Abstract
L-ficolin, one of the complement lectins found in human serum, is a novel pattern recognition molecule that can specifically bind to microbial carbohydrates, thereby activating the lectin complement pathway and mounting a protective innate immune response. However, little is known about the role of L-ficolin during viral infections in vivo. In the present study, we used a mouse model of influenza A virus infection to demonstrate that the administration of exogenous L-ficolin or ficolin A (FCNA – an L-ficolin-like molecule in the mouse) is protective against the virus. Furthermore, FCNA-null mice have a greatly increased susceptibility to infection with the influenza A virus. Moreover, we found recombinant human L-ficolin inhibited influenza A virus entry into Madin-Darby canine kidney cells. More importantly, L-ficolin can recognize and bind hemagglutinin (HA) and neuraminidase (NA) glycoproteins and different subtypes of influenza A virus, and these interactions can be competitively inhibited by N-acetyl-D-glucosamine. In addition, the binding of L-ficolin and FCNA may lead to the activation of the lectin complement pathway. To our knowledge, this is the first report demonstrating that L-ficolin can block influenza virus infections both in vitro and in vivo using FCNA-knockout mice, possibly by interacting with the carbohydrates of HA and NA. Therefore, these data may provide new immunotherapeutic strategies based on the innate immune molecule L-ficolin against the influenza A virus.
Key Words: Complement, L-ficolin, Influenza infection, Hemagglutinin, Neuraminidase, Glycoprotein
Introduction
The innate immune system prevents or limits the early stages of an infection and involves many different recognition and effector mechanisms, including the complement system. Complement can be activated via three pathways, the classical, the alternative and the lectin pathway [1]. The complement lectins in human serum are important innate immune molecules. Mannan-binding lectin (MBL) and the recently identified ficolins are two kinds of complement lectins [1]. These lectins are capable of recognizing microbial carbohydrates and can activate the lectin complement pathway by a mechanism similar to the classical pathway, but they use MBL-associated serine proteases instead of C1r and C1s [1,2].
Three members of the human ficolin family have been characterized, L-ficolin/P35 (ficolin-2) [3], H-ficolin (ficolin-3) [4] and M-ficolin (ficolin-1) [5]. L-ficolin/P35 (with a molecular weight of 35 kDa) was first cloned and described as a type of lectin (carbohydrate-binding proteins) with a similar structure and function to MBL [1,2]. L-ficolin, similar to MBL, contains a collagen-like stem structure. Unlike MBL, however, it has a fibrinogen-like domain and has a common binding specificity for N- acetyl-D-glucosamine (GlcNAc), 1,3-β-D-glucan, lipoteichoic acid and various acetylated compounds [6,7,8]. Both L-ficolin and MBL are carbohydrate recognition molecules that can recognize the surface molecules of microorganisms and subsequently trigger the activation of the lectin complement system, which plays a pivotal role in innate immunity [9]. In mice, the ficolin A (FCNA) molecule has been identified as the L-ficolin homologue [10].
Influenza A virus is an important human pathogen that causes yearly epidemics and sporadic pandemics worldwide [11]. There are three types of influenza viruses: A, B, and C. To date, 16 subtypes of hemagglutinin (HA) (H1–H16) have been identified; however, only H1, H2 and H3 subtypes have achieved sustained transmission in human populations [12]. Moreover, the highly pathogenic avian influenza H5N1 virus continues to be enzootic in poultry in parts of Asia and Africa and can be transmitted zoonotically to humans. From 2003 to November 2009, the influenza H5N1 virus caused 444 confirmed human cases, and 262 of them were fatal [13]. Thus, the development of an immunological defense strategy or of new drugs against the influenza A virus remains a high priority goal. The genome of influenza A viruses is composed of 8 RNA segments (0.9–2.3 kb) that span together approximately 13.5 kb and encode 11 proteins [14]. Segment 4 encodes the major surface glycoprotein (HA), which is responsible for attaching the virus to sialic acid residues on the host cell surface and for fusing the virus membrane envelope with the host cell membrane, thus delivering the viral genome to the cell. Segment 6 encodes another surface glycoprotein, neuraminidase (NA), which cleaves terminal sialic acid residues from glycoproteins and glycolipids on the host cell surface, thus releasing budding viral particles from an infected cell [15]. L-ficolin can also serve as an opsonin and enhance the clearance of pathogens [16]. L-ficolin has been reported to bind specifically to clinically important bacteria, including Salmonella typhimurium, Streptococcus pneumoniae and Staphylococcus aureus, and to function as an opsonin when binding to certain types of carbohydrates on the surface of pathogens in vitro [3,7,17]. In this study, we firstly show that L-ficolin can recognize and bind to HA and NA of the influenza A virus. We also demonstrate that L-ficolin or FCNA plays an important role in conferring protection to mice infected with the influenza A virus.
Materials and Methods
Viruses, Plasmids and Animals
The influenza A viruses H5N1 A/chicken/Hubei/489/2004 (GeneBank Accession No. AY770078), H1N1 A/Yamagata/120/86 and H1N1 A/PR/8/34 [18,19,20] were used in this study. The conditions for infection of embryonated hen eggs and purification of the viruses have been described by Zhang et al. [20]. Eukaryotic expression plasmids pCMVTag2B, pVAX-1 and pcDNA3.1 (–)/Myc-His A were purchased from Invitrogen, and the prokaryotic expression plasmid pGEX-KG was purchased from Amersham Biosciences. All DNA preparations were produced using endotoxin-free purification columns (Qiagen). Eight-week-old male BALB/c and C57BL/6 mice were purchased from the Center of Animal Experiments of the Wuhan University, China. The animal protocols were performed in compliance with all guidelines and were approved by the Institutional Animal Care and Use Committee of the Wuhan University.
Recombinant Plasmid Construction
The human L-ficolin cDNA was amplified and subcloned in frame into pGEX-KG and pcDNA3.1 (–)/Myc-His A, respectively [21,22]. The pVAX-1-FCNA plasmid construction was described by Fujimori et al. [23]. The cDNAs encoding HA and NA were amplified from influenza A virus H5N1 A/chicken/Hubei/ 489/2004 by RT-PCR, and then subcloned into pCMV-Tag or pGEX-KG.
Western Blot Analysis
Muscle and lung tissue from the injected mice were harvested 0, 4, 7 and 10 days after injection. The harvested tissues were homogenized, and L-ficolin was detected via Western blot analysis using anti-L-ficolin monoclonal antibody (mAb) GN5 (Hycult Biotechnology). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG was used as a secondary Ab.
Measurement of L-Ficolin Concentrations in Mouse Serum
The sandwich enzyme-linked immunosorbent assay (ELISA) method was used to measure the concentrations of mouse serum L-ficolin according to the methods described in a previous publication [22]. Briefly, 96-well ELISA plates were coated with 100 µl of rabbit anti-GST-L-ficolin polyclonal antibody. After incubation at room temperature (RT) for 1 h, the solution was removed and the plates were rinsed. After washing three times with 0.2% Tween-20 in PBS, 100 µl of mouse serum was added and the plates were incubated at 37°C for 2 h. The plates were washed three times, and the nonspecific binding sites on the ELISA plates were blocked with 5% BSA and incubated overnight at 4°C. Mouse monoclonal anti-human L-ficolin GN5 (1:1,000 dilution; HyCult Biotechnology) was added to each well and incubated at 37°C for 1 h. After washing three times, 100 µl of labeled HRP-conjugated goat anti-mouse IgG (1:1,000 dilution) were added. Color development was achieved by adding 100 µl/well of ready-to-use tetramethylbenzidine chromogen substrate (Sigma), and the optical density (OD) at 450 nm was measured using an ELISA reader.
Influenza Virus Challenge
Male BALB/C, C57BL/6 or FCNA knockout (KO) mice (7–8 weeks old) were randomly divided into groups, and pcDNA3.1A-L-ficolin, pcDNA3.1A, pVAX-1-FCNA or pVAX-1 plasmid was intramuscularly injected into the mice (20 µg per mouse) using a gene gun with an Electric Square Porator (Scientz Biotechnology) [24]. Each injection (125 µl) consisted of a 1:4 mixture of amethocaine (20 mg/ml) and the respective plasmids (20 µg/ml). The quadriceps femoris muscle was stimulated using an electrode to allow for simultaneous DNA injection and electrotransfer at the same site. Two days after plasmid injection, the mice were submitted to intranasal infection with either lethal [1 × 105 50% tissue culture infective doses (TCID50)] or sublethal (5 × 104 TCID50) doses of influenza A virus (H1N1 A/PR/8/34) or 2.8 × 105 TCID50 of influenza A virus (H1N1 A/Yamagata/120/86) [25].
The lethal dose groups were used for observing survival rates, whereas the sublethal dose groups were used for virus replication and histological analysis, and mouse lungs were harvested at different times after the infection.
Hematoxylin and eosin (HE)-stained lung tissue sections were examined light-microscopically [26] for evidence of injury in a blinded manner by a skilled pathologist. The sections were evaluated for overall cellularity and consolidation, alveolar wall thickness, amount of edema fluid present and the number of neutrophils and macrophages infiltrating the airways (alveoli and bronchioles) and interstitium. Each parameter was scored subjectively from 0 to 5: 0 was considered normal or unaffected, and 5 indicated that there was a marked change [27]. Average values for the parameters were added to obtain the final inflammatory score for each group.
Preparation of FCNA-KO Mice
A construct targeting gene disruption (the FcnA gene) was produced by homologous recombination. The targeted ES cells (129SVJ) were implanted into mouse C57BL/6J blastocysts to generate chimeric mice. Chimeras were mated with wild-type C57BL/6J to produce heterozygous mice. Heterozygous mice were screened using PCR and Southern blot hybridization, and were backcrossed with C57BL/6J. Homozygous mice (FcnA–/–) were produced by the intercross of F2 heterozygous offspring. FcnA–/– was identified by PCR-based genotyping using tail DNA. PCR was performed using the primer set 5′-TACTATTTAGCCCTGACTGACTTGA-3′ (U1971) and 5′-TCTCCACCTTCCTCTTCCTCCTCTA-3′ (L3865) for the wild-type allele, and another set, U1971 and 5′-CATCGCCTTCTATCGCCTTCTTGACGA-3′ (NeoU1), for the mutant allele. All DNA recombination and animal studies were conducted in accordance with the guidelines of the Fukushima Medical University and the Osaka University.
Virus Capture Assay
For this vial assay, 100 µl of influenza virus stock (800 HA units determined by the hemagglutination assay) were coated onto 96-well plates and incubation was performed at 4°C overnight. After washing, recombinant GST-L-ficolin and GST were added separately to the wells. After incubation at 37°C for 1 h, the plate was washed and monoclonal anti-L-ficolin antibody GN5 (Hycult Biotechnology) at a 1/2,000 dilution was added. HRP-labeled conjugate (rabbit anti-mouse IgG) was added at 1/2,000 dilution. Tetramethylbenzidine solution was added for color development, and absorbance was read at 450 nm. The binding ability of L-ficolin to viruses was determined as a ratio: OD value (GST-L-ficolin + influenza virus)/OD value (GST + influenza virus).
Neutralization Assay
In order to measure the virus-neutralizing activity, L-ficolin was serially diluted with DMEM and then mixed with 100 µl of virus (H1N1 A/Yamagata/120/86) suspension (1.4 × 105 TCID50). Prior to inoculating into Madin-Darby canine kidney (MDCK) cells, the mixtures were incubated for 30 min at RT. After 2 h of incubation, the supernatants were discarded and MDCK cells were washed with DMEM. After 48 h of incubation, the infected MDCK cells were collected and subjected to fluorescence quantitative (FQ)-RT-PCR to detect the expression of the M gene of the H1N1 virus. GAPDH was used as the reference gene. To further identify the anti-H1N1 activities of L-ficolin, different concentrations of mouse monoclonal anti-human L-ficolin GN5 (HyCult Biotechnology) were added and incubated with 120 µM L-ficolin. Then the L-ficolin/mAb mixture was incubated with 100 µl of virus suspension for 30 min at RT before inoculating into MDCK cells. The neutralization activity by L-ficolin was determined in terms of inhibition (%): (M gene copies in the absence of L-ficolin – M gene copies with L-ficolin)/M gene copies in the absence of L-ficolin.
Stable Transfections
Mammalian CT26 colon tumor cells in 6-well plates were transfected with 2 µg of eukaryotic expression plasmid pCMV-Tag2B-HA or pCMV-Tag2B-NA, using Lipofectamine™ 2000 (Invitrogen) in accordance with the manufacturer's instructions. For stable transfections, G418 was added to the cell culture media at a final concentration of 0.6 mg/ml 48 h after transfection. The culture medium was exchanged every 2 days. After 2 weeks of selection, HA- or NA-expressing cell clones were obtained.
Expression Assay Using Flow Cytometry
Individual stably transfected CT26 cell clones were analyzed for HA and NA expression. A culture of 1 × 105 cells/ml was incubated at RT with mouse anti-flag antibodies (Invitrogen) for 30 min. The cells were washed three times in PBS to remove unbound antibodies, followed by the addition of FITC-labeled anti-mouse IgG, and cells were again incubated for an additional 30 min at RT. The stained cells were washed again three times and analyzed using a Beckman Coulter EPICS ALTRA II flow cytometer. All experiments were performed in triplicate.
GST-Pull-Down Assay
Lysates of HA-CT26 cells or NA-CT26 cells with the Sepharose 4B-GST-L-ficolin complex were incubated at 37°C for 30 min, followed by washing three times in PBS. SDS loading dye (2%) was added, and the samples were boiled at 100°C for 5 min. The supernatant was used to perform SDS-PAGE and Western blot analysis using anti-flag mAb, anti-HA mAb and anti-NA mAb (Sigma-Aldrich).
Hemagglutination Assay
Hemagglutination activity [28,29] was expressed as an HA titer. Mice lungs were homogenized in 1 µl 0.9% NaCl for every 0.1 g lung tissue. The homogenate was then centrifuged at 5,000 rpm for 4 min, and the supernatants were collected into new tubes and serially diluted with normal saline into a 96-well plate. Virus stock was used as the positive control, and normal saline was used as the negative control. Chicken erythrocytes were added to each well, and the incubation was conducted at RT for 40 min. The wells were visually inspected for the presence or absence of HA. All assays were performed in triplicate.
Plaque Assay
The plaque assay was used to determine the lung viral titer and TCID50. In this assay, a total of 100 µl of a serially diluted lung homogenate sample were added to a confluent monolayer of MDCK cells in 24-well plates and incubated at 37°C for 1 h. After virus adsorption, the cells were washed with serum-free DMEM and the wells were overlaid with 1 ml/well of DMEM (prepared from a 2× stock) containing 1.95% w/v agarose and 0.0005% trypsin. After incubation at 37°C in 5% CO2 for 5–7 days, cells were fixed, and then the agar overlays were removed. The fixed monolayers were stained with 0.1% crystal violet to visualize the plaques. The virus titer was calculated as the number of TCID50 per lung and was expressed as the mean ± SEM for each mouse group.
RNA Extraction and cDNA Synthesis
Total RNA was extracted using the Trizol-chloroform-based method [30], and cDNA was synthesized using a RevertAid™ first-strand synthesis kit (Fermentas Life Science), in accordance with the manufacturer's instructions. The retrotranscription step was performed for 60 min at 42°C in a final volume of 20 µl containing the following: 300 pg of total RNA, 200 U RevertAid- M-MuLV reverse transcriptase, 20 U RiboLock™ RNase inhibitor, 0.2 g of random hexamer primer and the deoxyribonucleotide mix (final concentration of 1 mM for each deoxyribonucleotide).
FQ-RT-PCR
FQ-RT-PCR was performed using a Rotor-Gene™ 3000 system (Corbett Research) with the Maxima™ SYBR Green qPCR master mix (2×) kit (Fermentas Life Science). Triplicate samples were analyzed according to the methods described by Gravelat et al. [31]. Influenza virus was quantified using a forward primer with the sequence 5′-CATGGAATGGCTAAAGACAAGACC-3′ and a reverse primer with the sequence 5′-AAGTGCACCAGCAGAATAACTGAG-3′ (i.e. specific for M gene of influenza A viruses). The reference gene GAPDH was quantified using a forward primer with the sequence 5′-ACCACAGTCCATGCCATCAC-3′ and a reverse primer with the sequence 5′-TCCACCACCCTGTTGCTGTA-3′. Cycle conditions were 15 min at 95°C, 40 cycles of 15 s at 95°C, 30 s at 56°C, 20 s at 72°C and 7 min at 72°C. Single PCR products were confirmed with the heat dissociation protocol at the end of the PCR cycles.
Complement C4 Activation and Deposition Assay
Lectin pathway activation was quantified using the C4 deposition assay as previously described [22]. MBL and ficolin-depleted serum was generated by incubating fresh healthy human donor serum with GlcNAc-agarose beads (Sigma). C4c deposition on the virus-coated plates was measured using the following method: 96-well microtiter plates were coated with 2 × 104 TCID50 of H1N1 virus in PBS buffer and were incubated overnight at 4°C. The plates were blocked with TBS buffer (10 mM Tris-Cl, 140 mM NaCl, 5 mM CaCl2, pH 7.4) containing 0.1% BSA at 37°C for 1 h and were subsequently washed twice in TBS/Tween-20 buffer (TBS, 0.05% Tween-20). After washing, the concentrations of purified recombinant L-ficolin protein and 20% human serum diluted in TBS buffer containing 0.1% BSA were added and incubated in buffer with TBS-T. After incubation at 4°C overnight, the plates were washed thoroughly with PBS; 0.1 g of purified human C4 protein (Diagnostic Biosystems) was then added, and the plates were incubated at 37°C for 1.5 h. The plates were washed again before adding FITC-conjugated rabbit anti human C4c (Diagnostic Biosystems; 1:100 dilution) and incubating at RT for 30 min. After washing with PBS, absorbance was measured at 485 nm.
Statistical Analysis
The data were analyzed with SPSS software. Experimental data were analyzed by ANOVA. Differences were considered to be statistically significant for p = 0.05.
Results
Human L-Ficolin Protected Mice from Influenza A Infection
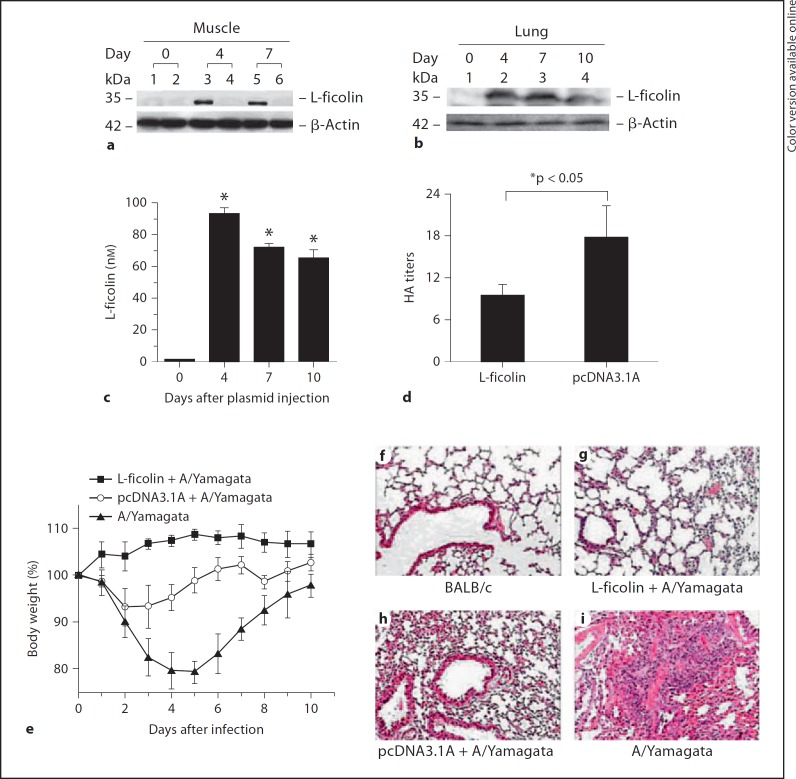
To demonstrate the roles of ficolin during influenza A virus infection in vivo, human L-ficolin cDNA was amplified and subcloned in frame into pcDNA3.1 (–)/Myc-His A and was administered intramuscularly to BABL/c mice by electroporation followed by an H1N1 virus (A/Yamagata/120/86) challenge. Before infection with the virus, we detected exogenous L-ficolin expression in BABL/c mice. As shown in figure 1a, 4 and 7days after plasmid injection, L-ficolin expression was detected in mouse muscle tissue. We also determined that the L-ficolin molecule was present in mouse lung tissue and serum after the injection of the L-ficolin-encoding plasmid (fig. 1b, c). L-ficolin was detected both in lung tissue and in serum starting 4 days after the plasmid injection. These data suggest that injection of the L-ficolin plasmid induced L-ficolin expression at the injection site and that the L-ficolin molecule was released into the circulation and lung tissue.
Fig. 1.
L-ficolin protects BABL/c mice against H1N1 (A/Yamagata/120/86) virus infection. Western blot analysis of L-ficolin in mouse muscle (a) and lung tissue (b) using the anti-L-ficolin mAb GN5. a BALB/c mice were injected with pcDNA3.1A-L-ficolin (lanes 1, 3 and 5) or control vector pcDNA3.1A (lanes 2, 4 and 6; 10 µg DNA per mouse) through intramuscular electroporation. Muscle was harvested from each mouse 0, 4 or 7 days after injection. b BALB/c mice were injected with pcDNA3.1A-L-ficolin and mouse lung was harvested from each mouse 0, 4, 7 or 10 days after injection. c L-ficolin concentrations in sera taken from mice injected with pcDNA3.1A-L-ficolin (* p < 0.05, day 0 group vs. other groups). d Viral hemagglutination assay of mouse lung tissue homogenates. Hemagglutination activity was expressed as a titer, i.e. the reciprocal of the highest dilution showing complete agglutination (* p < 0.05, L-ficolin group vs. pcDNA3.1A group). Data are shown as the mean ± SEM of at least 3 independent experiments. e A graph of the body weights of infected mice after the administration of L-ficolin plasmid treatment. f–i Histopathologic analyses of lung tissue. Lung tissue sections were analyzed using HE and were evaluated by light microscopy (×400).
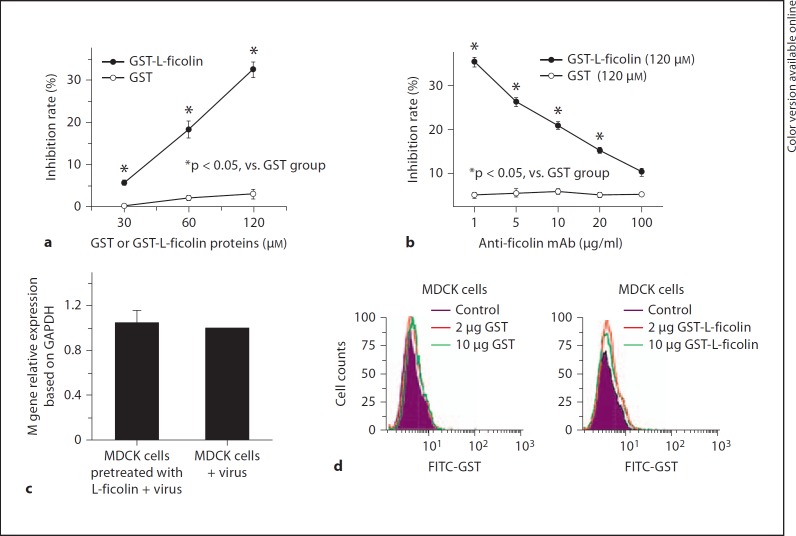
Fig. 4.
Neutralization of H1N1 A/Yamagata/120/86 infectivity. a Virus was incubated with L-ficolin for 30 min. The mixture was added to MDCK cells. The infected MDCK cells were collected for FQ-RT-PCR to detect the expression of the M gene of the H1N1 virus. The neutralization activity of L-ficolin was determined by percent inhibition: (M gene copies in the absence of L-ficolin – M gene copies with L-ficolin)/M gene copies in the absence of L-ficolin (* p < 0.05, L-ficolin + H1N1 virus vs. control group). This experiment was repeated 3 times. b FQ-RT-PCR analysis showed that neutralization effects of L-ficolin were reversed in cell culture by adding different doses of anti-L-ficolin mAb. FQ-RT-PCR (c) and flow-cytometric (d) analysis showed that L-ficolin did not bind to other elements or molecules on the cell surface and inhibition effects of L-ficolin on viral infection were not due to the binding of L-ficolin to MDCK cells. FITC anti-GST mAb was used in the flow-cytometric analysis.
A/Yamagata/120/86 is a variant of the A/PR/8/34 (H1N1) virus and can cause infection in the mouse [18]. The hemagglutination assay was used for measuring HA titers based on their ability to attach to molecules present on the surface of red blood cells. After infection with the A/Yamagata/120/86 virus (2.8 × 105 TCID50/mouse) [18], the HA titers of lung homogenates in the L-ficolin treatment group were significantly decreased compared with those injected with the empty vector (fig. 1d, * p < 0.05). All mice appeared hunched and had lost their appetite (classic flu-like symptoms) during the first 7 days after the challenge, but all had survived 10 days after the virus challenge. The A/Yamagata/120/86 virus did not cause severe disease in the mice, probably because humans are its natural host, but it did produce temporary flu-like symptoms. Loss of body weight did not occur in L-ficolin-treated mice (fig. 1e), whereas body weight was reduced in mice that did not receive L-ficolin treatment. Mice without L-ficolin treatment were found to be lethargic and exhibited other general disease-like symptoms, such as shivering (data not shown).
Histological analysis of the lung tissue, performed 6 days after infection, showed that the alveolar tissue of L-ficolin-treated mice appeared to be intact with only mild signs of alveolitis, a small amount of lymphocyte infiltration and a few red blood cells present in connective tissue. The mean pathology score was 2.3 (fig. 1g). In contrast, in the lungs of H1N1 virus-infected mice, multifocal, severe necrotizing broncho-interstitial pneumonia that was almost completely covering the lobes, and severe inflammatory cell infiltration was observed (fig. 1f–i; average pathology score, 4.8). These data suggest that L-ficolin protected BABL/c mice from A/Yamagata/120/86 virus infection. A moderate broncho-interstitial pneumonia was seen in the empty plasmid-treated group that was likely due to the empty vector injection nonspecifically enhancing the hosts' immunity against infection (fig. 1h; average pathology score, 3.7). In other studies, the control DNA vector pcDNA3.1 also elicited a low level of inflammatory response [32]. Moreover, studies conducted by Ma et al. [24] suggested that the pcDNA3.1 injection alone could introduce a certain level of interferon-γ, which is consistent with our results. We speculate that the pcDNA3.1A vehicle may contain bacterial DNA or a small amount of lipopolysaccharide/endotoxin. Bacterial DNA with a CpG structure and bacterial lipopolysaccharides, which are TLR9 and TLR4 ligands of mammalian antigen-presenting cells, respectively, could potentially stimulate the production of inflammatory factors.
Human L-Ficolin and Mouse FCNA Protect FCNA-KO Mice from Influenza A Virus Infection
To further assess the protective effects of L-ficolin against influenza A virus infection in FCNA-KO mice, both human L-ficolin and mouse FCNA were used.
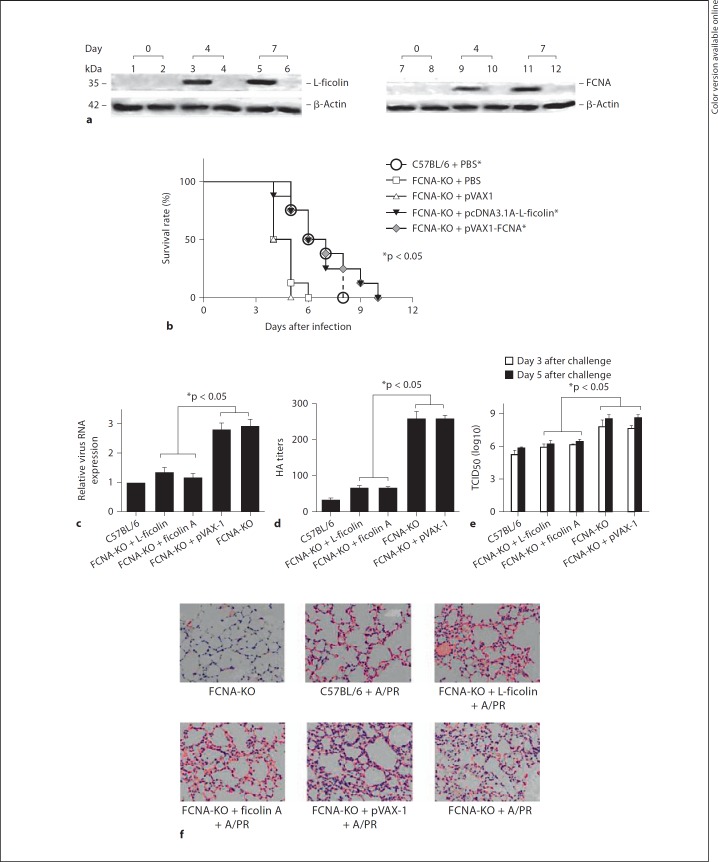
Before virus infection, 4 and 7 days after the intramuscular administration of pcDNA3.1-L-ficolin or pVAX-1-FCNA plasmids into mice using electroporation, the expression of both L-ficolin and FCNA was evaluated in mouse muscle tissue (fig. 2a). After the lethal infection with influenza virus H1N1 (A/PR/8/34), the FCNA-KO mice were more susceptible than the C57BL/6 (wild-type) mice (fig. 2b). Four days after the infection, 87.5 or 100% of the FCNA-KO mice that were injected with L-ficolin or FCNA survived, whereas only 50% of the FCNA-KO mice that were injected with PBS alone or empty vector survived (fig. 2b). Six days after infection, 50% of the FCNA-KO mice that were injected with L-ficolin and FCNA survived, whereas all of the FCNA-KO mice that were injected with PBS alone or empty vector died (fig. 2b). The L-ficolin and FCNA injection groups exhibited a prolonged survival time (T50) that was 2 days longer than that of the control or empty vector injection groups (fig. 2b). In a virus challenge using a sublethal dose, the administration of L-ficolin or FCNA plasmids significantly reduced the expression of viral RNA (fig. 2c) and the HA titer (fig. 2d) in FCNA-KO mice 5 days after infection. The TCID50 of mouse lung tissue 3 and 5 days after infection also showed less virus replication in L-ficolin- and FCNA-treated FCNA-KO mice compared with PBS-treated or empty vector-treated FCNA-KO mice (fig. 2e). Five days after infection, histological analysis of the lung tissue showed severe inflammation and significant lymphocyte infiltration in the FCNA-KO mice (average pathology score, 4.6), whereas mild inflammation and less lymphocyte infiltration occurred in the FCNA-KO mice administered the L-ficolin plasmid (average pathology score, 3.6) or its mouse analogue (FCNA plasmid; fig. 2f; average pathology score, 3.3). Our data suggest that the administration of L-ficolin and its analogue (FCNA plasmid) can, to some extent, reverse the pathology of influenza A virus infection in FCNA-KO mice.
Fig. 2.
L-ficolin protects FCNA-KO mice against H1N1 (A/PR/8/34) virus infection. a Western blot analysis of L-ficolin or FCNA expression in the muscle of FCNA-KO mice using monoclonal anti-L-ficolin antibody GN5 or polyclonal anti-FCNA antibody. Male FCNA-KO mice in a C57BL/6 background (8 weeks old) were injected with pcDNA3.1A-L-ficolin (lanes 1, 3 and 5), pcDNA3.1A (lanes 2, 4 and 6), pVAX-1-FCNA (lanes 7, 9 and 11) or pVAX-1 (lanes 8, 10 and 12; 10 µg DNA/mouse) through intramuscular electroporation. Muscle tissue was harvested from each mouse 0, 4 or 7 days after injection. b Survival rate of mice infected with H1N1 A/PR/8/34 (* p < 0.05, vs. FCNA-KO + PBS and FCNA-KO + pVAX-1). The Kaplan-Meier method was used to plot survival curves for each infected group of mice. A Breslow test was used for statistical analysis of the survival curves. Differences were considered to be statistically significant for p = 0.05. c Real-time RT-PCR analysis of viral RNA expression (* p < 0.05, FCNA-KO + L-ficolin or FCNA-KO + FCNA group vs. other FCNA-KO control groups). Data are shown as the mean ± SEM of at least 3 independent experiments. d Viral hemagglutination assays from mouse lung tissue homogenates 3 days after infection. Hemagglutination activity was expressed as HA titer, i.e. the reciprocal of the highest dilution showing complete agglutination (*p < 0.05, FCNA-KO + L-ficolin or FCNA-KO + FCNA group vs. other FCNA-KO control groups). Data are shown as the mean ± SEM of at least 3 independent experiments. e TCID50 of mouse lung tissue homogenates 3 and 5 days after infection (* p < 0.05, FCNA-KO + L-ficolin or FCNA-KO + FCNA group vs. other FCNA-KO control groups). The plaque assay was used to determine the lung viral titer and TCID50. f Histopathological analysis of lung tissue. The lung tissue sections were stained with HE and light-microscopically evaluated (×400).
Recombinant Human L-Ficolin Bound to Influenza A Virus
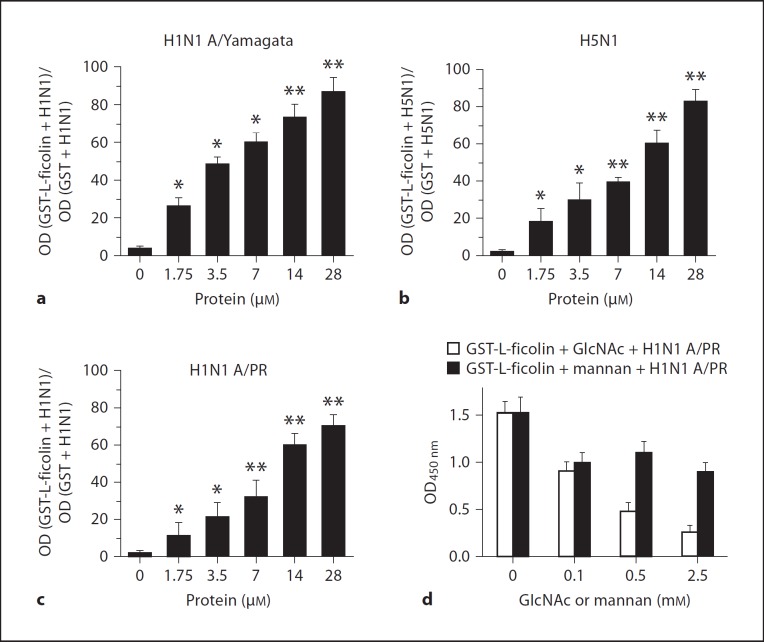
To assess whether recombinant L-ficolin can bind to influenza virus particles, a virus capture assay was employed. Virus samples, including H1N1 (A/Yamagata/120/86), H1N1 (A/PR/8/34) and H5N1 (A/chicken/Hubei/489/2004), were coated onto ELISA plates. Recombinant GST-L-ficolin or GST alone was added to the plates, and incubation was followed by extensive washing to remove any unbound protein. The bound viral particles were revealed using a HRP-labeled anti-L-ficolin mAb GN5, as described in the Materials and Methods. In order to minimize the interference from different ELISA plates, the binding ability of L-ficolin to the viruses was determined as a ratio: OD value (GST-L-ficolin + influenza virus)/OD value (GST + influenza virus). We found that GST-L-ficolin protein could bind significantly to 2 strains of H1N1 and to 1 strain of H5N1 virus when compared with the GST control groups (* p < 0.05, ** p < 0.01, fig. 3a–c). Increasing the amount of L-ficolin added to the plates resulted in an increase in the amount of L-ficolin bound to the virus-coated plates. Next, we attempted to disrupt these binding reactions using GlcNAc and mannan. The binding between GST-L-ficolin and H1N1 A/PR/8/34 was competitively inhibited by both GlcNAc and mannan (fig. 3d), and the inhibition was proportional to the concentration of these added sugars. This result is consistent with other reports that show L-ficolin binds to GlcNAc more strongly than it binds to mannan [33]. These data show that the L-ficolin can bind to different strains of influenza A virus, suggesting that L-ficolin may act as an innate immune molecule that recognizes the GlcNAc carbohydrate structure of the surface glycoproteins HA and NA of the influenza virus.
Fig. 3.
Recombinant L-ficolin bound to influenza virus particles. Influenza viruses (A/chicken/Hubei/489/2004, A/Yamagata/ 120/86 and A/PR/8/34) were coated onto 96-well plates, and recombinant GST-L-ficolin or GST was added. The binding ability of L-ficolin to the viruses was determined as a ratio: OD value (GST-L-ficolin + influenza virus)/OD value (GST + influenza virus). a Recombinant L-ficolin bound to H1N1 A/Yamagata/120/86 virus (** p < 0.01, * p < 0.05, 0 µM GST-L-ficolin group vs. various GST-L-ficolin concentration groups). b Recombinant L-ficolin bound to H5N1 A/chicken/Hubei/489/2004 virus (** p < 0.01, * p < 0.05, 0 µM GST-L-ficolin group vs. various L-ficolin-GST concentration groups). c Recombinant L-ficolin bound to H1N1 A/PR/8/34 virus (** p < 0.01, * p < 0.05, 0 µM GST-L-ficolin group vs. various GST-L-ficolin concentration groups). d Effects of GlcNAc and mannan on the binding between recombinant L-ficolin-GST and H1N1 A/PR/8/34 virus. 0.28 mM recombinant GST-L-ficolin or GST were added, along with GlcNAc or mannan, to a plate coated with H1N1 A/PR/8/34 virus. All data are shown as the mean ± SEM of at least 3 independent experiments.
Human L-Ficolin Inhibits Influenza A Virus Entry into MDCK Cells
To better understand the contribution of L-ficolin to virus entry, we used L-ficolin protein to interfere with H1N1 infection in vitro, and measured the neutralization activity of L-ficolin. MDCK cells were infected with H1N1 virus in the presence of L-ficolin, and FQ-RT-PCR was used to detect expression of the M gene of the H1N1 virus. As shown in figure 4a, H1N1 RNA replication was reduced after L-ficolin treatment compared with the GST control. A higher inhibition rate was observed when the L-ficolin concentration was increased. Further, the neutralization effects of L-ficolin could be reversed by adding different mAb doses against L-ficolin (fig. 4b). In addition, we incubated MDCK cells with L-ficolin recombinant protein, followed by performing the infections after having removed the L-ficolin from the media, and we observed that this treatment had no inhibition effects on the virus infection (fig. 4c). Flow-cytometric analysis also showed that different doses of L-ficolin did not bind to MDCK cells (fig. 4d). These data indicate that there are no interactions between L-ficolin and other elements or molecules on the cellular surface, and that L-ficolin can neutralize the influenza A virus infection and inhibit influenza A virus entry into MDCK cells.
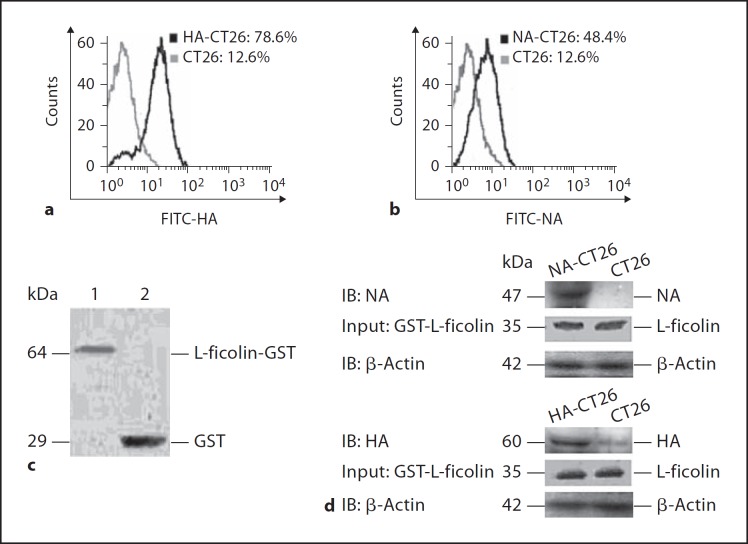
Recombinant Human L-Ficolin Specifically Recognizes and Binds to HA and NA
To explore the possible interaction between HA or NA proteins and L-ficolin, cDNAs encoding HA and NA were amplified from influenza A virus H5N1 A/chicken/Hubei/489/2004 using RT-PCR and were subcloned into the eukaryotic expression vectors, pCMV-Tag or pGEX-KG. The resulting recombinant pCMV-Tag-HA and pCMV-Tag-NA plasmids were transfected into CT26 cells using G418 to select for stable transfectants. We found that the surface expression of HA and NA in transfected CT26 cells was 78.6 and 48.4%, respectively, while the background fluorescence in control cells was only 12.6% (fig. 5a, b). Recombinant GST-L-ficolin was expressed in Escherichia coli under the induction of IPTG. The GST-L-ficolin protein was purified using glutathione Sepharose 4B, and its identity was confirmed using SDS-PAGE and Western blotting with an anti-GST antibody. The results showed that GST-L-ficolin and control GST had molecular weights of approximately 64 and 29 kDa, respectively (fig. 5c), which is consistent with the predicted sizes.
Fig. 5.
Binding assay between L-ficolin and HA or NA. Flow-cytometric analyses of HA expression in HA-CT26 (a) and NA expression in NA-CT26 (b) using anti-flag antibody. c Western blot analysis of recombinant GST-L-ficolin fusion proteins using anti-GST antibody. Lane 1 = GST-L-ficolin; lane 2 = GST. d Binding assay between L-ficolin and HA or NA by GST pull-down and Western blot analysis using anti-HA, anti-NA and anti-L-ficolin mAbs. β-Actin, a housekeeping gene with a constant expression, was used as an internal control.
A GST pull-down assay was performed to further demonstrate the physical association between L-ficolin and HA or NA. The HA-CT26 and NA-CT26 cell lysates were incubated with purified GST-L-ficolin, and complexes containing GST-L-ficolin-HA or GST-L-ficolin-NA were isolated using glutathione Sepharose 4B beads and analyzed using SDS-PAGE and Western blotting. The results confirmed that L-ficolin recognized and bound to HA and NA (fig. 5d). There was no binding detected between L-ficolin and the control CT26 cells lysates (fig. 5d).
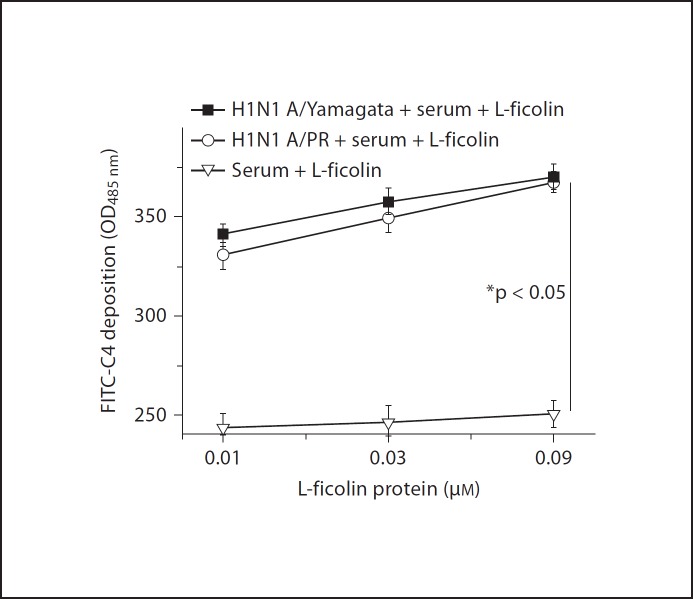
Binding of L-Ficolin to Influenza A Virus Triggers the Lectin Complement Pathway
We assessed whether the binding of L-ficolin to the influenza A virus in the presence of MBL-associated serine proteases from serum leads to the activation of the lectin pathway and C4 deposition. Activation of the lectin pathway was measured using C4 deposition assays (see Materials and Methods) in which the classical complement pathway was blocked by the use of 1 M NaCl. The high ionic strength of this buffer blocks the binding of C1q to immune complexes and disrupts the C1 complex. C4 is not involved in the alternative complement pathway. As shown in figure 6, in the presence of serum, higher levels of C4c deposition were observed after mixing L-ficolin with the influenza virus H1N1 A/PR/8/34 and A/Yamagata/120/86, when compared with the non-virus control group. Activation of the lectin pathway was L-ficolin dose dependent (fig. 6). The data indicate that the binding of L-ficolin to influenza A virus triggers the lectin complement pathway.
Fig. 6.
Binding of L-ficolin to influenza A virus leads to the activation of the complement lectin pathway.
Discussion
Influenza A viruses express two surface glycoproteins, HA and NA, which both play a critical role in the ability of the virus to replicate in susceptible target cells. HA is an envelope glycoprotein that mediates the attachment of virions to the cell surface and the fusion of the viral and endosomal membranes, thereby delivering the viral nucleocapsid into the cytoplasm to initiate viral replication [34]. The NA protein acts to cleave terminal N-acetyl neuraminic acid moieties from cell surface oligosaccharides and aids in the ability of newly synthesized virions to detach from infected cells and spread to neighboring target cells [35]. HA and NA have also been shown to serve as targets for recognition by C-type lectins of the collectin family, including serum MBL, bovine serum proteins conglutinin and collectin-43 and lung surfactant proteins (SP) A and SP-D [36]. Our results show that L-ficolin recognizes and binds to HA and NA glycoproteins (fig. 5). We also demonstrated that L-ficolin bound to both H1N1 and H5N1 in vitro by ELISA analysis (fig. 3a, c). We speculated that L-ficolin might bind to these differently glycosylated proteins from different strains (both H1 and H5). During the influenza challenge, both wild-type mice and KO mice that were injected with the L-ficolin plasmid had lower HA titers or less viral gene expression in lung homogenates and less pathological changes compared with other groups. These data demonstrated that L-ficolin protected the mice against influenza A virus infection. We speculate that following the injection of the L-ficolin plasmid, the L-ficolin molecule was produced and secreted into the circulation where it acted as an anti-influenza agent. The recombinant L-ficolin protein could bind to influenza A virus H1N1 A/Yamagata/120/86, H1N1 A/PR/8/34 and H5N1 A/chicken/Hubei/489/2004, and this binding could be disrupted by GlcNAc, and to a lesser extent by mannan, in vitro. More importantly, we are the first to demonstrate that in the presence of the influenza A virus, L-ficolin recognizes and binds to the envelope glycoproteins HA and NA directly and can trigger the lectin complement pathway. We propose that L-ficolin binds to HA and NA proteins to block virion entry and activates the lectin complement pathway, resulting in virus or virus-infected cell lysis.
Our ELISA and Western analyses showed that the L-ficolin concentration in the lung in the mouse model (fig. 1) was much lower than the 120 µM needed in vitro for 30% inhibition or neutralization in cell culture (fig. 4a). We speculate that the mechanisms inducing the anti-influenza response by L-ficolin may be more complicated in neutralization assays in vivo than in vitro. One possibility is that L-ficolin in vivo not only can neutralize virus infection but also can induce the activation of lectin complement in vivo, or might also activate phagocytosis to the virus-infected cells. Another possibility is that due to probable impurity of in vitro prepared L-ficolin recombinant protein, L-ficolin recombinant protein effects might be lower than its eukaryotic expression plasmid in vivo.
Leikina et al. [37] showed that carbohydrate-binding molecules inhibited viral fusion and entry by crosslinking membrane glycoproteins. Chang et al. [38] showed that recombinant chimeric lectins, consisting of an L-ficolin collagen domain and an MBL carbohydrate recognition domain, triggered a dose-dependent activation of the lectin complement pathway in the presence of influenza A virus, an observation that is consistent with our results.
It has been reported that other lectins, such as MBL and SP-D, bind to both HA and NA glycoproteins of the influenza virus with the majority of binding to the viral HA [39,40]. However, because there is a lack of mannosylated carbohydrates present on the envelope proteins of the A/PR/8/34/H1N1 strain, MBL and SP-D can bind only weakly to HA or NA and are unable to inhibit the infectivity of this strain of influenza [41]. However, our ELISA results demonstrate that L-ficolin can bind to the A/PR/8/34 virus, and this binding is attenuated in the presence of GlcNAc (fig. 3d). We speculate that the envelope proteins of the A/PR/8/34/H1N1 strain might contain GlcNAc carbohydrates but less mannosylated carbohydrates. L-ficolin can recognize the GlcNAc carbohydrate structure of the A/PR/8/34 virus. The interaction between the A/PR/8/34 virus and L-ficolin activated the lectin complement pathway (fig. 6).
Recently, we have shown that the L-ficolin protein recognizes and binds to the envelope glycoproteins E1 and E2 of HCV, thus activating the lectin complement pathway-mediated cytolytic activity in HCV-infected hepatocytes [22]. Krarup et al. [21] showed that L-ficolin binds to some capsulated Streptococcus pneumoniae serotypes. Cedzynski et al. [42] estimated serum L-ficolin concentrations in children with recurrent respiratory infections and predicted that high L-ficolin concentrations could confer enhanced protection from some infectious disorders. In this study, we found that the collectin molecule L-ficolin bound the envelope glycoproteins HA and NA and that L-ficolin enhanced mouse resistance to the influenza A virus. We hypothesize that L-ficolin, similar to other collectins, may inhibit influenza virus entry by binding to HA and NA, and may initiate not only the lectin pathway of complement activation but also other biological activities, such as the opsonization of pathogens. This report is the first to demonstrate that L-ficolin can directly bind to HA and NA and has a protective in vivo effect against the infection of influenza A viruses using FCNA-KO mice. Therefore, this study will contribute immensely to the limited information available regarding these highly pathogenic viruses and their relationship to the immune system.
Disclosure Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Acknowledgments
This work was supported by grants from the National Outstanding Youth Foundation of China (81025008), the 973 Program of China (2009CB522507), the National Natural Science Foundation of China (30921001 and 30800038), the National Special Fund of China for Important Infectious Disease (2012ZX10003-002), the Program for Changjiang Scholars and Innovative Research Team at University, the 211 program (303-581045), the Science and Technology Program of Wuhan and DFG Transregio 60. We thank Drs. Zhe Chen and Jianguo Wu for kindly providing H1N1 A/PR/8/34, H5N1 A/chicken/Hubei/489/2004 and H1N1 A/Yamagata/120/86.
References
- 1.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 2.Fujita T, Matsushita M, Endo Y. The lectin-complement pathway – its role in innate immunity and evolution. Immunol Rev. 2004;198:185–202. doi: 10.1111/j.0105-2896.2004.0123.x. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita M, Endo Y, Taira S, Sato Y, Fujita T. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–2454. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto R, Yae Y, Akaiwa M, Kitajima S, Shibata Y. Cloning and characterization of the Hakata antigen, a member of the ficolin/opsonin p35 lectin family. J Biol Chem. 1998;273:20721–20727. doi: 10.1074/jbc.273.33.20721. [DOI] [PubMed] [Google Scholar]
- 5.Endo Y, Sato Y, Matsushita M, Fujita T. Cloning and characterization of the human lectin P35 gene and its related gene. Genomics. 1996;36:515–521. doi: 10.1006/geno.1996.0497. [DOI] [PubMed] [Google Scholar]
- 6.Lynch NJ, Roscher S, Hartung T, Morath S, Matsushita M. L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol. 2004;172:1198–1202. doi: 10.4049/jimmunol.172.2.1198. [DOI] [PubMed] [Google Scholar]
- 7.Nahid AM, Sugii S. Binding of porcine ficolin-alpha to lipopolysaccharides from Gram-negative bacteria and lipoteichoic acids from Gram-positive bacteria. Dev Comp Immunol. 2006;30:335–343. doi: 10.1016/j.dci.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XL, Ali MA. Ficolins: structure, function and associated diseases. Adv Exp Med Biol. 2008;632:105–115. [PubMed] [Google Scholar]
- 9.Runza VL, Schwaeble W, Mannel DN. Ficolins: novel pattern recognition molecules of the innate immune response. Immunobiology. 2008;213:297–306. doi: 10.1016/j.imbio.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Kwon S, Kim MS, Kim D, Lee KW, Choi SY. Identification of a functionally relevant signal peptide of mouse ficolin A. J Biochem Mol Biol. 2007;40:532–538. doi: 10.5483/bmbrep.2007.40.4.532. [DOI] [PubMed] [Google Scholar]
- 11.Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikramaratna PS, Gupta S. Influenza outbreaks. Cell Microbiol. 2009;11:1016–1024. doi: 10.1111/j.1462-5822.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith JH, Nagy T, Barber J, Brooks P, Tompkins SM. Aerosol inoculation with a sub-lethal influenza virus leads to exacerbated morbidity and pulmonary disease pathogenesis. Viral Immunol. 2011;24:131–142. doi: 10.1089/vim.2010.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghedin E, Sengamalay NA, Shumway M, Zaborsky J, Feldblyum T. Large-scale sequencing of human influenza reveals the dynamic nature of viral genome evolution. Nature. 2005;437:1162–1166. doi: 10.1038/nature04239. [DOI] [PubMed] [Google Scholar]
- 15.McHardy AC, Adams B. The role of genomics in tracking the evolution of influenza A virus. PLoS Pathog. 2009;5:e1000566. doi: 10.1371/journal.ppat.1000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taira S, Kodama N, Matsushita M, Fujita T. Opsonic function and concentration of human serum ficolin/P35. Fukushima J Med Sci. 2000;46:13–23. doi: 10.5387/fms.46.13. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita M, Fujita T. Ficolins and the lectin complement pathway. Immunol Rev. 2001;180:78–85. doi: 10.1034/j.1600-065x.2001.1800107.x. [DOI] [PubMed] [Google Scholar]
- 18.Asahi Y, Yoshikawa T, Watanabe I, Iwasaki T, Hasegawa H. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol. 2002;168:2930–2938. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- 19.Scull MA, Gillim-Ross L, Santos C, Roberts KL, Bordonali E. Avian influenza virus glycoproteins restrict virus replication and spread through human airway epithelium at temperatures of the proximal airways. PLoS Pathog. 2009;5:e1000424. doi: 10.1371/journal.ppat.1000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Wang CY, Yang ST, Qin C, Hu JL. Inhibition of highly pathogenic avian influenza virus H5N1 replication by the small interfering RNA targeting polymerase A gene. Biochem Biophys Res Commun. 2009;390:421–426. doi: 10.1016/j.bbrc.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Krarup A, Sorensen UB, Matsushita M, Jensenius JC, Thiel S. Effect of capsulation of opportunistic pathogenic bacteria on binding of the pattern recognition molecules mannan-binding lectin, L-ficolin, and H-ficolin. Infect Immun. 2005;73:1052–1060. doi: 10.1128/IAI.73.2.1052-1060.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Ali MA, Shi Y, Zhao Y, Luo F. Specifically binding of L-ficolin to N-glycans of HCV envelope glycoproteins E1 and E2 leads to complement activation. Cell Mol Immunol. 2009;6:235–244. doi: 10.1038/cmi.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujimori Y, Harumiya S, Fukumoto Y, Miura Y, Yagasaki K. Molecular cloning and characterization of mouse ficolin-A. Biochem Biophys Res Commun. 1998;244:796–800. doi: 10.1006/bbrc.1998.8344. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Chen H, Wang Q, Luo F, Yan J. IL-24 protects against Salmonella typhimurium infection by stimulating early neutrophil Th1 cytokine production, which in turn activates CD8+ T cells. Eur J Immunol. 2009;39:3357–3368. doi: 10.1002/eji.200939678. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Matsuo K, Asanuma H, Takahashi H, Iwasaki T. Enhanced protection against a lethal influenza virus challenge by immunization with both hemagglutinin and neuraminidase-expressing DNAs. Vaccine. 1999;17:653–659. doi: 10.1016/s0264-410x(98)00247-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang QL, Pan Q, Ma Y, Wang K, Sun P. An attenuated Salmonella-vectored vaccine elicits protective immunity against Mycobacterium tuberculosis. Vaccine. 2009;27:6712–6722. doi: 10.1016/j.vaccine.2009.08.096. [DOI] [PubMed] [Google Scholar]
- 27.Sukumar N, Sloan GP, Conover MS, Love CF, Mattoo S. Cross-species protection mediated by a Bordetella bronchiseptica strain lacking antigenic homologs present in acellular pertussis vaccines. Infect Immun. 2010;78:2008–2016. doi: 10.1128/IAI.01142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee GC, Jeon ES, Kim WS, Le DT, Yoo JH. Evaluation of a rapid diagnostic test, NanoSign® Influenza A/B Antigen, for detection of the 2009 pandemic influenza A/H1N1 viruses. Virol J. 2010;7:244. doi: 10.1186/1743-422X-7-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McSharry JJ, Weng Q, Brown A, Kulawy R, Drusano GL. Prediction of the pharmacodynamically linked variable of oseltamivir carboxylate for influenza A virus using an in vitro hollow-fiber infection model system. Antimicrob Agents Chemother. 2009;53:2375–2381. doi: 10.1128/AAC.00167-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drakulovski P, Carcy B, Moubri K, Carret C, Depoix D. Antibodies raised against Bcvir15, an extrachromosomal double-stranded RNA-encoded protein from Babesia canis, inhibit the in vitro growth of the parasite. Infect Immun. 2003;71:1056–1067. doi: 10.1128/IAI.71.3.1056-1067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gravelat FN, Doedt T, Chiang LY, Liu H, Filler SG. In vivo analysis of Aspergillus fumigatus developmental gene expression determined by real-time reverse transcription-PCR. Infect Immun. 2008;76:3632–3639. doi: 10.1128/IAI.01483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verfaillie T, Cox E, Goddeeris BM. Immunostimulatory capacity of DNA vaccine vectors in porcine PBMC: a specific role for CpG-motifs? Vet Immunol Immunopathol. 2005;103:141–151. doi: 10.1016/j.vetimm.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Lu J, Teh C, Kishore U, Reid KB. Collectins and ficolins: sugar pattern recognition molecules of the mammalian innate immune system. Biochim Biophys Acta. 2002;1572:387–400. doi: 10.1016/s0304-4165(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 34.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 35.Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol. 2002;12:159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- 36.Reading PC, Tate MD, Pickett DL, Brooks AG. Glycosylation as a target for recognition of influenza viruses by the innate immune system. Adv Exp Med Biol. 2007;598:279–292. doi: 10.1007/978-0-387-71767-8_20. [DOI] [PubMed] [Google Scholar]
- 37.Leikina E, Delanoe-Ayari H, Melikov K, Cho MS, Chen A. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat Immunol. 2005;6:995–1001. doi: 10.1038/ni1248. [DOI] [PubMed] [Google Scholar]
- 38.Chang WC, Hartshorn KL, White MR, Moyo P, Michelow IC. Recombinant chimeric lectins consisting of mannose-binding lectin and L-ficolin are potent inhibitors of influenza A virus compared with mannose-binding lectin. Biochem Pharmacol. 2011;81:388–395. doi: 10.1016/j.bcp.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Job ER, Deng YM, Tate MD, Bottazzi B, Crouch EC. Pandemic H1N1 influenza A viruses are resistant to the antiviral activities of innate immune proteins of the collectin and pentraxin superfamilies. J Immunol. 2010;185:4284–4291. doi: 10.4049/jimmunol.1001613. [DOI] [PubMed] [Google Scholar]
- 40.Hartshorn KL, White MR, Voelker DR, Coburn J, Zaner K. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem J. 2000;351:449–458. [PMC free article] [PubMed] [Google Scholar]
- 41.van Eijk M, White MR, Batenburg JJ, Vaandrager AB, van Golde LM. Interactions of influenza A virus with sialic acids present on porcine surfactant protein D. Am J Respir Cell Mol Biol. 2004;30:871–879. doi: 10.1165/rcmb.2003-0355OC. [DOI] [PubMed] [Google Scholar]
- 42.Cedzynski M, Nuytinck L, Atkinson AP, St Swierzko A, Zeman K. Extremes of L-ficolin concentration in children with recurrent infections are associated with single nucleotide polymorphisms in the FCN2 gene. Clin Exp Immunol. 2007;150:99–104. doi: 10.1111/j.1365-2249.2007.03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]