Abstract
Antimicrobial peptides (AP) are important components of the innate immune system, yet little is known about their expression and function in the brain. Our previous work revealed upregulated gene expression of cathelicidin-related AP (CRAMP) following bacterial meningitis in primary rat glial cells as well as bactericidal activity against frequent meningitis-causing bacteria. However, the effect of cathelicidin expression on the progression of inflammation and mortality in bacterial meningitis remains unknown. Therefore, we used CRAMP-deficient mice to investigate the effect of CRAMP on bacterial growth, inflammatory responses and mortality in meningitis. Meningitis was induced by intracerebral injection of type 3 Streptococcus pneumoniae. The degree of inflammation was analyzed in various brain regions by means of immunohistochemistry and real-time RT-PCR. CRAMP deficiency led to a higher mortality rate that was associated with increased bacterial titers in the cerebellum, blood and spleen as well as decreased meningeal neutrophil infiltration. CRAMP-deficient mice displayed a higher degree of glial cell activation that was accompanied by a more pronounced proinflammatory response. Taken together, this work provides insight into the important role of CRAMP as part of the innate immune defense against pathogens in bacterial CNS infections. © 2013 S. Karger AG, Basel
Key Words: Antimicrobial peptides, Bacterial meningitis, Cathelicidin, CRAMP, Glial cells, Innate immunity, Streptococcus pneumoniae
Introduction
Bacterial meningitis is a neurological emergency that is still associated with a high mortality rate and long-term sequelae. Streptococcus pneumoniae is the leading cause of meningitis in adults [1, 2, 3]. Despite advances in medical management, childhood vaccination and the development of effective antimicrobial agents, mortality ranges from 16 to 37% [4], and 1 of 3 survivors suffer from neurological sequelae such as epileptic seizures, hearing loss, cognitive impairment and focal neurological deficits [5, 6]. Therefore, advances in its treatment are urgently needed. During the past decades, experimental animal models have shown that the outcome of bacterial meningitis is related to the severity of inflammation in the subarachnoid space. This reaction is characterized by an intensive inflammatory response of the meninges and the subarachnoid space which results in a breakdown of the blood-brain barrier. Consequently, large numbers of neutrophils are recruited to the cerebrospinal fluid, which liberate proinflammatory and chemotactic agents, thereby exaggerating and perpetuating the inflammatory reaction. Among the factors released by neutrophils are numerous cytotoxic agents such as oxidants that can cause collateral damage of the brain tissue [7].
Glial cells such as astrocytes and microglia are also involved in pathogen recognition and the activation of innate immune responses. They are the key regulators of innate immune responses within the brain because of their ability to release various factors supporting the immune defense of the host and helping to coordinate the activation of different immune cells [8]. Astrocytes are furthermore required for structural support and the maintenance of the blood-brain barrier. Microglial cells are considered to be CNS-resident macrophages and sensor cells that function as principal innate immune effector cells [9].
The activation of the innate immune system of the CNS leads to the synthesis of antimicrobial peptides (AP) [10], which have distinct anti-infective and proinflammatory properties [11]. Our own previous investigations revealed increased levels of the AP cathelicidin LL-37 and psoriasin in the cerebrospinal fluid of patients suffering from bacterial meningitis [12, 13]. In humans, one gene is known for cathelicidin. The mature peptide LL-37 is cleaved from a precursor peptide [14]. Furthermore, bacterial infection and the stimulation of glial cells by bacterial supernatants and virulence factors resulted in increased expression of the rat cathelicidin-related AP (CRAMP) by astrocytes and microglia as well as meningeal cells in vivo and in vitro [12, 15]. CRAMP and rat CRAMP are the active peptides from the only known cathelicidin gene present in mice [16] and rats [17], respectively.
This study was designed to determine the role of CRAMP in inflammation and mortality in a mouse model of pneumococcal meningitis. We determined bacterial growth and mortality of infected CRAMP-deficient and wild-type (WT) mice and characterized the inflammatory response by evaluating neutrophil infiltration, glial cell activation and cytokine/chemokine expression by means of immunohistochemistry and real-time RT-PCR. Furthermore, basal gene expression of inflammatory candidates was determined by PCR array analysis in CRAMP-deficient and WT mice.
Materials and Methods
Mouse Model of Experimental Pneumococcal Meningitis [18]
Male CRAMP-WT and CRAMP-deficient (knockout, KO) mice (weight 19-23 g, age 2-3 months; CRAMP-WT mice were backcrossed on the C57BL/6J background for at least 10 generations [19]) were anesthetized with ketamine (100 mg/kg) and xylazine (20 mg/kg), infected by injecting 104 colony-forming units (CFU) of an S. pneumoniae type 3 strain in the subarachnoid space through the right frontolateral skull. Control animals without infection were injected with 10 μl of a sterile saline solution. All infected mice developed clinical signs of infection within 24 h. The clinical state and survival time was closely monitored. In a second set of experiments with the same infection regime, male CRAMP-KO and -WT mice were infected and sacrificed 36 h after induction of meningitis without treatment with antibiotics and were either perfused with 4% formalin for immunohistochemical analysis or with 0.9% NaCl solution for RNA isolation. Bacterial titers were evaluated 36 h after infection in tissue homogenates of the cerebellum and spleen and in blood samples by plating 10-fold dilutions on blood agar plates and incubation for 24 h at 37°C with 5% CO2 (detection limit 102 CFU/ml in tissue homogenates and 103 CFU/ml in blood samples) [20]. All animal experiments were approved by the Animal Care Committee of the University Hospital of Aachen and by the District Government in Recklinghausen, North Rhine-Westphalia, Germany.
Meningeal Inflammation Score
Meningeal inflammation was estimated by the invasion of granulocytes into the frontal interhemispheric region, the whole hippocampal fissure (both sides), 3 superficial meningeal regions over the convexities and the third ventricle (complete distribution). One high-power field (diameter: 250 mm) was scored in each region: no granulocytes = 0; <10 granulocytes = 1; 10-50 granulocytes = 2, and >50 granulocytes = 3. The scores of the individual regions were added (range of the score: 0-21) [21].
RNA Isolation and Real-Time RT-PCR and PCR Array
Total RNA was isolated using the peqGold Trifast reagent (Peqlab, Erlangen, Germany) according to the manufacturer's instructions. RNA samples were reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Fermentas, Burlington, Ont., Canada) and random hexamer primers (Invitrogen, Darmstadt, Germany). The cDNA products were used immediately for SYBR green (Applied Biosystems, Darmstadt, Germany) real-time RT-PCR. Gene expression was monitored using the StepOne Plus apparatus (Applied Biosystems) according to manufacturer's protocol [22]. Relative quantification was performed using the ΔCt method which results in ratios between target genes and a housekeeping reference gene (TATA box binding protein). The primer for glial fibrillary acid protein (GFAP), integrin α M (Itgam) and β-defensin 4 (Defb4) were provided by Qiagen (QT00101143, QT00156471 and QT00257656; QuantiTect Primer Assay; Qiagen, Hilden, Germany). The primers for caspase-1, caspase-4, interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α) were manufactured by Eurofins MWG Operon (Ebersberg, Germany; please refer to table 1 for primer sequences). The specificity of the amplification reaction was determined by melting curve analysis. For PCR array analysis, we used the RT2 Profiler PCR Array Mouse Innate and Adaptive Immune Response (PAMM-052A; Qiagen) according to manufacturer's protocol.
Table 1.
Primer sequences for real-time RT-PCR gene analysis
| Primer | Sequence | Annealing temperature, ° C | |
|---|---|---|---|
| mTBP | forward reverse |
5′-AGAACAATCCAGACTAGCAGCA-3′ 5′-GGGAACTTCACATCACAGCTC-3′ |
58 |
| mCaspase-1 | forward reverse |
5′-GCTTCAATCAGCTCCATCAGC-3′ 5′-CAGTCAGTCCTGGAAATGTGCC-3 ‘ |
57 |
| mCaspase-4 | forward reverse |
5′-TAGACTCATTTCCTGCTTCCGG-3′ 5′-ATATCTCGTCAAGGTTGCCCG-3′ |
57 |
| mIL-1β | forward reverse |
5′-TCTGGGATCCTCTCCAGCCAAGC-3′ 5′-AAGCAGCCCTTCATCTTTTGGGGT-3′ |
58 |
| mIL-6 | forward reverse |
5′-TGCAAGAGACTTCCATCCAGTTGCC-3′ 5′-AAGCCTCCGACTTGTGAAGTGGT-3′ |
59 |
| mTNF-α | forward reverse |
5′-AGGGGCCACCACGCTCTTCT-3′ 5′-TGGTTTGCTACGACGTGGGCT-3′ |
59.5 |
Immunohistochemistry
Formalin-fixed and paraffin-embedded 5-μm whole coronary brain sections were examined. For immunofluorescence staining, sections were deparaffinized, pretreated for 3 × 7 min with microwaves in citric acid buffer, permeabilized with 0.1% Triton X in PBS for 10 min at room temperature and after blocking with 1.5% BSA in Tris incubated with either monoclonal mouse anti-GFAP (1:250; ab10062; Abcam, Cambridge, UK) or polyclonal goat anti-ionized calcium binding adaptor molecule 1 (Iba1; 1:100; ab5076; Abcam) overnight at 4°C. This was followed by incubation with the biotinylated secondary antibody (1:400; DAKO, Hamburg, Germany) and peroxidase-labeled streptavidin-biotin staining. For staining, aminoethyl carbazole was used. After counterstaining with hematoxylin, the slides were finally mounted with Aquatex (Boehringer, Mannheim, Germany). For neutrophil granulocyte staining, slices were stained with naphthol AS-D chloroacetate esterase (91-C Kit, Sigma-Aldrich, Taufkirchen, Germany) according to the manufacturer's protocol. As a negative control, primary antibodies were omitted.
Quantification of Immunoreactive Cells
Sections were examined blinded using objectives from ×2 up to ×60. Only immunoreactive cells within the granule cell layer and subgranular zone of the dentate gyrus were counted. An analysis software imaging system (microscope BX51; Olympus; software AnalySIS 3.2; Soft Imaging System GmbH, Münster, Germany) was used to measure the area of the dentate granule cell layer. The densities of immunolabeled cells were expressed as the number of marked cells per square millimeter of the area measured. The density of labeled cells was evaluated in 3 coronal sections from each mouse. Immunohistochemical data were expressed as medians and interquartile ranges. For statistical comparisons, the nonparametric Mann-Whitney U test was used.
Statistical Analysis
Survival time was expressed in hours and evaluated by generating a Kaplan-Meier plot that was statistically analyzed using the log-rank test. Bacterial titers were converted into log CFU/ml and compared by nonparametric U test. All real-time RT-PCR experiments were performed in duplicate. The values were expressed as means ± SEM. For statistical comparisons, ANOVA followed by a Bonferroni multiple comparison test was used. A value of p < 0.05 was considered statistically significant. For statistical calculation, GraphPad Prism 5.0 was used (GraphPad Software, San Diego, Calif., USA).
Results
CRAMP Deficiency Resulted in Increased Bacterial Burden, Decreased Neutrophil Infiltration and Increased Mortality in Pneumococcal Meningitis
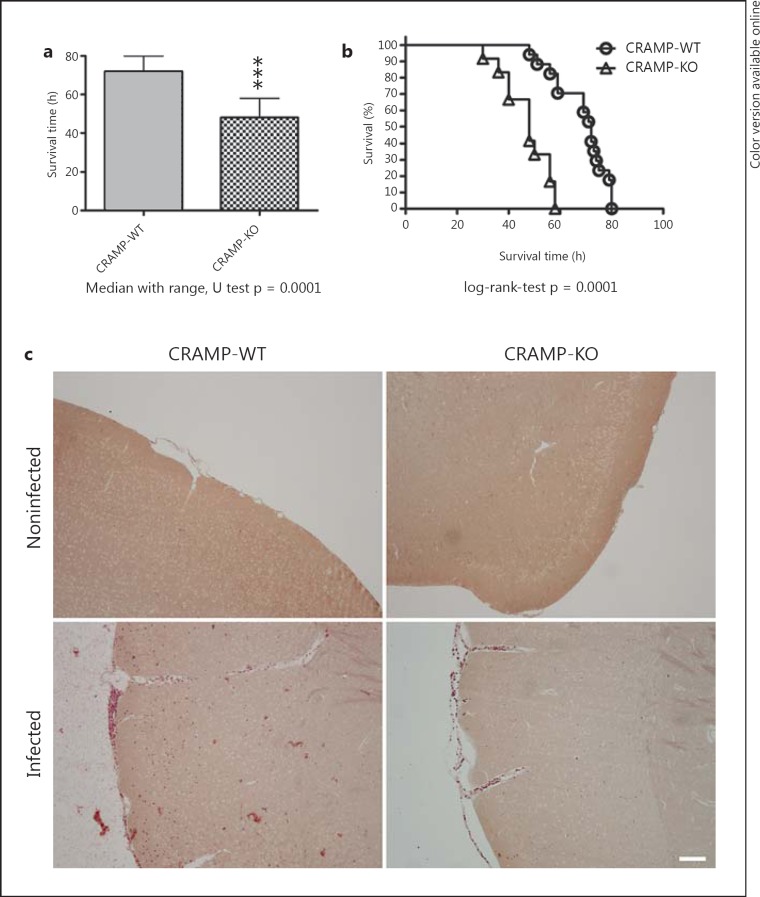
In a first set of experiments, mortality in pneumococcal meningitis was investigated. The survival time after infection was significantly shorter in CRAMP-deficient (KO) compared to WT mice (47.1 ± 8.8 vs. 68.6 ± 10.3 h, p = 0.0001; Mann-Whitney U test; fig. 1a). Fifty hours after infection, 94.1% of the WT animals were still alive compared to 33.3% of CRAMP-KO mice (fig. 1b). In addition, we determined bacterial titers in homogenates of the cerebellum and spleen and in blood samples from a separate set of CRAMP-KO and -WT mice 36 h after infection (no treatment with antibiotics). The results showed that CRAMP-KO animals had a statistically significantly higher bacterial burden in all investigated tissue compartments compared to WT mice (table 2). Furthermore, neutrophil granulocyte invasion was histochemically evaluated by chloroacetate esterase staining. Forty-eight hours after infection, WT mice displayed a higher degree of neutrophil granulocyte infiltration than CRAMP-deficient mice (fig. 1c). To verify the results, meningeal inflammation was determined by a semiquantitative score: a strong granulocytic invasion of the meninges was observed in all infected mice 48 h after inoculation, whereas infiltration of granulocytes was absent in noninfected animals. For CRAMP-WT mice, the meningeal inflammation score was 14 ± 1.6 (n = 6), whereas for CRAMP-KO mice the score was 9 ± 2.5 (n = 6). The difference between infected CRAMP-KO and -WT mice was significant (p = 0.0357, Mann-Whitney U test).
Fig. 1.
Mortality in pneumococcal meningitis. CRAMP-WT and CRAMP-KO mice were infected by injection of 104 CFU of type 3 S. pneumoniae in the subarachnoid space.a Comparison of survival times revealed that CRAMP-WT mice lived significantly longer (CRAMP-WT, n = 17; CRAMP-KO, n = 13; medians and ranges; *** p = 0.0001; Mann-Whitney U test). b Mortality was significantly increased in CRAMP-KO animals (Kaplan-Meier curve; p = 0.0001, log-rank test). c Detection of neutrophil granulocytes by the naphthol AS-D chloroacetate esterase reaction in the meninges and parenchyma revealing neutrophil infiltration in CRAMP-WT and -KO mice 48 h after pneumococcal infection. The figures show representative results from 1 of 3 mice per group (scale bar = 100 μm).
Table 2.
Bacterial titers after pneumococcal meningitis
| Bacterial titer, log CFU/ml |
|||
|---|---|---|---|
| cerebellum | blood | spleen | |
| CRAMP-WT | 5.7 (5.1/6.2) | 4.28 (3.8/5.39) | 4.92 (2.9/5.2) |
| CRAMP-KO | 6.54 (6/7.5)* | 6 (5.1/7)* | 5.5 (5.1/6)* |
Data are presented as medians (25th/75th percentiles). Please note significantly increased bacterial titers in infected CRAMPKO compared to infected CRAMP-WT mice. n = 8/group; * p = 0.0117 (cerebellum), 0.0113 (blood) and 0.0384 (spleen); Mann-Whitney U test.
Increased Glial Cell Activation following Pneumococcal Meningitis in CRAMP-Deficient Mice
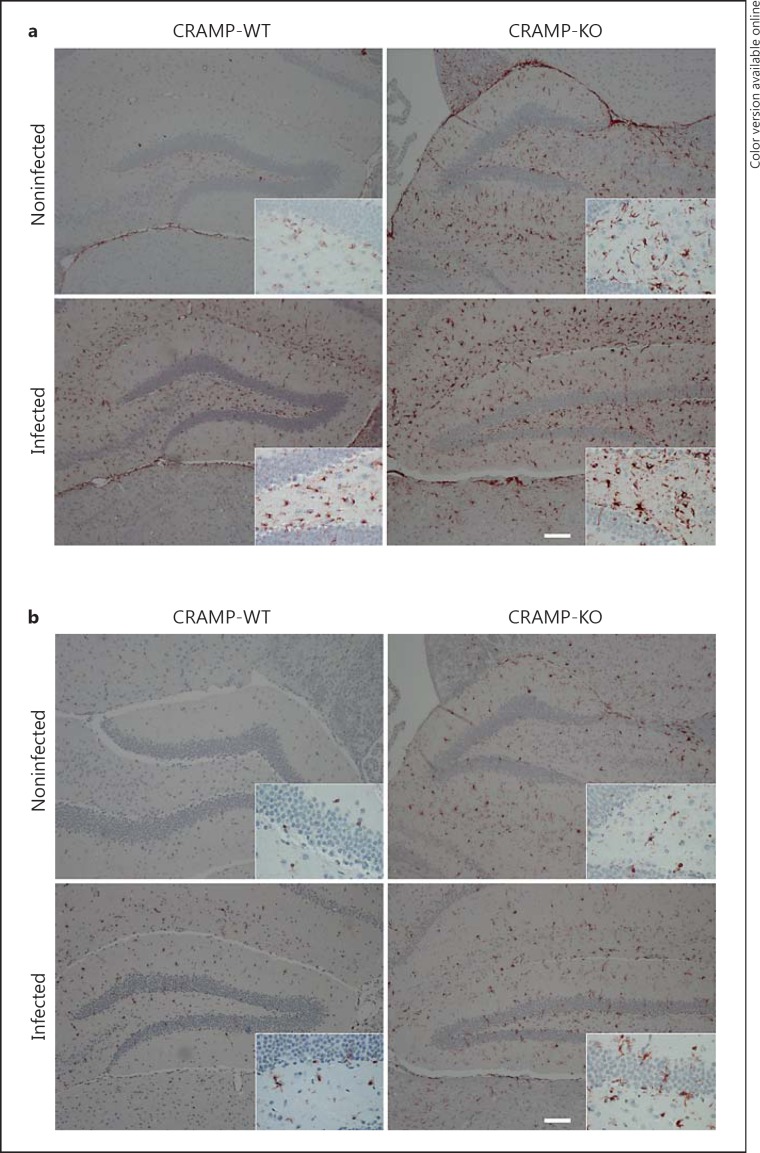
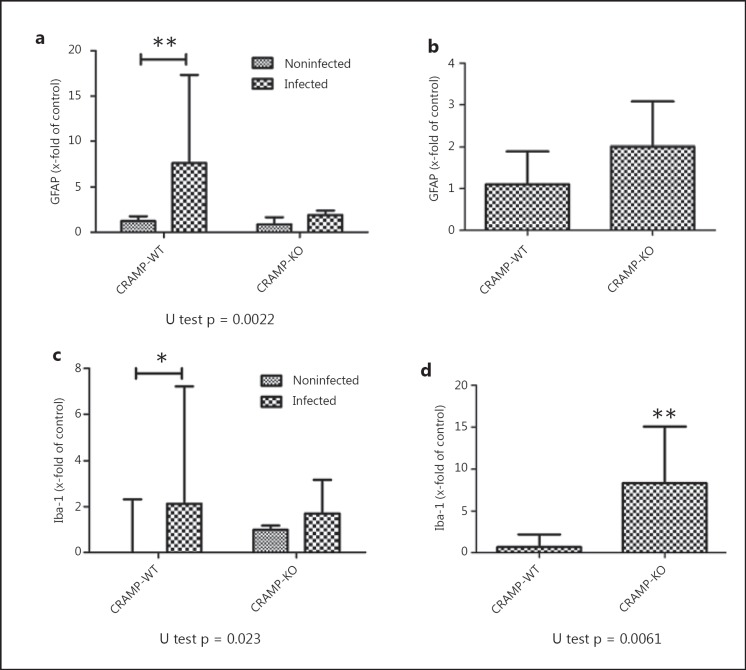
Thirty-six hours after infection, the degree of glial cell activation was determined by staining with GFAP, a marker of astrocytes, and Iba-1, a marker for activated microglia, in the hippocampal formation as a clearly definable brain region especially involved in the pathogenesis of bacterial meningitis. As shown in figure 2, the hippocampal formation of noninfected WT mice showed only very few GFAP- or Iba-1-positive cells, whereas infection with S. pneumoniae resulted - as expected - in a strong increase in the number of GFAP- or Iba-1-immunoreactive cells. Interestingly, healthy CRAMP-KO mice already displayed a high basal rate of GFAP-positive cells in the hippocampal formation in comparison to CRAMP-WT animals. Quantification of astrocytes in the subgranular zone and the granule cell layer of the dentate gyrus revealed significantly more astrocytes (n/mm2) in CRAMP-WT mice exposed to S. pneumoniae than in NaCl-treated WT mice [noninfected CRAMP-WT 1.24 (0.13/1.75)/mm2 vs. infected WT 7.65 (6.88/11.81)/mm2; medians (25th/75th percentiles), Mann-Whitney U test, p = 0.0022; fig. 3a]. Induction of bacterial infection in the CRAMP-KO mice did not result in a significant increase in GFAP-immunoreactive cells in comparison to noninfected CRAMP-KO [noninfected CRAMP-KO 0.87 (0.63/1.5)/mm2 vs. infected KO 1.9 (1.38/2.29)/mm2; medians (25th/75th percentiles), Mann-Whitney U test, p = 0.067; fig. 3a]. Next, the density of GFAP-immunoreactive cells of the dentate gyrus of infected CRAMP-KO mice was normalized to infected WT mice. Previously, basal expression of astrocytes of uninfected mice was subtracted. The results showed no significant difference between infected WT and infected CRAMP-KO mice [2.01 (0.84/2.88)-fold increase; median (25th/ 75th percentile), p = 0.3810, Mann-Whitney U test; fig. 3b].
Fig. 2.
Glial cell activation in the hippocampus after pneumococcal meningitis. Coronal brain sections stained with anti-GFAP to discover astrocytes (a) or anti-Iba-1 to identify activated microglial cells (b) 36 h after infection with S. pneumoniae. The figures show representative results from 1 of 3 independent experiments (scale bar = 100 μm). A detailed section was included. Please note that noninfected CRAMP-KO mice showed a stronger endogenous GFAP and Iba-1 immunoreactivity compared to noninfected WT mice.
Fig. 3.
Quantification of glial cells in the dentate gyrus of the hippocampus after pneumococcal meningitis. GFAP- and Iba-1-immunoreactive cells were quantified per mm2 area of the subgranular and granule cell layer of the dentate gyrus (see results) of infected and healthy CRAMP-KO and -WT mice 36 h after induction of meningitis. Immunoreactive cells were normalized to noninfected controls and fold increases are presented in the graph (a, c). Data from 4 mice/group are presented as medians with ranges/mm2. Density of GFAP (b)- and Iba-1 (d)-immunoreactive cells within the subgranular and granule cell layer of the dentate gyrus of infected CRAMP-KO mice were normalized to infected -WT mice. Previously, basal expression of astrocytes or microglial cells of the uninfected mice was subtracted. Comparison revealed significantly more activated microglia in CRAMP-KO mice in pneumococcal meningitis (* p < 0.05, ** p = 0.0061, infected vs. noninfected mice, Mann-Whitney U test).
Similarly to astrocytes, the noninfected CRAMP-KO mice had already a higher basal rate of microglial cell activation within the hippocampal formation in comparison to WT mice (fig. 3a). As expected, the number of microglial cells strongly increased in WT mice in the hippocampal formation 36 h after infection [noninfected CRAMP-WT 0.0 (0.0/2)/mm2 vs. infected WT 2.13 (1.85/4.09)/mm2; median (25th/75th percentile), Mann-Whitney U test, p = 0.023; fig. 3c]. The increase following infection with S. pneumoniae was not significant [noninfected CRAMP-KO 0.99 (0.85/1.16)/mm2 vs. infected CRAMP-KO 1.71 (0.94/1.53)/mm2; medians (25th/75th percentiles), p = 0.1143, Mann-Whitney U test; fig. 3c]. However, comparing only the infected CRAMP-KO mice normalized to infected WT mice (basal expression of microglial cells of the uninfected mice was subtracted), the density of Iba-1-immunoreactive cells was significantly increased in the CRAMP-deficient mice [8.34 (3.56/ 13.56)-fold increase in infected CRAMP-KO mice; median (25th/75th percentile), p = 0.0061; fig. 3d].
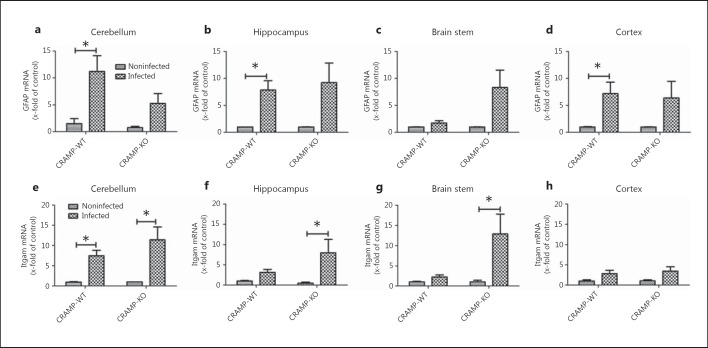
We analyzed mRNA expression of GFAP (as a marker for activated astrocytes) as well as Itgam (or CD11b [23], as a marker of microglial cell activation) in the cerebellum, hippocampal formation, brain stem and frontal cortex of CRAMP-KO and -WT mice 36 h after infection with S. pneumoniae. As shown in figure 4a, b, c, d, WT mice showed a significant increase in GFAP mRNA expression in the cerebellum, hippocampal formation and frontal cortex (11.2 ± 3, 7.8 ± 1.7 and 7.2 ± 2.1 times, respectively; all p < 0.05; fig. 4a, b, d), whereas no significant increase in GFAP mRNA expression was detected in any region of CRAMP-KO mice. In WT mice, Itgam mRNA expression was only significantly increased in the cerebellum (7.5 ± 1.4 times, p < 0.05; fig. 4e), whereas CRAMP-deficient mice showed a significant increase in activated microglial cell gene expression in the cerebellum, hippocampus and brain stem (11.4 ± 3.1, 8 ± 2.9 and 12.9 ± 4.9 times, respectively; p < 0.05; fig. 4e-g).
Fig. 4.
Gene expression of glial cell markers after pneumococcal meningitis. Analysis of mRNA expression of the astrocyte marker GFAP (a-d) and the activated microglial marker Itgam (e-h) in the cerebellum, hippocampal formation, brain stem and frontal cortex of CRAMP-KO and -WT mice 36 h after induction of pneumococcal meningitis by real-time RT-PCR. Data were assessed in 6 independent experiments in duplicate. * p < 0.05 infected CRAMP-WT vs. CRAMP-KO mice (ANOVA followed by a Bonferroni test).
Comparison of General Immune Response after Bacterial Meningitis in the Hippocampal Formation of CRAMP-KO and -WT Mice by PCR Array and Real-Time RT-PCR
In a next set of experiments, we analyzed mRNA expression of genes generally involved in innate and adaptive immune responses in the hippocampal formation of CRAMP-KO and -WT mice 36 h after infection with S. pneumoniae. As shown in online supplementary figure 1A (for all online supplementary material, see www.karger.com/doi/10.1159/000353645), 24 genes showed increased expression while 4 genes were downregulated in infected WT mice compared to noninfected WT mice. In 59 genes, the expression rate did not change. Table 3 shows the genes with a change in the expression (increase or decrease). However, the differences between infected and noninfected WT animals were not significant (n = 3). In CRAMP-deficient mice, infection resulted in an increased expression of 33 genes, whereas the expression of only 1 gene was decreased. In 14 genes, mRNA expression was significantly increased compared to noninfected mice. Altogether, the results showed a stronger immune response in CRAMP-KO mice than -WT mice after pneumococcal infection.
Table 3.
Results of PCR array mouse innate and adaptive immune responses
| Gene | x-fold of control |
|
|---|---|---|
| CRAMP-WT | CRAMP-KO | |
| Adora2a (adenosine A2A receptor) | 5.5895 | 4.4838 |
| Casp1 (caspase-1) | 4.3058 | 4.314* |
| Casp 4 (caspase-4) | 22.9138 | 38.8959* |
| Ccl2 (CCL2) | 106.4495 | 2,875.7481 |
| Ccr3 (C-C chemokine receptor type 3) | – | 4.0441 |
| Cd14 | 34.4855 | 29.3627 |
| Clec7a (C-type lectin domain family 7 member A/dectin) | – | 5.7234* |
| Cybb (cytochrome b-245) | 14.0473 | 29.3954* |
| Defb4 | – | 4.1689 |
| Dmbt1 (deleted in malignant brain tumors 1) | – | 4.0212 |
| Hmox1 [heme oxygenase (decycling) 1] | 5.4103 | 5.4396 |
| Ifnb1 (interferon-β1) | 9.1583 | 4.0817 |
| Il10 (IL-10) | 4.4772 | 4.7127 |
| Il1a (IL-1α) | 11.614 | 14.019 |
| Il1b (IL-1β) | 52.6794 | 66.8595 |
| Il1f9 (IL-1 family member 9) | 24.1392 | 25.3921 |
| Il1r2 (IL-1 receptor, type II) | 8.5841 | 6.4109 |
| Il1rn (IL-1 receptor antagonist) | 6.6392 | 9.6017 |
| Il6 (IL-6) | 55.0987 | 48.4762 |
| Irf1 (interferon regulatory factor 1) | 6.3225 | 12.0827* |
| Lyz1 (lysozyme 1) | 5.4183 | 13.1175 |
| Myd88 [myeloid differentiation primary response gene (88)] | - | 6.5557* |
| Ncf4 (neutrophil cytosolic factor 4) | 5.3611 | 9.4759* |
| Nfkbia [nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor, alpha (http://www.genenames.org/data/hgnc_data.php?hgnc_id=7797)] | 4.1035 | |
| Nlrc4 (NLR family CARD domain-containing protein 4) | – | 5.5342* |
| Pglyrp1 (peptidoglycan recognition protein 1) | – | 4.5003* |
| Pglyrp3 (peptidoglycan recognition protein 3) | 4.4648 | – |
| Ptafr (platelet-activating factor receptor gene) | – | 11.5002* |
| Serpine1 (plasminogen activator inhibitor-1) | 18.6022 | 23.5001* |
| Tlr1 (TLR1) | – | 5.0086* |
| Tlr2 (TLR2) | 7.9732 | 26.2452 |
| Tlr8 (TLR8) | – | 6.2569* |
| Tlr9 (TLR9) | – | 4.1669 |
| Tnf (TNF-α) | 25.0953 | 82.921 |
| Trem1 (triggering receptor expressed on myeloid cells 1) | 7.759 | 8.4433 |
| Fn1 (fibronection 1) | –18.7371 | – |
| Ifngr2 (interferon-γ receptor 2) | –33.3823 | – |
| Nos2 (nitric oxide synthase 2) | –13.9407 | – |
| Serpina1a [serine (or cysteine) peptidase inhibitor member 1A] | – | –4.5967 |
Expression levels of different genes in the hippocampal formation of CRAMP-KO and -WT mice. Only genes with increased or decreased expression rates are listed. – = No change in expression. Each group represents the analysis of 3 mice. Data are presented as means. * p < 0.05 vs. noninfected controls (t test).
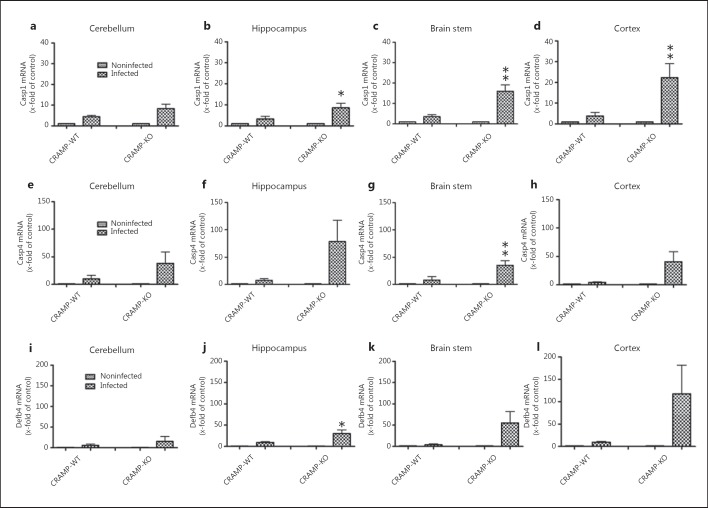
To verify the results of the PCR array, we evaluated gene expression of candidates both in the hippocampal formation as well as in the cerebellum, brain stem and frontal cortex from CRAMP-deficient and -WT mice. First we examined gene expression of caspase-1, which proteolytically cleaves other precursors of inflammatory cytokines IL-1β and IL-18 into active mature peptides [24]. As shown in figure 5a, b, c, d, expression analysis revealed only a low but significant increase in infected WT mice (maximum in the cerebellum; 4.4 ± 0.7 induction compared to noninfected CRAMP-WT; p < 0.05) whereas the increase in the expression in three of four brain regions of the CRAMP-deficient mice was statistically significant with a maximum increase in the frontal cortex (22.4- ± 6.6-fold induction compared to noninfected CRAMP-KO mice, p < 0.05 between infected CRAMP-WT and -KO mice). Similarly, gene expression of the cysteine protease caspase-4 was only slightly increased by infection in all four brain regions in WT mice (fig. 5e, f, g, h; maximum in the cerebellum: 9.8 ± 5.1 increase in induction; nonsignificant compared to noninfected CRAMP-WT mice), whereas in CRAMP-KO mice the expression increase was more pronounced with the gene expression rate reaching statistical significance within the brain stem (35.3- ± 8.2-fold increase compared to non-infected CRAMP-KO mice, p < 0.05 CRAMP-WT vs. -KO mice; fig. 5g). Furthermore, table 3 shows that in the hippocampal formation of CRAMP-deficient mice Defb4 expression was increased. Defb4 is the mouse homologue of the human Defb2 [25]. To verify the hypothesis that Defb4 compensates the lack of CRAMP, we compared Defb4 expression in the different brain regions of CRAMP-deficient and -WT mice. While in WT mice Defb4 expression was only slightly increased in the hippocampal formation and frontal cortex with maximum expression in the cortex (9.5- ± 2-fold increase vs. noninfected WT mice; p < 0.05; fig. 5l), CRAMP-deficient mice displayed a strong increase in Defb4 expression in all pathological brain regions studied. The increase in the hippocampal formation was significant compared to infected WT mice (30.3- ± 8.5-fold increase vs. noninfected CRAMP-deficient mice, p < 0.05 CRAMP-WT vs. -KO mice).
Fig. 5.
Expression of different inflammation markers after pneumococcal meningitis in different brain regions. 36 h after subarachnoid infection with S. pneumoniae, mRNA expression levels of caspase-1 (a-d), caspase-4 (e-h) and Defb4 (i-l) were determined in the cerebellum, hippocampal formation, brain stem and cortex of CRAMP-KO and -WT mice by real-time RT-PCR. Please note the stronger increase in expression in infected CRAMP-KO compared to infected-WT mice. Data were assessed in 6 independent experiments in duplicate. * p < 0.05, ** p < 0.01, infected CRAMP-WT vs. infected CRAMP-KO mice (ANOVA followed by a Bonferroni test). Casp1/4 = Caspase-1/4.
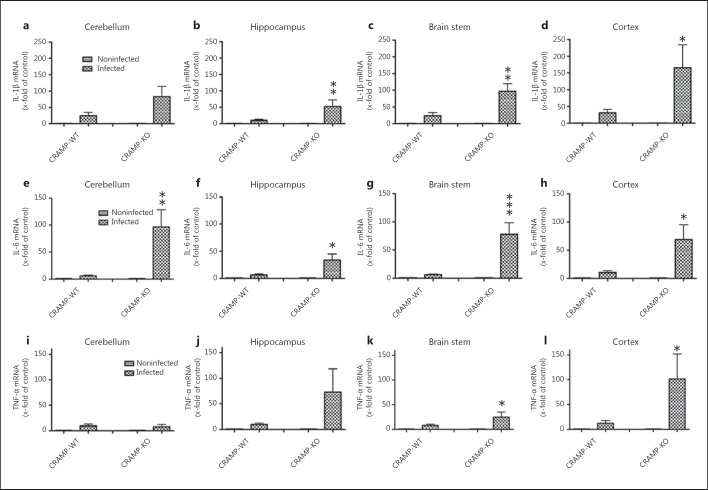
Next, we compared the expression of the proinflammatory cytokines IL-1β, IL-6 and TNF-α, which play an important role during the course of pneumococcal meningitis. The infection resulted in a strong increase in IL-1β mRNA expression in both strains with highest increases in the cerebellum and cortex of CRAMP-WT mice (24.2- ± 10- and 30.2- ± 11-fold induction of expression vs. non-infected WT mice, respectively; p < 0.05; fig. 6a-d), whereas the expression within the hippocampus, brain stem and cortex was significantly higher in CRAMP-deficient mice compared with infected WT mice (52.3- ± 19-, 97.1- ± 23- and 166- ± 68-fold increase in expression, p < 0.05 or p < 0.01 between infected CRAMP-WT and -KO mice). The expression of both IL-6 and TNF-α were increased in both strains but generally more pronounced in CRAMP-deficient mice. The maximum increase in both IL-6 and TNF-α mRNA expression in the WT mice was detected in the cortex (10.4- ± 2.2-fold induction in expression vs. noninfected WT mice; p < 0.01; fig. 6h; 12.1- ± 4.2-fold induction in expression compared to noninfected WT mice; p < 0.05; fig. 6l). In infected CRAMP-deficient mice, the maximum increase in expression was reached for IL-6 in the cerebellum (96.5- ± 31.7-fold increase in expression vs. noninfected CRAMP-KO mice, p < 0.01 between infected CRAMP-WT and -KO mice; fig. 6e) and for TNF-α in the cortex (101.7- ± 50.4-fold increase in expression compared to noninfected CRAMP-KO mice, p < 0.05 between infected CRAMP-WT and -KO mice; fig. 6l).
Fig. 6.
Expression of proinflammatory cytokines after pneumococcal meningitis in different brain regions. 36 h after subarachnoid infection with S. pneumoniae, mRNA expression levels of IL-1β (a-d), IL-6 (e-h) and TNF-α (i-l) were determined in the cerebellum, hippocampal formation, brain stem and cortex of CRAMP-KO and -WT mice by real-time RT-PCR. Please note the stronger increase in expression in infected CRAMP-KO compared to infected WT mice. Data were assessed in 6 independent experiments in duplicate. * p < 0.05, ** p < 0.01, *** p < 0.001, infected CRAMP-WT vs. infected CRAMP-KO mice (ANOVA followed by a Bonferroni test).
Discussion
In the present study, the consequences of the lack of CRAMP were investigated in a very well-established and -characterized mouse model of pneumococcal meningitis [18]. CRAMP deficiency resulted in a higher mortality rate that was associated with increased bacterial burden and decreased neutrophil granulocyte infiltration in the CNS as well as in distinct changes in inflammatory immune reactions. Our results suggest that CRAMP is an important component of innate immune responses in the defense of the CNS against S. pneumoniae invasion and that the lack of this protein results in an unfavorable outcome. These results are supported by other studies using infection models in CRAMP-deficient mice. Chromek et al. [26] showed that cathelicidin protects the urinary tract of children against invasive bacterial infection by Escherichia coli and Huang et al. [27] reported an increased susceptibility to Pseudomonas aeruginosa keratitis in CRAMP-deficient mice. Reduced bacterial clearance and delayed neutrophil influx were observed in CRAMP-KO mice infected with P. aeruginosa[28]. Mice lacking CRAMP were more susceptible to meningococcal infection and exhibited increased bacterial growth in the blood, liver, and spleen [29]. In a previous work, we emphasized the importance of AP in CNS infections by revealing increased synthesis of the human cathelicidin LL-37 and S100 protein psoriasin in the cerebrospinal fluid of patients suffering from bacterial meningitis [12, 13]. In addition, our in vitro studies revealed an increase in rat CRAMP in glial and meningeal cells after exposure to bacterial components [12, 15]. Other work showed a direct antimicrobial activity of cathelicidins against S. pneumonia and Neisseria meningitidis as the most important meningitis pathogens [29, 30]. With respect to insertion into membrane bilayers, the cathelicidins form pores in the bacterial membrane which resulted in increased permeability with subsequent rapid cell death. A second mechanism for bacterial killing is the binding of intracellular targets to influence bacterial proteins (e.g. the activation of autolytic enzymes, inhibition of cell wall biosynthesis and synthesis of DNA, RNA and protein). Another important feature of these peptides is their capacity to bind and neutralize bacterial lipopolysaccharides in vitro, which may account for the ability of exogenously administered peptides to protect against sepsis in vivo [31]. Taken together, AP represent an important part of the first-line defense against invading bacteria in the CNS such as the important pathogen S. pneumoniae. In addition to its antimicrobial activity, CRAMP also acts as a potent chemoattractant for myeloid cells engaging FPR2 which may be a contributing factor to reduced neutrophil infiltration in CRAMP-deficient mice observed in this study [32, 33]. Furthermore, CRAMP enhanced neutrophil host defense via an increase in IL-8 expression under the control of MAPK p38 and extracellular signal-regulated kinase, and stimulated the generation of reactive oxygen species time and dose dependently. Furthermore, CRAMP exhibits its immunoregulatory properties by stimulating IL-6 cytokine production and glial cell activation and its potential neuroprotective properties via the induction of neurotrophic factors [34, 35].
In general, the meningitis-induced systemic inflammatory reaction is dependent on the release of both bacterial toxins and its interaction with host pattern recognition receptors as well as host-derived cytotoxic agents. This physiologic process can be harmful because it results in the recruitment of neutrophils that are considered an important part of the host-derived detrimental effects by releasing cytotoxic agents that may lead to direct brain tissue damage [7]. However, our results showed decreased neutrophil infiltration in infected KO mice associated with increased bacterial titers and an increased inflammatory reaction.
Several studies have demonstrated the importance of glial cells in the development and regulation of inflammatory reactions following the infection of the CNS or in neurodegenerative diseases [36, 37]. The brain is protected from penetrating pathogens by the blood-brain barrier as well as by glial cells such as astrocytes and microglia. Astrocytes belong to the well-characterized innate immune neuroglia, and in addition to many other roles, their main function is the synthesis and regulation of inflammatory cytokines such as IL-1ß, IL-6 and different chemokines [38] as well as the synthesis of anti-inflammatory cytokines [39]. The mediatory functions of astrocytes are compromised during inflammatory reactions and thereby potentially worsening outcome [36]. Microglial cells are the macrophages of the brain. Microglial cells are being activated after the invasion of pathogens into the brain and similar to astrocytes release various mediators such as cytokines and chemokines to activate other immune cells. They can also function as antigen-presenting cells in the brain for invading immune cells such as T lymphocytes [9]. In bacterial meningitis, increased activation of astrocytes and microglia may aggravate neuronal cell death in the hippocampal formation by both induction of apoptosis and necrosis [40]. However, our findings revealed a strong increase in the astrocyte marker GFAP in infected WT mice, whereas the CRAMP-deficient animals displayed an already increased basal rate of astrocyte activation with only small further increase under pathological conditions. Comparison of infected CRAMP-deficient and -WT mice revealed that the density of GFAP-immunoreactive cells was about the same in both groups. This shows that CRAMP deficiency results per se in a higher activation state of astrocytes whose extent is comparable to that of ‘physiological inflammation’ after bacterial invasion. Similar results were obtained for microglial activation in healthy animals: CRAMP deficiency alone resulted in a higher degree of Iba-1 immunoreactivity in comparison to noninfected controls. In contrast to the observations of astrocytes, the density of Iba-1-positive cells/Itgam gene expression of infected CRAMP-deficient mice was significantly higher than in WT mice suggesting enhanced microglial activation by CRAMP deficiency after pneumococcal meningitis. The observation of enhanced endogenous glial cell activation in CRAMP-deficient mice in comparison to WT mice was not published in other studies yet and therefore needs further investigation.
The hippocampal formation is one of the most vulnerable brain regions in general and in bacterial meningitis in particular. Neuronal damage within the dentate gyrus is due to both bacteremia and inflammatory reactions and closely related to long-term sequelae, such as memory impairment [41, 42]. Therefore, we compared the general immune response after pneumococcal meningitis between CRAMP-deficient and -WT mice. Using a PCR array for markers of innate and adaptive immune responses, we found increased expression of the pattern recognition receptors Toll-like receptors (TLR) 1, 2, 8 and 9 in the hippocampal formation of infected CRAMP-deficient mice. TLR play a major role in triggering the immune response. They are able to detect a variety of pathogens and initiate inflammatory immune responses [9]. Interestingly, TLR1 and TLR2 are involved in the detection of components of Gram-positive bacteria such as S. pneumoniae while TLR8 and TLR9 are responsible for the detection of ssRNA and dsDNA from various pathogens [43]. Previous findings suggest an involvement of TLR9 in innate immune responses after pneumococcal meningitis by influencing CRAMP expression in mice [44]. In addition, an important signaling protein of the TLR pathways, the adapter protein MyD88, was increased only in the infected CRAMP-deficient mice. The increase in TLR receptor expression was accompanied by an increase in proinflammatory cytokine expression. Recent studies underlined the importance of TNF-α, for example, for inflammatory processes [45], but also for neuronal apoptosis in the hippocampus [46]. The higher expression rate of TNF-α in infected CRAMP-deficient mice may be regarded as the expression of a stronger immune reaction in those animals and may have influenced or even caused the worse outcome in the form of higher mortality. The generally stronger inflammatory response was also accompanied by increased expression of caspase-1 and −4. As a third class of caspases, they are involved in inflammation [47]. After the recognition of pathogens, caspase-1 molecules group together to form an oligomeric inflammasome [48]. The activated caspase-1 is now able to process proIL-1β and is thereby involved in immune responses [24]. Our findings showed a strong increase of IL-1β expression in infected CRAMP-deficient compared to the -WT mice associated with increased caspase-1 expression. Furthermore, caspase-4 - as well as caspase-1 - has immunomodulatory effects. It is believed that caspase-4 is required to splice caspase-1 and thus to activate it [49]. Taken together, all these changes in the inflammatory reaction caused by CRAMP deficiency accompanied the unfavorable outcome. However, it cannot be concluded from these data whether all these changes in the inflammatory cascade are only caused by the lack of CRAMP alone or whether the higher bacterial burden may also in part be responsible for these changes.
Interestingly, the PCR array and real-time RT-PCR revealed increased expression of the AP Defb4 in infected CRAMP-KO mice. Mouse Defb4 is the homologue of the human β-defensin 2 [25]. Defb4 acts synergistically with antimicrobial lysozyme against bacteria [50]. Lysozyme is an enzyme which hydrolyses the sugar backbone of peptidoglycans in the cell wall of Gram-positive bacteria and thus disassembles the highly immunogenic peptidoglycan into less inflammable components [51]. In patients suffering from pneumococcal meningitis, the amount of lysozyme is increased [52]. Our results showed a stronger increase in infected CRAMP-deficient compared to -WT mice. We propose that by increasing Defb4 expression, the lack of CRAMP may be compensated. The lack of CRAMP resulted in an increased number of bacteria and an increased inflammatory response. This might be a consequence of the imbalance in the innate immune system due to the lack of CRAMP which results in an imbalance in pro- and anti-inflammatory mediators. It can be hypothesized that CRAMP physiologically exerts anti-inflammatory effects. Additional studies need to explore this topic in further detail.
In conclusion, our results reveal the importance of the CRAMP in host defense in a mouse model of pneumococcal meningitis. Although pneumococci are pathogens for rodents [18, 53], the immune response in the mouse is partly different from the immune response in humans [54]. These limitations must be considered in the interpretation of the results. However, despite these limitations, our results provide interesting insights into the function of the innate immune system in the course of bacterial meningitis. A balanced immune response includes the presence of soluble components of innate immunity such as AP. Thus, the lack of APs leads to greater damage and increased mortality. As a next step, adjunctive treatment of bacterial meningitis with CRAMP will help to evaluate the potential therapeutic use of these peptides in bacterial infections.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgments
We thank Susanne Echterhagen, Lian Shen, Michaela Nicolau and Sabine Hamm for excellent technical assistance. This study was supported by the Else Kröner-Fresenius-Stiftung (L.-O.B.) and START Program of the RWTH Aachen University (S.C.T. and L.-O.B.).
References
- 1.Brouwer MC, McIntyre P, de Gans J, Prasad K, van de Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2010;9:CD004405. doi: 10.1002/14651858.CD004405.pub3. [DOI] [PubMed] [Google Scholar]
- 2.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849–1859. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
- 3.Arda B, Sipahi OR, Atalay S, Ulusoy S. Pooled analysis of 2,408 cases of acute adult purulent meningitis from Turkey. Med Princ Pract. 2008;17:76–79. doi: 10.1159/000109595. [DOI] [PubMed] [Google Scholar]
- 4.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24:557–591. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh V, Tucci V, Galwankar S. Infections of the nervous system. Int J Crit Illn Inj Sci. 2012;2:82–97. doi: 10.4103/2229-5151.97273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Beek D, Schmand B, de Gans J, Weisfelt M, Vaessen H, Dankert J, Vermeulen M. Cognitive impairment in adults with good recovery after bacterial meningitis. J Infect Dis. 2002;186:1047–1052. doi: 10.1086/344229. [DOI] [PubMed] [Google Scholar]
- 7.Koedel U, Klein M, Pfister HW. New understandings on the pathophysiology of bacterial meningitis. Curr Opin Infect Dis. 2010;23:217–223. doi: 10.1097/QCO.0b013e328337f49e. [DOI] [PubMed] [Google Scholar]
- 8.Mariani MM, Kielian T. Microglia in infectious diseases of the central nervous system. J Neuroimmune Pharmacol. 2009;4:448–461. doi: 10.1007/s11481-009-9170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konat GW, Kielian T, Marriott I. The role of Toll-like receptors in CNS response to microbial challenge. J Neurochem. 2006;99:1–12. doi: 10.1111/j.1471-4159.2006.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su Y, Zhang K, Schluesener HJ. Antimicrobial peptides in the brain. Arch Immunol Ther Exp (Warsz) 2010;58:365–377. doi: 10.1007/s00005-010-0089-7. [DOI] [PubMed] [Google Scholar]
- 11.Brandenburg LO, Merres J, Albrecht LJ, Varoga D, Pufe T. Antimicrobial peptides: multifunctional drugs for different applications. Polymers. 2012;4:539–560. [Google Scholar]
- 12.Brandenburg LO, Varoga D, Nicolaeva N, Leib SL, Wilms H, Podschun R, Wruck CJ, Schroder JM, Pufe T, Lucius R. Role of glial cells in the functional expression of LL-37/rat cathelin-related antimicrobial peptide in meningitis. J Neuropathol Exp Neurol. 2008;67:1041–1054. doi: 10.1097/NEN.0b013e31818b4801. [DOI] [PubMed] [Google Scholar]
- 13.Jansen S, Podschun R, Leib S, Grotzinger J, Oestern S, Michalek M, Pufe T, Brandenburg LO. Expression and function of psoriasin (S100A7) and koebnerisin (S100A15) in the brain. Infect Immun. 2013;81:1788–1797. doi: 10.1128/IAI.01265-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kai-Larsen Y, Agerberth B. The role of the multifunctional peptide LL-37 in host defense. Front Biosci. 2008;13:3760–3767. doi: 10.2741/2964. [DOI] [PubMed] [Google Scholar]
- 15.Brandenburg LO, Varoga D, Nicolaeva N, Leib SL, Podschun R, Wruck CJ, Wilms H, Lucius R, Pufe T. Expression and regulation of antimicrobial peptide rCRAMP after bacterial infection in primary rat meningeal cells. J Neuroimmunol. 2009;217:55–64. doi: 10.1016/j.jneuroim.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–13093. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 17.Termen S, Tollin M, Olsson B, Svenberg T, Agerberth B, Gudmundsson GH. Phylogeny, processing and expression of the rat cathelicidin rCRAMP: a model for innate antimicrobial peptides. Cell Mol Life Sci. 2003;60:536–549. doi: 10.1007/s000180300045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerber J, Raivich G, Wellmer A, Noeske C, Kunst T, Werner A, Bruck W, Nau R. A mouse model of Streptococcus pneumoniae meningitis mimicking several features of human disease. Acta Neuropathol. 2001;101:499–508. doi: 10.1007/s004010000326. [DOI] [PubMed] [Google Scholar]
- 19.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 20.Liebetanz D, Gerber J, Schiffner C, Schutze S, Klinker F, Jarry H, Nau R, Tauber SC. Pre-infection physical exercise decreases mortality and stimulates neurogenesis in bacterial meningitis. J Neuroinflammation. 2012;9:168. doi: 10.1186/1742-2094-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tauber SC, Stadelmann C, Spreer A, Bruck W, Nau R, Gerber J. Increased expression of BDNF and proliferation of dentate granule cells after bacterial meningitis. J Neuropathol Exp Neurol. 2005;64:806–815. doi: 10.1097/01.jnen.0000178853.21799.88. [DOI] [PubMed] [Google Scholar]
- 22.Slowik A, Merres J, Elfgen A, Jansen S, Mohr F, Wruck CJ, Pufe T, Brandenburg LO. Involvement of formyl peptide receptors in receptor for advanced glycation end products (RAGE)- and amyloid beta 1-42-induced signal transduction in glial cells. Mol Neurodegener. 2012;7:55. doi: 10.1186/1750-1326-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 24.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–651. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 26.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 27.Huang LC, Reins RY, Gallo RL, McDermott AM. Cathelicidin-deficient (Cnlp−/−) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007;48:4498–4508. doi: 10.1167/iovs.07-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovach MA, Ballinger MN, Newstead MW, Zeng X, Bhan U, Yu FS, Moore BB, Gallo RL, Standiford TJ. Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in gram-negative bacterial pneumonia. J Immunol. 2012;189:304–311. doi: 10.4049/jimmunol.1103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergman P, Johansson L, Wan H, Jones A, Gallo RL, Gudmundsson GH, Hokfelt T, Jonsson AB, Agerberth B. Induction of the antimicrobial peptide CRAMP in the blood-brain barrier and meninges after meningococcal infection. Infect Immun. 2006;74:6982–6991. doi: 10.1128/IAI.01043-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucki R, Pastore JJ, Randhawa P, Vegners R, Weiner DJ, Janmey PA. Antibacterial activities of rhodamine B-conjugated gelsolin-derived peptides compared to those of the antimicrobial peptides cathelicidin LL37, magainin II, and melittin. Antimicrob Agents Chemother. 2004;48:1526–1533. doi: 10.1128/AAC.48.5.1526-1533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- 32.Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, Bidzhekov K, Rottenberg ME, Weber C, Lindbom L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wantha S, Alard JE, Megens RT, van der Does AM, Doring Y, Drechsler M, Pham CT, Wang MW, Wang JM, Gallo RL, von Hundelshausen P, Lindbom L, Hackeng T, Weber C, Soehnlein O. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res. 2013;112:792–801. doi: 10.1161/CIRCRESAHA.112.300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157:1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 35.Baertling F, Kokozidou M, Pufe T, Clarner T, Windoffer R, Wruck CJ, Brandenburg LO, Beyer C, Kipp M. ADAM12 is expressed by astrocytes during experimental demyelination. Brain Res. 2010;1326:1–14. doi: 10.1016/j.brainres.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 36.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 39.Nash B, Thomson CE, Linington C, Arthur AT, McClure JD, McBride MW, Barnett SC. Functional duality of astrocytes in myelination. J Neurosci. 2011;31:13028–13038. doi: 10.1523/JNEUROSCI.1449-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerber J, Nau R. Mechanisms of injury in bacterial meningitis. Curr Opin Neurol. 2010;23:312–318. doi: 10.1097/WCO.0b013e32833950dd. [DOI] [PubMed] [Google Scholar]
- 41.Meli DN, Christen S, Leib SL, Tauber MG. Current concepts in the pathogenesis of meningitis caused by Streptococcus pneumoniae. Curr Opin Infect Dis. 2002;15:253–257. doi: 10.1097/00001432-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Ostergaard C, Leib SL, Rowland I, Brandt CT. Bacteremia causes hippocampal apoptosis in experimental pneumococcal meningitis. BMC Infect Dis. 2010;10:1. doi: 10.1186/1471-2334-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 44.Brandenburg LO, Jansen S, Albrecht LJ, Merres J, Gerber J, Pufe T, Tauber SC. CpG oligodeoxynucleotides induce the expression of the antimicrobial peptide cathelicidin in glial cells. J Neuroimmunol. 2013;255:18–31. doi: 10.1016/j.jneuroim.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Gerber J, Bottcher T, Hahn M, Siemer A, Bunkowski S, Nau R. Increased mortality and spatial memory deficits in TNF-alpha-deficient mice in ceftriaxone-treated experimental pneumococcal meningitis. Neurobiol Dis. 2004;16:133–138. doi: 10.1016/j.nbd.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Bogdan I, Leib SL, Bergeron M, Chow L, Tauber MG. Tumor necrosis factor-alpha contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J Infect Dis. 1997;176:693–697. doi: 10.1086/514092. [DOI] [PubMed] [Google Scholar]
- 47.Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 48.McIntire CR, Yeretssian G, Saleh M. Inflammasomes in infection and inflammation. Apoptosis. 2009;14:522–535. doi: 10.1007/s10495-009-0312-3. [DOI] [PubMed] [Google Scholar]
- 49.Sollberger G, Strittmatter GE, Kistowska M, French LE, Beer HD. Caspase-4 is required for activation of inflammasomes. J Immunol. 2012;188:1992–2000. doi: 10.4049/jimmunol.1101620. [DOI] [PubMed] [Google Scholar]
- 50.Garcia JR, Krause A, Schulz S, Rodriguez-Jimenez FJ, Kluver E, Adermann K, Forssmann U, Frimpong-Boateng A, Bals R, Forssmann WG. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819–1821. [PubMed] [Google Scholar]
- 51.Hoijer MA, Melief MJ, Debets R, Hazenberg MP. Inflammatory properties of peptidoglycan are decreased after degradation by human N-acetylmuramyl-L-alanine amidase. Eur Cytokine Netw. 1997;8:375–381. [PubMed] [Google Scholar]
- 52.Klockars M, Reitamo S, Weber T, Kerttula Y. Cerebrospinal fluid lysozyme in bacterial and viral meningitis. Acta Med Scand. 1978;203:71–74. doi: 10.1111/j.0954-6820.1978.tb14834.x. [DOI] [PubMed] [Google Scholar]
- 53.Leib SL, Clements JM, Lindberg RL, Heimgartner C, Loeffler JM, Pfister LA, Tauber MG, Leppert D. Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain. 2001;124:1734–1742. doi: 10.1093/brain/124.9.1734. [DOI] [PubMed] [Google Scholar]
- 54.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data