Abstract
Type I interferons (IFNs) promote natural killer (NK) and CD8+ T-cell responses, which play a role not only in the resolution of infection but also in the induction of acute lung injury following influenza A virus infection. We show here that IFN-α receptor knock-out (Ifnar1−/−) mice exhibited impaired cytotoxic activity as well as an increased ability of NK and CD8+ T cells to produce IFN-γ after infection with influenza virus A/FM/1/47 (H1N1, a mouse-adapted strain). A deficiency in IFNAR signaling significantly impaired IL-10 production in influenza virus-infected lungs and enhanced IFN-γ production by NK cells, which were suppressed by exogenous IL-10. Depletion of NK cells but not CD8+ T cells in Ifnar1−/− mice improved the survival rate after A/FM/1/47 infection, indicating that NK cells are responsible for acute lung injury in Ifnar1−/− mice following influenza A virus infection, although the depletion of IFN-γ did not improve the outcome. Thus, type I IFN signaling plays a role not only in the upregulation of cytotoxicity but also in the downregulation of some effector mechanisms including IFN-γ production by NK and CD8+ T cells via IL-10 production.
Key Words: Natural killer cell, CD8+ T cell, Interferon-γ, Type I interferon, Influenza, Cytotoxicity
Introduction
The resolution of acute influenza A virus infection depends on interferon (IFN)-γ production and cytotoxic functions of effector cells such as natural killer (NK) and CD8+ T cells. However, excessive immune responses often induce acute lung injury following infection with influenza A viruses [1, 2, 3, 4, 5, 6]. We have recently demonstrated with IL-15 knock-out mice that IL-15-dependent CD8+ T cells are at least partly responsible for the pathogenesis of acute pneumonia caused by influenza A viruses [1]. Similarly, Abdul-Careem et al. [2] have recently reported that IL-15-dependent NK cells also contribute to the pathogenesis of influenza infection. Thus, both NK cells and CD8+ T-cell responses can be responsible for the pathogenesis of acute lung injury caused by influenza A viruses. Major effector molecules of NK and CD8+ T cells are IFN-γ and cytotoxic molecules such as granzyme B and perforin [3, 4]. However, it remains unknown which effector molecules produced by these cells play a role in the induction of acute lung injury following infection with influenza A viruses.
Type I IFNs play an important role in the protection against influenza A virus infection [5] not only by direct inhibition of viral replication through IFN-stimulated gene-encoded proteins [6, 7] but also by enhancing NK cell activity [8] and antiviral CD8+ T-cell responses [9, 10]. Meanwhile, type I IFNs have recently been shown to exert anti-inflammatory activity including inhibition of interleukin (IL)-1, IL-18 or IL-12 production [11, 12, 13]. We have found that type I IFNs are responsible not only for direct resolution of the viral load but also for suppression of immunopathology caused by influenza A viruses, at least partly by enhancement of IL-10 production [14]. However, it remains unknown which effector molecules in NK and CD8+ T cells are affected by type I IFNs in the induction of acute lung injury following infection with influenza A viruses.
Here, we examined the role of type I IFNs on effector immune cells during influenza virus A/FM/1/47 (H1N1, a mouse-adapted strain) infection. Our data show that type I IFNs enhanced cytotoxic activities of NK and CD8+ T cells but rather suppressed IFN-γ production by these cells via the induction of IL-10. NK cells, which were the main producer of IFN-γ in the lungs of IFNAR knock-out (Ifnar1−/−) mice after influenza virus infection, partly contributed to influenza immunopathology in the absence of IFNAR signaling, although the depletion of IFN-γ did not improve the outcome. Our data suggest that type I IFNs act as a modulator of some effector molecules especially in NK cells which can contribute to acute lung injury caused by influenza virus infection.
Materials and Methods
Mice
IFN-α receptor knock-out 129/Sv (Ifnar1−/−) mice from B&K Universal Ltd. were backcrossed with C57BL/6J mice (The Jackson Laboratory, Bar Harbor, Me., USA) for more than seven generations. β2-Microglobulin knock-out (β2m−/−) mice were purchased from Taconic (Germantown, N.Y., USA). IFN-γ knock-out (Ifng−/−) mice were purchased from The Jackson Laboratory. C57BL/6-Ly5.1-congenic mice were purchased from Charles River Japan (Hino, Japan). The mice were maintained in specific pathogen-free conditions and used at 7-12 weeks of age. The study design was approved by the Committee of Ethics on Animal Experiments of the Faculty of Medicine, Kyushu University, Fukuoka, Japan. Experiments were carried out under the Guidelines for Animal Experiments. Laboratory animals were cared for and used in accordance with the experimental animal standards of Japan.
Reagents
Fluorescein isothiocyanate-conjugated anti-T-cell receptor β (TCRβ; H57-597) and anti-CD45.2 (Ly5.2; 104) monoclonal antibodies (mAbs), phycoerythrin-conjugated anti-F4/80 (BM8), anti-TCRγδ (UC7) and anti-Ly-6G (Gr-1; RB6-8C5) mAbs, allophycocyanin-conjugated anti-CD8α (53-6.7) and anti-CD45.1 (Ly5.1; A20) mAbs, and PerCP-Cy5.5-conjugated CD44 (IM7) mAb were purchased from eBioscience (San Diego, Calif., USA). Fluorescein isothiocyanate-conjugated CD11b (M1/70), CD11c (HL3) and Ly-6G (1A8) mAbs, allophycocyanin-conjugated IFN-γ (XMG1.2) mAb, PerCP-Cy5.5-conjugated anti-CD3ε (145-2C11), anti-CD4 (L3T4 RM4-5), anti-CD8α (53-6.7), and NK1.1 (PK136) mAbs as well as purified anti-NK1.1 (PK136) mAb were purchased from BD Biosciences (San Jose, Calif., USA). H-2Db tetramers were purchased from MBL (Nagoya, Japan). Murine recombinant IL-10 was purchased from GenScript (Piscataway, N.J., USA), IFN-β was purchased from PBL Interferon Source (Piscataway, N.J., USA), and IL-12 was purchased from PeproTech (Rocky Hill, N.J., USA).
Virus
Influenza virus A/FM/1/47 was provided by the Osaka Prefectural Institute of Public Health, Osaka, Japan [15, 16], and intranasally infected on day 0 with 20 μl of fluid containing 25-50 plaque-forming units (pfu) of influenza virus dropped into each nostril. For viral titer, Madin-Darby canine kidney cells were plated at 1 × 106 cells in a flat-bottomed 6-well plate 24 h before infection. Supernatants from serially diluted lung homogenates were used at 37°C for 2 h to infect the Madin-Darby canine kidney cells. The cells were subsequently overlaid with DMEM (MP Biomedicals) mixed with 0.75% agarose (Lonza) in the presence of 1 μg/ml N-acetyltrypsin (Sigma-Aldrich, St. Louis, Mo., USA). Plaques were counted 3 days after infection.
Histology
Lung tissues were removed and fixed with 10% neutral buffered formalin and then embedded in paraffin. After the tissues were cut into round slices, the tissue sections were stained with hematoxylin and eosin and examined microscopically.
Enzyme-Linked Immunosorbent Assay
The levels of IFN-γ, IL-6, IL-1β, and IL-10 were measured by enzyme-linked immunosorbent assay (ELISA) using a DuoSet ELISA kit (R&D Systems), and IL-15 was measured using an ELISA Ready-SET-Go! (eBioscience), according to the manufacturer's instructions.
Cell Preparation
Lung tissues were minced and incubated in 1.5 h of stirring at 37°C in RPMI-1640 with 10% fetal bovine serum (FBS), 150 units/ml collagenase (Invitrogen) and 60 units/ml DNase (Roche Applied Science). The resulting suspension was centrifuged to isolate cells, resuspended in RPMI-1640 with 10% FBS layered on 33% Percoll and centrifuged at 600 g. Cells at the bottom of the tube were harvested and washed extensively before use. Splenocytes were prepared by centrifugation and resuspended in RPMI-1640 supplemented with 10% FBS, penicillin (100 units/ml) and streptomycin (100 μg/ml). NK cells were sorted from spleen cells of wild-type (WT) mice using anti-magnetic microbeads and an autoMACS cell separator (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions at >90% purity.
In vitro Stimulation
The lung cells from WT mice (1 × 106) were incubated with various doses of recombinant IFN-β (rIFN-β) for 24 h and with 10 μg/ml brefeldin A for the last 4 h at 37°C. IL-10-producing cells were detected by intracellular staining and analyzed by flow cytometry. NK1.1+ cells from WT splenocytes (1 × 106) were incubated with 10 ng/ml rIL-12 and various doses of rIL-10 for 24 h and with 10 μg/ml brefeldin A for the last 4 h at 37°C. IFN-γ-producing cells were detected by intracellular staining and analyzed by flow cytometry, and IFN-γ in supernatants was detected by ELISA.
In vivo Cytotoxicity Assay
Analysis of in vivo cytolytic activity was carried out using a protocol similar to that previously reported [1]. B6-Ly5.1+ splenocytes were divided into two populations and labeled with a high (10 μM) and a low concentration (1 μM) of carboxyfluorescein succinimidyl ester (CFSE). Next, CFSEhigh cells were pulsed with 5 μg/ml nuclear protein (NP)-derived ASNENMDTM peptide for 1 h at 37°C, while CFSElow cells were not pulsed. After washing, these groups were mixed in equal proportions and then injected intravenously into Ly5.2+ mice infected with the influenza virus 6 days earlier. Lungs were obtained from the recipients 12 h later for flow cytometric analysis to measure in vivo killing activity. Percent specific lysis was calculated according to the formula [1 - (ratio primed/ratio unprimed) × 100], where the ratio unprimed = % CFSElow/% CFSEhigh cells remaining in non-infected recipients, and the ratio primed = % CFSElow/% CFSEhigh cells remaining in infected recipients. WT and β2m−/− splenocytes were labeled with a low (1 μM) and a high concentration (10 μM) of CFSE, respectively. These groups were mixed in equal proportions and then injected intravenously into mice. Lung cells were obtained from the recipients 24 h later for flow cytometric analysis to measure in vivo killing activity. Percent specific lysis was calculated according to the formula [1 - % CFSEhigh/% CFSElow × 100].
Ex vivo Cytotoxicity Assay
YAC-1 cells were labeled with 1 μM CFSE. 1 × 104 YAC-1 cells were incubated with 1 × 105 splenocytes (X), no splenocytes (Y) and 0.05% NP-40 (Z) for 4 h at 37°C. The numbers of living (propidium iodide-negative) CFSE-labeled YAC-1 cells were counted by flow cytometry. Percent killing was calculated according to the formula [1 - X/(Y - Z) × 100]; X, Y and Z indicate the living YAC-1 cell numbers of each group.
In vivo Depletion of Cells
Anti-CD8 mAb 200 µg (clone 2.43), 50 µg anti-NK1.1 mAb (clone PK136) or 500 µg anti-IFN-γ mAb (clone R4-6A2) were intraperitoneally injected into mice on the indicated days after influenza virus infection.
Flow Cytometric Analysis
Cells were surface stained with various combinations of mAbs and then subjected to intracellular cytokine staining that followed the manufacturer's instructions (BD Biosciences). The stained cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed with CellQuest software (BD Biosciences). For the intracellular cytokine staining (Cytokine FACS), lung cells were incubated with 10 μg/ml NP-specific peptide (ASNENMDTM or ARSALILRGSVAHK) and 10 μg/ml brefeldin A (Sigma-Aldrich) for 4 h at 37°C in 96-well flat-bottom plates at a concentration of 1 × 106/well in a volume of 200 μl of RPMI-1640 containing 10% FCS. After culture, cells were surface stained with various combinations of mAbs and then subjected to intracellular cytokine staining using the manufacturer's instructions (BD Biosciences). In brief, 100 µl of BD Cytofix/Cytoperm solution (BD Biosciences) was added to the cell suspension with mild mixing and placed for 20 min at 4°C. Fixed cells were washed twice with 250 µl of BD Perm/Wash solution (BD Biosciences) and stained intracellularly with anti-IFN-γ mAb for 15 min at 4°C. Samples were acquired in a FACSCalibur flow cytometer and analyzed by CellQuest software.
Statistical Analysis
The difference in survival rates was evaluated by the log-rank test (Mantel-Cox). Differences in parametric data were evaluated by Student's t test. A p value <0.05 was considered significant.
Results
Susceptibility of Ifnar1−/− Mice to Infection with Influenza Virus A/FM/1/47
We have recently reported the increased susceptibility of Ifnar1−/− mice to infection with influenza virus A/FM/1/47 as assessed by mortality and morbidity [14]. To confirm our previous findings, we monitored the survival of Ifnar1−/− mice daily after influenza virus infection. All mice in the Ifnar1−/− group died within 10 days after intranasal infection with 25 pfu of influenza virus A/FM/1/47, while half of the WT mice survived beyond day 15 after infection (online suppl. fig. 1A; for all online suppl. material, see www.karger.com/doi/10.1159/000356824). The Ifnar1−/− mice also had a higher morbidity than the WT mice as assessed by body weight (online suppl. fig. 1A). The viral titers in the lungs were significantly higher at an early stage in Ifnar1−/− mice than in WT mice, but the viruses were mostly eliminated in both groups by day 9 after infection (online suppl. fig. 1B). Lung histopathology of Ifnar1−/− mice after hematoxylin and eosin staining revealed more severe inflammation and tissue damage in comparison with WT mice (online suppl. fig. 1C). Thus, Ifnar1−/− mice were highly susceptible to influenza A virus infection.
We have also reported that proinflammatory cytokine levels following influenza virus A/FM/1/47 infection were significantly higher in the lung homogenates of Ifnar1−/− mice during the course of infection. Consistently, the levels of IL-1β, IL-6 and IFN-γ were significantly higher in Ifnar1−/− mice than in WT mice after influenza virus infection (online suppl. fig. 1D). Conversely, the levels of IL-10 and IL-15 in the lungs were significantly lower in Ifnar1−/− mice than in WT mice on days 6 and 9 after infection despite the increased viral load (online suppl. fig. 1D). There was no difference in the level of TNF-α or IL-12p70 between the two groups after infection [14].
Activity of NK Cells in Ifnar1−/− Mice Inoculated Intranasally with Influenza Virus A/FM/1/47
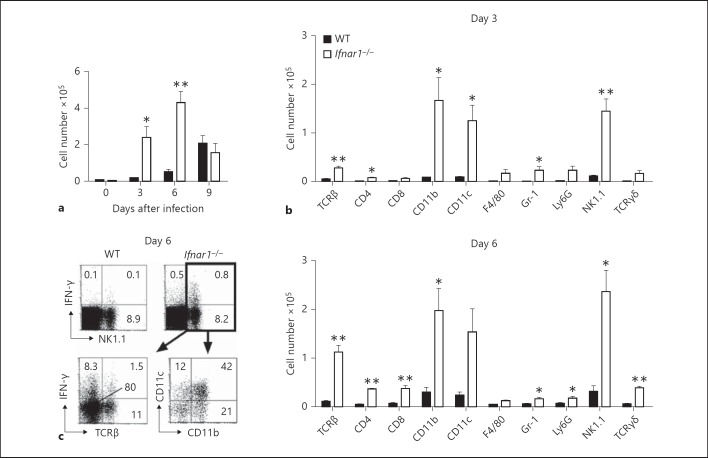
Several proinflammatory cytokines including IL-1β and IL-6 have been reported to play an important role in influenza immunopathology [17, 18], yet the role of IFN-γ remains unclear. Therefore, we examined the role of IFN-γ in influenza-infected Ifnar1−/− mice. To determine the main source of IFN-γ, we analyzed the phenotype of IFN-γ-producing cells in the lungs of mice infected with influenza virus by flow cytometric analysis. IFN-γ-producing cells were significantly higher in Ifnar1−/− mice on days 3 and 6 after infection (fig. 1a), and the major surface markers of these cells were CD11b+, CD11c+ and NK1.1+ (fig. 1b). Further examination of these cells revealed that more than 80% of the NK1.1+ cells were TCRβ-, and more than half of the NK1.1+ cells expressed CD11b+ and/or CD11c+ (fig. 1c). These results suggest that, except in the late phase of infection, the main producer of IFN-γ after influenza virus infection was NK cells.
Fig. 1.
IFN-γ-producing cells in the lungs of Ifnar1−/− mice after infection with influenza virus A/FM/1/47. Lung cells were harvested on days 3, 6 and 9 after infection with 25 pfu of influenza virus A/FM/1/47. The samples were incubated with 10 μg/ml brefeldin A for 4 h at 37°C. Expression of IFN-γ was detected by intracellular staining and analyzed by flow cytometry. Each group consists of 3 mice. The data are representative of at least 3 independent experiments. Error bars represent the mean ± SEM. * p < 0.05; ** p < 0.01. a Total numbers of IFN-γ-producing cells in the lungs of WT and Ifnar1−/− mice after infection. b Numbers of surface marker-positive IFN-γ-producing cells of each surface marker in the lungs of WT and Ifnar1−/− mice on days 3 and 6 after infection. c Percentage of IFN-γ-producing NK1.1+ cells in the lungs of WT and Ifnar1−/− mice (above), and percentage of TCRβ+, CD11b+ and CD11c+ cells in NK1.1+ gated lung cells of Ifnar1−/− mice (below) on day 6 after infection. The numbers indicate the percentage of cells in the corresponding quadrants.
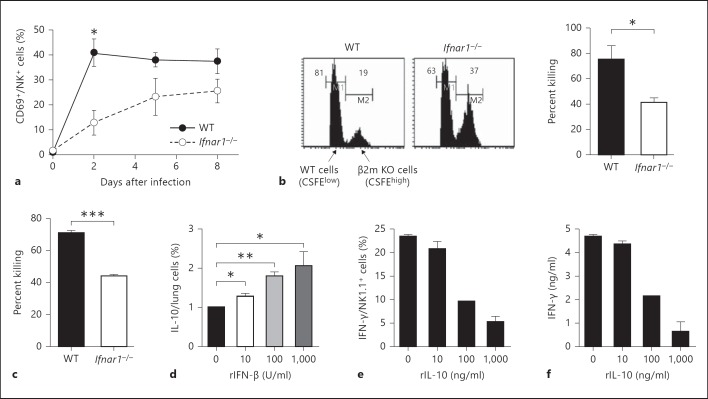
The total number of NK cells in the lungs was not significantly different between WT and Ifnar1−/− mice (data not shown). As shown in figure 2a, CD69 expression as an activation marker for cytolysis in NK cells increased after influenza virus infection and was significantly lower in Ifnar1−/− mice than in WT mice at an early stage of infection. The cytolytic activity of NK cells against β2m−/− cells in vivo was significantly lower in Ifnar1−/− mice than in WT mice (fig. 2b). Similarly, the cytolytic activity of NK cells in Ifnar1−/− mice was significantly impaired as assessed by ex vivo cytotoxicity assay using YAC-1 cells (fig. 2c). Thus, NK cells in the influenza virus-infected Ifnar1−/− mice exhibited an increased ability for IFN-γ production but an impaired cytotoxicity ability. These results suggest that IFNAR signaling is important to NK cell cytotoxicity but may rather inhibit IFN-γ production by NK cells.
Fig. 2.
Activity of NK cells in Ifnar1−/− mice after infection with influenza virus A/FM/1/47. Each group consists of 3 mice. The data are representative of at least 3 independent experiments. Error bars represent the mean ± SEM. * p < 0.05; ** p < 0.01; *** p < 0.001. a CD69 expression in NK cells (NK1.1+CD3e-) in the lungs of WT and Ifnar1−/− mice was analyzed by flow cytometry after infection with 25 pfu of influenza virus A/FM/1/47. b CFSElow-labeled WT and CFSEhigh-labeled β2m−/− splenocytes were co-injected equally into WT or Ifnar1−/− mice infected with influenza virus A/FM/1/47 6 days earlier. Transferred cells were analyzed in the lungs of recipient mice 24 h after injection (histograms), and percent killing was calculated as shown in Methods (graph). Values in each histogram represent the percentage of CFSElow and CFSEhigh cells. c WT and Ifnar1−/− lung cells were harvested on day 5 after infection with 25 pfu of influenza virus A/FM/1/47. NK1.1+ cells were positively purified to >90% using autoMACS. 1 × 105 NK1.1+ cells were incubated with 1 × 104 CFSE-labeled YAC-1 cells. The number of living (propidium iodide-negative) YAC-1 cells was counted by flow cytometry, and percent killing was calculated as shown in Methods. d 1 × 106 lung cells from WT mice were incubated with the indicated dose of rIFN-β for 24 h and with 10 μg/ml brefeldin A for the last 4 h at 37°C. IL-10-producing cells were detected by intracellular staining and analyzed by flow cytometry. e, f NK1.1+ cells from WT splenocytes were positively purified to >90% using autoMACS. The cells were incubated with 10 ng/ml rIL-12 and the indicated dose of rIL-10 for 24 h and with 10 μg/ml brefeldin A for the last 4 h at 37°C. IFN-γ-producing cells were detected by intracellular staining and analyzed by flow cytometry (d), and IFN-γ in supernatants was detected by ELISA (e).
IFNAR signaling has been reported to enhance IL-10 production, although the precise signaling pathways remain elusive [12]. We stimulated the lung cells of WT mice with exogenous IFN-β and confirmed that IFN-β induced IL-10 production in a dose-dependent manner (fig. 2d). We then speculated that decreased IL-10 production in the absence of IFNAR signaling might enhance IFN-γ production by NK cells. To confirm that IL-10 can directly suppress IFN-γ production by NK cells, we sorted NK1.1+ cells from the spleens of WT mice and measured IFN-γ production by stimulation of IL-12 production after treatment with IL-10. As shown in figure 2e and f, IL-10 could directly suppress IFN-γ production by NK1.1+ cells in a dose-dependent manner.
Antigen-Specific T Cells in Ifnar1−/− Mice Inoculated Intranasally with Influenza Virus A/FM/1/47
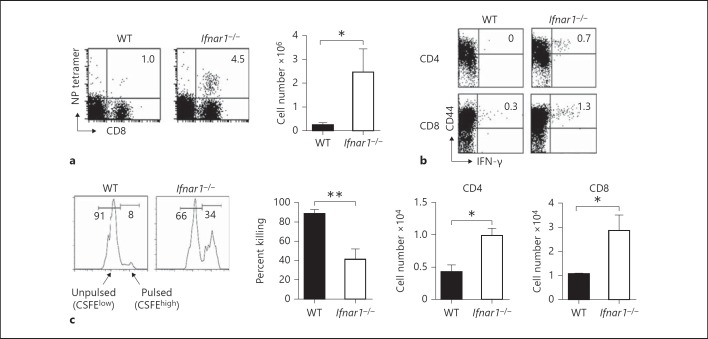
Virus-specific T-cell responses have been reported to play an important role during influenza virus infection [19, 20, 21]. Therefore, we also examined the number of antigen (Ag)-specific CD8+ T cells in the lungs of Ifnar1−/− mice on day 6 after infection, as assessed by staining with an H-2Db tetramer coupled with NP peptide. The number of NP-specific CD8+ T cells was significantly higher in Ifnar1−/− mice than in WT mice (fig. 3a). We next compared the functional activity of NP-specific T cells between Ifnar1−/− and WT mice infected with influenza virus A/FM/1/47, as assessed by intracellular cytokine flow cytometric analysis and in vivo cytotoxicity assay. After in vitro stimulation with NP-derived peptides, the percentage and absolute number of CD4+ and CD8+ T cells producing IFN-γ were significantly higher in the lungs of Ifnar1−/− mice (fig. 3b).
Fig. 3.
Ag-specific T cells in the lungs of Ifnar1−/− mice after infection with influenza virus A/FM/1/47. Each group consists of 3 mice. The data are representative of at least 3 independent experiments. Error bars represent the mean ± SEM. * p < 0.05; ** p < 0.01. a NP-specific CD8+ T cells on day 6 after infection with 25 pfu of influenza virus A/FM/1/47. The lung cells were stained with αCD8 mAb and NP-major histocompatibility complex class I tetramer. Samples were analyzed by flow cytometry. Dot plots are shown after lymphocyte gating. The numbers indicate the percentage of NP tetramer-positive cells in CD8+ cells. Absolute cell numbers were counted by multiplying the percentage of tetramer-positive cells by the absolute number of lung cells. b IFN-γ-producing T cells on day 6 after infection with 25 pfu of influenza virus A/FM/1/47. The lung cells were incubated with 10 μg/ml NP-derived ASNENMDTM peptide for CD8+ cells or 10 μg/ml NP-derived ARSALILRGSVAHK peptide for CD4+ cells and with 10 μg/ml brefeldin A for 4 h at 37°C, and expression of IFN-γ was detected by intracellular staining. Dot plots are shown after CD4+ (above) and CD8+ (below) cell gating. The numbers indicate the percentage of cells in the corresponding quadrants. The absolute numbers of IFN-γ-producing CD4+ and CD8+ cells were counted by multiplying the percentage by the absolute number of lung cells. c In vivo cytotoxic activity of NP-specific CD8+ T cells on day 6 after infection with 25 pfu of influenza virus A/FM/1/47. Histograms are gated on Ly5.1+ cells in the lung 12 h after co-injection with equal numbers of CFSEhigh-labeled NP peptide-pulsed splenocytes and CFSElow-labeled unpulsed splenocytes into mice infected with influenza virus A/FM/1/47 6 days earlier. The percentage of specific lysis was calculated as shown in Methods.
We next measured the ability of CD8+ T cells to eliminate fluorescently labeled spleen cells pulsed with NP peptides after infection, by directly detecting the cytotoxic activity of CD8+ T cells in vivo. As shown in figure 3c, the elimination of the NP-pulsed target cells was severely impaired in the lungs of Ifnar1−/− mice on day 6 after infection, indicating severely reduced in vivo cytotoxic activity of the NP-specific CD8+ T cells in Ifnar1−/− mice in contrast to the increased number of NP-specific CD8+ T cells producing IFN-γ after infection. These results indicate that IFNAR signaling also enhances cytolytic activity of Ag-specific cytotoxic T cells but rather suppresses IFN-γ production by these cells as in NK cells.
Susceptibility of NK1.1+ and CD8+ Cell-Depleted Ifnar1−/− Mice to Infection with Influenza Virus A/FM/1/47
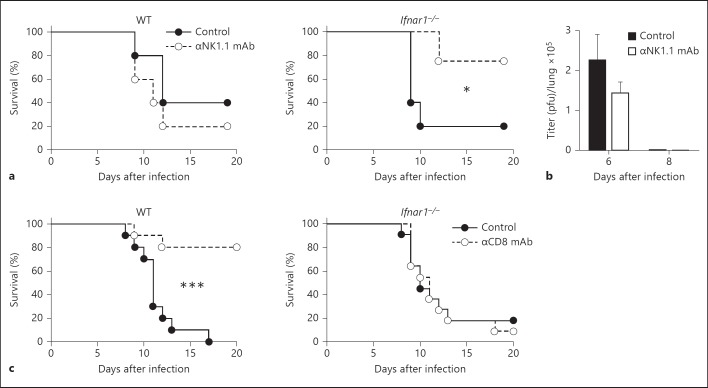
To examine the in vivo role of NK cells on the survival of Ifnar1−/− mice, we administered anti-NK1.1 mAb to deplete NK1.1+ cells after influenza virus infection. As shown in figure 4a, the survival rate of the Ifnar1−/− mice improved after the depletion of NK1.1+ cells, compared with the control mAb-treated mice. However, the protective effect of anti-NK1.1 mAb was not seen in WT mice. There were no significant differences in the viral titers in the lungs of Ifnar1−/− mice after depletion of NK1.1+ cells (fig. 4b). These results suggest that NK cells were at least partly responsible for the acute lung injury caused by influenza A virus in Ifnar1−/− mice. In other words, NK cell activity could be detrimental without IFNAR signaling during influenza virus infection.
Fig. 4.
Susceptibility of NK1.1+ and CD8+ cell-depleted Ifnar1−/− mice after infection with influenza virus A/FM/1/47. Each group consists of 10-11 mice. The data are representative of at least two independent experiments. Error bars represent the mean ± SEM. * p < 0.05; *** p < 0.001. a, b 50 μg anti-(α)NK1.1 mAb (clone PK136) or isotype-matched control mAb was intraperitoneally injected into the respective Ifnar1−/− mice on day 5 after infection with 25 pfu of influenza virus A/FM/1/47. a Survival rates of αNK1.1 mAb-treated WT and Ifnar1−/− mice. Each group consists of 5 mice. b Viral titers in the lungs on days 6 and 8 after infection. Each group consists of 3 mice. c Survival rates of αCD8 mAb (clone 2.43)-treated WT and Ifnar1−/− mice after infection with influenza virus A/FM/1/47. 200 μg αCD8 mAb or isotype-matched control mAb was intraperitoneally injected into WT mice infected with 50 pfu influenza virus A/FM/1/47 and into Ifnar1−/− mice infected with 25 pfu influenza virus A/FM/1/47 on days 1, 4 and 7 after infection.
We recently reported that IL-15-dependent CD8+ T cells contributed to lung injury and mortality in C57BL/6 mice following infection with influenza virus A/FM/1/47 [1]. Next, we administered anti-CD8 mAb to WT and Ifnar1−/− mice to deplete CD8+ T cells. Consistent with our previous report [1], 80% of the CD8+ T-cell-depleted WT mice survived after infection with A/FM/1/47, while all the control Ab-treated WT mice died by day 17 (fig. 4c). However, there was no significant difference between the anti-CD8 mAb-treated group and the control Ab-treated group in the Ifnar1−/− mice. These results suggest that CD8+ T cells could act detrimentally mainly on the WT hosts during influenza A virus infection, but other cells such as NK cells may have contributed more greatly to the acute lung injury caused by influenza A virus infection in Ifnar1−/− mice.
Susceptibility of IFN-γ-Depleted Ifnar1−/− Mice to Infection with Influenza Virus A/FM/1/47
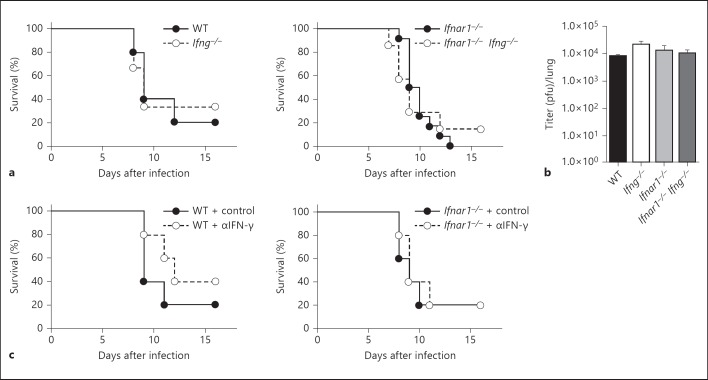
Multiple studies of influenza virus infection have demonstrated little requirement for IFN-γ in efficient viral clearance or mounting an effective immune response [22, 23]. However, little is known about the effect of this cytokine on influenza immunopathology. From the data above, we considered the possibility that increased IFN-γ in the absence of IFNAR signaling might contribute to the acute lung injury induced by influenza virus infection. To examine it, we generated mice lacking both IFNAR and IFN-γ (Ifnar1−/−Ifng−/−). We compared the survival rates of WT, Ifng−/−,Ifnar1−/− and Ifnar1−/−Ifng−/− mice infected with influenza virus A/FM/1/47. Unexpectedly, there were no significant differences between the survival rate of Ifnar1−/− and Ifnar1−/−Ifng−/− mice as well as WT and Ifng−/− mice (fig. 5a). Viral titers in the lungs also showed no significant differences between these four groups (fig. 5b).
Fig. 5.
Susceptibility of IFN-γ-depleted Ifnar1−/− mice after infection with influenza virus A/FM/1/47. The data are representative of at least two independent experiments. Error bars represent the mean ± SEM. a Survival rates of WT, Ifng−/−, Ifnar1-/- and Ifnar1−/−Ifng−/− mice after infection with 25 pfu of influenza virus A/FM/1/47. Each group consists of 5-12 mice. b Viral titers of WT, Ifng−/−,Ifnar1−/− and Ifnar1−/−Ifng−/− mice on day 6 after infection with 25 pfu of influenza virus A/FM/1/47. Each group consists of 3 mice. c Survival rates of αIFN-γ mAb (R4-6A2)-treated WT and Ifnar1−/− mice after infection with influenza virus A/FM/1/47. 500 μg αIFN-γ mAb or isotype-matched control mAb was intraperitoneally injected into WT and Ifnar1−/− mice infected with 25 pfu influenza virus A/FM/1/47, respectively, on days 5-8 after infection. Each group consists of 5 mice.
To directly examine the effect of IFN-γ at the peak of lung inflammation, we administered anti-IFN-γ mAb to WT and Ifnar1−/− mice on day 5 after A/FM/1/47 infection. As is the case with mice lacking IFN-γ, there were no significant differences in the survival rates between anti-IFN-γ mAb and control Ab-treated mice either in WT and Ifnar1−/− mice (fig. 5c). These results suggest that increased IFN-γ in the lungs after infection was not responsible for lethal lung injury in Ifnar1−/− mice following influenza A virus infection.
Discussion
Type I IFNs play critical roles in the protection against influenza A virus infection directly through inhibition of viral duplication and indirectly through enhancement of antiviral immunity [7]. We found here that the numbers of cells capable of producing IFN-γ including NK cells and CD8+ T cells were significantly higher in the lungs of Ifnar1−/− mice than in WT mice after infection. Innate immunity recognizes viruses as pathogen-associated molecular patterns (PAMPs), resulting in the release of proinflammatory cytokines such as IL-12. Therefore, it is possible that increased IL-12 due to increased viral load is responsible for increased IFN-γ production in Ifnar1−/− mice after influenza A virus infection. However, we did not detect increased IL-12 production in Ifnar1−/− mice after infection. Instead, we found that the production of anti-inflammatory cytokine IL-10 was significantly reduced in Ifnar1−/− mice, and IL-10 directly suppressed IFN-γ production by NK cells. IL-10 is a suppressor cytokine which inhibits immune responses via STAT3 and SOCS in IL-10 signal transduction [24]. The inhibition of nuclear factor-κB, MAPKs and/or the activation of the inhibitory PI3K/AKT pathway by IL-10 would also explain the suppression of a large number of inflammatory genes [24]. Thus, these results suggest that increased IFN-γ production in Ifnar1−/− mice during the course of infection with influenza A virus was due not to increased viral load as PAMPs but to decreased IL-10 production in the absence of IFNAR signaling. Viral PAMPs recognized by Toll-like receptors can also induce IL-10 production [25], while IFNAR signaling has recently been reported to enhance IL-10 production [12, 13, 26]. Considering our data, IFNAR signaling seems to be more critical for IL-10 production than Toll-like receptor signaling during lethal influenza virus infection.
There are several reports concerning the role of IL-10 during acute influenza A virus infection. Neutralization of IL-10 signaling during an ongoing influenza virus infection resulted in exacerbation of inflammation and increased mortality [27]. On the contrary, deficiency in IL-10 before infection enhanced survival after influenza virus infection [28]. IL-10 may be detrimental at an early stage of infection because it can inhibit effective viral clearance, but it may be beneficial at a late stage because it can suppress excessive immune responses which cause tissue injury.
As opposed to the enhanced production of IFN-γ, the cytolytic activity of NK cells and Ag-specific CD8+ T cells was significantly impaired in Ifnar1−/− mice following influenza virus infection. It has recently been reported that type I IFN signaling enhances the cytotoxic activity of NK cells and Ag-specific CD8+ T cells via induction of granzymes in a STAT1-dependent but Ag-independent manner [29, 30]. Defective IFNAR signaling may decrease the cytolytic activity of NK cells and Ag-specific CD8+ T cells because of impaired induction of effector molecules. In addition, IL-15 has been reported to upregulate the expression of cytotoxic molecules such as granzyme B and perforin that are closely correlated with the cytotoxic effector function of NK and CD8+ T cells [31, 32]. Thus, in addition to the direct defect of IFNAR signaling, decreased IL-15 production may also be responsible for impaired cytotoxicity on NK and CD8+ T cells in Ifnar1−/− mice after influenza A virus infection.
Influenza A viruses often cause acute severe pneumonia induced by an excessive inflammation called a ‘cytokine storm’ [33, 34]. Several kinds of cytokines or chemokines have been reported to contribute to lung injury caused by influenza virus infection [17, 18, 35, 36, 37]. IL-15 is responsible for the pathogenesis of acute pneumonia caused by influenza A virus via activation of NK and CD8+ T cells [1, 2], suggesting that both NK cells and CD8+ T-cell responses may be responsible for the influenza immunopathology. However, it remains unknown which effector molecules produced by these cells play a role in the induction of acute lung injury following infection with highly pathogenic influenza A viruses. In the present study, a defect of IFN-γ did not affect the susceptibility of WT and Ifnar1−/− mice. Furthermore, in our preliminary experiments, perforin-deficient (perforin−/−) mice and Ifnar1−/− perforin−/− mice also showed the same susceptibility as control WT or Ifnar1−/− mice, suggesting that cytotoxic activity may not contribute to the lethal lung injury induced by influenza virus infection. Further studies such as the investigation of other effector molecules may shed light on the complicated mechanism of immunopathology induced by lethal influenza A virus infection.
It is reported that NK cell-mediated cytotoxicity can interfere with pathways which limit virus-induced disease by negatively regulating the magnitude of T-cell responses to infection [38]. This raises the possibility that an impaired NK-mediated cytotoxicity may upregulate T-cell responses to influenza virus infection. However, CD8+ T-cell cytotoxicity was also impaired in Ifnar1−/− mice after infection, although IFN-γ production by T cells was enhanced. More recently, it is reported that innate lymphoid cells play roles in either promoting disease or promoting tissue repair in the lungs during influenza virus infection [39, 40]. Therefore, it is possible that the lack of IFNAR signaling may alter innate lymphoid cell functions besides NK cell functions in Ifnar1−/− mice. Further experiments are required to elucidate this possibility.
In conclusion, in this study, we demonstrated the opposing role of type I IFNs in IFN-γ production and cytotoxic activities of NK and CD8+ T cells. Although the involvement of this mechanism in influenza immunopathology remains unclear, it may lead to the new outlook on lung homeostasis and resolution of excessive immune responses.
Supplementary Material
Supplementary data
Acknowledgements
We would like to thank Akiko Yano, Mihoko Ookubo, Miki Kijima, and Kiyomi Akasaki for their secretarial assistance and the members of our laboratory for their helpful discussions.
This work was supported in part by funding from the Yakult Bioscience Foundation, the Urakami Foundation and the Takeda Science Foundation to Y. Yoshikai.
References
- 1.Nakamura R, Maeda N, Shibata K, Yamada H, Kase T, Yoshikai Y. Interleukin-15 is critical in the pathogenesis of influenza A virus-induced acute lung injury. J Virol. 2010;84:5574–5582. doi: 10.1128/JVI.02030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdul-Careem MF, Mian MF, Yue G, Gillgrass A, Chenoweth MJ, Barra NG, Chew MV, Chan T, Al-Garawi AA, Jordana M, Ashkar AA. Critical role of natural killer cells in lung immunopathology during influenza infection in mice. J Infect Dis. 2012;206:167–177. doi: 10.1093/infdis/jis340. [DOI] [PubMed] [Google Scholar]
- 3.Lettau M, Schmidt H, Kabelitz D, Janssen O. Secretory lysosomes and their cargo in T and NK cells. Immunol Lett. 2007;108:10–19. doi: 10.1016/j.imlet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Billiau A, Matthys P. Interferon-gamma: a historical perspective. Cytokine Growth Factor Rev. 2009;20:97–113. doi: 10.1016/j.cytogfr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 6.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 7.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 9.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 10.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billiau A. Anti-inflammatory properties of type I interferons. Antiviral Res. 2006;71:108–116. doi: 10.1016/j.antiviral.2006.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Arimori Y, Nakamura R, Yamada H, Shibata K, Maeda N, Kase T, Yoshikai Y. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Res. 2013;99:230–237. doi: 10.1016/j.antiviral.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Brown EG. Increased virulence of a mouse-adapted variant of influenza A/FM/1/47 virus is controlled by mutations in genome segments 4, 5, 7, and 8. J Virol. 1990;64:4523–4533. doi: 10.1128/jvi.64.9.4523-4533.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuno Y, Matsumoto K, Isegawa Y, Ueda S. Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J Virol. 1994;68:517–520. doi: 10.1128/jvi.68.1.517-520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 20.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 21.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen HH, van Ginkel FW, Vu HL, Novak MJ, McGhee JR, Mestecky J. Gamma interferon is not required for mucosal cytotoxic T-lymphocyte responses or heterosubtypic immunity to influenza A virus infection in mice. J Virol. 2000;74:5495–5501. doi: 10.1128/jvi.74.12.5495-5501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price GE, Gaszewska-Mastarlarz A, Moskophidis D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J Virol. 2000;74:3996–4003. doi: 10.1128/jvi.74.9.3996-4003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Brown J, Garcia CA, Tang Y, Benakanakere MR, Greenway T, Alard P, Kinane DF, Martin M. The role of glycogen synthase kinase 3 in regulating IFN-beta-mediated IL-10 production. J Immunol. 2011;186:675–684. doi: 10.4049/jimmunol.1001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKinstry KK, Strutt TM, Buck A, Curtis JD, Dibble JP, Huston G, Tighe M, Hamada H, Sell S, Dutton RW, Swain SL. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CK, Rao DT, Gertner R, Gimeno R, Frey AB, Levy DE. Distinct requirements for IFNs and STAT1 in NK cell function. J Immunol. 2000;165:3571–3577. doi: 10.4049/jimmunol.165.7.3571. [DOI] [PubMed] [Google Scholar]
- 30.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yajima T, Nishimura H, Sad S, Shen H, Kuwano H, Yoshikai Y. A novel role of IL-15 in early activation of memory CD8+ CTL after reinfection. J Immunol. 2005;174:3590–3597. doi: 10.4049/jimmunol.174.6.3590. [DOI] [PubMed] [Google Scholar]
- 32.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 33.Doherty PC, Turner SJ, Webby RG, Thomas PG. Influenza and the challenge for immunology. Nat Immunol. 2006;7:449–455. doi: 10.1038/ni1343. [DOI] [PubMed] [Google Scholar]
- 34.Maines TR, Szretter KJ, Perrone L, Belser JA, Bright RA, Zeng H, Tumpey TM, Katz JM. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol Rev. 2008;225:68–84. doi: 10.1111/j.1600-065X.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 35.Deng R, Lu M, Korteweg C, Gao Z, McNutt MA, Ye J, Zhang T, Gu J. Distinctly different expression of cytokines and chemokines in the lungs of two H5N1 avian influenza patients. J Pathol. 2008;216:328–336. doi: 10.1002/path.2417. [DOI] [PubMed] [Google Scholar]
- 36.Dawson TC, Beck MA, Kuziel WA, Henderson F, Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156:1951–1959. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 39.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data