Abstract
Streptococcus pneumoniae is one of the most common causes of sepsis. Sepsis is associated with the release of ‘damage-associated molecular patterns’ (DAMPs). The receptor for advanced glycation end products (RAGE) is a multiligand receptor, abundantly expressed in the lungs, that recognizes several of these DAMPs. Triggering of RAGE leads to activation of the NF-κB pathway and perpetuation of inflammation. Earlier investigations have shown that the absence of RAGE reduces inflammation and bacterial dissemination and increases survival in sepsis caused by S. pneumoniae pneumonia. We hypothesized that the detrimental role of RAGE depends on the level of RAGE expression in the primary organ of infection. By directly injecting S. pneumoniae intravenously, thereby circumventing the extensive RAGE-expressing lung, we here determined whether RAGE contributes to an adverse outcome of bacteremia or whether its role is restricted to primary lung infection. During late-stage infection (48 h), rage−/− mice had an attenuated systemic inflammatory response, as reflected by lower plasma levels of proinflammatory cytokines, reduced endothelial cell activation (as measured by E-selectin levels) and less neutrophil accumulation in lung tissue. However, RAGE deficiency did not influence bacterial loads or survival in this model. In accordance, plasma markers for cell injury were similar in both mouse strains. These results demonstrate that while RAGE plays a harmful part in S. pneumoniae sepsis originating from the respiratory tract, this receptor has a limited role in the outcome of primary bloodstream infection by this pathogen.
Key Words: Receptor for advanced glycation end products, Streptococcus pneumoniae, Sepsis
Introduction
Sepsis is a leading cause of death and represents a major challenge in the care of critically ill patients [1, 2]. Streptococcus pneumoniae is a frequent cause of sepsis that, in the majority of cases, originates from a respiratory focus [3, 4]. At particular risk for developing pneumococcal sepsis is the expanding group of high-aged and immunocompromised patients. Together with the emergence of antibiotic resistance the burden of this disease is expected to grow in the future [4]. Hence, new treatment strategies must be explored to improve care of infections caused by S. pneumoniae.
Immunomodulating agents designed to attenuate the systemic hyperinflammation syndrome are a widely studied topic in sepsis research. In this field the receptor for advanced glycation end products (RAGE) has been implicated as a possible target considering its role in perpetuating inflammatory responses [5, 6]. RAGE is a member of the immunoglobulin superfamily of cell surface molecules [7, 8] and is expressed on a wide array of cell types. RAGE binds to Mac-1 on neutrophils [9], contributing to neutrophil recruitment, and to a number of endogenous molecules that are released upon cell stress or cell damage known as damage-associated molecular patterns (DAMPs). DAMPs that are recognized by RAGE include high-mobility group box 1 (HMGB1) and S100 proteins [5, 6]. Engagement of RAGE activates the NF-κB pathway, which in turn upregulates expression of RAGE, thus inducing sustained cellular inflammation and promoting cellular dysfunction and tissue damage [10, 11].
Several animal studies have shown that RAGE is critically involved in the deleterious effects of acute inflammatory disorders, including sepsis [12, 13, 14, 15, 16]. Deletion of the rage gene was initially found to protect against the lethal effects of septic shock in polymicrobial sepsis, which was associated with a strongly reduced activation of NF-κB in the peritoneum and lungs [12]. Our group previously investigated the role of RAGE in sepsis derived from S. pneumoniae pneumonia [14], showing increased RAGE expression in the lungs upon infection and a relatively protected phenotype of RAGE deficient (rage−/−) mice [14]. In accordance, independent investigations showed that anti-RAGE treatment improved outcome in invasive pneumococcal pneumonia [15]. However, RAGE inhibition may be ineffective or even harmful in other infectious diseases [13, 16], suggesting that detrimental effects of RAGE might depend on the pathogen and/or level of RAGE expression in the primary infected organ.
In the setting of pneumonia caused by a highly virulent strain of S. pneumoniae, RAGE may contribute to enhanced pulmonary inflammation as a consequence of its abundant expression in the lungs [17], impairing the integrity of the blood-lung barrier [18], leading to enhanced bacterial dissemination and a worsened survival [14]. To investigate the role of RAGE in host defense during primary bacteremia, we directly injected S. pneumoniae into the tail vein in order to ‘bypass’ the established role of RAGE in the lungs during pneumonia-derived sepsis. Even though direct pneumococcal infection of the bloodstream (e.g. through blood transfusion) is not common [19], this study may help identify whether RAGE's harmful role in pneumococcal sepsis is restricted to the pulmonary compartment. To investigate this, we challenged mice with the same pneumococcal strain - serotype 3, ATCC 6303 - as in the pneumonia study, allowing better comparisons. We demonstrate that RAGE amplifies the systemic inflammatory response, but does not impact on bacterial loads or survival during fulminant systemic infection with S. pneumoniae. These data demonstrate that RAGE is not critically involved in the outcome of lethal nonfocal pneumococcal sepsis and suggest that the detrimental effect of RAGE is primarily exerted in the pulmonary compartment.
Methods
Ethics Statement
Experiments were carried out in accordance with the Dutch Experiment on Animals Act and approved by the Animal Care and Use Committee of the University of Amsterdam (Permit No. DIX101223).
Mice
C57Bl/6 wild-type (Wt) mice were purchased from Charles River Laboratories Inc. (Maastricht, The Netherlands). Rage−/− mice, backcrossed >10 times to a C57Bl/6 background, were generated as described [12] and bred in the animal facility of the Academic Medical Center (Amsterdam, The Netherlands). The Animal Care and Use Committee of the University of Amsterdam approved all experiments.
Design
Sepsis was induced as previously described [20]. Mice were intravenously injected in the tail vein with 5 × 105 of S. pneumoniae serotype 3 (American Type Culture Collection 6303, Rockville, Md., USA) in a 200-μl saline solution (n = 7-8 per strain) and sacrificed 24 or 48 h thereafter or monitored in a survival study. Collection and handling of samples were done as previously described [20, 21]. In brief, blood was drawn into heparinized tubes and organs were removed aseptically and homogenized in 4 volumes of sterile isotonic saline using a tissue homogenizer (Biospec Products, Bartlesville, UK). To determine bacterial loads, 10-fold dilutions were plated on blood agar plates and incubated at 37°C for 16 h. Organ homogenates were processed for cytokine measurements as described [21].
Assays
Tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, RAGE and E-selectin concentrations were measured in tissue homogenates using ELISAs (all R&D Systems, Minneapolis, Minn., USA). The detection limit of the RAGE ELISA was 62.5 pg/ml. Plasma TNF-α, IL-6 and IL-1β were measured by cytometric bead array flex set assay (BD Biosciences, San Jose, Calif., USA). Lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) were measured in plasma with kits from Sigma-Aldrich (St. Louis, Mo., USA), using a Hittachi analyzer (Boehringer Mannheim, Mannheim, Germany).
Histology
Lungs, spleens and livers were harvested for histologic examination after 24 and 48 h, fixed in 10% formaldehyde and embedded in paraffin. Four-micrometer-thick sections were used for stainings. For granulocyte staining, slides were deparaffinized and rehydrated using standard procedures. Endogenous peroxidase activity was quenched by a solution of 0.3% H2O2 in methanol. Slides were then digested by a solution of pepsin 0.025% (Sigma-Aldrich) in 0.1 M HCl. After being rinsed, the sections were incubated in Ultra V Block (Thermo Scientific, Fremont, Calif., USA) and then exposed to FITC-labeled anti-mouse Gr-1 monoclonal antibody (BD PharMingen, San Diego, Calif., USA). After washes, slides were incubated with a rabbit anti-FITC antibody (Nuclilab, Ede, The Netherlands) followed by further incubation with Brightvision poly-horseradish peroxidase anti Rabbit IgG (Immunologic, Duiven, The Netherlands), rinsed again and developed using Bright DAB (Immunologic). The sections were counterstained with methyl green (Sigma-Aldrich), hydrated and mounted in Pertex (Histolab, Gothenburg, Sweden). Gr-1-stained slides were photographed with a microscope equipped with a digital camera (Leica CTR500, Leica Microsystems, Wetzlar, Germany). Ten random pictures were taken per slide. In these images Gr-1 positivity and total surface area were measured using Image J software (US National Institutes of Health, Bethesda, Md., USA; http://rsb.info.nih.gov/ij); the amount of Gr-1 positivity was expressed as a percentage of the total surface area. Immunostaining for RAGE was performed as previously described [14]. In short, paraffin slides were deparaffinized and rehydrated using standard procedures. Endogenous peroxidase activity was quenched using 1.5% H2O2 in PBS. Primary antibodies used were goat anti-mouse RAGE polyclonal antibodies (Neuromics, Edina, Minn., USA) and secondary antibodies were biotinylated rabbit anti-goat antibodies (DakoCytomation, Glostrup, Denmark). ABC solution (DakoCytomation) was used as the detection enzyme. DAB peroxidase (Sigma-Aldrich) was used as substrate for visualization. Counterstaining was performed with methyl green (Sigma-Aldrich).
HMGB1 Western Blot
For Western blotting of HMGB1, plasma samples were diluted 8× in sodium dodecyl sulfate buffer, 2% 2-mercaptoethanol. After heating, samples were run on a 15% polyacrylamide sodium dodecyl sulfate gel and subsequently transferred to blotting membrane polyvinylidene difluoride membranes (Pharmacia, Piscataway, N.J., USA). Following blocking with 5% nonfat dry milk proteins (Protifar from Nutricia, Zoetermeer, The Netherlands) in 0.1% Tween phosphate-buffered saline (PBS-T), membranes were washed and incubated overnight in 1 µg/ml primary rabbit anti-HMGB1 polyclonal antibody (ab18256, Abcam, Cambridge, UK) in 1% nonfat dry milk proteins in PBS-T at 4°C. After washing with PBS-T, membranes were probed with peroxidase-labeled secondary antibodies (Cell Signaling Technology, Danvers, Mass., USA) for 1 h at room temperature in 1% bovine serum albumin in PBS-T. After washing with PBS-T, membranes were incubated with Lumi-Light Plus Western Blotting Substrate (Roche, Mijdrecht, The Netherlands) and positive bands were detected using an LAS3000 Luminescent Image Analyzer dark box (Fujifilm, Tokyo, Japan). Image quantification was performed using AIDA Image analyzer software (Raytest, Straubenhardt, Germany).
Statistical Analysis
Data are presented as means ± standard error of the mean, unless indicated otherwise. Differences between rage−/− and Wt mice were analyzed by Mann-Whitney U test. Survival was compared by Kaplan-Meier analysis followed by a logrank test. Analyses were done using GraphPad Prism version 5.0 (Graphpad Software, San Diego, Calif., USA). Values of p < 0.05 were considered statistically significantly different.
Results
Expression of RAGE and HMGB1 in S. pneumoniae Sepsis
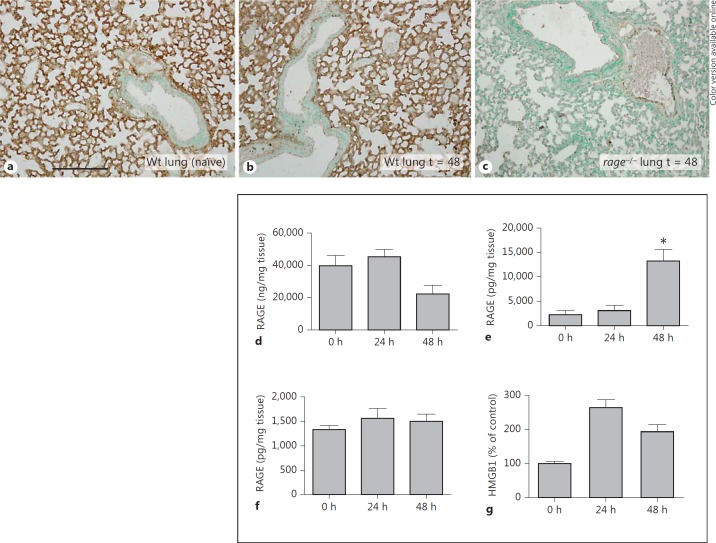
Previous investigations have established that RAGE is constitutively expressed in pulmonary tissue and that RAGE expression is enhanced during pneumonia [14, 17]. To determine whether primary bloodstream infection alters RAGE expression, we performed immunohistochemical stainings of RAGE in mouse lungs and spleens after intravenous infection with S. pneumoniae. In accordance with earlier investigations [13, 17], uninfected lungs extensively expressed RAGE, which was primarily present in the interalveolar septa showing an endothelial pattern (fig. 1a). RAGE was not present in bronchial epithelium. Forty-eight hours after infection, lungs showed similar expression of RAGE compared to lungs from naïve mice (fig. 1b). Immunohistochemical analysis of RAGE in lung tissue from rage−/− mice (used as a negative control) confirmed the specificity of RAGE staining (fig. 1c). There was minimal RAGE staining in normal healthy spleens, which did not change after infection (data not shown).
Fig. 1.
Expression of RAGE in mouse organs and of its ligand HMGB1 in plasma after systemic pneumococcal infection. a Representative picture of a lung sample from a normal, uninfected Wt mouse, displaying extensive RAGE staining. b Lung sample from a Wt mouse 48 h after intravenous injection of 5 × 105 CFU of S. pneumoniae. c Absence of RAGE positivity in the lung of a rage−/− mouse. Scale bar = 200 μm. RAGE concentrations in lungs (d), spleens (e) and livers (f) from naïve Wt mice and 24 and 48 h after intravenous injection of 5 × 105 CFU of S. pneumoniae (5-8 mice per group at each time point). * p < 0.05 versus naïve mice (0 h). g Densitometric analysis of HMGB1 blot expressed as a percentage of the mean density measured in naïve control plasma samples. HMGB1 detection was enhanced at 24 and 48 h compared to naïve control samples (0 h; n = 3 per time point). Bars represent mean ± standard error of the mean.
As well as immunohistochemical stainings, we measured RAGE concentrations in lung, spleen and livers by ELISA. Interestingly, pulmonary RAGE concentrations tended to be lower 48 h after infection (median 22 μg/mg tissue) compared to naïve lungs (median 40 μg/mg tissue; fig. 1d). Uninfected spleens and livers displayed relatively low concentrations of RAGE (median of 1.2 and 1.6 ng/mg tissue, respectively). Sepsis enhanced RAGE protein concentrations tenfold in livers, but hardly in spleens (fig. 1f). Plasma levels of soluble (s)RAGE remained below detection limits (data not shown).
To obtain insight into the systemic release of HMGB1, a ligand for RAGE [22], we performed a Western blot on plasma samples harvested 24 and 48 h after infection. At both time points, circulatory HMGB1 was detected and elevated compared to uninfected mice (fig. 1g).
RAGE Does Not Affect Bacterial Outgrowth in a Lethal Model of Pneumococcal Sepsis
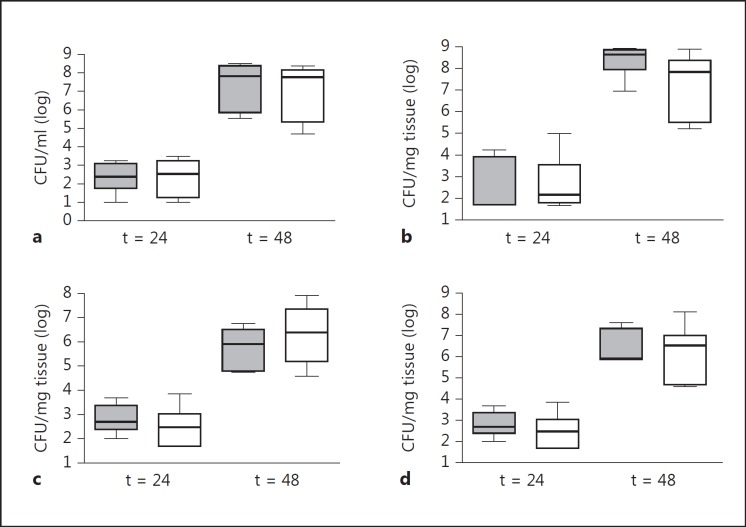
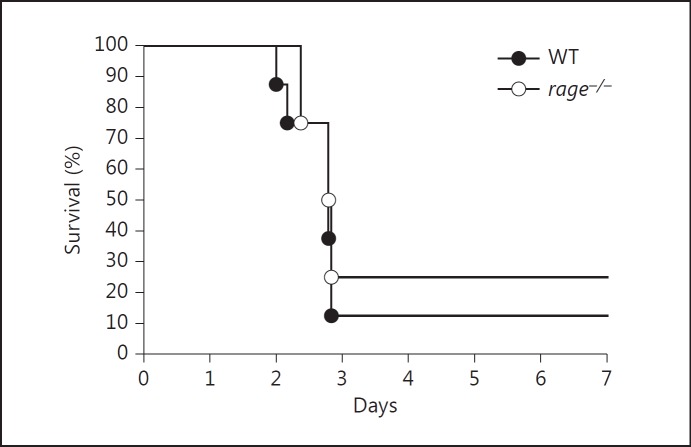
Earlier studies have shown that RAGE impairs the outcome of pneumococcal pneumonia: mice lacking RAGE had an improved survival together with lower pulmonary inflammation and bacterial loads [14]. Since RAGE is primarily expressed in the lungs, we wondered whether this detrimental role of RAGE is restricted to pneumococcal pneumonia. To examine this, we injected 500,000 colony-forming units (CFU) of the same pneumococcal strain into the tail vein of Wt and rage−/− mice and harvested lungs, blood, spleens and livers to determine bacterial loads. At 24 and 48 h postinfection, bacterial burdens were similar in all organs of both mouse strains (fig. 2). Forty-eight hours after infection, however, 3 out of 8 Wt mice had already died, suggesting that RAGE contributed to lethality in this model. Monitoring survival in a separate experiment did not reveal a statistically significant difference between Wt and rage−/− mice, although the latter mouse strain displayed a modest survival advantage (fig. 3).
Fig. 2.
Wt and rage−/− mice display similar bacterial outgrowth after systemic infection with S. pneumoniae. Bacterial loads in the blood (a), lung (b), spleen (c) and liver (d) after intravenous injection of 5 × 105 CFU of S. pneumoniae in Wt (grey) and rage−/− mice (white). Data are expressed as box-and-whisker diagrams depicting the smallest observation, lower quartile, median, upper quartile and largest observation (n = 5-8 mice per group at each time point).
Fig. 3.
Wt and rage−/− mice display a similar survival after systemic infection with S. pneumoniae. Survival of Wt and rage−/− mice after intravenous injection of 5 × 105 CFU of S. pneumoniae (8 mice per group).
RAGE Enhances Systemic Cytokine Production in S. pneumoniae Bacteremia
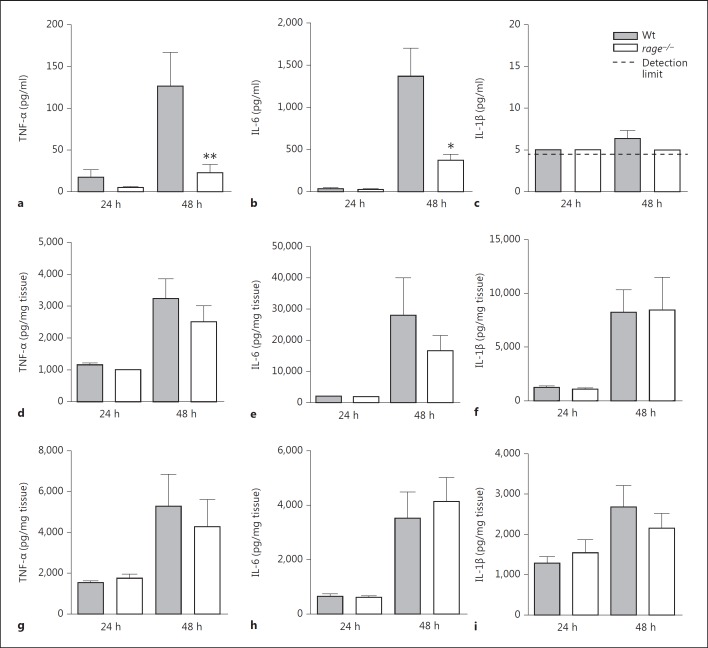
Upon binding of ligands, RAGE leads to sustained activation of NF-κB and upregulation of the RAGE receptor, thereby perpetuating inflammatory responses [11]. To determine the role of RAGE in inflammation during pneumococcal sepsis, we measured circulatory as well as organ cytokine (TNF-α, IL-1β, IL-6) levels 1 and 2 days postinfection. Twenty-four hours after infection, no differences were observed between mouse strains, while 48 h after infection, rage−/− mice had significantly lower levels of plasma TNF-α and IL-6 (fig. 4a, b, c). Although RAGE is especially expressed in the lungs [17], no significant differences in pulmonary cytokine levels were observed between Wt and rage−/− mice (fig. 4d, e, f). In addition, RAGE did not influence cytokine concentrations in the spleen (fig. 4g, h, i).
Fig. 4.
Rage−/− mice show lower plasma TNF-α and IL-6 levels after systemic infection with S. pneumoniae. Cytokine (TNF-α, IL-6 and IL-1β) levels in plasma (a-c), lungs (d-f) and spleen (g-i) 24 and 48 h after intravenous injection of 5 × 105 CFU of S. pneumoniae in Wt and rage−/− mice (5-8 mice per group at each time point). Bars represent mean ± standard error of the mean. * p < 0.05, ** p < 0.01 versus Wt mice at the same time point.
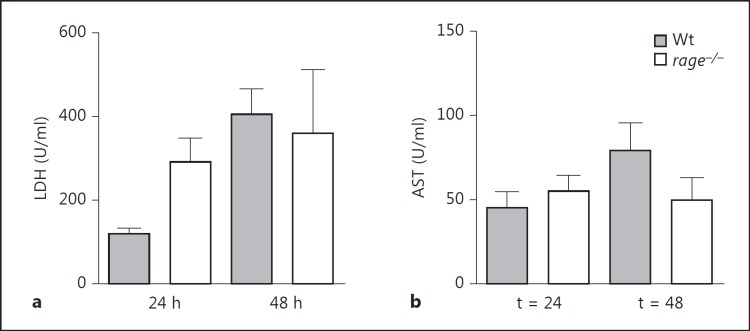
RAGE Does Not Impact on Cell Injury after Systemic Infection of S. pneumoniae
Sustained cellular activation by RAGE may lead to cellular dysfunction and tissue damage [11]. To evaluate the role of RAGE in cellular injury in S. pneumoniae bacteremia, we measured plasma levels of LDH, a general cell injury marker and AST, a marker specific for liver injury. Relative to rage−/− mice, Wt mice did not differ in cell injury markers, suggesting that RAGE does not contribute to tissue damage in this model of pneumococcal infection (fig. 5).
Fig. 5.
No difference in damage markers between Wt and rage−/− mice. LDH (a) and AST (b) were measured in plasma of Wt and rage−/− mice (5-8 mice per group at each time point). Bars represent mean ± standard error of the mean.
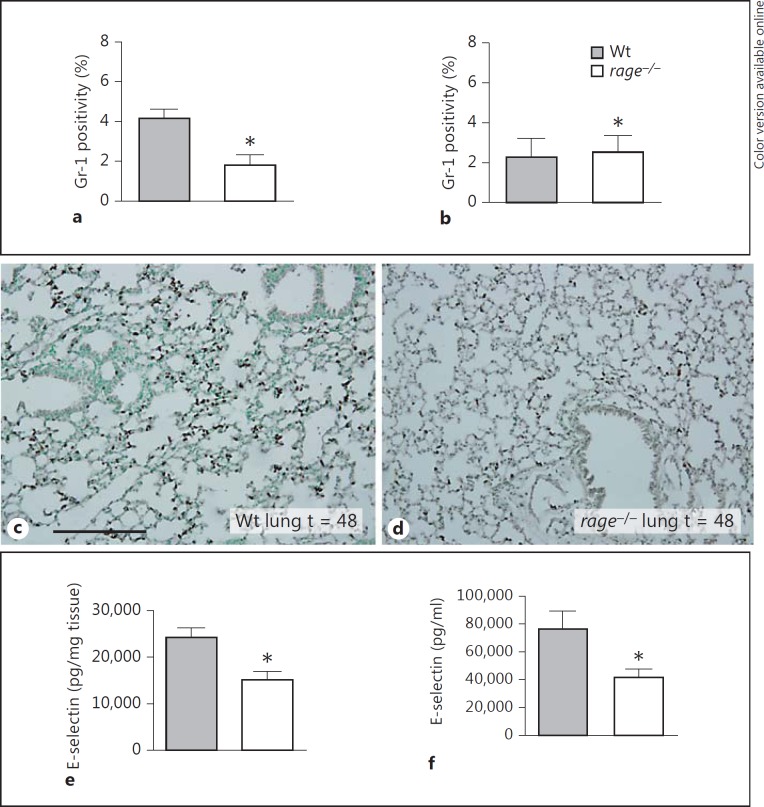
RAGE Enhances Neutrophil Recruitment to the Lungs
Recruitment of leukocytes to infectious sites is an essential step in host defense. RAGE has been implicated to play a role in neutrophil recruitment possibly mediated by Mac-1 on neutrophils [9]. To investigate the role of RAGE in leukocyte recruitment to specific organs in nonfocal sepsis, we analyzed Gr-1 stainings in lung and spleen sections 48 h after infection. Lungs from rage−/− mice had a lower percentage of Gr-1 staining compared to Wt mice indicating lower pulmonary neutrophil numbers (fig. 6a, c, d). Gr-1 staining in the spleens was similar in Wt and rage−/− mice (fig. 6b). Together these data suggest that pulmonary RAGE is involved in directing neutrophils to the lungs in pneumococcal sepsis.
Fig. 6.
RAGE deficiency diminishes neutrophil accumulation in the lung. Quantification of pulmonary Gr-1 positivity 48 h after infection in lungs (a) and spleens of (b) of Wt and rage−/− mice (5-8 mice per group). Bars represent mean ± standard error of the mean. * p < 0.05 versus Wt mice at the same time point. Representative neutrophil stainings (brown; color online version only) of Wt (c) and rage−/− (d) lungs 48 h after induction of pneumococcal sepsis. Scale bar = 200 μm. E-selectin was measured in lungs (e) and soluble E-selectin in plasma (f) 48 h after intravenous injection of 5 × 105 CFU of S. pneumoniae in Wt and rage−/− mice (5-8 mice per group). Bars represent mean ± standard error of the mean. * p < 0.05 versus Wt mice at the same time point.
RAGE Contributes to Endothelial Cell Activation
To obtain insight in the contribution of RAGE to endothelial cell activation during sepsis, we measured E-selectin levels in plasma, lungs and spleen. Forty-eight hours after infection, rage−/− mice displayed lower E-selectin levels in plasma and lungs when compared with Wt mice (fig. 6e, f). E-selectin levels did not differ between mouse strains in spleens at any time point (data not shown).
Discussion
S. pneumoniae is the most commonly isolated pathogen in pneumonia and an important causative organism in severe sepsis [3]. Invasive infection and accompanying inflammatory mechanisms can cause tissue damage that is associated with release of DAMPs, which are recognized by pattern recognition receptors (PRRs) and perpetuate inflammatory responses [23]. RAGE is a PRR that is primarily expressed in the lung and has been implicated to interact with several DAMPs including HMGB1. In the setting of sepsis originating from pneumococcal pneumonia, RAGE enhanced pulmonary inflammation, resulting in enhanced bacterial growth and dissemination, and a worsened survival [14]. In the current study, we directly infected Wt and rage−/− mice with pneumococci into the bloodstream. Although most cases of pneumococcal sepsis have a pulmonary focus [3, 4], this experimental model was used to investigate the role of RAGE in S. pneumoniae sepsis independent from respiratory dissemination. We show that the presence of RAGE is associated with enhanced systemic inflammation, increased endothelial cell activation and higher neutrophil numbers in the lungs. However, RAGE did not influence bacterial loads or survival in this model of fulminant systemic S. pneumoniae infection. These results imply that while RAGE plays a harmful part in pneumococcal pneumonia [14, 15], this receptor does not play a critical role in the outcome of sepsis during primary bloodstream infection with this clinically relevant pathogen.
RAGE is abundantly expressed in normal healthy lungs and upregulated during S. pneumoniae pneumonia [14, 17, 24, 25]. In contrast to these earlier findings, we here observed a decreased expression of pulmonary RAGE in a model of intravenously induced pneumococcal sepsis. The pattern of RAGE staining, however, remained unchanged. Similarly to our finding, the level of pulmonary RAGE expression was found to decrease in other lung injury and pulmonary fibrosis models [26, 27]. In addition, RAGE was hardly detected in naïve or infected spleens and livers.
sRAGE, lacking the transmembrane and cytoplasmic domains [28], has been detected in human plasma. sRAGE levels are elevated and suggested to be a marker of outcome in patients with sepsis [29], pneumonia [30] and acute lung injury [31]. In this study, we were unable to detect sRAGE in plasma, which is in accordance with an earlier study measuring circulating sRAGE levels in diabetic mice [32]. To the best of our knowledge, plasma sRAGE levels have never been documented in mice. The commercially available ELISA kit we used in our study is designed to measure any murine form of extracellular domain of sRAGE species with a detection limit of 62.5 pg/ml. Our measurements in plasma samples did not exceed this detection limit, which could be a limitation of the method we used to measure this protein. We could, however, detect plasma HMGB1. The expression of HMGB1 was increased in S. pneumoniae-infected mice compared to uninfected mice. Together with several other endogenous ligands, HMGB1 interacts with RAGE [11]. When bound to other DAMPs, such as extracellular cell-free DNA, HMGB1 is able to produce an inflammatory stimulus through RAGE and other PRRs [22]
The binding of RAGE triggers multiple intracellular signaling pathways which result in the translocation of NF-κB and the transcription of proinflammatory proteins. Activation of the NF-κB pathway upregulates expression of RAGE, leading to sustained cellular inflammation and the promotion of cellular dysfunction and tissue damage [11]. In accordance, harmful effects of RAGE have been shown in models of endotoxic shock and sepsis [12, 33]. In line with these results, RAGE enhanced lung inflammation and worsened survival in sepsis originated from pneumococcal pneumonia [14]. In the present study, we found a reduced inflammatory response in rage−/− mice, as reflected by lower TNF-α and IL-6 levels in plasma, 48 h after infection. The effect of RAGE was likely underestimated since at this time point 3 out of 8 Wt mice had already died. Although this finding suggests that the presence of RAGE increases lethality in this model, in a formal survival study a statistically significant difference could not be demonstrated. It should be noted that the severity of this intravenous challenge model varies from experiment to experiment. Indeed, in preliminary pilot studies we observed an approximate 50% lethality in Wt mice after intravenous injection of the S. pneumoniae dose used in the current manuscript. This variance can be partly explained by the fact that for each experiment a fresh bacterial inoculum is prepared. This is why all experiments were strictly controlled, i.e. Wt and rage−/− mice were infected with the exact same inoculum at the same time. In accordance with the survival data presented here, the plasma concentrations of cell damage markers such as LDH and AST were similar in Wt and rage−/− mice after systemic pneumococcal infection. The discrepancy between lung and bloodstream infection suggests that the pulmonary compartment has a somewhat harmful role in pneumococcal sepsis pathophysiology. A similar finding was observed earlier in the outcome of a rabbit model of pneumosepsis [34]. Here it was demonstrated that pulmonary infection with a cytotoxic strain of Pseudomonas aeruginosa damaged the alveolar epithelium, allowing for proinflammatory mediators to leak into the systemic compartment, responsible for the signs of septic shock. Intriguingly, shock did not occur after systemic infection, even though bacteria were evidently present, establishing the crucial importance of tissue infection in the pathogenesis in this type of septic shock.
Targeting RAGE in sepsis models may impair antibacterial defense, as demonstrated in Escherichia coli peritonitis [13, 16] where rage−/− mice had higher bacterial loads compared to Wt mice. In pneumococcal pneumonia, conflicting results were observed, as rage−/− mice demonstrated lower bacterial loads [14], while the opposite was true when Wt mice were treated with an anti-RAGE antibody [15]. While using the same strain of the S. pneumoniae as in these former pneumonia studies, RAGE did not affect bacterial outgrowth after intravenous injection of bacteria. The majority of bacteria entering the bloodstream are taken up by the liver [35], and several studies have shown that the spleen is particularly important in the systemic clearance of encapsulated S. pneumoniae [36, 37]. The fact that RAGE did not influence bacterial outgrowth in the systemic model of infection could be due to the fact that RAGE, in contrast to the lungs, is hardly detected in these organs. Of note, the pneumococcal strain used is highly virulent in mice, which corresponds with the fact that serotype 3 pneumococci are associated with severe infections in humans as well [38].
Several studies have implicated RAGE in leukocyte recruitment. RAGE has been identified as a binding partner for Mac-1 on neutrophils in static and flow in vitro conditions [9, 12, 39, 40, 41]. In vivo, rage−/− mice displayed a diminished number of inflammatory cells in the peritoneum after cecal legation and puncture [12] and thioglycollate-induced peritonitis [9] and in the bronchoalveolar compartment after S. pneumoniae pneumonia [14]. Although there was no infectious focus in the current study, rage−/− mice remarkably demonstrated a reduced number of Gr-1-positive cells in lung tissue, indicating lower neutrophil counts in this organ. However, spleens demonstrated similar Gr-1 expression between Wt and rage−/− mice. Clearly, these different roles of RAGE in neutrophil accumulation in lungs and spleen may be related to the extent of RAGE expression, which is much higher in the former organ. RAGE in addition enhanced the extent of endothelial cell activation, as reflected by higher E-selectin levels in whole lung and plasma. The reduced expression of E-selectin in the lungs may have contributed to the diminished neutrophil recruitment in rage−/− mice [42].
In conclusion, our results show that rage−/− mice had a reduced systemic cytokine response, reduced endothelial cell activation and lower neutrophil numbers in the lungs in response to systemic S. pneumoniae infection. RAGE did not influence bacterial loads. The finding that RAGE deficiency did not impact on survival in spite of attenuated inflammation suggests that in this model systemic inflammation is not the cause of death or that inflammation was not reduced enough in rage−/− mice to alter survival. Taken together with previous studies on the role of RAGE in host defense against infection [5, 6], these data suggest that the impact of RAGE on the outcome of infectious disease depends on both the pathogen and the primary site of infection. In addition, the current data suggest that the harmful role of RAGE in S. pneumoniae infection is primarily exerted in the pulmonary compartment and not systemically.
Disclosure Statement
The authors declare no conflict of interests.
Acknowledgements
We thank Joost Daalhuisen and Marieke S. ten Brink for expert technical assistance. We would like to thank Drs. Peter Nawroth and Angelika Bierhaus (Department of Internal Medicine and Clinical Chemistry, University of Heidelberg, Heidelberg, Germany) for generously providing rage−/− mice. This work is supported by a grant from the Landsteiner Foundation for Blood transfusion Research (project LSBR 0706).
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laterre PF, Garber G, Levy H, Wunderink R, Kinasewitz GT, Sollet JP, Maki DG, Bates B, Yan SC, Dhainaut JF. Severe community-acquired pneumonia as a cause of severe sepsis: data from the PROWESS study. Crit Care Med. 2005;33:952–961. doi: 10.1097/01.ccm.0000162381.24074.d7. [DOI] [PubMed] [Google Scholar]
- 4.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–1556. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 5.Christaki E, Lazaridis N, Opal SM. Receptor for advanced glycation end products in bacterial infection: is there a role for immune modulation of receptor for advanced glycation end products in the treatment of sepsis? Curr Opin Infect Dis. 2012;25:304–311. doi: 10.1097/QCO.0b013e3283519b82. [DOI] [PubMed] [Google Scholar]
- 6.van Zoelen MA, Achouiti A, van der Poll T. The role of receptor for advanced glycation endproducts (RAGE) in infection. Crit Care. 2011;15:208. doi: 10.1186/cc9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 8.Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- 9.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 12.Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Zoelen MA, Schmidt AM, Florquin S, Meijers JC, de BR, de Vos AF, Nawroth PP, Bierhaus A, van der Poll T. Receptor for advanced glycation end products facilitates host defense during Escherichia coli-induced abdominal sepsis in mice. J Infect Dis. 2009;200:765–773. doi: 10.1086/604730. [DOI] [PubMed] [Google Scholar]
- 14.van Zoelen MA, Schouten M, de Vos AF, Florquin S, Meijers JC, Nawroth PP, Bierhaus A, van der Poll T. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J Immunol. 2009;182:4349–4356. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- 15.Christaki E, Opal SM, Keith JC, Jr, Kessimian N, Palardy JE, Parejo NA, Tan XY, Piche-Nicholas N, Tchistiakova L, Vlasuk GP, Shields KM, Feldman JL, Lavallie ER, Arai M, Mounts W, Pittman DD. A monoclonal antibody against RAGE alters gene expression and is protective in experimental models of sepsis and pneumococcal pneumonia. Shock. 2011;35:492–498. doi: 10.1097/SHK.0b013e31820b2e1c. [DOI] [PubMed] [Google Scholar]
- 16.van Zoelen MA, Achouiti A, Schmidt AM, Yang H, Florquin S, Tracey KJ, van der Poll T. Ligands of the receptor for advanced glycation end products, including high-mobility group box 1, limit bacterial dissemination during Escherichia coli peritonitis. Crit Care Med. 2010;38:1414–1422. doi: 10.1097/CCM.0b013e3181de18bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley ST, Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J Biomed Biotechnol. 2010;2010:917108. doi: 10.1155/2010/917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas R, Verin AD, Black SM, Catravas JD. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochem Pharmacol. 2009;77:1763–1772. doi: 10.1016/j.bcp.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polizzotto MN, Neo H, Spelman D, Shortt J, Cole-Sinclair MF, Borosak M, Wong P, Wood EM. Streptococcus pneumoniae septicemia associated with red blood cell transfusion. Transfusion. 2008;48:1520–1521. doi: 10.1111/j.1537-2995.2008.01764.x. [DOI] [PubMed] [Google Scholar]
- 20.van der Windt GJ, Blok DC, Hoogerwerf JJ, Lammers AJ, de Vos AF, Van't Veer C, Florquin S, Kobayashi KS, Flavell RA, van der Poll T. Interleukin 1 receptor-associated kinase M impairs host defense during pneumococcal pneumonia. J Infect Dis. 2012;205:1849–1857. doi: 10.1093/infdis/jis290. [DOI] [PubMed] [Google Scholar]
- 21.Wieland CW, van Lieshout MH, Hoogendijk AJ, van der Poll T. Host defence during Klebsiella pneumonia relies on haematopoietic-expressed Toll-like receptors 4 and 2. Eur Respir J. 2011;37:848–857. doi: 10.1183/09031936.00076510. [DOI] [PubMed] [Google Scholar]
- 22.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 24.van Zoelen MA, van der Sluijs KF, Achouiti A, Florquin S, Braun-Pater JM, Yang H, Nawroth PP, Tracey KJ, Bierhaus A, van der Poll T. Receptor for advanced glycation end products is detrimental during influenza A virus pneumonia. Virology. 2009;391:265–273. doi: 10.1016/j.virol.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zoelen MA, Wieland CW, van der Windt GJ, Florquin S, Nawroth PP, Bierhaus A, van der Poll T. Receptor for advanced glycation end products is protective during murine tuberculosis. Mol Immunol. 2012;52:183–189. doi: 10.1016/j.molimm.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Ramsgaard L, Englert JM, Manni ML, Milutinovic PS, Gefter J, Tobolewski J, Crum L, Coudriet GM, Piganelli J, Zamora R, Vodovotz Y, Enghild JJ, Oury TD. Lack of the receptor for advanced glycation end-products attenuates E. coli pneumonia in mice. PLoS One. 2011;6:e20132. doi: 10.1371/journal.pone.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Englert JM, Hanford LE, Kaminski N, Tobolewski JM, Tan RJ, Fattman CL, Ramsgaard L, Richards TJ, Loutaev I, Nawroth PP, Kasper M, Bierhaus A, Oury TD. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am J Pathol. 2008;172:583–591. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10) FASEB J. 2008;22:3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 29.Bopp C, Hofer S, Weitz J, Bierhaus A, Nawroth PP, Martin E, Buchler MW, Weigand MA. sRAGE is elevated in septic patients and associated with patients outcome. J Surg Res. 2008;147:79–83. doi: 10.1016/j.jss.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Narvaez-Rivera RM, Rendon A, Salinas-Carmona MC, Rosas-Taraco AG. Soluble RAGE as a severity marker in community acquired pneumonia associated sepsis. BMC Infect Dis. 2012;12:15. doi: 10.1186/1471-2334-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J. 2009;23:1766–1774. doi: 10.1096/fj.08-117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto Y, Harashima A, Saito H, Tsuneyama K, Munesue S, Motoyoshi S, Han D, Watanabe T, Asano M, Takasawa S, Okamoto H, Shimura S, Karasawa T, Yonekura H, Yamamoto H. Septic shock is associated with receptor for advanced glycation end products ligation of LPS. J Immunol. 2011;186:3248–3257. doi: 10.4049/jimmunol.1002253. [DOI] [PubMed] [Google Scholar]
- 34.Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest. 1999;104:743–750. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benacerraf B, Sebestyen MM, Schlossman S. A quantitative study of the kinetics of blood clearance of P32-labelled Escherichia coli and staphylococci by the reticuloendothelial system. J Exp Med. 1959;110:27–48. doi: 10.1084/jem.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lammers AJ, de Porto AP, Florquin S, de Boer OJ, Bootsma HJ, Hermans PW, van der Poll T. Enhanced vulnerability for Streptococcus pneumoniae sepsis during asplenia is determined by the bacterial capsule. Immunobiology. 2011;216:863–870. doi: 10.1016/j.imbio.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Kang YS, Kim JY, Bruening SA, Pack M, Charalambous A, Pritsker A, Moran TM, Loeffler JM, Steinman RM, Park CG. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2004;101:215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinberger DM, Harboe ZB, Sanders EA, Ndiritu M, Klugman KP, Ruckinger S, Dagan R, Adegbola R, Cutts F, Johnson HL, O'Brien KL, Scott JA, Lipsitch M. Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis. 2010;51:692–699. doi: 10.1086/655828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frommhold D, Kamphues A, Hepper I, Pruenster M, Lukic IK, Socher I, Zablotskaya V, Buschmann K, Lange-Sperandio B, Schymeinsky J, Ryschich E, Poeschl J, Kupatt C, Nawroth PP, Moser M, Walzog B, Bierhaus A, Sperandio M. RAGE and ICAM-1 cooperate in mediating leukocyte recruitment during acute inflammation in vivo. Blood. 2010;116:841–849. doi: 10.1182/blood-2009-09-244293. [DOI] [PubMed] [Google Scholar]
- 40.Zen K, Chen CX, Chen YT, Wilton R, Liu Y. Receptor for advanced glycation endproducts mediates neutrophil migration across intestinal epithelium. J Immunol. 2007;178:2483–2490. doi: 10.4049/jimmunol.178.4.2483. [DOI] [PubMed] [Google Scholar]
- 41.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–1139. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lebensburger JD, Howard T, Hu Y, Pestina TI, Gao G, Johnson M, Zakharenko SS, Ware RE, Tuomanen EI, Persons DA, Rosch JW. Hydroxyurea therapy of a murine model of sickle cell anemia inhibits the progression of pneumococcal disease by down-modulating E-selectin. Blood. 2012;119:1915–1921. doi: 10.1182/blood-2011-08-374447. [DOI] [PMC free article] [PubMed] [Google Scholar]