Abstract
Purpose:
Preclinical data established Interleukin-15 as a homeostatic factor and powerful stimulator of NK and CD8+ T cell function, the basis for clinical testing.
Experimental Design:
A first-in-human outpatient phase I dose escalation trial of subcutaneous (SC) rhIL-15 was conducted in refractory solid tumor cancer patients. Therapy consisted of daily (Monday - Friday) SC injections of rhIL-15 for two consecutive weeks (10 total doses/cycle). Clinical response was assessed by RECIST. Pharmacokinetics of rhIL-15 and immune biomarkers were evaluated.
Results:
Nineteen patients were treated with rhIL-15 at dose levels of 0.25, 0.5, 1, 2 and 3 mcg/kg/day. Fourteen patients completed ≥ 2 cycles of therapy that was well tolerated. One serious adverse event (SAE), grade 2 pancreatitis, required overnight hospitalization. Enrollment was halted after a patient receiving 3 mcg/kg/day developed a dose limiting SAE of grade 3 cardiac chest pain associated with hypotension and increased troponin. No objective responses were observed; however, several patients had disease stabilization including a renal cell carcinoma patient who continued protocol treatment for 2 years. The treatment induced profound expansion of circulating NK cells, especially among the CD56bright subset. A proportional but less dramatic increase was found among circulating CD8+ T cells with maximal 3-fold expansion for the 2 and 3 mcg/kg patients.
Conclusions:
SC rhIL-15 treatment was well tolerated, producing substantial increases in circulating NK and CD8+ T cells. This protocol establishes a safe outpatient SC rhIL-15 regimen of 2 mcg/kg/day dosing amenable to self-injection and with potential as a combination immunotherapeutic agent.
Keywords: Phase 1, Subcutaneous, IL-15, Outpatient, Solid Tumor
TRANSLATIONAL RELEVANCE
Preclinical experiments demonstrated that Interleukin-15 (IL-15) can control homeostasis and stimulate natural killer (NK) and antigen-specific CD8+ T cell activity without causing activation induced cell death (AICD) or promoting T regulatory (Treg) cell function. Recognition of these properties led to the designation of IL-15 as the immunotherapeutic with highest potential for clinical development by the 2007 NCI Immunotherapy Workshop. Unexpected toxicities encountered in the first-in-human clinical trial of recombinant human (rh)IL-15 given as daily 30-minute intravenous bolus (IVB) infusions severely limited dose escalation. Preclinical and non-human primate toxicology experiments suggested that subcutaneous (SC) administration should lower peak concentrations and improve clinical tolerance. This is a first-in-human experience with outpatient subcutaneous rhIL-15, allowing 6-fold more drug delivery than IVB, and inducing robust levels of immune activation. These results will allow the export of IL-15 immunotherapy to an outpatient setting and testing of combinatorial strategies to improve cancer treatment.
INTRODUCTION
Positive reports from clinical trials evaluating immune checkpoint inhibitors, antitumor monoclonal antibodies and adoptive cellular therapies have refocused oncologic drug development on immune-based investigational agents(1–7). While immune checkpoint inhibitors have appreciable activity in several solid tumor types not typically considered immunosensitive(8–10) and whereas cellular therapies using cells with genetically manipulated chimeric antigen receptor cells (CARs) have impressive activity in several leukemias, these therapies have ultimately been demonstrated to be effective in only a minority of advanced cancer patients seeking therapy(7, 11–14). NK cell therapy is also promising in acute myeloid leukemia with 30–50% remission reported after NK cell infusions(15, 16). Our evolving understanding of a productive anti-tumor immune response hypothesizes that infiltration of tumors by activated tumor antigen (Ag)-specific lymphocytes capable of sustained activity is critical for clinical activity(17, 18). Continued support or stimulation of these effector cells requires sustained production of stimulatory cytokines and mitigation of the immunosuppressive effects of CD4+ CD25+ FoxP3+ T regulatory (Treg) cells and myeloid-derived suppressor cells (MDSC)(19–22).
IL-15 is a homeostatic factor for NK and T cells and is required for NK cell development. Like IL-2, IL-15 potentiates NK cell antitumor activity in vitro and in vivo(23–30). Experiments demonstrated that Interleukin-15 (IL-15) improved the survival of mice in established models of MC38 and CT-25 colorectal carcinomas(31, 32). In the transgenic murine melanoma Pmel model, IL-15 was shown to stimulate a potentially curative antigen-specific CD8+ T cell response that was also synergistic with other common gamma chain cytokines(33). Co-administration of IL-15 with the fowlpox TRICOM and gp160 vaccines further demonstrated synergistic activity producing long-lasting antigen-specific CD8+ T cell responses against renal cell carcinoma and HIV, respectively, that was superior to these vaccines plus IL-2(34). These experiments, among others, have established IL-15 as an immunotherapeutic that activates NK cells and CD8+ T cells, sustains long-term memory T cells, inhibits activation-induced cell death (AICD) and does not promote the activity of regulatory T cells (Tregs)(26–29). The biologic effects of IL-15 compare very favorably with Interleukin-2 (IL-2), the prototypic immunotherapeutic cytokine that is occasionally administered to metastatic melanoma and renal cell carcinoma patients. Despite durable and sometimes complete responses, the small percentage of responders and significant clinical toxicities of high dose IV bolus IL-2 (HDIL-2) treatment limit its use(35, 36). In the first-in-human phase I clinical trial of recombinant human (rh) IL-15, treatment was given as a 30-minute infusion (IVB) once daily for 12 consecutive days(37). Dose escalation was constrained by post-infusion toxicities of fevers, rigors and transiently decreased blood pressure, though less problematic than similar HDIL-2 toxicities. Moreover, rhIL-15 IVB resulted in lower maximum tolerated dose (MTD of 0.3 mcg/kg IVB) and immune activation than was anticipated based on the non-human primate toxicology experiments. Coincidental to this first-in-human rhIL-15 clinical trial, additional non-human primate experiments demonstrated that sustained administration regimens of rhIL-15 by either continuous intravenous infusion [CIV] or subcutaneous [SC] injection produced substantially greater immune activation with less toxicity and possibly greater clinical potential than the initial IVB regimen(30). Based these preclinical data and the clinical experience in the first-in-human rhIL-15 IVB trial, this phase I dose escalation trial of SC rhIL-15 administered by daily injection Monday through Friday for 2 consecutive weeks of a 28-day cycle, was initiated. The goal of this study was to design a safe outpatient regimen that could be used alone or in combinatorial strategies. The safety, pharmacokinetics, correlative immunologic laboratory analyses, and clinical activity of this treatment regimen are reported.
MATERIALS AND METHODS
Patients
Patients with advanced metastatic melanoma (MM), renal cell carcinoma (RCC), non-small cell lung (NSCLC) and squamous cell head and neck carcinoma (SCCH&N) were enrolled in this phase I open-label, non-randomized dose escalation study. Eligible patients were required to be age ≥ 18 years, have histologically confirmed metastatic solid tumors, failed at least 1 prior standard treatment regimen, have ECOG performance status 0 or 1, absolute lymphocyte count (ALC) >500/mcL, absolute neutrophil count (ANC) >1,000/mcL, platelets >100,000/mcL, total bilirubin within normal institutional limits, PT/PTT <1.5 x institutional upper limit of normal (ULN), non-transfused hemoglobin >9 g/dL, alkaline phosphatase ≤2.5 x ULN, AST /ALT <2 ULN, serum creatinine <1.5 x ULN, absence of CNS metastases, no history of clinically significant autoimmune disease or hematopoietic malignancy, no history of severe asthma, no use of systemic corticosteroid treatment or inhaled steroids, no evidence of clinically active infection, no history of or serology positive for HIV or hepatitis B or C or HTLV-1, and no clinically significant congestive (NYHA class II or greater) heart disease. Pregnant female patients were excluded, and patients must have been more than 4 weeks from their most recent treatment, 6 weeks for nitrosoureas/mitomycin, 8 weeks for anti-CTLA4 or anti-PD1, more than 2 weeks from radiation therapy, have recovered from previous treatment, not receiving any other investigational treatment and able to give informed consent.
Study Design
This trial was sponsored and overseen by the Cancer Immunotherapy Trials Network (CITN) and conducted at 5 clinical centers in the United States [University of Wisconsin (Madison, WI), University of Minnesota (Minneapolis, MN), Stanford University (Stanford, CA), Seattle Cancer Care Alliance (Seattle, WA) and the National Cancer Institute/National Institute of Health (NCI/NIH Bethesda MD)] between July 2013 and March 2016. This protocol was approved and monitored by the Cancer Treatment Evaluation Program (CTEP)/NCI/NIH). The Fred Hutchinson Cancer Research Center Institutional Review Board (IRB) functioned as the Central IRB for this study and for 3 of the respective enrolling institutions. The primary objective was to define the maximum tolerated dose (MTD) for this SC rhIL-15 regimen. A standard 3 + 3 phase I design was employed that enrolled at least 3 patients at each dose level, with dose escalation proceeding in the absence of dose limiting toxicity (DLT) occurring during the first treatment cycle. If a DLT occurred in one of the first 3 patients enrolled at a dose level, the cohort size was expanded to 6 patients. If ≥ 2 out of 3 or 6 patients experienced DLTs, dose escalation would be halted and the prior level considered the MTD. The NCI Common Toxicity Criteria version 4 (CTCv4) was used to assess adverse events (AEs) with DLTs being defined as any ≥ grade 3 toxicity with non-hematologic exceptions based on previous clinical studies with rhIL-15 that included grade 3 fatigue or anorexia, grade 3 hypocalcemia, hypokalemia, hypomagnesemia, hyponatremia, hypophosphatemia that responded to medical intervention, temperature > 400 C for < 48 hours, febrile neutropenia not requiring urgent intervention; hematologic exceptions were grade 3 or 4 lymphopenia, grade 3 neutropenia and grade 3 lymphocyte increase. ALC > 25,000/mm3 was also not considered a DLT, but was designated the “maximum desired effect” and would prompt interruption of treatment until the lymphocyte count dropped without precluding additional subsequent treatment. However, ALC > 35, 000/mm3 was considered a DLT.
Study Treatment
The investigational agent used in this trial was E. coli-derived rhIL-15 manufactured by the Biopharmaceutical Development Program (BDP) of the Division of Cancer Treatment and Diagnosis (DCTD)/ NCI using current Good Manufacturing Practices (cGMP). Study drug was provided to the treating centers by the Pharmaceutical Management Branch/DCTD. Treatment cycles were 28 days in length with patients receiving daily SC rhIL-15 on days 1 through 5 and 8 through 12 at dose levels of 0.25, 0.5, 1, 2 and 3 mcg/kg/day. Injection sites were rotated to different areas of the body (upper and lower extremities, each of the 4 quadrants of the abdomen) to minimize the summated local effects of drug administration. Routine premedication with antipyretics or non-steroidal anti-inflammatory agents such as acetaminophen, ibuprofen, naproxen or aspirin at established doses and schedules were given at the treating physician’s discretion. Additional concomitant anti-emetics, anti-diarrheals, IV fluids or electrolyte replacement based on clinical or laboratory assessments and blood transfusions while on treatment based on individual institutional guidelines were allowed.
Clinical and Investigational Assessments
Standard clinical assessment of the patients included routine monitoring of vital signs, appraisal of adverse events (AEs), injection site reactions, routine chemistry panels and CBC on each treatment day. A detailed history and physical (H&P) exam was performed on day 1 and day 8 and focused H&P on all other treatment days. Serum samples to detect anti-IL-15 antibodies were collected prior to day 1 dosing of each cycle of treatment and 6 months after treatment was completed, from a select group of patients. Limited rhIL-15 pharmacokinetic (PK) analysis was performed during cycle 1 with serum samples obtained immediately prior to the first dose of study drug (baseline), then at 10 minutes, 1, 4, and 24 hours after the initial treatment to assess serum IL-15 levels, as well as inflammatory cytokines. Heparinized whole blood samples were also obtained at baseline, day 11, and day 15 (72 hours after completion of treatment) during each cycle for immunophenotyping of peripheral blood mononuclear cells (PBMC), and NK cellular function assessment at cycle 1 time points.
Specimen handling and processing
Heparinized whole blood samples collected at each clinical site were shipped by overnight express mail in insulated shippers that contained LogTag temperature recorders (Northcote, Auckland, New Zealand) to continuously record ambient temperatures during shipment. Samples were received at the University of Washington CITN Central Laboratory an average of 28 hours later. Aliquots of fresh whole blood were immediately used for real-time antibody labeling for flow cytometric analyses and the remainder of the samples processed to plasma and PBMC using standard Ficoll-Hypaque isolation immediately upon receipt. PBMC were cryopreserved in 10% DMSO (Sigma, St. Louis, MO) and 12.5% HSA (Gemini, Atlanta, GA) at −80°C and subsequently maintained in vapor phase liquid nitrogen freezers. Cryopreserved PBMC samples were shipped to the University of Minnesota for functional lymphocyte testing in Cryoport liquid nitrogen shippers (Irvine, CA). Serum was collected at the clinical sites within 4 hours of blood draw and frozen at −80°C. Batched samples were later shipped on dry ice to the CITN Central Laboratory and then subsequently to the NCI for testing(38).
Correlative Flow Cytometry and Cellular Cytotoxicity Analyses
Immunophenotyping
Whole blood flow cytometric analyses were performed initially using day 1 and 11 time point samples. The protocol was amended in April 2014 to add flow cytometric testing on day 15 of each cycle to better assess the post-treatment lymphocytosis suggested by other studies to be maximal 3 days after completion of the rhIL-15 injections. Fresh whole blood samples were labeled with fluorescently-labeled antibodies to cell surface molecules CD45 (2D1), CD3 (UCHT1), CD8 (SK1), CD56 (NCAM16.1), CD16 (3G8), CD14 (MOP9), CD123 (9FS) (all BD Biosciences, San Jose, CA) and CD4 (RPA-T4), CD19 (HIB19), and HLA-DR (L243) (all Biolegend, San Diego, CA) after overnight shipping to the CITN Central Lab, using a method adapted from Hensley et al(38). Samples were treated with BD FACS™ Lysing Solution (BD Biosciences) and immediately frozen at −80°C for later batch testing on a BD LSRII flow cytometer. Absolute cell numbers were obtained using Trucount tubes (BD Biosciences). Data analysis was performed using FlowJo software (Treestar, Ashland, OR).
Functional Lymphocyte Evaluation
Cryopreserved PBMC were thawed, washed once in PBS + 0.3% bovine serum albumin (BSA), then resuspended in RPMI 1640 with 10% fetal calf serum (FCS) without cytokines at a cellular concentration of 2×106/mL and incubated at 37oC in 5% CO2 until the functional assays were performed. After an 18 to 24-hour incubation, NK cell activity was tested against K562 targets at an effector to target ratio of 2:1 in a 5-hour assay that assessed CD107a expression and intracellular TNF-α production using the Transcription Factor Fixation/Permeabilization Concentrate and Diluent (eBioscience Thermo Fisher, Waltham, MA). The same buffer was used in the unstimulated Ki67 expression assay. Fluorescently-labeled anti-human mAbs utilized were PE-Cy7-conjugated CD56 (HCD56), FITC or BV605-conjugated CD45 (H130), BV785-conjugated CD3 (OKT3), PerCP Cy5.5-conjugated CD107a (LAMP-1), BV421-conjugated TNF-α (Mab11) and BV711-conjugated Ki67 (Ki67; all Biolegend)). Cells were fixed with 2% paraformaldehyde and analyzed at one time on a BD LSRII flow cytometer. All results were analyzed using FlowJo software.
Pharmacokinetic (PK) Analyses and Detection of anti-rhIL-15 antibodies
PK and anti-rhIL-15 antibody analyses of frozen serum samples were conducted at the Clinical Support Laboratory, Frederick National Laboratory for clinical research, (Leidos Biomedical Research , Frederick, MD). Serum rhIL-15 concentrations were assessed using a human IL-15 specific ELISA kit (R & D Systems, Minneapolis, MN) according to manufacturer’s directions. Serum IL-15 levels were analyzed using SoftMax® Pro software version 5.2 or higher(37).
An ELISA developed by the Waldmann laboratory (NCI, Bethesda, MD) and previously used to monitor the development of anti-IL-15 antibodies in NCI rhIL-15 clinical trials(37) was used for this same purpose in baseline and pretreatment day 1 patient sera from all cycles. For this test, 100 ng/mL of rhIL-15 was used to precoat 96-well microliter plates, then plates washed and blocked with PBS/3% BSA. An affinity purified goat anti-human IL-15 (R&D Systems) was used to define the standard curve. After overnight incubation with sera and controls at 4ºC and washing, biotinylated IL-15 was added for 2 hours at 37ºC. Plates were washed, then streptavidin-alkaline phosphatase was added for 2 hours at 37ºC. Plates were washed and then developed with p-nitrophenol phosphate for 1 hour at 37ºC. Results were determined using SoftMax Pro Version 5.2 or higher. The lower limit of quantitation in undiluted serum is 156 ng/mL for this ELISA.
Evaluation of the Neutralizing Capacity of anti-IL-15 Antibodies
To detect antibodies that could specifically neutralize E. coli rhIL-15 but not rhIL-2 or endogenous heterodimeric IL-15 (HetIL-15), inhibition of IL-15 or IL-2 induced NK-92 proliferation by 3H-thymidine incorporation was measured(39). Briefly, serial dilutions of affinity-purified goat anti-human-IL-15 were added to rhIL-15-treated NK-92 cell cultures to produce a standard curve. To assess the neutralizing capability of anti-IL-15 antibodies present in patient sera, serial dilutions of individual sera were added to rhIL-15-treated NK-92 cell cultures and neutralizing antibody levels (ng/mL) calculated by comparison to the standard antibody curve.
Assay for Serum Inflammatory Cytokines
The Meso Scale Discovery (MSD) V-PLEX immunoassay system (Rockville, MD) was used to quantify serum concentrations of human interferon-gamma (IFN-γ), IL-1β, IL-6, IL-10, IL-12p70 and tumor necrosis factor-alpha (TNF-α). Assays were performed according to manufacturer’s instructions as previously described(37).
Statistical Analysis
Characteristic statistics for a “3 + 3” phase I dose escalation trial where 2 of 3 patients in a dosing cohort (proportion 0.67 with 95% confidence intervals [CI] 21 to 94%) or 2 of 6 patients in a dosing cohort (proportion 0.33 with 95% CI 10 to 70%) demonstrate that the MTD has been exceeded and the previous tolerable dose level represents the true MTD(40). For the laboratory studies, descriptive statistics were used as indicated.
RESULTS
Patients and Treatment
Twenty eligible patients were enrolled (one refused treatment after signing consent) and 19 patients were treated with SC rhIL-15, including 9 with RCC, 6 with NSCLC, and 3 each with MM or SCCH&N (Table 1). The median age of the patients was 61 years (range 38 to 78) and ~two-thirds were male. Patients enrolled into this trial were heavily pretreated and had progressed or not responded following one or more systemic therapies. Six of nineteen patients completed 4 treatment cycles; two of these patients received additional cycles as permitted by the study protocol and approved by CTEP. Most patients discontinued protocol therapy due to disease progression, but 3 patients stopped due to a treatment-related adverse event (AE), and 3 other patients completed the protocol therapy without significant toxicity or disease progression. Of those who stopped for an AE, one patient discontinued treatment when their pre-existing mild psoriasis became worse, a second patient discontinued treatment for an SAE of pancreatitis and the third patient for an SAE of DLT grade 3 cardiac chest pain. Approximately one-third of treated patients (N=7) had disease stabilization and continued their outpatient treatment beyond 2 cycles, including a patient who remained on treatment for 2 years. The protocol was amended twice to increase the maximum number of treatment cycles when anti-IL-15 antibodies were first identified in the absence of safety concerns, and 2nd after it was determined that prolonged treatment of patients with disease stabilization may have resulted from study treatment. The decision to terminate the protocol before the MTD was formally defined was made by the study principal investigator in conjunction with the CITN Safety Committee after review of the AE profile for the 3 mcg/kg dose level, concluding that 2 mcg/kg most likely represented the maximum tolerated dose that could be administered safely as an outpatient regimen.
Table 1.
Subject Demographics
| Dose (mcg/kg) | Subject | Age | Gender | Cancer Type | Cycles Completed | Off-Study for |
|---|---|---|---|---|---|---|
| 0.25 | 1 | 75 | M | Renal | 2 | Progression |
| 0.25 | 2 | 61 | M | Lung | 4 | Completed |
| 0.25 | 3 | 55 | M | Renal | 4 | Progression |
| 0.5 | 4 | 38 | F | Lung | 4 | Progression |
| 0.5 | 5 | 74 | M | Renal | 24 | Completed |
| 0.5 | 6 | 58 | F | Melanoma | 2 | Progression |
| 1.0 | 7 | 71 | M | SCCHN | 1 | Discontinued* |
| 1.0 | 8 | 69 | F | Lung | 4 | Completed |
| 1.0 | 9 | 61 | F | Melanoma | 2 | Progression |
| 2.0 | 10 | 62 | M | Melanoma | 1 | Adverse Event |
| 2.0 | 11 | 59 | M | Renal | 2 | Progression |
| 2.0 | 12 | 77 | M | Renal | 2 | Adverse Event |
| 2.0 | 13 | 60 | M | Lung | 6 | Completed |
| 2.0 | 14 | 44 | M | SCCHN | 2 | Progression |
| 2.0 | 15 | 53 | M | Renal | 2 | Progression |
| 3.0 | 16 | 78 | F | Renal | 4 | Progression |
| 3.0 | 17 | 75 | M | SCCHN | 1 | Progression |
| 3.0 | 18 | 59 | F | Lung | 1 | Adverse Event |
| 3.0 | 19 | 61 | F | Lung | 2 | Progression |
Response assessment not performed; patient discontinued study participation
Completed=Completed 4 cycles (or more) without disease progression
Dose Escalation and Treatment-Related Adverse Events
Daily SC injections of rhIL-15 were generally well tolerated, especially at the first 3 dose levels. The most common symptoms associated with treatment were as expected: fevers, chills, decreased blood pressure (BP) and injection site reactions (Table 2: Adverse Events occurring in 5 or more subjects). Importantly, all injection site reactions that occurred at any dose level were grade 1 (2 to 4 mm of erythema) and no suggestion of recall events or increases in the severity of injection site reactions occurred during subsequent treatment cycles. Patients with decreased BP were all in the 2 or 3 mcg/kg dose cohorts except for 2 patients at the 0.5 mcg/kg dose level. Fatigue was noted in 9 of 19 patients, all grade 1, with the exception of 1 patient with grade 2 fatigue. Nausea and/or vomiting occurred in < half of patients, was mild, and was neither more common nor severe in the higher dose levels. The most common laboratory abnormalities were anemia, hypophosphatemia, thrombocytopenia and hypoalbuminemia, in that order of frequency. Some level of mild anemia was present in 12 of 19 patients. Seven of 19 patients experienced transient mild thrombocytopenia, defined as <150,000/mcL, with a range of 112–134,000 (mean 125,000). Transient lymphopenia was reported only in the 2 and 3 mcg/kg dose cohorts, and mild neutropenia only occasionally. Elevations in aspartate and/or alanine aminotransferase occurred in ~¼ of patients treated in this protocol and was generally mild, but more common in the higher dose level cohorts.
Table 2.
Adverse Events
| Highest AE Grade/Dose Cohort | |||||||
|---|---|---|---|---|---|---|---|
| Adverse Event | # Subjects Affected, Total (%) | # Subjects Affected, 2 & 3 mcg/kg (%) | 0.25 mcg/kg | 0.5 mcg/kg | 1 mcg/kg | 2 mcg/kg | 3 mcg/kg |
| Injection site reaction | 14 (74) | 7 (70) | 1 | 1 | 1 | 1 | 1 |
| Chills | 13 (68) | 8 (80) | 1 | 1 | 1 | 2 | 2 |
| Anemia | 12 (63) | 7 (70) | 2 | 2 | 2 | 2 | 2 |
| Fever | 11 (58) | 10 (100) | 1 | 2 | 3 | ||
| Hypotension | 10 (53) | 8 (80) | 1 | 2 | 2 | ||
| Fatigue | 9 (47) | 6 (60) | 1 | 1 | 1 | 2 | |
| Hypophosphatemia | 9 (47) | 5 (50) | 3 | 3 | 3 | 2 | |
| Tachycardia | 7 (37) | 5 (50) | 1 | 1 | 1 | 1 | |
| Vomiting | 7 (37) | 4 (40) | 2 | 1 | 1 | 1 | 1 |
| Hypoalbuminemia | 7 (37) | 4 (40) | 3 | 2 | 1 | 2 | |
| Hypertension | 7 (37) | 3 (30) | 2 | 3 | 3 | 1 | |
| Dry skin | 6 (32) | 5 (50) | 1 | 1 | 2 | ||
| Nausea | 6 (32) | 3 (30) | 2 | 1 | 1 | 1 | |
| Lymphopenia | 5 (26) | 5 (50) | 3 | 3 | |||
| Elevated AST | 5 (26) | 4 (40) | 1 | 1 | 2 | ||
| Diarrhea | 5 (26) | 3 (30) | 3 | 1 | 1 | 1 | |
Adverse events occurring in 5 more subjects is shown
One SAE of pancreatitis began approximately 2 days after the patient’s last rhIL-15 dose of cycle 1, requiring overnight hospitalization, pain medications and acute alteration of his diet. Study treatment was discontinued and the patient recovered fully within a few weeks without sequelae. The third patient enrolled at the 3 mcg/kg dose level had grade 3 cardiac chest pain, an SAE and DLT. After supportive care and hospitalization for 1 day, this SAE resolved without sequelae. When two other patients treated at this dose level experienced grade 2 or 3 fevers, the decision was made to discontinue protocol treatment with the conclusion that 2 mcg/kg was likely the MTD for outpatient therapy.
Clinical Response
The objective response rate was assessed according to RECIST 1.1 criteria guidelines by following marker lesions defined in baseline CT scans with radiographic restaging after every second cycle of treatment. Patients with suspicious physical findings or complaints were restaged early as clinically indicated. Consistent with the fact that most patients discontinued treatment for disease progression after 2 or fewer treatment cycles, no objective responses were observed. The median time to progression (TTP) was 8 weeks, but several of the NSCLC and RCC patients had disease stabilization beyond initial restaging. One of the RCC patients treated at the 0.5 mcg/kg dose level had stabilization of small volume lung disease for 2 years. Interestingly, this patient’s lesions were growing prior to treatment, and he experienced regrowth of his lung metastases within 8 months of cessation of treatment, suggesting that rhIL-15 antitumor effects played a role in his disease stabilization.
Pharmacokinetics and Production of Inflammatory Cytokines
The time of maximum drug concentration (Tmax) was found to occur 4 hours following SC administration of rhIL-15 (Figure 1A), which was noticeable beginning at the 1 mcg/kg dose level. The maximum drug concentration (Cmax) at this time point increased proportionally with higher dose levels of rhIL-15 so that the arithmetic mean value for the Cmax (± 1 SD) was <30 (± 0) pg/mL, 87 (± 50) pg/mL, 624 (± 714) pg/mL, 1632 (± 2049) pg/mL, and 6459 (± 2180) pg/mL for the 0.25, 0.5, 1.0, 2.0 and 3.0 mcg/kg/day dose cohorts respectively. By 24 hours post dose, the mean serum rhIL-15 concentration had fallen more than one log to <30, 38, 36, 70, and 113 pg/mL for the 5 dose levels. The non-linear dose-response for the Cmax value is most consistent with a PK model of more complete clearance of serum rhIL-15 at the lower dose levels due to greater availability of high-affinity IL-15 receptors.
Figure 1. rhIL-15 pharmacokinetics and cytokine responses.
Blood samples were collected from enrolled subjects before, and 10 minutes, 1, 4 and 24 hours after the first dose of rhIL-15 given subcutaneously during cycle 1. Serum was frozen until batch ELISA testing was performed for IL-15 (A), IL-6 (B), and IFN-γ (C) levels, as described in Materials and Methods. Mean results from each dose cohort are shown with error bars (±1SD); the y-axis is shown in log (A) or linear scales (B, C). Error bars for panels (B) and (C) were omitted to facilitate viewing of the data because error bars for all dose cohorts overlapped, even at the 4-hour peak time point.
Changes in serum concentrations of several important inflammatory cytokines were also evaluated. There were no consistent changes in IL-1β, IL-10, and IL-12p70 (data not shown). Small increases in mean IL-6, IFN-γ (Figures 1B and 1C, respectively), and TNF-α (data not shown) were seen in the 3 highest dose cohorts, mirroring the pharmacokinetics of rhIL-15 and peaking at 4 hours after dosing. However, peak levels were not statistically different among dose cohorts. IL-6 levels were slightly elevated but significantly lower than IL-6 levels seen with cytokine release syndrome from chimeric antigen receptor gene modified T cells (41). Given the overall higher levels of IFN-γ detected, it is more likely that IFN-γ levels corresponded with the post-injection onset of fevers in treated patients.
Lymphocyte Expansion and Immune Activation in Lymphocyte Subsets
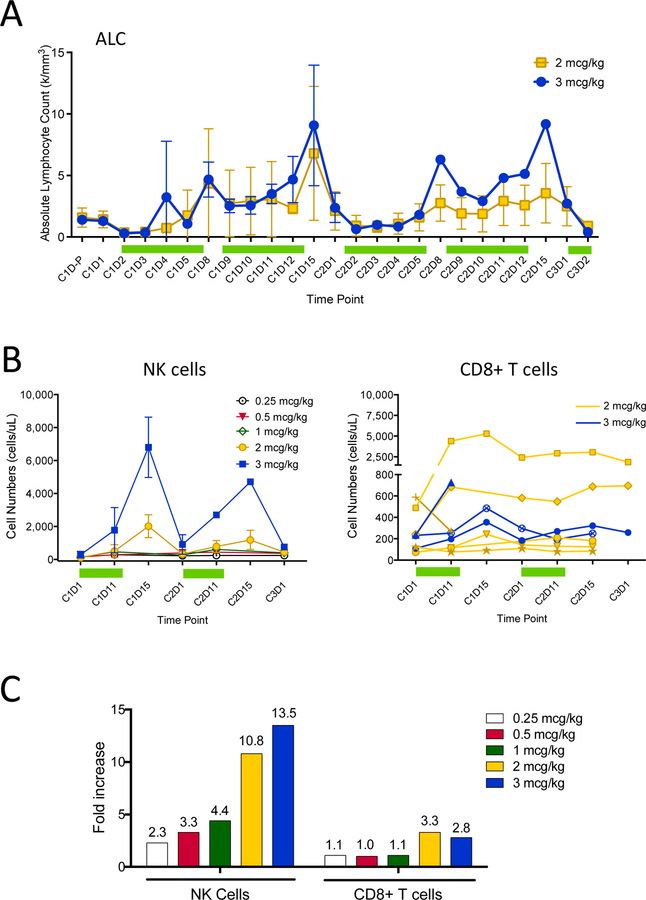
Serial analyses of absolute lymphocyte counts (ALC) and flow cytometric assessment of lymphocyte subsets revealed significant increases in ALC and circulating NK cell numbers respectively beginning with the initial dose level (Figure 2A, 2B). The mean maximum post-treatment ALC increase during cycle 1 was 1.5, 2.4, 2.3, 3.9 and 8.2-fold respectively for the 5 dose levels, whereas the maximum post-treatment WBC increase was 1.1, 1.9, 1.3, 1.4, and 2.0-fold. A dramatic increase in the number of circulating NK cells produced mean 2.3, 3.3, 4.4, 10.8, and 13.5-fold increases respectively for the 5 dose levels (Figure 2C). Increases in the number of circulating CD8+ effector cells were modest, with mean maximal 3.3 and 2.8-fold increases over baseline at the 2 and 3 mcg/kg dose levels, respectively. Maximal increases in NK and CD8+ T cell numbers occurred during the second week of treatment during each cycle, with peak numbers consistently on day 15, three days after the last dose of rhIL-15. This observation is consistent with return of NK cells into the peripheral circulation after withdrawal from activation. Maximal increases in NK cell numbers during cycle 2 were consistently lower than in cycle 1, suggesting tachyphylaxis of the rhIL-15 induced NK cell lymphocytosis but less evident for the ALC and CD8+ T cell responses. The finding of tachyphylaxis for NK cells but not for CD8+ T cells is in agreement with observations from both mouse and nonhuman primate studies(30).
Figure 2. Circulating lymphocyte, NK and CD8+ T cell numbers before and during rhIL-15 treatment.
Absolute lymphocyte counts were calculated from CBC data obtained daily from individual subjects’ local labs for the 2 mcg/kg/day (yellow, N=6) and 3 mcg/kg/day (blue, N=3) dose cohorts, and includes a pre-cycle 1 day1 (C1D-P) time point (A). Each line/symbol represents mean results from each dose cohort with error bars (±1SD). Green bars represent periods of daily rhIL-15 treatment. Absolute cell frequencies (cells/mcL) of CD45+CD3-CD56+ NK cells in fresh whole blood were measured using Trucount tubes and are shown as means (±1SD) grouped by dose cohort (B, left). Absolute cell frequencies (cells/mcL) of CD45+CD3+CD8+ T cells among subjects treated with 2 or 3 mcg/kg/day of rhIL-15 is shown (B, right). Each line/symbol represents results from a single individual from the 2 and 3 mcg/kg/day dose cohorts as shown in (A). Mean fold-increases for whole blood NK and CD8+ T cell frequencies during cycle 1 at days 11 or 15 (whichever was available and/or maximal) compared to baseline (day 1) for all treated subjects is shown, grouped by dose cohort (C). Mean CD56+ NK cell fold increases (± 1SD) for the 0.25 (N=3), 0.5 (N=3), 1 (N=3), 2 (N=6), and 3 (N=3) mcg/kg dose cohorts were 2.3 (± 1.2), 3.3 (± 2.3), 4.4 (± 3.2), 10.8 (± 8.2), and 13.5 (± 6.6) respectively. Mean CD8+ T cell fold increases (± 1SD) for the 0.25, 0.5, 1, 2, and 3 mcg/kg dose cohorts were 1.1 (±0.2), 1.0 (±0.2), 1.1 (±0.2), 3.3 (± 3.8), and 2.8 (± 0.6) respectively.
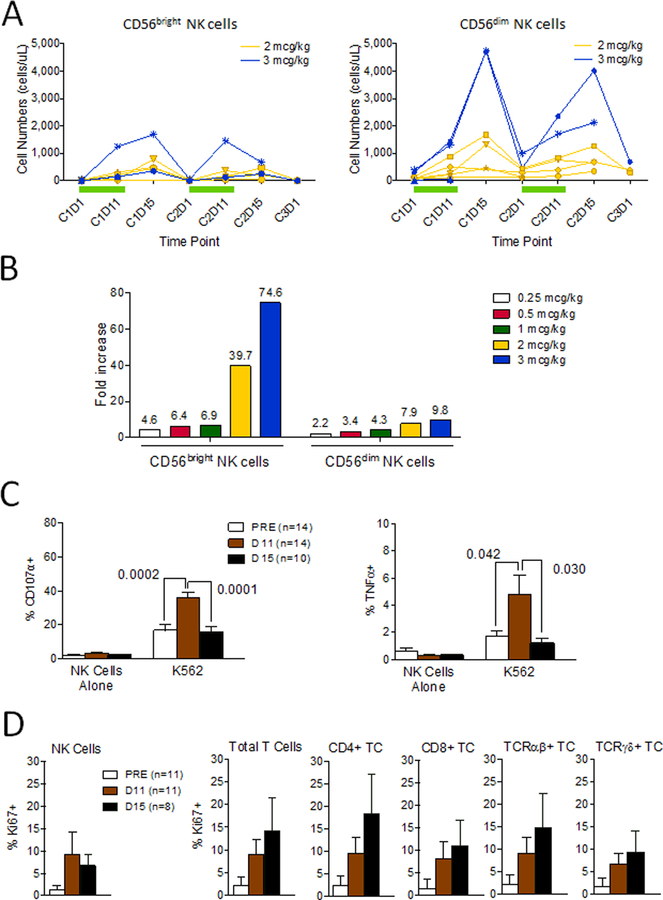
A deeper analysis indicated that both CD56bright and CD56dim NK cells increased in number (Figure 3A). However, the larger CD56dim subset had the highest absolute numbers post-treatment, while the smaller CD56bright subset demonstrated a greater fold-increase in cell numbers (Figure 3A). This is not unexpected as CD56bright NK cells are known to proliferate at a higher rate than CD56dim NK cells. NK cytotoxic capacity stimulated by K562 cells in vitro, and measured by CD107a+ degranulation or TNF-α+ production (Figure 3C) was maximal at the end of treatment rather than 3 days after rhIL-15 cessation when NK cell numbers peaked in the peripheral circulation. Increased expression of intracellular Ki67, a specific marker of cellular proliferation, among multiple lymphocyte subsets (Figure 3D) corroborates the NK cell increases, but also suggests that rhIL-15-stimulated increases in T cell subsets may be underestimated in the circulation.
Figure 3. Circulating NK cell subset expansion and NK cell function during rhIL-15 treatment.
Whole blood samples were analyzed for CD3-CD56+ NK cell subset frequencies of CD56bright (left) and CD56dim (right) NK cells using multiparametric flow cytometry as described (A). Individual subject data are represented by a single line/symbol, in yellow for the 2 mcg/kg/day (N=6) and blue for the 3 mcg/kg/day (N=4) dose cohorts (A). Green bars represent periods of daily rhIL-15 treatment. Mean fold-increases during cycle 1 at days 11 or 15 (whichever was available and/or maximal) compared to baseline (day 1) for treated subjects, grouped by dose cohort, are indicated by each column for whole blood CD56bright and CD56dim NK cells (B). Mean CD56bright NK cell fold increases (± 1SD) for the 0.25 (N=3), 0.5 (N=3), 1 (N=3), 2 (N=6), and 3 (N=4) mcg/kg dose cohorts were 4.6 (± 1.6), 6.4 (± 4.1), 6.9 (± 2.2), 39.7 (± 54.4), and 74.6 (± 74.4) respectively. Mean CD56dim NK cell fold increases (± 1SD) for the 0.25, 0.5, 1, 2, and 3 mcg/kg dose cohorts were 2.2 (± 1.2), 3.4 (± 2.5), 4.3 (± 3.6), 7.9 (± 4.7), and 9.8 (± 7.0) respectively. Mean values are shown above each column. Cryopreserved samples obtained before rhIL-15 initiation (N=11) and at Day 11 (N=11) and Day 15 (N=8) during/after treatment were assessed for evidence of active proliferation by intracellular Ki67 labeling of NK cells, T cells and T cell subsets as described (C). NK cell degranulation (C, left panel) or intracellular TNF-α (C, right panel) measured after no stimulation or stimulation with K562 for 5 hours is shown. Cryopreserved samples obtained before rhIL-15 initiation (N=11) and at Day 11 (N=11) and Day 15 (N=8) during/after treatment were assessed for evidence of active proliferation by intracellular Ki67 labeling of NK cells, T cells and T cell subsets as described (D).
Assessment of Anti-rhIL-15 Antibodies and Neutralizing Activity
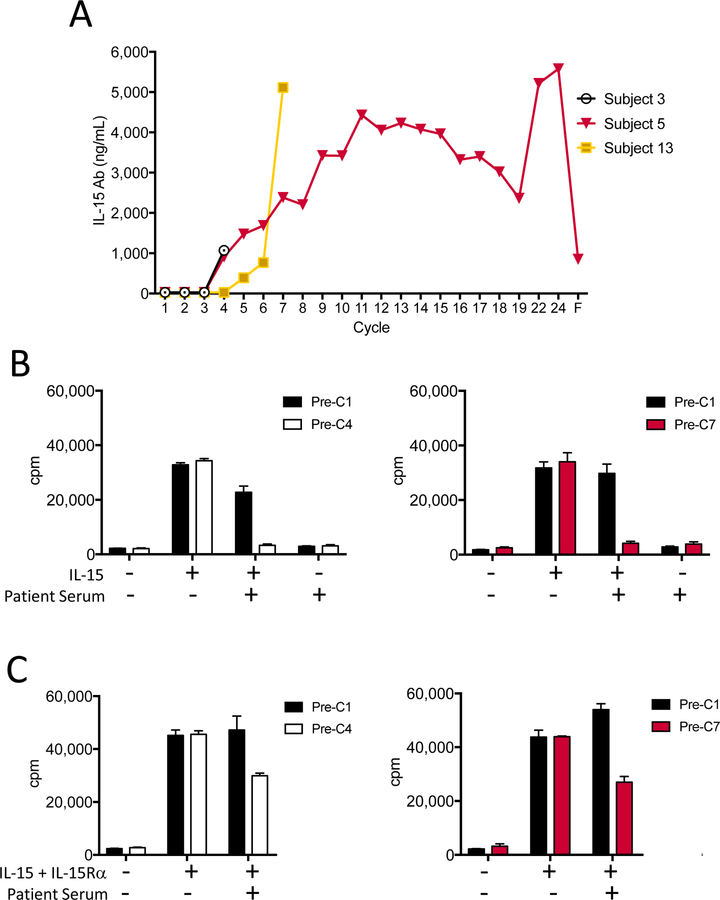
Only 3 of the 19 enrolled patients developed anti-rhIL-15 antibodies (Figure 4A); all were first detected after the third cycle of treatment. These anti-rhIL-15 antibody titers continued to rise in 2 patients who continued treatment and for whom subsequent samples were available. The detection of anti-IL-15 antibodies was not associated with any clinical toxicity.
Figure 4. Development of anti-rhIL-15 antibodies and assessment of neutralizing capacity.
All enrolled subjects were tested for the presence of anti-rhIL-15 serum antibodies at baseline, at the start of each cycle, and at their final blood draw for correlative testing. Of 19 subjects, only 3 developed antibodies against rhIL-15, and serum antibody levels for these 3 subjects re shown (A). For the graph x-axis in panel A, “F” refers to the 6-month follow-up visit after study withdrawal. Serum from two subjects, Subject 3 (0.25mcg/kg/day, white and black bars) and Subject 5 (0.5mcg/kg/day, red and black bars) were tested for their ability to inhibit the proliferation of NK92, an IL-15 dependent cell line, in the presence of rhIL-15 (B) or rhIL-15 + IL-15 receptor-α (C) in a 3H-thymidine incorporation assay. Means of triplicate values ±1 SD are shown.
The neutralizing capacity of anti-rhIL-15 antibodies that developed in treated patients was evaluated using the IL-15/IL-2 dependent cell line NK-92 (Figure 4B and C). The addition of anti-rhIL-15 antibody-containing patient sera to cultures of NK-92 cells and exogenous rhIL-15 resulted in inhibition of NK-92 cell proliferation (Figure 4B). These results indicate that the anti-rhIL-15 antibodies that developed were neutralizing for the clinical agent. However, when heterodimeric rhIL-15/IL-15Rα was added to the NK-92 proliferation assay, patient serum exhibited only a partial functional suppression of proliferation (Figure 4C). These results demonstrate the relative specificity of the neutralizing anti-IL-15 antibodies against the single chain E.coli rhIL-15 used for treatment compared to the physiologic heterodimeric IL-15/IL15Rα that is active in vivo.
DISCUSSION
The primary goal of this phase I dose escalation trial, to identify the maximum safe and tolerable dose of SC rhIL-15 that could be administered on an outpatient basis, was achieved. Subject 10 (2 mcg/kg) experienced an SAE of pancreatitis that resolved fully within a few weeks without sequelae and Subject 18 (3 mcg/kg) had an SAE and dose-limiting grade 3 cardiac chest pain that also resolved quickly after discontinuation of treatment with supportive care. Apart from these two events, the spectrum of adverse events was generally found to be mild and reversible. Dose dependent adverse events were most often immunomodulatory cytokine toxicities such as mild chills, fevers, fatigue, nausea/vomiting and skin changes that could be lessened or eliminated with standard antipyretics or antiemetics. Mild and/or transient decreases in blood pressure, red blood cells, platelets and white blood cells also seemed dose dependent, but did not result in consequential clinical adverse events, exemplifying a good composite safety profile. The primary goal of this study was to establish a safe outpatient dose for subcutaneous rhIL-15, and we determined 2 mcg/kg to be the MTD.
Demonstration of a clinical effect in single agent phase I trials is rare, and was not seen in this trial. The patients entered in this trial were generally older (median age 61 and 32% >70 years) and heavily pretreated. Seven of the patients had stable disease (SD) and continued treatment beyond initial restaging, including a RCC patient (Subject 5) who remained on treatment for 2 years with SD and had continued stable disease for an additional 8 months before beginning other treatment. The SC IL-15 treatment produced only a modest circulating CD8+ T lymphocytosis, although Ki67 analyses suggested that these data may underestimate the effect of rhIL-15 on T cells. More encouraging, SC rhIL-15 proved to be a forceful stimulator of human NK cells, generating a 10-fold expansion of highly functional NK cells at the 2 highest doses that was equivalent to the level of NK cell expansion in non-human primates treated with 20 mcg/kg (37). The fold-increase in NK cells was greater in the small population of CD56bright NK cells relative to CD56dim NK cells, with absolute numbers greatest in both subsets 3 days after the last dose of rhIL-15 in cycle 1. By contrast, NK cell function peaked shortly after the last IL-15 dose and rapidly diminished several days thereafter, possibly due to cytokine withdrawal terminating a cumulative effect on activation and/or trafficking of activated cells into tissues from the peripheral blood.
Preliminary PK analyses indicated the time to maximum rhIL-15 concentration post-injection is ~4 hours. Even in the two highest dose cohorts, the 24-hour serum rhIL-15 concentrations had decreased more than one log from the 4-hour peak value, therefore supporting a daily treatment schedule for SC rhIL-15. The similar kinetics of rhIL-15 Cmax and inflammatory cytokines IL-6 and IFN-γ was not surprising.
For patients treated with recombinant human protein agents, the development of anti-drug antibodies (ADA) is not uncommon. Further, SC administration of these drugs would potentially make them more immunogenic and thus more likely to elicit an ADA response. Although these antibodies are often clinically inconsequential with regard to efficacy or toxicity, E. coli-derived non-glycosylated proteins have high potential for inducing consequential ADAs because of their dissimilarity from mammalian glycoproteins. The original rhIL-7 formulation which required a new mammalian cell line production method to address neutralizing ADAs that prohibited repeat dosing in patients is a cautionary tale(42). While three intermediate dose level patients treated with multiple cycles of rhIL-15 in this study developed progressively increasing titers of neutralizing ADAs, these antibodies had no apparent clinical consequence. This is best exemplified by Subject 5, who seemingly experienced the greatest clinical benefit from rhIL-15 treatment (SD for 2 years), but also had the highest ADA levels. Although more attention to this phenomenon will still be required in future SC rhIL-15 trials with patients evaluated over multiple cycles, results from this trial suggest that ADA seen in this study are not an obstacle for the physiologic activity of IL-15 when trans-presented as a complex with IL-15Rα.
While preclinical animal model testing identifies the basic immunologic events anticipated to occur in humans, initial clinical efforts with new agents often identify new toxicities or demonstrate important dissimilarities among murine, macaque and human physiology. The initial first-in-human clinical trial with rhIL-15 administered as an IVB was unexpectedly and severely limited in dose escalation, produced diminutive immune activation, and thereby demonstrated little potential for use in combination with other agents. In the current study, new or problematic toxicities were not identified and subcutaneous dosing allowed 6-fold more drug administration compared to IVB. In fact, adverse effects associated with daily SC rhIL-15 injection were mild, well-tolerated, and manageable on an outpatient basis, making rhIL-15 amenable to future combination with other therapies. IL-15 may also be broadly applied to immunotherapy for other diseases, although we need to cautious about hematologic malignancies that might actually be stimulated by IL-15. In addition to defining the basic safety goals, a deeper understanding of treatment-related functional changes in responsive effector cell subsets was a critical objective for this research. In summary, using an outpatient regimen of SC IL-15, these analyses revealed robust effects on immune effector NK cells primarily, with lesser effects on CD8+ T cells, which will inform the design of future combination rhIL-15 treatment regimens including NK(15, 16, 43) or T cell infusions(4–7), checkpoint inhibitors(1–3) and multiple FDA-approved cancer targeting antibodies.
ACKNOWLEDGMENTS
The authors would like to acknowledge the contributions of the following individuals to this work: Minjun Apodaca, ASCP, and other members of the CITN Central Laboratory for their technical contributions, Stephen C. De Rosa MD and Tiffany Hensley-McBain of the HIV Vaccine Trials Network for their help in establishing the flow cytometric methods, the Fred Hutchinson Cancer Research Center Flow Cytometry Facility for their support in conducting testing, Valarie McCullar from the Miller laboratory at the University of Minnesota for functional assay testing, and Angela Riggins for her help in preparation of the manuscript figures.
Study support: Cancer Immunotherapy Trials Network (CITN): NIH 1U01 CA154967–01 (ClinicalTrials.gov ). This study was also partially supported by the Intramural Research Program of the National Cancer Institute and P01 CA111412 (JSM) and NCI R35 CA197292 (JSM).
Footnotes
Conflict of Interest Statement: DG McNeel reported holding ownership interest (including patents) in and being a consultant/advisory board member for Madison Vaccines Inc. No potential conflicts of interest were disclosed by other authors.
REFERENCES
- 1.Borghaei H et al. , Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 373, 1627–1639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer JR et al. , Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366, 2455–2465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL et al. , Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366, 2443–2454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber J et al. , White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clin Cancer Res 17, 1664–1673 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA et al. , Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17, 4550–4557 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW et al. , T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maude SL et al. , Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371, 1507–1517 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley JC, Lin MT, Le DT, Eshleman JR, Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 22, 813–820 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Gatalica Z et al. , Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 23, 2965–2970 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Le DT et al. , PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New Engl J Med 372, 2509–2520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Teachey DT, Porter DL, Grupp SA, CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 125, 4017–4023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed N et al. , Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol 33, 1688–1696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins PF et al. , A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 21, 1019–1027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran E et al. , Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JS et al. , Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Bachanova V et al. , Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 123, 3855–63, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maccalli C, Parmiani G, Ferrone S, Immunomodulating and Immunoresistance Properties of Cancer-Initiating Cells: Implications for the Clinical Success of Immunotherapy. Immunol Invest 46, 221–238 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Wang M, Busuttil RA, Pattison S, Neeson PJ, Boussioutas A, Immunological battlefield in gastric cancer and role of immunotherapies. World J Gastroenterol 22, 6373–6384 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Vella AT, Regulatory T Cells and Cancer: A Two-Sided Story. Immunol Invest 45, 797–812 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Tao JH et al. , Foxp3, Regulatory T Cell, and Autoimmune Diseases. Inflammation 40, 328–339 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Gabrilovich DI, Nagaraj S, Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9, 162–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soliman H, Mediavilla-Varela M, Antonia S, Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J 16, 354–359 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldmann TA et al. , Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood 117, 4787–4795 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen Bernard, Zhang Meili, Dilillo David, Ravetch Jeffrey V., Waldmann Thomas A., Interleukin-15 enhances rituximab-dependent cytotoxicity ex vivo and in vivo against a mouse lymphoma expressing human CD20. [abstract]. In: Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18–22; Philadelphia, PA Philadelphia (PA): AACR; 10.1158/1538-7445. [DOI] [Google Scholar]
- 25.Waldmann TA, The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res 3, 219–227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldmann TA, Tagaya Y, The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 17, 19–49 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Waldmann TA, The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6, 595–601 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Fehniger TA, Caligiuri MA, Interleukin 15: biology and relevance to human disease. Blood 97, 14–32 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Marks-Konczalik J et al. , IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci U S A 97, 11445–11450 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sneller MC et al. , IL-15 administered by continuous infusion to rhesus macaques induces massive expansion of CD8+ T effector memory population in peripheral blood. Blood 118, 6845–6848 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi H et al. , Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood 105, 721–727 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Zhang M et al. , Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A 106, 7513–7518 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klebanoff CA et al. , IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A 101, 1969–1974 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudo-Saito C et al. , Combination therapy of an orthotopic renal cell carcinoma model using intratumoral vector-mediated costimulation and systemic interleukin-2. Clin Cancer Res 13, 1936–1946 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Fyfe G et al. , Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 13, 688–696 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg SA et al. , Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst 85, 622–632 (1993). [DOI] [PubMed] [Google Scholar]
- 37.Conlon KC et al. , Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 33, 74–82 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hensley TR et al. , Enumeration of major peripheral blood leukocyte populations for multicenter clinical trials using a whole blood phenotyping assay. J Vis Exp, e4302 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meager A, Wadhwa M, Detection of anti-cytokine antibodies and their clinical relevance. Expert Rev Clin Immunol 10, 1029–1047 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Iasonos A, Wilton AS, Riedel ER, Seshan VE, Spriggs DR, A comprehensive comparison of the continual reassessment method to the standard 3 + 3 dose escalation scheme in Phase I dose-finding studies. Clin Trials 5, 465–477 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DW et al. , Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sportes C et al. , Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res 16, 727–735 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Martinez A et al. , A phase I/II trial of interleukin-15--stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy 17, 1594–1603 (2015). [DOI] [PubMed] [Google Scholar]