Abstract
Francisella tularensis causes the zoonotic disease tularemia. Arthropod vectors are important transmission routes for the disease, although it is not known how Francisella survives the efficient arthropod immune response. Here, we used Drosophila melanogaster as a model host for Francisella infections and investigated whether the bacteria are resistant to insect humoral immune responses, in particular to the antimicrobial peptides (AMPs) secreted into the insect hemolymph. Moreover, we asked to what extent such resistance might depend on lipopolysaccharide (LPS) structure and surface characteristics of the bacteria. We analyzed Francisella novicida mutant strains in genes, directly or indirectly involved in specific steps of LPS biosynthesis, for virulence in wild-type and RelishE20 immune-deficient flies, and tested selected mutants for sensitivity to AMPs in vitro. We demonstrate that Francisella is sensitive to specific fly AMPs, i.e. Attacin, Cecropin, Drosocin and Drosomycin. Furthermore, six bacterial genes, kpsF, manB, lpxF, slt, tolA and pal, were found to be required for resistance to Relish-dependent immune responses, illustrating the importance of structural details of Francisella lipid A and Kdo core for interactions with AMPs. Interestingly, a more negative surface charge and lack of O-antigen did not render mutant bacteria more sensitive to cationic AMPs and did not attenuate virulence in flies.
Key Words: Francisella tularensis, Drosophila melanogaster, Antimicrobial peptides, Lipopolysaccharide, Host-pathogen interactions
Introduction
Francisella tularensis is a gram-negative facultative intracellular bacterium and the causative agent of the zoonotic disease tularemia. Worldwide about 250 mammalian wildlife species such as rodents, rabbits, squirrels or deer are known carriers of tularemia agents. Humans contract infections through direct contact with carrier animals, inhalation or ingestion of contaminated dust or water, as well as through arthropod bites. Two subspecies of F. tularensis, subsp. tularensis (type A) and subsp. holartica (type B) are of clinical importance. Another species, Francisella novicida, is an environmental isolate that is not infectious for healthy humans, but causes a tularemia-like disease in wildlife. F. novicida is more than 97% genetically identical to F. tularensis[1] and is a widely used laboratory model for pathogenic Francisella species.
Hematophagous arthropods like hard ticks, horse flies or mosquitoes have been recognized as vectors of tularemia, and mosquito-borne infection has been linked to some of the largest epidemics of tularemia ever reported [2]. The bacteria have been detected in the midgut, salivary glands and in hemolymph of ticks [3, 4] and in midgut and Malpighian tubule cells of mosquitoes [5, 6], but it is not clear how F. tularensis survives in these hosts that are known to possess efficient cellular and humoral immune responses.
To date there is no laboratory model available using a natural arthropod vector of Francisella. We recently demonstrated that Drosophila melanogaster can be employed as an in vivo model of Francisella infections that enables analysis of bacterial survival and virulence mechanisms in an arthropod host [7]. Similar to Francisella infections of mammalian hosts, the bacteria are phagocytosed by the fly's macrophage-like hemocytes, and they proliferate in these cells relying on nearly the same set of virulence genes. In addition, Francisella propagates extracellularly in the open circulatory system (hemolymph) of infected flies and eventually kills them.
One of the immediate immune mechanisms induced by bacterial infections is the secretion of antimicrobial peptides (AMPs) into the extracellular space. Many of these mostly small and cationic peptides electrostatically interact with negative charges on the bacterial surface and integrate into the membrane by pore formation leading to disruption of the permeability barrier and the transmembrane potential. Moreover, certain peptides can also inhibit critical intracellular targets [8].
F. tularensis is known to circumvent important innate immune mechanisms in mammalian hosts. The O-antigen of Francisella lipopolysaccharide (LPS) does not activate the complement system; the lipid A has weak endotoxic activity and is not proinflammatory [9]. In contrast to the classical endotoxin of enterobacteriaceae, Francisella lipid A is not phosphorylated and carries only four acyl chains. Moreover, the majority of lipid A occurs in a free form without O-antigen. This free lipid A is monophosphorylated in the 1′-position with an adjacent galactosamine; in addition, it can be mannosylated or glycosylated in the 4′- or 6′ position [10, 11]. Interestingly, these changes in the lipid A structure, more specifically the removal of a 3′-acyl chain and a 4′-phosphate, are also responsible for Francisella's natural resistance to the cationic AMP polymyxin B [12].
Here, we investigated whether Francisella is resistant to insect humoral immune responses, in particular to D. melanogaster AMPs, and asked to what extent such resistance might depend on the Francisella LPS structure and outer membrane composition.
Materials and Methods
Bacterial Cultures
The live vaccine strain (LVS) of F. tularensis subspecies holarctica was originally supplied by the US Army Medical Research Institute of Infectious Diseases (Fort Detrick, Frederick, Md., USA). The F. novicida wild-type strain U112 was obtained from C. Manoil together with the two-allele library of U112-derived transposon insertion strains from which most mutants originated [13]. Other strains used were an LVS-derived wbtA deletion mutant [14] and U112-derived deletion mutants of lpxF[12], manB and manC[15]. If not otherwise noted, bacteria were grown on modified GC agar (GC II agar base complemented with hemoglobin and ISO-VITALEX) supplemented with 50 µg/ml polymyxin B or 15 µg/ml kanamycin (for mutant strains) at 37°C and 5% CO2 for 24 h. Enterobacter cloacae was obtained from Dan Hultmark's laboratory and grown on Luria broth agar plates.
Fly Strains
All fly strains were grown on standard potato meal agar at 25°C and 60% humidity. The D. melanogaster Oregon R strain served as wild type. For expression of single AMP genes in immune deficient background we crossed w1118; b pr imd; daughterless-GAL4 spzrm7/TM6c to w1118; UAS-AMP imd/CyO; UAS-AMP spzrm7/TM6c Tb Sb flies [16]. AMP stands for either Attacin A (AttA), Diptericin (Dpt), Defensin (Def), Drosocin (Drc), Drosomycin (Drs) or Cecropin A (CecA). For expression of cecropin genes in wild-type background we crossed w1118; +; UAS-CecA and w1118; +; UAS-CecB (3 different lines; all generated by S. Ekengren/D. Hultmark, unpublished) to w1118; +; da-GAL4 flies. The da>AMP offspring used in experiments was incubated at 29°C for approximately 24 h prior to experiments to obtain a strong activity of the UAS-GAL4 system. Other fly strains used were w; +; RelishE20[17].
Quantitative RT-PCR
Total RNA from 8 flies per sample was isolated using Trizol reagent (Invitrogen). cDNA synthesis was performed on DNase-I treated total RNA (2.5 µg) by oligo (dT) priming using the Superscript III preamplification system (Invitrogen). PCR was performed using a 7900 HT Fast Real-Time PCR. Primer sequences are listed in online supplementary table S1 (for all online suppl. material, see www.karger.com?doi=10.1159/000342468).
D. melanogaster Infections
Flies were infected either by injection of resuspended bacteria using a glass capillary (method A) or by pricking with plated bacteria using a glass needle (method B). For method A bacteria were resuspended in phosphate-buffered saline to OD600 nm 1.0 for F. tularensis LVS derivatives and to OD600 nm 0.01 for F. novicida derivatives, corresponding to approximately 3 × 109 CFU/ml and 3 × 107 CFU/ml, respectively. For method B bacteria were grown on plates. Both methods were performed as previously described [7, 18]. Inhibition of phagocytosis with latex beads and viable count analysis was also performed as described previously [7].
Inhibition Zone Assay
This assay was adapted for Francisella from Hultmark [19]. Bacteria were grown to early log-phase (OD600 nm 0.1, corresponding to approx. 3 × 108 CFU/ml) in trypticase soy broth supplemented with 0.1% L-cysteine and 0.2% dextrose. Thin-layer agarose plates were prepared from 10 µl bacterial suspension and 0.8% agarose in supplemented trypticase soy broth medium. Fine glass needles were loaded with 2 µl protease inhibitor solution (Roche) and then used to collect hemolymph from the heads of 5 flies per sample. Plates were incubated at 29°C until bacterial lawns and inhibition zones were clearly visible. For each combination of fly genotype and bacterial strain 3–18 hemolymph samples were tested. Protease inhibitor solution alone did not inhibit bacterial growth.
Data Analysis
We calculated differences in median life length (ΔMLL) between test and control flies to determine the effect of treatment on fly survival [7, 18]. For bacterial growth in flies original data from viable count analysis was logarithmically transformed and the absolute increase in bacterial load per fly from injection (day 0) to day ‘X’ after infection was used for comparisons between test and control (in vivo proliferation, proliferation index). To identify statistically significant differences we used Student's two-sample t test. Probability values below <0.05 were considered significant.
Results
F. tularensis Is Sensitive to Drosophila Antimicrobial Peptides in vivo
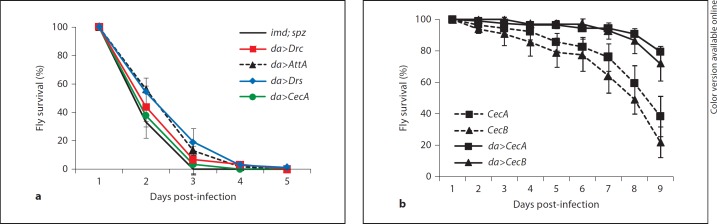
In the fruit fly induction of AMP genes upon bacterial infection is controlled by the NF-ĸB-dependent Toll and Imd/Relish signaling pathways. We previously demonstrated that in RelishE20 mutant flies, which almost completely lack antimicrobial peptides, Francisella proliferates much faster than in wild-type flies [7]. Although Relish also regulates a number of other genes with less known or unknown relation to the immune system [20, 21, 22], the above-mentioned observation suggests that Francisella is sensitive to Drosophila antimicrobial peptides despite its overall virulence in flies. In order to test this hypothesis we utilized a genetic approach, the UAS/GAL4 system to ectopically express single D. melanogaster AMPs in immune deficient genetic background [16]. In contrast to commercially available synthetic AMPs, this approach offers in vivo testing and all putative post-translational modifications of the peptides that might be required for full antimicrobial activity. The fly strains used in these experiments were deficient in both the Toll (by mutation in the spätzle gene) and the Imd/Relish pathways, and are considered to completely lack AMP gene expression. Individual AMP genes representative of the major types of D. melanogaster AMPs were constitutively and ubiquitously expressed in this imd; spätzle double mutant background using the ubiquitously active daughterless promoter (da>AMP flies). We then injected da>AMP flies with LVS bacteria and monitored fly survival. LVS-infected imd; spätzle control flies died within 3 days, similar to infected RelishE20 flies. Expression of Attacin A, Drosocin or Drosomycin significantly prolonged the survival of LVS-infected flies with 27, 8 and 37%, respectively (fig. 1a). We did not see a significant effect of Cecropin A, Diptericin or Defensin although overexpression of the respective AMP gene was confirmed using quantitative RT-PCR (table 1). However, the ectopic expression levels in da>AMP flies reached only about 20% of the levels induced by LVS bacteria in wild-type flies, except in the case of Defensin.
Fig. 1.
Protective effect of antimicrobial peptides on survival of flies infected with F. tularensis LVS. Antimicrobial peptides were ectopically expressed using the UAS/GAL4 system with the ubiquitously active daughterless (da) promoter indicated by ‘da>'. UAS-constructs were Attacin A (AttA), Cecropin A (CecA), Cecropin B (CecB), Drosocin (Drc), Drosomycin (Drs). a Survival of LVS-injected imd; spz immune-deficient flies which overexpress single antimicrobial peptides. Control flies were w1118; b pr imd; da-GAL4 spzrm7 with an MLL of 1.7 ± 0.1 days. The difference in MLL (ΔMLL) was 0.5 ± 0.2 days for da>AttA, 0.3 ± 0.1 days for da>CecA, 0.1 ± 0.0 days for da>Drc and 0.6 ± 0.1 days for da>Drs. b Survival of LVS-injected wild-type flies overexpressing Cecropin A or B with an MLL of 9.9 ± 0.5 and 10.3 ± 0.4 days, respectively, in comparison to 7.9 ± 0.4 days for LVS-infected genotype controls; results shown are from one fly strain transgenic for either UAS-CecA or UAS-CecB; for each UAS-construct similar results were obtained from two additional independent fly strains. Median values of three independent experiments with 20–80 flies per experiment are shown, error bars show standard error of mean.
Table 1.
Relative expression of AMP genes in noninfected da>AMP flies and in wild-type flies (OR) infected with E. cloacae, compared to LVS-infected OR Gene F old expression LVS-infect ed OR
| Gene | Fold expression |
||
|---|---|---|---|
| LVS-infected OR | da>AMP (mean ± SEM) | E. cloacae-infected OR (median) | |
| AttA | 1 | 0.23±.07 | 803.38 |
| CecA | 1 | 0.26±.04 | 1.94 |
| Def | 1 | 3.15 | 1.94 |
| Dpt | 1 | 0.51±.29 | 1.47 |
| Drc | 1 | 0.20 | 1.35 |
| Drs | 1 | 0.12±.08 | 0.70 |
The relative expression of AMP genes was determined by quantitative real-time PCR of whole flies. Infected flies were harvested 6–20 h after infection. Mean values of 2–3 independent experiments are shown.
Upon microbial infection, very high steady-state concentrations of various AMPs can be detected in the hemolymph of wild-type flies. In addition, AMPs are thought to act in synergy with each other. Tzou et al. [16] have shown that combined expression of two AMP genes prolongs fly survival for longer than expression of only one AMP gene. We therefore tested whether overexpression of Cecropin genes in a wild-type background provides flies with a greater survival advantage than if expressed in the immune-deficient background. Three different fly strains transgenic for expression of Cecropin A and three strains for Cecropin B were crossed and the survival of LVS-injected da>CecA and da>CecB offspring was monitored. Constitutive expression of either Cecropin A or Cecropin B in wild-type flies significantly prolonged fly survival with 23 or 30% of the lifespan of infected control flies, respectively (p < 0.001; fig. 1b). Taken together we found that overexpression of several antimicrobial peptides prolonged the survival of LVS-infected flies.
Modifications in Lipid A and an Intact Kdo Core Are Important for Virulence in D. melanogaster
We then wanted to investigate the importance of LPS and other membrane components for Francisella interaction with the fly humoral immune response. For this purpose we turned to F. novicida and made use of a well-characterized library of F. novicida U112 transposon insertion mutants [13] from which we chose roughly one hundred mutants in genes that had been annotated as being involved in ‘cell wall/LPS/capsule’, ‘motility/attachment/secretion structure’ or were simply ‘unknown’ [23], and that had been reported with attenuated mutant phenotypes in flies in at least one of three recent studies [18, 24, 25]. In addition, we chose a number of genes already known to be required for LPS biosynthesis in F. novicida or F. tularensis [10, 11, 15, 26, 27]: ten genes required for O-antigen synthesis, two genes reported to affect the Kdo core structure and five genes involved in modifying lipid A. In our experiments we also included slt, a gene encoding a putative soluble lytic murein transglycosylase. Mutations in this gene were expected to affect the structure of peptidoglycan rather than that of LPS.
First, we analyzed the LPS phenotype of the transposon insertion or deletion mutant strains and confirmed the absence of O-antigen in mutant strains for FTN 1256, the wbt and the man genes (online suppl. fig. S1, S2) [15, 27], as well as for FTN 1222/kpsF. To our surprise, even the lpxF, flmK and slt mutants appeared to have severely reduced O-antigen.
Second, we determined the surface charge (zeta potential) of the mutant strains, since this feature might correlate to their sensitivity to cationic antimicrobial peptides. The F. novicida U112 wild type had an almost neutral zeta potential of approximately −3 mV. Most mutant strains lacking O-antigen and/or the Kdo core demonstrated clearly more negative surface charges, while slt, lpxF and other mutant strains showed little but significant differences compared to the wild type (online suppl. tables S2, S3).
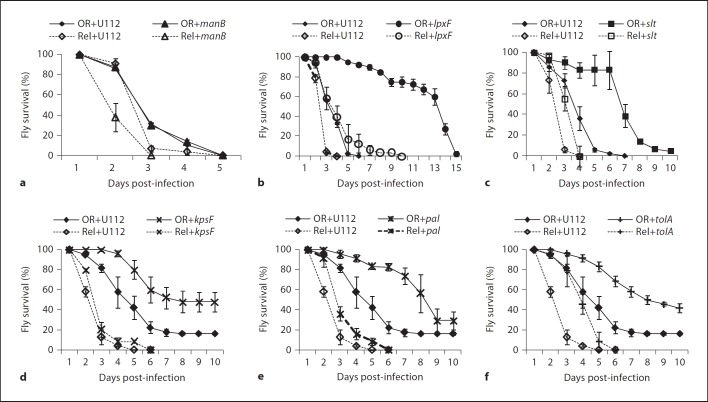
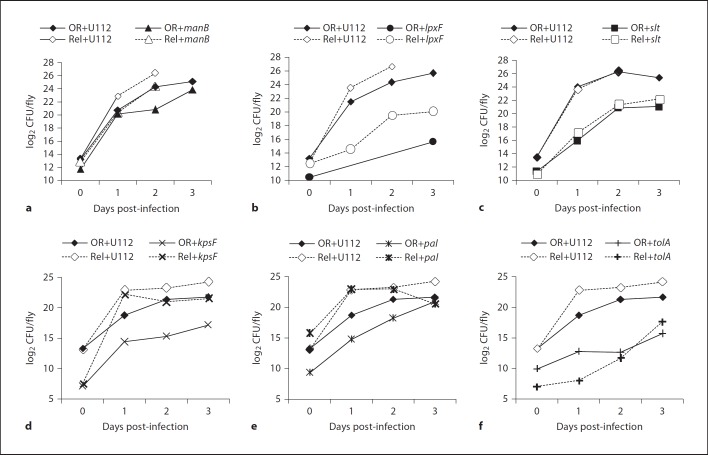
We then infected wild-type D. melanogaster flies either by pricking with mutants selected from the list of interesting candidates in online supplementary table S2 or by injection with mutants listed in online supplementary table S3. Fly survival and bacterial proliferation in flies was monitored (fig. 2, 3, online suppl. tables S2, S3). Among the mutations affecting O-antigen biosynthesis only transposon insertion in FTN 1222/kpsF led to significantly prolonged fly survival (fig. 2d, online suppl. table S2). In a previous study this mutant had shown a defect in intracellular proliferation in cultured Drosophila cells [24], but we did not observe reduced proliferation in flies (fig. 3d, online suppl. table S2). In Escherichia coli, kpsF encodes an arabinose-5-phosphate isomerase, which is involved in Kdo biosynthesis. Neisseria meningitides KpsF is required for the addition of core glycosyl residues like Kdo to lipid A and for the presence of a capsule [28]. Together, these findings suggest that KpsF has a similar role in F. novicida and that the kpsF mutant not only lacks O-antigen, but is also deficient in its core.
Fig. 2.
Survival of wild-type (OR) and RelishE20 immune deficient (Rel) D. melanogaster flies infected with F. novicida U112 or U112-derived mutant strains as indicated. a–c 30–45 wild-type or 40–55 RelishE20 mutant flies per experiment were infected by injection. d–f 25 wild-type or RelishE20 mutant flies per experiment were infected by pricking. Median values of three independent experiments are shown, error bars show standard error of the mean. For statistical analysis of the data see online supplementary tables S2 and S3.
Fig. 3.
In vivo proliferation of F. novicida U112 and U112-derived mutant strains in wild-type (OR) or in RelishE20 immune deficient (Rel) D. melanogaster flies. a–c Flies were infected by injection. d–f Flies were infected by pricking. Median values of three independent experiments are shown based on homogenates from 5 flies per sample and time point. For statistical analysis of the data see online supplementary tables S2 and S3.
The other O-antigen mutants were not attenuated in flies, thus extending and confirming our previous results (online suppl. table S2) [18]. The sugar composition of O-antigen differs between F. novicida and F. tularensis, therefore we also tested an LVS wbtA deletion in flies. Similar to the F. novicidawbtA mutant, the LVS wbtA mutant was not significantly attenuated in the fly model (not shown).
ManB, a phosphomannomutase, and ManC, a guanylyltransferase, are consecutively required for the addition of mannose to the Kdo core; mutant bacteria in either of these genes contain a ‘naked’ Kdo moiety without O-antigen[15]. The manB mutant was as virulent as the U112 wild type with regard to fly survival, but proliferated less well in flies than U112 bacteria, which has also been observed by others (fig. 2a, 3a, online suppl. table S3) [25]. Surprisingly, neither the manC transposon insertion strain nor manC deletion strains demonstrated attenuation in flies (online suppl. table S3).
Among the mutations affecting lipid A only the lpxF mutant strain was significantly attenuated. For this mutant, which lacks the lipid A 4′-phosphatase and retains not only the 4′-phosphate but also a 3′-acyl chain, prolonged fly survival correlated with strongly reduced bacterial proliferation (fig. 2b, 3b, online suppl. table S3). However, it has to be noted that this mutant grows much slower in vitro than the U112 wild-type or other transposon insertion strains [12]. Other modifications of the lipid A component as caused by mutations in lpxE or in either of the flm genes did not affect bacterial virulence in flies. Similar to our previous observations, the slt mutant strain was clearly attenuated in both virulence and in vivo proliferation (fig. 2c, 3c, online suppl. table S3) [18].
Considering the zeta potential of the various mutant strains we tested in flies, there was no overall correlation between bacterial surface charge and attenuated mutant phenotypes in flies. Our results demonstrate that the LPS O-antigen of Francisella does not significantly contribute to bacterial survival and virulence in flies. The results rather suggest that small changes in outer membrane composition like the specific structure and charge distribution of the Kdo core and the lipid A component of F. novicida LPS are important for bacterial resistance to fly immunity.
tolA and pal Mutants Are Attenuated in D. melanogaster
In addition to the above-identified LPS genes, we also found FTN 0354/tolA and FTN 0357/pal (peptidoglycan-associated lipoprotein) to be required for virulence in flies, although only the tolA mutant was reduced in its in vivo proliferation (fig. 2e, f, 3e, f, online suppl. table S2). In E. coli TolA and Pal are part of a multiprotein complex, the Tol-Pal system, that bridges between the peptidoglycan and the outer membrane and that is important for proper structure and function of the outer membrane [reviewed in [29]]. Mutations in tol and pal genes result in hypersensitivity to detergents and several antibiotics, leakage of periplasmic proteins, impaired motility and aberrant cell division [30]. Interestingly, TolA and Pal are necessary for correct surface polymerization of O-antigen chains that are assembled in a wzy (polymerase)-dependent manner. Although such a mechanism has been suggested for Francisella[31], we did not detect an O-antigen defect in pal or tolA mutants (online suppl. table S2).
F. novicida LPS Genes Required for Resistance to the Fly's Humoral Immune Response
We further asked whether the attenuated virulence and/or reduced growth of the various mutant strains were due to increased sensitivity to humoral immune responses in the hemolymph of D. melanogaster. We infected immune deficient RelishE20 mutant flies with mutant bacteria and determined whether the attenuated phenotype was reversed in such flies (phenotype ‘rescue’). Loss of Relish function almost completely eliminates expression of the inducible AMP genes, but does not affect the phagocytic function of the hemocytes [17]. The F. novicida U112 wild-type strain killed RelishE20 flies more rapidly and proliferated faster than in wild-type flies, similarly as we have shown before for LVS, and others for U112 [7, 25]. The manB mutant also proliferated faster and killed RelishE20 flies more rapidly than wild-type flies (fig. 2a, 3a, online suppl. table S3). A comparison of proliferation and fly survival in RelishE20 versus wild-type flies revealed a small but significant rescue of the attenuated manB phenotype (not shown). The attenuation of the lpxF, pal, tolA and kpsF mutant strains was rescued in RelishE20 flies indicating that these mutants are indeed more sensitive to Relish-dependent defense mechanisms than the U112 wild type (fig. 2, 3, online suppl. tables S2, S3). Despite the severely reduced immune responses in these flies, lpxF mutant bacteria still grew more slowly than wild-type bacteria, which is probably due to this mutant's general growth defect. The slt mutant demonstrated some rescue of attenuation with regard to fly survival, but it's in vivo proliferation was similar in wild-type and in RelishE20 flies (fig. 2c, 3c, online suppl. table S3).
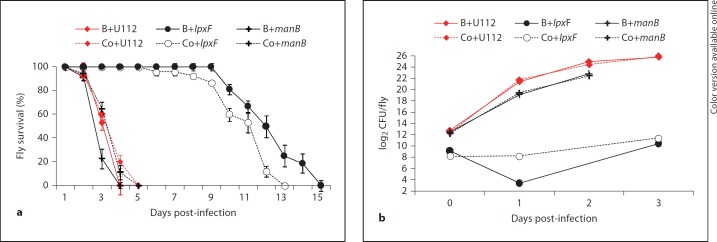
During Francisella infections of flies a growing proportion of the bacteria is localized in hemocytes, where they are protected from humoral immune responses in the hemolymph [7, 25]. We therefore forced the bacteria to remain in the extracellular compartment by blocking phagocytosis prior to the infection. This treatment did not affect the virulence and proliferation of the U112 wild-type strain or of the manB mutant, but significantly prolonged fly survival after infection with the lpxF mutant and delayed proliferation of this mutant (fig. 4, online suppl. table S4). The results from these two approaches demonstrate that the LpxF, ManB, Pal and KpsF proteins contribute to F. novicida resistance against Relish-dependent immune responses, in particular against the humoral responses in the hemolymph. Such a function can also be concluded for TolA [this study, [25]].
Fig. 4.
Blocking phagocytosis improved the survival of lpxF mutant-infected flies but not that of manB mutant-infected flies. a Survival of wild-type flies injected with either F. novicida U112 or U112-derived mutant strains as indicated. Prior to infection flies were injected with latex beads (B) to block phagocytosis of bacteria; control flies were injected with buffer (Co). b Growth of Francisella in wild-type flies. Flies were treated as described in a. Median values of three independent experiments with at least 35 flies per survival experiment and 4 flies per sample for viable count analysis are shown, error bars show standard error of the mean. For statistical analysis of the data see online supplementary table S4.
In vitro Sensitivity of Francisella to AMPs
To reduce the complexity of host-pathogen interactions and to test whether antimicrobial peptides directly inhibit Francisella growth, we utilized an inhibition zone assay in combination with pooled hemolymph samples of noninfected da>AMP flies. Overall, hemolymph from nonchallenged control flies (wild type or imd; spz double mutant) did not inhibit bacterial growth. However, occasionally hemolymph from imd; spz flies generated inhibition zones in lawns of the lpxF and slt mutants indicating that these strains are sensitive to humoral immune mechanisms, which might be dysregulated in the double mutant flies since hemolymph from RelishE20 flies did not generate inhibition zones (table 2 and data not shown). Analysis of hemolymph samples enriched in single AMPs showed increasing inhibition of wild-type and mutant bacterial growth. Since neither the amount of hemolymph nor the concentration of AMPs could be measured exactly in these experiments, we refrained from a quantitative analysis. Nevertheless, the results show that hemolymph from Drosocin- or Drosomycin-expressing flies inhibited the growth of the U112 wild-type strain, and that manB or lpxF mutant bacteria were sensitive to additional da>AMP hemolymph samples. Attacin and Defensin samples generated larger or more consistent inhibition zones than did control samples (not shown). LVS bacteria were more sensitive than the U112 wild type, and the slt mutant did not demonstrate increased sensitivity to da>AMP hemolymph (not shown).
Table 2.
Growth inhibition of F. novicida U112 and U112-derived mutants by D. melanogaster hemolymph
| Fly genotype | U112 | manB | lpxF |
|---|---|---|---|
| imd; spz | – | – | + |
| da>Attacin A | – | – | + |
| da>Cecropin A | – | + | + + |
| da>Cecropin B | + | + | + + |
| da>Defensin | – | – | + + |
| da>Diptericin | – | + | + + |
| da>Drosocin | + | + | + + |
| da>Drosomycin | + | – | + |
Results are based on 3–18 (on average 9) independent replicates for each combination of fly genotype and bacteria.
Discussion
We have demonstrated here that overexpression of AMPs like Attacin A, Cecropin, Drosocin and Drosomycin aid to protect Drosophila against the persistence of Francisella in vivo and/or inhibit bacterial growth in vitro. These antimicrobial peptides belong to three different structural groups: (i) linear α-helical peptides (cecropins, human LL-37), (ii) peptides forming disulphide bonds (Drosomycin, defensins) and (iii) peptides rich in particular amino acids (attacins, drosocins, pig PR-39, cow indolicidin) [32, 33]. It is likely that the various peptides interact differently with bacterial membranes, which is supported by our findings that wild-type Francisella was not sensitive to all peptides tested and that specific changes in the LPS core and lipid A extended sensitivity to additional AMPs. Specificity in the interactions between AMPs and bacteria was also demonstrated by Tzou et al. [16], who tested activity of various AMPs against a number of different microbes.
Cecropins probably act by binding to LPS and disrupting the bacterial membrane via pore formation, leading to release of cytoplasmic contents [34]. Drosocin and Attacin belong to the group of proline-/glycine-rich peptides. Like Cecropin, Drosocin is known to interact with LPS. In addition, it binds to cytoplasmic bacterial proteins like the heat shock protein DnaK and the chaperonin GroEL, both involved in protein folding [35]. In E. coli it has been shown that Attacin, which is a rather long peptide, partially integrates into the outer membrane via hydrophobic interactions with lipid A, and subsequently inhibits the synthesis of outer membrane proteins [36]. Rough E. coli strains are more sensitive to Attacin than strains carrying long O-antigen chains. In our experiments, the mere absence of O-antigen did not render Francisella mutants in manB or lpxF more sensitive to Attacin. But, with the 4′-phosphate and the 3′-acyl chain retained, the lipid A of the lpxF mutant is structurally similar to that of E. coli. This can explain this mutant's sensitivity to Attacin in contrast to the resistance of the manB mutant. These structural alterations in lpxF mutant lipid A seem also to trigger TLR2 and TLR4 signaling, whilst wild-type F. novicida lipid A does not [37]. Surprisingly, we found Drosomycin, which is considered an antifungal peptide, to be effective against Francisella. However, Tzou et al. [16] showed that the combined overexpression of Drosomycin and Drosocin increased resistance to the gram-positive bacterium Micrococcus luteus. In line with these findings it is interesting to note that although Drosomycin contains an additional disulfide bond, it is structurally similar to insect defensins and to human β-defensins. The inducible human β-defensin hBD-3 exhibits antimicrobial activity against F. tularensis LVS, while hBD-1 and hBD-2 do not seem to affect the bacteria [38].
It is noteworthy that lack of O-antigen and higher negative surface charge did not per se render the bacteria attenuated in flies, and more sensitive to humoral immune responses. In mouse models of Francisella infection O-antigen mutant strains are attenuated. Such mutants are serum-sensitive since their LPS variant activates complement, which wild-type Francisella LPS does not [39]. In D. melanogaster, however, a homologue to the mammalian complement system has not been found.
Taken together, the unique structure of Francisella LPS, specifically the structure and charge distribution of the Kdo core and of lipid A appear to be essential for virulence and resistance to humoral immune responses in flies. Apart from genes directly involved in LPS biosynthesis, we also found components of the Tol-Pal outer membrane protein complex to be important, probably because of an overall effect on membrane integrity in the corresponding mutants. Interestingly, the Tol-Pal system also functions as receptor for certain AMPs like colicins. However, from our results it is not likely that the Tol-Pal system of Francisella is involved in the uptake of insect AMPs, since the tolA mutant was attenuated rather than more virulent in flies.
Arthropod-transmitted tularemia is a worldwide problem. Still not much is known about the interactions between Francisella and its arthropod vectors. Like D. melanogaster, mosquitoes express Attacins, Cecropins and Defensins, but also a fourth group of AMP genes, gambicins, which are only found in mosquitoes [40]. The small number of mosquito AMPs in contrast to Drosophila may reflect adaptations to their respective environment and the bacteria they encounter. Similarly, sequence variations in AMPs between mosquito species may account for the fact that only few wild-caught species tested positive for Francisella and that so far no competent mosquito vector has been established as a laboratory model [5, 6]. The ability of Francisella to survive in the presence of various insect AMPs indicates that the bacteria in turn are well adapted to survive in the hemolymph of an insect host. Even though we have demonstrated here that Francisella is sensitive to Drosophila AMPs, the peptides may only delay the outcome of the infection, death of the fly. In a competent arthropod vector of tularemia, however, such a delay might allow for Francisella transmission at the next blood meal.
Supplementary Material
Supplemental Video
Acknowledgements
We thank Karin Borge Renberg, Linda Junfors, Olena Rzhepishevska and Madeleine Ramstedt for technical help. We are grateful to Sofia Ekengren and Dan Hultmark for unpublished UAS-Cecropin transgenic fly strains, to Bruno Lemaitre for fly strains and to Alain Charbit, Tina Guina and Colin Manoil for bacterial strains. This work was supported by grants from the Swedish Research Council, the County Council of Västerbotten, Sweden, and from the Medical Faculty at Umeå University.
References
- 1.Larsson P, Elfsmark D, Svensson K, Wikstrom P, Forsman M, Brettin T, Keim P, Johansson A. Molecular evolutionary consequences of niche restriction in Francisella tularensis, a facultative intracellular pathogen. PLoS Pathog. 2009;5:e1000472. doi: 10.1371/journal.ppat.1000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen JM, Mead PS, Schriefer ME. Francisella tularensis: an arthropod-borne pathogen. Vet Res. 2009;40:7. doi: 10.1051/vetres:2008045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goethert HK, Telford SR., 3rd Nonrandom distribution of vector ticks (dermacentor variabilis) infected by Francisella tularensis. PLoS Pathog. 2009;5:e1000319. doi: 10.1371/journal.ppat.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reese SM, Dietrich G, Dolan MC, Sheldon SW, Piesman J, Petersen JM, Eisen RJ. Transmission dynamics of Francisella tularensis subspecies and clades by nymphal Dermacentor variabilis (Acari: Ixodidae) Am J Trop Med Hyg. 2010;83:645–652. doi: 10.4269/ajtmh.2010.10-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triebenbach AN, Vogl SJ, Lotspeich-Cole L, Sikes DS, Happ GM, Hueffer K. Detection of Francisella tularensis in alaskan mosquitoes (Diptera: Culicidae) and assessment of a laboratory model for transmission. J Med Entomol. 2010;47:639–648. doi: 10.1603/me09192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundström JO, Andersson A-C, Bäckman S, Schäfer ML, Forsman M, Thelaus J. Detection of Francisella tularensis holarctica in adult mosquitoes hatched from field collected larvae, suggest a novel transmission cycle originating in aquatic larval habitats. Emerg Infect Dis. 2011;17:794–799. doi: 10.3201/eid1705.100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vonkavaara M, Telepnev MV, Ryden P, Sjöstedt A, Stöven S. Drosophila melanogaster as a model for elucidating the pathogenicity of Francisella tularensis. Cell Microbiol. 2008;10:1327–1338. doi: 10.1111/j.1462-5822.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 8.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 9.Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, Edebro H, Forsman M, Bystrom M, Pelletier M, Wilson CB, Miller SI, Skerrett SJ, Ernst RK. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by toll-like receptors. Infect Immun. 2006;74:6730–6738. doi: 10.1128/IAI.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunn JS, Ernst RK. The structure and function of Francisella lipopolysaccharide. Ann NY Acad Sci. 2007;1105:202–218. doi: 10.1196/annals.1409.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Raetz CR. A two-component Kdo hydrolase in the inner membrane of Francisella novicida. Mol Microbiol. 2010;78:820–836. doi: 10.1111/j.1365-2958.2010.07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Ribeiro AA, Guan Z, Abraham SN, Raetz CR. Attenuated virulence of a Francisella mutant lacking the lipid a 4′-phosphatase. Proc Natl Acad Sci USA. 2007;104:4136–4141. doi: 10.1073/pnas.0611606104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher LA, Ramage E, Jacobs MA, Kaul R, Brittnacher M, Manoil C. A comprehensive transposon mutant library of Francisella novicida, a bioweapon surrogate. Proc Natl Acad Sci USA. 2007;104:1009–1014. doi: 10.1073/pnas.0606713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raynaud C, Meibom KL, Lety MA, Dubail I, Candela T, Frapy E, Charbit A. Role of the wbt locus of Francisella tularensis in lipopolysaccharide O-antigen biogenesis and pathogenicity. Infect Immun. 2007;75:536–541. doi: 10.1128/IAI.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai XH, Shirley RL, Crosa L, Kanistanon D, Tempel R, Ernst RK, Gallagher LA, Manoil C, Heffron F. Mutations of Francisella novicida that alter the mechanism of its phagocytosis by murine macrophages. PLoS One. 2010;5:e11857. doi: 10.1371/journal.pone.0011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzou P, Reichhart J-M, Lemaitre B. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient drosophila mutants. Proc Natl Acad Sci USA. 2002;99:2152–2157. doi: 10.1073/pnas.042411999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedengren M, Åsling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, Hultmark D. Relish, a central factor in the control of humoral, but not cellular immunity in Drosophila. Mol Cell. 1999;4:827–837. doi: 10.1016/s1097-2765(00)80392-5. [DOI] [PubMed] [Google Scholar]
- 18.Åhlund MK, Ryden P, Sjöstedt A, Stöven S. Directed screen of Francisella novicida virulence determinants using drosophila melanogaster. Infect Immun. 2010;78:3118–3128. doi: 10.1128/IAI.00146-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hultmark D. Fair Haven: SOS Publications; 1998. Quantification of Antimicrobial Activity, Using the Inhibition Zone Assay. [Google Scholar]
- 20.Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002;3:711–722. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 21.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal S, Wu J, Wu LP. Microarray analyses reveal distinct roles for Rel proteins in the Drosophila immune response. Dev Comp Immunol. 2008;32:50–60. doi: 10.1016/j.dci.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohmer L, Fong C, Abmayr S, Wasnick M, Larson Freeman TJ, Radey M, Guina T, Svensson K, Hayden HS, Jacobs M, Gallagher LA, Manoil C, Ernst RK, Drees B, Buckley D, Haugen E, Bovee D, Zhou Y, Chang J, Levy R, Lim R, Gillett W, Guenthener D, Kang A, Shaffer SA, Taylor G, Chen J, Gallis B, D'Argenio DA, Forsman M, Olson MV, Goodlett DR, Kaul R, Miller SI, Brittnacher MJ. Comparison of Francisella tularensis genomes reveals evolutionary events associated with the emergence of human pathogenic strains. Genome Biol. 2007;8:R102. doi: 10.1186/gb-2007-8-6-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asare R, Akimana C, Jones S, Abu Kwaik., Y Molecular bases of proliferation of Francisella tularensis in arthropod vectors. Environ Microbiol. 2010;12:2587–2612. doi: 10.1111/j.1462-2920.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moule MG, Monack DM, Schneider DS. Reciprocal analysis of Francisella novicida infections of a drosophila melanogaster model reveal host-pathogen conflicts mediated by reactive oxygen and imd-regulated innate immune response. PLoS Pathog. 2010;6:e1001065. doi: 10.1371/journal.ppat.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebastian S, Dillon ST, Lynch JG, Blalock LT, Balon E, Lee KT, Comstock LE, Conlan JW, Rubin EJ, Tzianabos AO, Kasper DL. A defined O-antigen polysaccharide mutant of Francisella tularensis live vaccine strain has attenuated virulence while retaining its protective capacity. Infect Immun. 2007;75:2591–2602. doi: 10.1128/IAI.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindemann SR, Peng K, Long ME, Hunt JR, Apicella MA, Monack DM, Allen LA, Jones BD. Francisella tularensis schu s4 O-antigen and capsule biosynthesis gene mutants induce early cell death in human macrophages. Infect Immun. 2011;79:581–594. doi: 10.1128/IAI.00863-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tzeng YL, Datta A, Strole C, Kolli VS, Birck MR, Taylor WP, Carlson RW, Woodard RW, Stephens DS. KpsF is the arabinose-5-phosphate isomerase required for 3-deoxy-D-manno-octulosonic acid biosynthesis and for both lipooligosaccharide assembly and capsular polysaccharide expression in Neisseria meningitidis. J Biol Chem. 2002;277:24103–24113. doi: 10.1074/jbc.M200931200. [DOI] [PubMed] [Google Scholar]
- 29.Godlewska R, Wisniewska K, Pietras Z, Jagusztyn-Krynicka EK. Peptidoglycan-associated lipoprotein (Pal) of Gram-negative bacteria: function, structure, role in pathogenesis and potential application in immunoprophylaxis. FEMS Microbiol Lett. 2009;298:1–11. doi: 10.1111/j.1574-6968.2009.01659.x. [DOI] [PubMed] [Google Scholar]
- 30.Vines ED, Marolda CL, Balachandran A, Valvano MA. Defective O-antigen polymerization in TolA and pal mutants of Escherichia coli in response to extracytoplasmic stress. J Bacteriol. 2005;187:3359–3368. doi: 10.1128/JB.187.10.3359-3368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TH, Sebastian S, Pinkham JT, Ross RA, Blalock LT, Kasper DL. Characterization of the O-antigen polymerase (Wzy) of Francisella tularensis. J Biol Chem. 2010;285:27839–27849. doi: 10.1074/jbc.M110.143859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 33.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 34.Silvestro L, Weiser JN, Axelsen PH. Antibacterial and antimembrane activities of cecropin A in Escherichia coli. Antimicrob Agents Chemother. 2000;44:602–607. doi: 10.1128/aac.44.3.602-607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otvos L Jr., Insug O, Rogers ME, Consolvo PJ, Condie BA, Lovas S, Bulet P, Blaszczyk-Thurin M. Interaction between heat shock proteins and antimicrobial peptides. Biochemistry. 2000;39:14150–14159. doi: 10.1021/bi0012843. [DOI] [PubMed] [Google Scholar]
- 36.Carlsson A, Nystrom T, de Cock H, Bennich H. Attacin – an insect immune protein – binds LPS and triggers the specific inhibition of bacterial outer-membrane protein synthesis. Microbiology. 1998;144:2179–2188. doi: 10.1099/00221287-144-8-2179. [DOI] [PubMed] [Google Scholar]
- 37.Kanistanon D, Hajjar AM, Pelletier MR, Gallagher LA, Kalhorn T, Shaffer SA, Goodlett DR, Rohmer L, Brittnacher MJ, Skerrett SJ, Ernst RK. A Francisella mutant in lipid a carbohydrate modification elicits protective immunity. PLoS Pathog. 2008;4:e24. doi: 10.1371/journal.ppat.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han S, Bishop BM, van Hoek ML. Antimicrobial activity of human beta-defensins and induction by Francisella. Biochem Biophys Res Commun. 2008;371:670–674. doi: 10.1016/j.bbrc.2008.04.092. [DOI] [PubMed] [Google Scholar]
- 39.Clay CD, Soni S, Gunn JS, Schlesinger LS. Evasion of complement-mediated lysis and complement C3 deposition are regulated by Francisella tularensis lipopolysaccharide O antigen. J Immunol. 2008;181:5568–5578. doi: 10.4049/jimmunol.181.8.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, Maccallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video