and mHTT are expressed throughout the body, the striatum is especially vulnerable and undergoes marked atrophy. Striatal astrocytes express mHTT in HD mouse models and in postmortem human tissue [88,89].
Selective expression of mHTT in astrocytes led to phenotypes related to HD in mice [88,90], and mHTT deletion from astrocytes in a HD mouse model slowed progression of some disease-related symptoms [91]. Normal and mHTT-expressing human glia delivered to the striatum of HD model and control mice, respectively, ameliorated and caused HD striatum-dependent cellular and behavioral phenotypes in mice [92], including alterations in MSNs. In terms of molecular mechanisms, the expression of two astrocyte-enriched proteins (Kir4.1 and GLT1) are reduced in HD mouse models [49,93–96]. Striatal astrocytes in HD model mice also display smaller territory sizes, reduced Ca2+ signaling, reduced cholesterol metabolism, and altered GABA transporter function [46,51,52,97–100]. Systems biology approaches are needed to shed light on underlying astrocyte mechanisms in HD and to evaluate how they contribute to pathophysiology. The function of HTT in astrocytes and the possible role of neurotoxic astrocytes in HD are both unknown [101]. Answering these questions may provide opportunities to exploit the basic biology of striatal astrocytes and to use HD as an exemplar neurodegenerative disease (see Outstanding Questions).
Outstanding Questions.
Are astrocytes locally heterogeneous in the striatum at molecular and functional levels?
Are specific striatal astrocytes organized anatomically in relation to either striosome or matrix compartments?
Which proteins are expressed within the plasma membrane of striatal astrocytes as well as within other physiological compartments, such as end-feet? What functions do such proteins serve?
Do subcompartment specific markers exist within branches, branchlets, and leaflets?
How do striatal astrocytes interact with striatal neurons, the vasculature, and microglia?
How do astrocytes and microglia interact to modify neuronal function during inflammatory cues?
How are striatal astrocytes affected by slow neuromodulators, such as dopamine?
Can striatal astrocytes be exploited as endogenous neuromodulators to treat disorders that involve striatal dysfunction?
Concluding Remarks
Water-breathing lamprey, the oldest group of vertebrates that diverged from mammals ~560 million years ago, appear to have putative GFAP-positive astrocytes [102] and a BG circuitry [103]. Thus, it is feasible that striatal neurons and astrocytes were in existence at about the same time as the dawn of vertebrate evolution [104] and coevolved to function in a synergistic manner. In accord, recent studies summarized herein provide evidence for striatal astrocyte–neuron bidirectional interactions with relevance to neural circuit function, behavior, and disease. Based on these insights, I suggest that astrocytes would be amenable to local pharmacological or genetic manipulation to produce desirable effects in the circuits that drive complex disease-related behaviors. This may be particularly relevant in complex psychiatric traits, including, but not limited to, those with a neuroinflammatory component. It is likely that such ideas will be tested in the years ahead and lead us into areas of physiology hitherto not considered and, potentially, into novel therapeutics for human disorders.
Highlights.
The striatum has been leveraged for studying astrocytes systematically in a defined circuitry, and for examining how their interactions with neurons relate to specific behaviors.
Morphologically complex ‘bushy’ astrocytes are integral to neural circuits and evenly tile the entire striatum, the largest nucleus of the basal ganglia.
Striatal astrocytes have several specific molecular features, such as high μ-crystallin expression, low GFAP expression, and striatal astrocyte-specific gene expression.
Striatal astrocytes make extensive contacts with striatal medium spiny neuron somata and additional finger-like connections with most excitatory synapses.
Striatal astrocytes modulate medium spiny neuron synapses to alter circuit function and mouse behavior, such as causing OCD-like excessive self-grooming, via slow homeostatic functions involving ambient GABA, K+, and synaptogenic cues.
Acknowledgments
Supported by the National Institutes of Health (NIH) (NS111583, DA047444, NS060677, and MH104069), a Paul G. Allen Foundation Distinguished Investigator Award, CHDI Foundation, and the Ressler Family Foundation. The author regrets that many papers could not be cited (especially early studies), because of space limits and the requirement to focus primarily on the past 5 years. Thanks to Vahri Beaumont, Jun Nagai, Xinzhu Yu, and Eiji Shigetomi for comments/help, to Janet Iwasa and Shraddha Nayak for help with Figure 1, and to Blanca Diaz-Castro for help with Figure 2.
Glossary
- μ-Crystallin
a protein thought to bind thyroid hormone and function as a ketimine reductase.
- Cre/ERT2
a tamoxifen-inducible Cre recombinase that allows controlled gene expression.
- Crym
gene encoding μ-crystallin that is highly expressed in striatal astrocytes.
- Glial fibrillary acid protein (GFAP)
an intermediate filament protein that is highly expressed in astrocytes in several brain regions (e.g., hippocampus) but is not well expressed in striatal astrocytes.
- GLT1
a plasma membrane glutamate transporter strongly enriched in astrocytes.
- hM4Di
a chemogenetic receptor used to stimulate the Gi pathway in striatal astrocytes in vivo. This has been used to study striatal astrocyte functions in vivo.
- Medium spiny neuron (MSN)
frequently also called striatal projection neurons or spiny projection neurons. These represent most neurons in the striatum and are GABAergic.
References
- 1.Kettenmann H and Verkhratsky A (2008) Neuroglia: the 150 years after. Trends Neurosci. 31,653–659 [DOI] [PubMed] [Google Scholar]
- 2.Allen NJ and Lyons DA (2018) Glia as architects of central nervous system formation and function. Science 362, 181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd GM and Grillner S (2010) Handbook of Brain Microcircuits, Oxford University Press [Google Scholar]
- 4.Khakh BS and Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci 18, 942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erö C et al. (2018) A cell atlas for the mouse brain. Front. Neuroinform 12, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Bartheld CS et al. (2016) The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J. Comp. Neurol 524, 3865–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barres BA (2008) The mystery and magic of glia: a perspective on their roles in health and disease. Neuron 60, 430–440 [DOI] [PubMed] [Google Scholar]
- 8.Ilieva H et al. (2009) Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol 187, 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khakh BS et al. (2017) Unravelling and exploiting astrocyte dysfunction in Huntington’s disease. Trends Neurosci. 40, 422–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Araque A et al. (2014) Gliotransmitters travel in time and space. Neuron 81,728–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira JF et al. (2015) Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 38, 535–549 [DOI] [PubMed] [Google Scholar]
- 12.Halassa MM and Haydon PG (2010) Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol 72, 335–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haydon PG and Nedergaard M (2014) How do astrocytes participate in neural plasticity? Cold Spring Harb. Perspect. Biol 7, a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedergaard M et al. (2010) Glial calcium and diseases of the nervous system. Cell Calcium 47, 140–149 [DOI] [PubMed] [Google Scholar]
- 15.Nedergaard M and Verkhratsky A (2012) Artifact versus reality-how astrocytes contribute to synaptic events. Glia 60, 1013–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eroglu C and Barres BA (2010) Regulation of synaptic connectivity by glia. Nature 468, 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adermark L and Lovinger DM (2006) Ethanol effects on electrophysiological properties of astrocytes in striatal brain slices. Neuropharmacology 51, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 18.Adermark L and Lovinger DM (2008) Electrophysiological properties and gap junction coupling of striatal astrocytes. Neurochem. Int 52, 1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graybiel AM and Grafton ST (2015) The striatum: where skills and habits meet. Cold Spring Harb. Perspect. Biol 7, a021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burguière E et al. (2015) Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr. Opin. Neurobiol 30, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalueff AV et al. (2016) Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci 17, 45–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graybiel AM (2008) Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci 31,359–387 [DOI] [PubMed] [Google Scholar]
- 23.Grillner S and Graybiel AM (2006) Microcircuits. The Interface between Neurons and Global Brain Function, The MIT Press [Google Scholar]
- 24.Nicola SM et al. (2000) Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu. Rev. Neurosci 23, 185–215 [DOI] [PubMed] [Google Scholar]
- 25.Gerfen CR and Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci 34, 441–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreitzer AC and Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60, 543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klaus A et al. (2017) The spatiotemporal organization of the striatum encodes action space. Neuron 95, 1171–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markowitz JE et al. (2018) The striatum organizes 3D behavior via moment-to-moment action selection. Cell 174,44–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tecuapetla F et al. (2016) Complementary contributions of striatal projection pathways to action initiation and execution. Cell 166, 703–715 [DOI] [PubMed] [Google Scholar]
- 30.Bates GP et al. (2015) Huntington disease. Nat. Rev. Dis. Primers 1, 15005. [DOI] [PubMed] [Google Scholar]
- 31.Esposito S et al. (2014) Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: an overview. Eur. J. Clin. Microbiol. Infect. Dis 33, 2105–2109 [DOI] [PubMed] [Google Scholar]
- 32.Chen SK et al. (2010) Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141, 775–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagarajan N et al. (2018) Corticostriatal circuit defects in Hoxb8 mutant mice. Mol. Psychiatry 23, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin D et al. (2009) Striatal volume differences between non-human and human primates. J. Neurosci. Methods 176, 200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen GD and Williams RW (2001) Complex trait analysis of the mouse striatum: independent QTLs modulate volume and neuron number. BMC Neurosci. 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foo LC et al. (2011) Development of a method for the purification and culture of rodent astrocytes. Neuron 71,799–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanz E et al. (2009) Cell-type-specific isolation of ribosome-associated mRNAfrom complex tissues. Proc. Natl. Acad. Sci. U. S. A 106, 13939–13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivasan R et al. (2016) New transgenic mouse lines for selectively targeting astrocytes and studying calcium signals in astrocyte processes in situ and in vivo. Neuron 92,1181–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.John Lin CC et al. (2017) Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci 20, 396–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boisvert MM et al. (2018) The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22,269–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelley KW et al. (2018) Variation among intact tissue samples reveals the core transcriptional features of human CNS cell classes. Nat. Neurosci 21, 1171–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itoh N et al. (2018) Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc. Natl. Acad. Sci. U. S. A 115, E302–E309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller SJ et al. (2019) Molecularly defined cortical astroglia subpopulation modulates neurons via secretion of Norrin. Nat. Neurosci 22, 741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morel L et al. (2017) Molecular and functional properties of regional astrocytes in the adult brain. J. Neurosci 37,8706–8717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morel L et al. (2019) Intracortical astrocyte subpopulations defined by astrocyte reporter mice in the adult brain. Glia 67, 171–181 [DOI] [PubMed] [Google Scholar]
- 46.Jiang R et al. (2016) Dysfunctional calcium and glutamate signaling in striatal astrocytes from Huntington’s disease model mice. J. Neurosci 36, 3453–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gokce O et al. (2016) Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-Seq. Cell Rep. 16, 1126–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borel F et al. (2014) Crystal structure of mouse mu-crystallin complexed with NADPH and the T3 thyroid hormone. FEBS J. 281, 1598–1612 [DOI] [PubMed] [Google Scholar]
- 49.Tong X et al. (2014) Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci 17, 694–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chai H et al. (2017) Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological and functional evidence. Neuron 95, 531–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Octeau JC et al. (2018)An optical neuron-astrocyte proximity assay at synaptic distance scales. Neuron 98, 49–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu X et al. (2018) Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 99, 1170–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagai J et al. (2019) Hyperactivity with disrupted attention by activation of an astrocyte synaptogenic cue. Cell 177, 1280–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shigetomi E et al. (2013) Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 141, 633–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang R et al. (2014) Imaging intracellular Ca2+ signals in striatal astrocytes from adult mice using genetically-encoded calcium indicators. J. Vis. Exp 93, e51972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerfen CR and Bolam JP (2016) The neuroanatomical organisation of the basal ganglia In Handbook of Basal Ganglia Structure and Function (2nd edn) (Steiner H and Tseng K, eds), pp. 3–32, Academic Press [Google Scholar]
- 57.Cui Y et al. (2018) Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554, 323–327 [DOI] [PubMed] [Google Scholar]
- 58.Ingham CA et al. (1998) Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J. Neurosci 18, 4732–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frotscher M et al. (2014) Fine structure of synapses on dendritic spines. Front. Neuroanat 8, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ventura R and Harris KM (1999)Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci 19, 6897–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witcher MR et al. (2007) Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia 55, 13–23 [DOI] [PubMed] [Google Scholar]
- 62.Wu L et al. (2019) PARIS, an optogenetic method for functionally mapping gap junctions. Elife 8, e43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J et al. (2019) Glutamate-releasing SWELL1 channel in astrocytes modulates synaptic transmission and promotes brain damage in stroke. Neuron 102, 813–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charles AC et al. (1991) Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6, 983–992 [DOI] [PubMed] [Google Scholar]
- 65.Dani JW et al. (1992) Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8, 429–440 [DOI] [PubMed] [Google Scholar]
- 66.Cornell-Bell AH et al. (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473 [DOI] [PubMed] [Google Scholar]
- 67.Smith S (1994) Neural signalling. Neuromodulatory astrocytes. Curr. Biol 4, 807–810 [DOI] [PubMed] [Google Scholar]
- 68.Smith SJ (1992) Do astrocytes process neural information? Prog. Brain Res 94, 119–136 [DOI] [PubMed] [Google Scholar]
- 69.Octeau JC et al. (2019)Transient, consequential extracellular potassium elevations accompany Channelrhodopsin excitation. Cell Rep. 27, 2249–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mederos S et al. (2019) Melanopsin for precise optogenetic activation of astrocyte–neuron networks. Glia 67, 915–934 [DOI] [PubMed] [Google Scholar]
- 71.Martin R et al. (2015) Circuit-specific signaling in astrocyte–neuron networks in basal ganglia pathways. Science 349, 730–734 [DOI] [PubMed] [Google Scholar]
- 72.Agarwal A et al. (2017) Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93, 587–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jackson JG and Robinson MB (2015) Reciprocal regulation of mitochondrial dynamics and calcium signaling in astrocyte processes. J. Neurosci 35, 15199–15213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gangarossa G et al. (2013) Spatial distribution of D1R- and D2R-expressing medium-sized spiny neurons differs along the rostro-caudal axis of the mouse dorsal striatum. Front Neural Circuits 7, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Molofsky AV and Deneen B (2015) Astrocyte development: a guide for the perplexed. Glia 63, 1320–1329 [DOI] [PubMed] [Google Scholar]
- 76.Tepper JM et al. (1998) Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev. Neurosci 20, 125–145 [DOI] [PubMed] [Google Scholar]
- 77.Sun W et al. (2013) Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haustein MD et al. (2014) Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82, 413–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiacco TA and McCarthy KD (2018) Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J. Neurosci 38, 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Savtchouk I and Volterra A (2018) Gliotransmission: beyond black-and-white. J. Neurosci 38, 14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khakh BS and McCarthy KD (2015) Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol 7, a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agulhon C et al. (2010) Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254 [DOI] [PubMed] [Google Scholar]
- 83.Petravicz J et al. (2014) Astrocyte IP3R2-dependent Ca(2+) signaling is not a major modulator of neuronal pathways governing behavior. Front. Behav. Neurosci 8, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petravicz J et al. (2008) Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci 28, 4967–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kang J et al. (1998) Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat. Neurosci 8, 683–692 [DOI] [PubMed] [Google Scholar]
- 86.Durkee CA et al. (2019) Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 67, 1076–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pierce KL et al. (2002) Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol 3, 639–650 [DOI] [PubMed] [Google Scholar]
- 88.Bradford J et al. (2009) Expression of mutant huntingtin in mouse brain astrocytes causes age-dependent neurological symptoms. Proc. Natl. Acad. Sci. U. S. A 106, 22480–22485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bradford J et al. (2010) Mutant huntingtin in glial cells exacerbates neurological symptoms of Huntington disease mice. J. Biol. Chem 285, 10653–10661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin JY et al. (2005) Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J. Cell Biol 171, 1001–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wood TE et al. (2018) Mutant huntingtin reduction in astrocytes slows disease progression in the BACHD conditional Huntington’s disease mouse model. Hum. Mol. Genet 28, 487–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benraiss A et al. (2016) Human glia can both induce and rescue aspects of phenotype in Huntington Disease. Nat. Commun 7, 11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Estrada-Sanchez AM et al. (2009) Glutamate toxicity in the striatum of the R6/2 Huntington’s disease transgenic mice is age-dependent and correlates with decreased levels of glutamate transporters. Neurobiol. Dis 34, 78–86 [DOI] [PubMed] [Google Scholar]
- 94.Lievens JC et al. (2001) Impaired glutamate uptake in the R6 Huntington’s disease transgenic mice. Neurobiol. Dis 8, 807–821 [DOI] [PubMed] [Google Scholar]
- 95.Miller BR et al. (2008) Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience 153, 329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sari Y et al. (2010) Ceftriaxone-induced up-regulation of cortical and striatal GLT1 in the R6/2 model of Huntington’s disease. J. Biomed. Sci 17, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skotte NH et al. (2018) Integrative characterization of the R6/2 mouse model of Huntington’s disease reveals dysfunctional astrocyte metabolism. Cell Rep. 23, 2211–2224 [DOI] [PubMed] [Google Scholar]
- 98.Dvorzhak A et al. (2016) Functional indicators of glutamate transport in single striatal astrocytes and the influence of Kir4.1 in normal and Huntington mice. J. Neurosci 16, 4959–4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vagner T et al. (2016) Systemic application of AAV vectors targeting GFAP-expressing astrocytes in Z-Q175-KI Huntington’s disease mice. Mol. Cell. Neurosci 77, 76–86 [DOI] [PubMed] [Google Scholar]
- 100.Wojtowicz AM et al. (2013)Reduced tonic inhibition in striatal output neurons from Huntington mice due to loss of astrocytic GABA release through GAT-3. Front Neural Circuits 7, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liddelow SA et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cinelli E et al. (2017) ATP and astrocytes play a prominent role in the control of the respiratory pattern generator in the lamprey. J. Physiol 595, 7063–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grillner S and Robertson B (2016) The basal ganglia over 500 million years. Curr. Biol 26, R1088–R1100 [DOI] [PubMed] [Google Scholar]
- 104.Freeman MR and Rowitch DH (2013) Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 80, 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]