Abstract
Neutrophils are recruited to a site of infection or injury where they help initiate the acute inflammatory response. In instances of sterile inflammation, where no microbial threats are present, this neutrophil recruitment is mediated by the release of danger signals or damage-associated molecular patterns (DAMPs) from disrupted cells and tissues. At basal state, many of these substances are sequestered and remain hidden within the cell, but are released following the rupture of the plasma membrane. In other instances, these DAMPs are undetected by the innate immune system unless chemically or proteolytically modified by tissue damage. DAMPs may be directly detected by neutrophils themselves and modulate their recruitment to sites of damage or, alternatively, they can act on other cell types which in turn facilitate the arrival of neutrophils to a site of injury. In this review, we outline the direct and indirect effects of a number of DAMPs, notably extracellular ATP, mitochondrial formylated peptides and mitochondrial DNA, all of which are released by necrotic cells. We examine the effect of these substances on the recruitment and behaviour of neutrophils to sites of sterile injury. We also highlight research which suggests that neutrophils are actively involved in triggering the resolution phase of an inflammatory response. This review brings to light a growing body of work that demonstrates that the release of DAMPs and the ensuing influx of neutrophils plays an important functional role in the inflammatory response, even when no pathogens are present.
Key Words: Inflammation, Sterile injury, Damage-associated molecular patterns, Chemotaxis, Neutrophils
Introduction
Following infection or tissue injury, neutrophils are among the first leukocytes to be recruited from the bloodstream. The ensuing inflammatory response depends on the ability of these potent innate immune effector cells to interact with activated endothelium in the proximity of the area of insult and, to move into the adjacent extravascular space. This process typically (but not always) occurs in the post-capillary venules and is characterized by the upregulation of adhesion molecules on the lumenal surface of endothelial cells, which allows neutrophils to roll and then adhere along the vessel wall. Following the detection of additional activating signals, neutrophils are able to follow directional cues, crawl along the vessel walls, exit the vasculature and home to the site of injury in the surrounding tissues. The substances that facilitate this process can be released by microbial pathogens themselves, secreted by the body's own cells, or generated by the cleavage of serum components. The intricacies of this canonical leukocyte recruitment cascade have been recently reviewed elsewhere [1, 2, 3, 4] and will therefore not be elaborated upon here. Neutrophils, once extravasated and present at a site of injury, help initiate the inflammatory response by releasing a number of pro-inflammatory mediators. These mediators recruit additional immune cells to the area, setting the stage for pathogen eradication and/or tissue repair. Neutrophils possess an arsenal of microbial killing mechanisms which allow them to quickly and efficiently eliminate invading pathogens. However, when tissues are damaged by trauma or non-infectious agents, neutrophils are also recruited to the area despite the absence of a microbial stimulus. The characteristic redness, pain, heat, swelling and loss of function associated with this inflammation is at least in part mediated by neutrophils. This response is called sterile inflammation due to the absence of pathogen and it is known to occur in numerous instances such as blunt trauma, ischemia-reperfusion injury, exposure to toxins or crystal particulates and auto-inflammatory diseases. Although a wide variety of cells are able to sense and respond to sterile injury, and are able to instigate a plethora of downstream effects, we will limit the discussion to the behaviour of neutrophils in direct response to such injuries, or to cell types which help orchestrate neutrophilic recruitment. The focus will be on the mechanisms by which these danger signals, when released by sterile injury, inform and instruct the neutrophil response in such situations.
DAMPs Mean ‘Danger’ - but Not Necessarily from Infection
The innate immune system is characterized by its ability to rapidly mount a response due to injury and infection. It is able to detect a broad range of conserved structures present amongst microbes, known as pathogen-associated molecular patterns or PAMPs, and can thus differentiate such targets from self tissues. It can therefore rapidly eliminate these dangerous foreigners while avoiding attack upon the body's own cells. In instances of sterile inflammation, the innate immune system must now recognize and respond to damaged self, but not healthy self. To allow for this response, the innate immune system is designed to recognize a number of self-derived molecules that are either altered or relocated from their normal cellular compartment. These structures allow the body to differentiate between healthy tissue and tissues that are stressed or damaged, a notion popularized by Polly Matzinger as the ‘danger hypothesis’, wherein the body's immune system is thought to be poised to recognize not necessarily a ‘stranger’ (a microbe), but ‘danger’ (the damage to host tissues resulting from injury or infection) [5]. The understanding that self molecules from damaged tissues summon the inflammatory response is largely accepted today. Commonly referred to as ‘damage-associated molecular patterns’ or DAMPs, these can be structures that are normally present within cells and remain hidden from the innate immune system. Once actively or passively released following cell damage or disruption, self molecules function as DAMPs [6]. Alternatively, certain components external to the cell can act as DAMPs following damage-induced chemical or proteolytic modification of their structures, enabling their detection by inflammatory cells [6].
The detection of DAMPs occurs via germ line-encoded pattern recognition receptors present in numerous cell types, many of which also have well-described roles in the detection of PAMPs. Most DAMPs and PAMPs exhibit a striking functional similitude as many bind the same pattern recognition receptors despite considerable differences in structure. For example, toll-like receptor (TLR) 4 binds the self molecules high mobility group box-1 (HMGB1) and heat shock proteins (amongst others), and yet is also responsible for the detection of lipopolysaccharide present in Gram-negative bacterial cell walls [7]. Regardless of the nature of the ligand, or its source, these receptors are responsible for inducing a pro-inflammatory phenotype within a responding cell. This habitually occurs in tissue resident macrophages and culminates with the production of interleukin-1 (IL-1), a highly pro-inflammatory cytokine, when the offending DAMPs are from necrotic cells [8]. This step appears to be critical in the induction of acute inflammation in response to sterile injury as mice deficient in components of the IL-1 pathway exhibited reduced neutrophil infiltration following peritoneal injections of necrotic cells [9].
The generation of functional IL-1β occurs downstream of the NLRP3 inflammasome. The NLRP3 inflammasome is a cytosolic protein complex that, following oligomerization, instigates a signalling cascade that culminates in caspase-1-dependent cleavage of functionally inert pro-IL-1β. This process occurs in response to a wide variety of inflammatory stimuli and DAMPs of broad origin and structure. The common trait is that all these stimuli induce some form of stress or damage to the cell which is sensed intracellularly by the NLRP3 protein complex. Furthermore, a non-conventional processing of pro-IL-1β can proceed via the cathepsin C protease [10], highlighting the complexity of signalling in response to sterile injury, yet its ultimate dependence on the generation of the pro-inflammatory cytokine IL-1.
A large number of DAMPs are implicated in the response to sterile injury and have been described in several recent reviews [6, 7, 11]. In general, DAMPs such as HMGB1 [12, 13], S100 proteins [14], heat shock proteins [15], the endogenous nucleic acids RNA [16] and DNA [17], and altered extracellular matrix components such as hyaluronan [18], trigger a variety of broad inflammatory effects such as pro-inflammatory cytokine release and the upregulation of adhesion molecules on endothelium. As a result, they promote acute inflammation and the recruitment of neutrophils to a site of damage. Table 1 lists a number of DAMPs and their putative receptors. In some cases DAMPs must activate protein synthesis to induce neutrophil recruitment (such as TLR ligands), while in others, neutrophils can be recruited directly and immediately (such as with formylated peptides, described below).
Table 1.
DAMPs are released by several methods and are detected by numerous overlapping pathways to promote inflammation
| DAMP | Release mechanism | Detection |
|---|---|---|
| ATP | Cellular necrosis Polarized release from membrane |
P2X7 NLRP3 inflammasome P2Y2' |
| Heat shock proteins | Cellular necrosis | TLR2, TLR4, CD91, CD24 |
| HMGB1 | Active release from monocytes, Mφ, non-immune cells Cellular necrosis |
RAGE, TLR2, TLR4, CD24 |
| Mitochondrial DNA | Cellular necrosis, mitochondrial release Defective mitophagy |
TLR9 NLRP3 inflammasome TLR9 |
| Mitochondrial formylated peptides | Cellular necrosis | FPR1, FPR2/ALX |
| Nucleic Acids DNA RNA |
Cellular necrosis, apoptosis Cellular necrosis |
TLR9 TLR3 |
| S100 proteins | Cellular necrosis | RAGE |
| Hyaluronan | Fragmentation to LMW HA | TLR2, TLR4, CD44 |
LMW HA = Low molecular weight hyaluronan; Mφ = macrophage; NLRP3 = NOD LRR and pyrin domain-containing 3.
It is worth noting that unlike in a simple in vitro system, where a single molecule can set up a chemotactic gradient to recruit neutrophils over a limited distance, in vivo there are multiple environments, including the intravascular site and the extravascular space separated by an endothelial barrier with dynamic blood flow and extensive distances. Recent studies have highlighted the complex nature of such recruitment [19, 20]. In fact multiple cell types and multiple signals are likely required to orchestrate the recruitment of neutrophils to sites of sterile injury in vivo. In the present review we will focus on necrosis-induced DAMPs that are instrumental in neutrophil recruitment to sterile injury.
DAMPs and Their Effects on Neutrophils
Extracellular ATP and mitochondria-derived molecules are released from necrotic cells and have numerous far-reaching effects on the acute inflammatory response, affecting both inflammatory and non-inflammatory cells. However, both of these types of DAMPs are detected directly by neutrophils. In the following section, we will highlight their roles specific to the recruitment of neutrophils to a site of sterile injury.
Extracellular ATP
Extracellular ATP has been identified as a potent DAMP. Studies indicate that ATP, acting through the cell surface P2Y2 receptor, may induce chemotactic activity or extension of cellular processes in certain cell types, such as brain microglia. In such instances of sterile injury in the brain, viable cells adjacent to the site of damage are thought to release ATP to help recruit immune cells [21]. Other reports also indicate that ATP is secreted by apoptotic cells where again, in a P2Y2-dependent manner, it acts as a ‘find me’ signal for monocytes and macrophages [22], although this appears not to be the case with neutrophils. Instead of acting as a chemoattractant for neutrophils, work by Chen et al. [23] has revealed that the P2Y2 receptor is in part responsible for an autocrine signal amplification loop at the leading edge of the neutrophil. ATP is released from the leading edge of neutrophils moving towards a pre-existing gradient of attractant, yet fails to provide actual directional instruction to the migrating cell, instead enhancing the response to the pre-existing chemoattractant gradient. In this context, ATP does not function exclusively as a DAMP, as it is undoubtedly implicated in migration to sites of both infectious and sterile inflammation.
If the ATP is not creating a chemotactic signal, then how is it functioning to aid in neutrophil recruitment? Intravital imaging experiments have revealed that in blood vessels neutrophils immediately adhered upon sterile injury. Inhibition of ATP reduced the number of adherent neutrophils, however, those cells that did manage to adhere independently of ATP showed no signs of impaired chemotaxis [20], further supporting the notion that ATP is not a chemotactic molecule but is required for recruitment. In this model of sterile injury, it is possible that ATP either emanates from a cell adjacent to the injury or is released passively from necrotic cells following the disruption of the plasma membrane. Millimolar concentrations of ATP are present within the cell and, following rupture, release into the extracellular space is quite likely. In this scenario, ATP would therefore be present at high concentration within the vicinity of the injured cells and could then diffuse through the tissue or blood and activate adjacent cells, causing neutrophils to adhere within the vasculature.
There is little evidence that ATP can directly activate integrins to induce neutrophil adhesion, but the presence of extracellular ATP at a sterile lesion can engage the P2X7 receptor and activate the Nlrp3 inflammasome in bone marrow-derived (macrophage) and non-bone marrow-derived cells (endothelium), which in turn results in the production of IL-1β [20, 24]. This IL-1β promotes the upregulation of adhesion molecules and chemokines on the surface of endothelium, and thereby facilitates the recruitment of neutrophils to the vicinity of the injury. Indeed, both MIP2 and KC, two important CXCR2 neutrophil chemokines, were critical to the recruitment of neutrophils by setting up a chemotactic gradient in the vasculature [20]. Clearly the indirect and direct effects of ATP on neutrophil recruitment are widespread, as the purine plays a role in activating resident cells surrounding a site of injury to instigate the adhesion and recruitment of neutrophils to an area of insult.
Mitochondria
Mitochondria are the energy-generating organelles of eukaryotic cells, thought to be evolutionarily linked to bacteria-like endosymbionts [25]. These organelles synthesize a number of peptides that are microbial in nature encoded by their ancestral genomes. Such peptides, which bare formyl groups on their N-terminus, resemble the formylated peptides which are ubiquitously produced by bacterial translational machinery. Indeed, the mammalian immune system has evolved to detect such protein structures through the formyl peptide receptors (FPRs), which are G-protein-coupled receptors that initiate a number of downstream effector functions. Most notably, substances detected by FPRs present on the membrane of neutrophils direct cell migration and instigate the oxidative burst [for a comprehensive review on FPRs, see [26]. In the case of the high affinity FPR1 receptor, it is critical for directing migrating neutrophils towards bacterial infections by binding microbial formylated peptides such as fMLF. It is also clear that formyl peptides released from damaged tissues are able to attract neutrophils through this receptor [27]. Indeed, purified synthetic mitochondrial peptides induced calcium flux and MAPK signalling in HL-60 neutrophil-like cell lines expressing different FPRs [28]. Interestingly, this latter study suggested that chemotactic responses towards mitochondrial peptides were not dependent on FPR1, relying instead on the lower affinity formyl peptide receptor-like 1 (FPRL-1; now referred to as FPR2/ALX [26]). Despite the potential role of FPR2/ALX more recent work has once again highlighted the importance of the high affinity receptor FPR-1 in facilitating neutrophil chemotaxis towards damaged tissues. In these studies, either treatment with cyclosporin H, a selective inhibitor of FPR1, or FPR1-specific antibody reduced neutrophil chemotaxis towards damaged mitochondria [29, 30]. These studies focussed on neutrophil chemotaxis within the context of patients with trauma-induced systemic inflammatory response syndrome (SIRS) and also described mitochondrial signatures present within the circulation of these patients. During SIRS, neutrophils are inappropriately recruited to distal organs, contributing to the development of multiple organ dysfunction, circulatory collapse and death [31]. Circulating mitochondrial DAMPs have been suggested to play a role in this inappropriate neutrophil recruitment to various organs and thus are, for a large part, responsible for the constellation of symptoms present in SIRS. Another study has demonstrated that cellular necrosis in the liver during acetaminophen induced hepatotoxicity releases the products of damaged mitochondria into the circulation [32]. These mitochondrial-derived DAMPs, in combination with chemokine signalling, were responsible for not only neutrophil-mediated liver pathology, but also for systemic inflammation and remote lung injury. Blockade of FPR1 and CXCR2, as well as TLR9, the receptor for CpG DNA (see below), reduced organ injury [32]. These findings are in line with previous evidence indicating that endogenous DNA from apoptotic sinusoidal endothelial cells is responsible for acetaminophen-induced hepatotoxicity via both TLR9 and the NLRP3 inflammasome [17]. It is possible that both molecules contribute to the neutrophil recruitment as well as instigating hepatic injury.
Recent assessments of neutrophil recruitment kinetics to a site of focal sterile injury in the mouse liver reveal that adherent neutrophils are attracted to the general vicinity of the damaged tissues by following intravascular gradients of chemokines [20]. However, FPR1 ligands, possibly mitochondrial formylated peptides, also figure prominently in this recruitment cascade. Whereas chemokines bring the neutrophils to the vicinity of the injury, formylated peptides appear to direct the neutrophils the final few hundred microns into the injury. For the neutrophils to arrive at the precise site of tissue necrosis, they must ignore gradients of chemokines expressed in the surrounding vasculature and start following hierarchically superior signals emanating from the injury itself. This chemotactic hierarchy has been previously described and suggests that bacterial formylated peptides are always dominant to endogenous chemo-attractants in vitro [33, 34, 35], and is observed even when dominant signals are present at 1/100th of their optimal concentration. Furthermore, neutrophils placed directly into environments containing high concentrations of endogenous chemokines will continue to migrate towards distal sources of formylated peptide demonstrating the dominance of this type of signal. The opposite is not true in as much as neutrophils, when migrating towards a chemokine gradient, will change direction and move towards a new gradient of formylated peptide [34, 35]. Formylated peptides of either bacterial or endogenous origin therefore serve as dominant signals in the recruitment of neutrophils, as migration towards these substances supersedes movement towards other intermediate level chemo-attractants, enabling neutrophils to home to the precise site of injury or infection, and thus sparing surrounding viable tissues. Recent experiments in our lab suggest that, in vitro, when presented with injured cells or chemokines, neutrophils preferentially migrated towards injured cells [20].
Interestingly, while neutrophils migrate within sinusoids towards a site of hepatic necrosis in response to damage in the skin, neutrophils immediately leave the vasculature and migrate through tissue towards the afflicted site [36]. This difference in paths taken by neutrophils could be due to the presence of extravascular sentinel cells in the skin such as macrophages or perhaps even tissue-resident neutrophils which immediately recruit subsequent waves of responding cells into surrounding tissue [36]. In the liver, Kupffer cells are present within the sinusoids, thus accounting for the intravascular recruitment observed in this organ. Such differences highlight the fact that there are undoubtedly numerous pathways a neutrophil may follow for recruitment to a site of sterile injury and the pathway utilized is likely a product of the tissue anatomy, position of other immune cells (sentinels), and the location and concentration of recruiting chemo-attractants.
Another DAMP found within mitochondria is the remnant microbial genome itself. The mammalian immune system is able to detect unmethylated CpG repeats present in microbial DNA and mitochondrial DNA (mtDNA). This detection occurs through TLR9, an endosomal pattern recognition receptor which binds evolutionary conserved unmethylated CpG repeats present in prokaryotic DNA. Zhang et al. [37] identified mtDNA in the circulation of trauma patients and found that at clinically relevant concentrations, mtDNA activated neutrophils, causing them to secrete the pro-inflammatory chemokine IL-8. Although the release of mtDNA has a direct effect on neutrophils themselves, it is also likely detected by numerous cell types such as endothelium and macrophages, which in turn facilitate the recruitment of neutrophils to a site of sterile injury. Furthermore, when mtDNA is injected into the knee joint, it produces inflammatory arthritis mediated primarily by mononuclear cell infiltrates [38], supporting the notion that mtDNA plays a role in localized inflammation and is able to induce the recruitment of other inflammatory cells in addition to neutrophils.
Interestingly, recent reports suggest that during apoptosis, mtDNA may also be released into the cytosol by stressed mitochondria, and that this helps trigger the oligomerization of the NLRP3 inflammasome via the direct binding of mtDNA to the cytosolic inflammasome components [39]. It is unclear whether cells detecting necrotic tissues are similarly able to activate inflammasome signalling via cell-autonomous mtDNA, or if apoptotic cells in the vicinity of a sterile injury might also activate such a pathway [40]. Furthermore, during dysfunctional autophagy, mtDNA that escapes from inappropriately removed mitochondria activates TLR9 and promotes inflammation [41]. These studies demonstrate that mtDNA, even when derived from within an individual cell, can have profound inflammatory effects which parallel those seen when released by trauma-induced cell disruption.
In addition to mitochondrial formylated peptides and mtDNA, mitochondria also appear to be able to induce acute inflammation in other ways. Recently, Iyer et al. [24] have demonstrated that mitochondria, when injected intraperitoneally, needed to be viable to induce NLRP3-dependent neutrophilic influx and IL-1β production. It is thought that this activation occurs via the production of ATP during oxidative respiration by these viable mitochondria. Accordingly, the inhibition of mitochondrial respiration and P2X7 receptor deficiency dampened subsequent inflammation. This suggests a partnership between two of the main DAMPs that are implicated in neutrophil recruitment, ATP and mitochondria.
Why Send a Neutrophil to a Site of Sterile Injury? Inflammation versus Resolution
Detecting tissue disruption is important because it could signify that nearby barriers, such as the epithelium, may be compromised. It makes sense that evolution would favour immune strategies that quickly send neutrophils to a site of potential microbial invasion thereby preventing the entry of noxious microbes. This effort to pre-empt impending infection would confer an advantage to the host that responds in a timely fashion, however, this response may come at the expense of inflammation and injury to self tissues when there are not pathogens present. In the case of such a false alarm, significant collateral damage induced by the inflammatory infiltrates could occur. This in turn could disrupt normal tissue function, potentially leading to permanent dysfunction due to aberrant deposition of collagen and the subsequent development of fibrosis [42]. This notion is supported by the fact that at early stages of development of the mammalian embryo, prior to the establishment of inflammatory cell lineages, injuries in tissues heal without scarring. Furthermore, in mice that lack multiple leukocyte cell lineages, wound healing following sterile injury proceeds normally [43].
This would suggest that neutrophils may not be able to discriminate between injured tissue and infection and treat both situations in a similar if not identical manner. Alternatively, it may be that neutrophils need to go to sites of sterile injury to play an active role in promoting the resolution phase of inflammation. It is commonly appreciated that neutrophils have the ability to engulf and remove cellular debris that accumulates at a site of damage, but it is likely that neutrophils play a much greater role in enabling resolution. In line with this model are studies that have shown that neutrophils are required for revascularization in instances of neoplasia [44] and tissue transplantation [45]. In addition to effects on the revascularization of a site of injury, neutrophils actively promote the recruitment of subsets of monocytes and trigger changes in the inflammatory microenvironment that are required for eventual resolution of the response. Numerous neutrophil instigated mechanisms are responsible for this active and regulated return to tissue homeostasis, including, but not limited to, the release of mediators that facilitate monocyte recruitment, the elaboration of pro-resolution lipid molecules and the inhibition of further neutrophil recruitment by apoptotic neutrophils, and the ability of the latter to reprogram macrophages upon phagocytic engulfment [46]. In addition, neutrophils release matrix metalloproteases and other enzymes that may clear the way for the remodelling of tissue [47]. With this in mind, the release of DAMPs at a site of damage may therefore be necessary to promote the neutrophil-triggered resolution phase and promote wound healing, even if the presence of these cells is detrimental to surrounding tissues in the short term.
It appears that some DAMPs have both pro-inflammatory and pro-resolution effects on neutrophils. The presence of a given DAMP is therefore as important as the context of its appearance in determining functional outcomes. For example, extracellular ATP, as described above, is potently inflammatory. However, cell-surface apyrases and nucleotidases degrade extracellular ATP to adenosine, which is then free to bind to cell surface adenosine receptors on the plasma membrane [48]. During oxygen imbalance, such as would occur during ischemia or inflammation, adenosine activates a receptor that causes the arrest of NF-κB-mediated inflammatory pathways [48]. Additional paradoxical reports of DAMP signalling effects on neutrophils have been shown for members of the formyl peptide receptor family. Two members of this family are known to be expressed in human neutrophils, FPR1 and FPR2/ALX, and mitochondrial formyl peptides have been shown to induce inflammatory processes such as chemotaxis in neutrophil-like cell lines expressing the low affinity human FPR2/ALX [28]. Interestingly, another ligand for this receptor, annexin A1, is released by neutrophils and macrophages at sites of inflammation, and is upregulated by anti-inflammatory mediators such as glucocorticoids [49]. In models of inflammation, the binding of annexin A1 to FPR2/ALX potently reduces the trafficking of neutrophils, and flow chamber experiments demonstrate a marked decrease in interactions between neutrophils and endothelium following exposure to annexin A1 and its N-terminal peptide [50]. Another report indicates that an N-terminal fragment from annexin A1 binds FPRs including FPR2/ALX in human monocytes, causing chemotaxis [51]. As both mitochondrial formyl peptides and annexin A1 could be produced at sites of sterile injury, they may each play important roles in respectively promoting or dampening inflammation. It is unclear how two ligands that bind the same receptor trigger seemingly different responses; however, it likely is affected by the type of responding cell, the action of these two ligands on other receptors (such as FPR1 which they have the capacity to bind as well), and the effect of other signals present within the complex inflammatory or pro-resolving microenvironments.
Despite the unwanted collateral damage induced by neutrophil recruitment, infiltration of these cells to a site of sterile injury may play a significant and active role in prompting the resolution of inflammation. DAMPs therefore serve an important purpose, not only in pre-empting invasion by possible microbial threats, but by bringing in neutrophils and other leukocytes to facilitate the eventual resolution of the inflammatory response and ensuing reparative processes. Understanding the effects of DAMPs on neutrophils and the resolution of acute inflammation is important because an inability to quench such a response encourages progression to a state of chronic inflammation which can lead to multiple deleterious effects to surrounding cells and tissues.
Conclusion and Future Directions
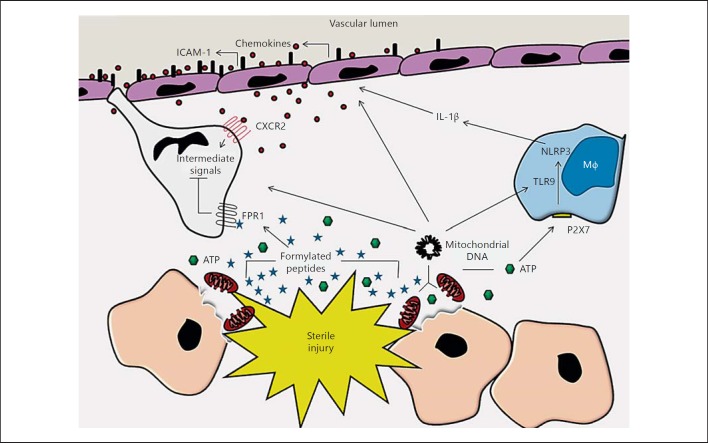
Neutrophil recruitment in response to sterile inflammation is governed by the release of DAMPs. These numerous altered or displaced host products bind to a multitude of receptors on the surface of neutrophils themselves. Alternatively, they may be detected by resident cells within the damaged tissue, which in turn alter the microenvironment to promote and facilitate neutrophil recruitment. DAMPs thus prime the innate immune system and trigger the influx of neutrophils to sites of tissue injury, despite the absence of microbes. It is clear that multiple parallel signalling cascades simultaneously triggered by numerous types of DAMPs, in a number of different cell types, underlie this recruitment. Figure 1 provides a general summary of these processes. Future research must elucidate how these responding cells behave in the complex, overlapping signals present in both the sterile inflammatory environment and in the presence of infectious microbes.
Fig. 1.
Sterile injury causes rupture of the plasma membrane and disruption of mitochondria. This releases ATP from damaged cells, and formylated peptides (which are normally sequestered within the mitochondria) and mtDNA. ATP, once released, activates the NLRP3 inflammasome in nearby sentinel immune cells such as macrophages (Mφ), generating functional IL-1β. Detection of this cytokine and of mtDNA by endothelial cells causes the upregulation of adhesion molecules on their lumenal surface and the production of chemokine gradients. Neutrophils are subsequently recruited from the vasculature to the general area of insult. There, they are guided to the site of tissue necrosis by dominant formyl peptide receptor signals, which not only direct migration, but also block signalling induced by distracting gradients of intermediate chemokines.
Though neutrophilic inflammation is widely held to be responsible for much of the collateral injury to adjacent healthy tissues when present near a site of tissue damage, it is becoming increasingly apparent that the neutrophil plays a critical role in triggering the onset of resolution in such tissues. Understanding how and why neutrophils interact with DAMPs present at a site of sterile injury, the dynamics of their recruitment and departure, and their ability to influence the recruitment of other cell types like monocytes is therefore vitally important. By deciphering the mechanisms that govern neutrophil behaviour at sites of sterile injury, we may identify potential therapeutic targets that would enable us to dampen inflammatory responses without reducing the potential to induce the pro-resolution processes that are responsible for returning damaged tissues to homeostasis. Such knowledge could be applied to solving complex inflammatory pathologies which have a neutrophilic component and could potentially reduce the damaging inflammation present in instances of ischemia, myocardial infarction, trauma and toxin-induced liver injury. Further studies will also help determine whether it is possible to modulate inflammation in response to sterile injury, without interfering with the host response to pathogens.
The fact that the systemic release of certain DAMPs such as mtDNA and mitochondrial formylated peptides occurs during traumatic injury is important in our understanding of traumatic injury-induced SIRS. The ability of such circulating mediators to induce damage in numerous organs, even those remote to the initial site of injury, helps explain the alarming similarity between SIRS and microbial sepsis. It will be important to determine whether potential therapies which target endogenous products released from cells by tissue damage have any translational value, and whether or not these endogenous products could be similarly targeted in sepsis.
Herein, we have highlighted a number of the sterile inflammatory mechanisms that orchestrate neutrophil recruitment either directly or indirectly to sites of tissue injury and necrotic cell death. DAMPs, like extracellular ATP, mitochondrial formylated peptides, and mtDNA, play a central role in recruiting neutrophils to sterile injury. We have outlined how the establishment of a potent pro-inflammatory environment, in most circumstances characterized by the generation of functional IL-1β, facilitates the initial recruitment of neutrophils, but further mediators, such as endogenous chemokine gradients and ligands released from the dead cells themselves, direct neutrophils into necrotic foci once they are in the general vicinity of an injury. These overlapping, yet non-redundant signals efficiently guide neutrophils to the precise site of injury and likely spare surrounding viable tissues from further damage, potentially setting the stage for the establishment of resolution phase programs.
Acknowledgements
The work in the authors' laboratory is supported by Canadian Institutes for Health Research operating grants and group grant, as well as the Canadian Foundation for Innovation. P.K. is an Alberta Heritage Foundation for Medical Research (AIHS) Scientist and the Snyder Chair in Critical Care Medicine. K.P. is supported by Alberta Innovates Health Solutions.
References
- 1.Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- 2.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 3.Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol. 2009;9:364–375. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- 4.Ward PA, Acute and chronic inflammation . Fundamentals of Inflammation. In: Serhan CN, Ward PA, Gilroy DW, editors. New York: Cambridge University Press; 2010. pp. pp 1–16. [Google Scholar]
- 5.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 6.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J Immunol. 2010;184:4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 10.Kono H, Orlowski GM, Patel Z, Rock KL. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J Immunol. 2012;189:3734–3740. doi: 10.4049/jimmunol.1200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock KL, Lai JJ, Kono H. Innate and adaptive immune responses to cell death. Immunol Rev. 2011;243:191–205. doi: 10.1111/j.1600-065X.2011.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 15.Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol. 2005;175:2777–2782. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- 16.Cavassani KA, Ishii M, Wen H, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imaeda AB, Watanabe A, Sohail MA, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheibner KA, Lutz MA, Boodoo S, et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 19.Chou RC, Kim ND, Sadik CD, et al. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33:266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 21.Davalos D, Grutzendler J, Yang G, Kim JV, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 22.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 24.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 26.Ye RD, Boulay F, Wang JM, et al. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. J Exp Med. 1982;155:264–275. doi: 10.1084/jem.155.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabiet MJ, Huet E, Boulay F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur J Immunol. 2005;35:2486–2495. doi: 10.1002/eji.200526338. [DOI] [PubMed] [Google Scholar]
- 29.Hauser C, Sursal T, Rodriguez EK, et al. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J Orthop Trauma. 2010;24:534–538. doi: 10.1097/BOT.0b013e3181ec4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma. 2010;68:1328–1334. doi: 10.1097/TA.0b013e3181dcd28d. [DOI] [PubMed] [Google Scholar]
- 31.Tsukamoto T, Chanthaphavong RS, Pape HC. Current theories on the pathophysiology of multiple organ failure after trauma. Injury. 2010;41:21–26. doi: 10.1016/j.injury.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Marques PE, Amaral SS, Pires DA. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology. 2012;56:1971–1982. doi: 10.1002/hep.25801. [DOI] [PubMed] [Google Scholar]
- 33.Campbell JJ, Foxman EF, Butcher EC. Chemoattractant receptor cross talk as a regulatory mechanism in leukocyte adhesion and migration. Eur J Immunol. 1997;27:2571–2578. doi: 10.1002/eji.1830271016. [DOI] [PubMed] [Google Scholar]
- 34.Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–1360. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heit B, Tavener S, Raharjo E, et al. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng LG, Qin JS, Roediger, et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J Invest Dermatol. 2011;131:2058–2068. doi: 10.1038/jid.2011.179. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Raoof M, Chen Y. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins LV, Hajizadeh S, Holme E, et al. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- 39.Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinon F. Dangerous liaisons: mitochondrial DNA meets the NLRP3 inflammasome. Immunity. 2012;36:313–315. doi: 10.1016/j.immuni.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Oka T, Hikoso S, Yamaguchi O, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stramer BM, Mori R, Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- 43.Martin P, DʼSouza D, Martin J, et al. Wound healing in the PU.1 null mouse - tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 44.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christoffersson G, Henriksnäs J, Johansson L, et al. Clinical and experimental pancreatic islet transplantation to striated muscle: establishment of a vascular system similar to that in native islets. Diabetes. 2010;59:2569–2578. doi: 10.2337/db10-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 47.Harty MW, Muratore CS, Papa EF. Neutrophil depletion blocks early collagen degradation in repairing cholestatic rat livers. Am J Pathol. 2010;176:1271–1281. doi: 10.2353/ajpath.2010.090527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khoury J, Ibla JC, Neish AS, Colgan SP. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Invest. 2007;117:703–711. doi: 10.1172/JCI30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 50.Hayhoe RPG, Kamal AM, Solito E, et al. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- 51.Ernst S, Lange C, Wilbers A, et al. An annexin 1 N-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J Immunol. 2004;172:7669–7676. doi: 10.4049/jimmunol.172.12.7669. [DOI] [PubMed] [Google Scholar]