Abstract
Paneth cell-derived enteric antimicrobial peptides significantly contribute to antibacterial host defense and host-microbial homeostasis. Regulation occurs by enzymatic processing and release into the small intestinal lumen, but the stimuli involved are incompletely understood. Here, the capacity of various microbial and immune stimuli to induce antimicrobial peptide release from small intestinal tissue was systematically evaluated using antibacterial activity testing, immunostaining for Paneth cell granules and mass spectrometry. We confirmed the stimulatory activity of the muscarinic receptor agonist carbachol and the nucleotide-binding oligomerization domain ligand muramyl dipeptide. In contrast, no release of antibacterial activity was noted after treatment with the Toll-like receptor ligands poly(I:C), lipopolysaccharide or CpG, and the cytokines interleukin (IL)-15, IL-22, IL-28 and interferon-γ. Rapid Paneth cell degranulation and antimicrobial activity release, however, was observed after stimulation with the endogenous mediators IL-4 and IL-13. This process required phosphatidylinositol 3-kinase and was associated with protein kinase B phosphorylation in Paneth cells. Flow cytometric analysis confirmed expression of the IL-13 receptor α1 on isolated Paneth cells. Our findings identify a novel role of IL-13 as inducer of Paneth cell degranulation and enteric antimicrobial peptide release. IL-13 may thus contribute to mucosal antimicrobial host defense and host microbial homeostasis.
Key Words: Intestine, Antimicrobial peptide, Interleukin-13, Paneth cell, Intestinal crypts
Introduction
Antimicrobial peptides represent a large family of evolutionary conserved small cationic molecules with broad range antibacterial, antiviral and antiprotozoal activity. These peptides can be divided into distinct families based on the amino acid sequence and secondary structure, and exhibit marked differences in their anatomical distribution, regulation and antibacterial spectrum [1]. In the small intestine, Paneth cells situated in the gland-like crypts secrete large quantities of antimicrobial peptides in addition to enzymes such as lysozyme and phospholipase A2, and the antibacterial lectin RegIIIγ [2, 3, 4]. Humans express two α-defensins, HD5 and HD6, encoded in two genomic copies per diploid genome [5]. Mouse Paneth cells express a large number of α-defensins (also named cryptdins) as well as a related family of the so-called cryptdin-related sequence (CRS) peptides [3, 6, 7].
Mice lacking Paneth cells or Paneth cell-derived antimicrobial peptides exhibit an enhanced susceptibility to infection [8, 9]. Conversely, transgenic expression of the human defensin HD5 by mouse Paneth cells confers enhanced resistance towards oral infection [10]. In addition to antibacterial host defense, Paneth cell-derived antimicrobial peptides also contribute to mucosal homeostasis and maintenance of the enteric microbiota [11]. Reduced α-defensin production has been associated with the translocation of commensal bacteria into the subepithelial tissue in mice and ileal Crohn's disease patients [12, 13, 14]. A number of conditions such as reduced activity of the transcription factor Tcf4 [15], lack of innate immune stimulation [16], endoplasmic reticulum stress or impaired Paneth cell survival/differentiation as a consequence of mutations in ATG16L1 or caspase 8 were associated with reduced antimicrobial peptide production and mucosal inflammation [17, 18, 19].
Paneth cell-derived defensins are mainly regulated at a posttranscriptional level [20, 21]. Degranulation of the peptide-containing vesicles and release into the crypt lumen appear to play a critical role, but this issue is still incompletely understood [1, 22, 23]. Both cholinergic stimulation and innate immune receptor stimulation by exposure to several microbial constituents have been suggested to induce antimicrobial peptide release [20, 23, 24, 25, 26]. In the present study we have extended these studies and systematically tested a number of microbial innate immune receptor ligands and endogenous immunological mediators with documented biological activity at the intestinal mucosa using an ex vivo model of small intestinal antimicrobial peptide secretion. Our results provide firm evidence that in addition to neurogenic and microbial stimuli, the endogenous mediators interleukin(IL)-4 and IL-13 stimulate Paneth cell degranulation and antimicrobial peptide release extending the list of biological functions of these cytokines.
Materials and Methods
Reagents
Endotoxin-free recombinant mouse IL-4, IL-13, IL-15, IL-22, IFN-γ and IL-28 (IFN-λ) was purchased from Peprotech (Rocky Hill, N.J., USA). Escherichia coli ultrapure Dm31 lipopolysaccharide (LPS) was obtained from List Biological Laboratories (Denver, Colo., USA) and stimulatory CpG Oligo 1668 with the sequence 5′-TCCATGACGTTCCTGATGCT-3′ from Eurofins MWG Operon (Ebersberg, Germany). Other reagents such as atropine, carbachol, wortmannin, Ly294002, poly(I:C) and synthetic muramyl dipeptide (MDP) were purchased from Sigma (Taufenkirchen, Germany) if not stated otherwise.
Ex vivo Mucosal Antimicrobial Peptide Release Assay
C3H/HeN mice were purchased from Charles River Breeding Laboratories (Wilmington, Mass., USA), housed under specific pathogen-free conditions, and treated in accordance with the local animal protection legislation (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit Oldenburg, 10/0143 and 12/0822). Animals were euthanized and small intestinal tissue was removed. Intestinal crypts were isolated as recently described [27] and incubated in the absence or presence of 100 µM of carbachol for 30 min. Cell-free supernatant of untreated and carbachol-treated crypts was used in an antibacterial activity assay (see below). In addition, it was lyophylized, extracted in ice-cold 60% aqueous acetonitrile containing 1% trifluoroacetic acid and centrifuged at 11,000 g for 20 min. Supernatants were freeze-dried and redissolved in 20% ethanol. Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry analysis was carried out with a Reflex III (Bruker Daltonics, Leipzig, Germany) [28]. The detection of prodefensins/pro-CRS peptides indicates secretion also of non-marker matrix metalloproteinase 7 (MMP7)-processed peptides. Prodefensins/pro-CRS peptides appear to be more readily detected by mass spectrometry as compared to mature defensins/CRS peptides and might be overrepresented in this type of analysis.
For the ex vivo Paneth cell release assay, ileal small intestinal mucosal tissue sections were flushed with PBS and mounted in Ussing chambers (World Precision Instruments Inc., Sarasota, Fla., USA) filled at the serosal and mucosal side with 0.5 ml of nonsupplemented RPMI1640. Carbachol (100 µM), carbachol plus atropine (1 mM), microbial stimuli (concentration as indicated) or endogenous mediators (10 ng/ml) with or without wortmannin (0.5 µM) or Ly294002 (50 µM) were added to the mucosal tissue surface and incubated for 45 min at 37°C. Mucosal fluid was removed and stored at −80°C. Antibacterial activity was measured using the indicator strain Bacillus megaterium 11 (Bm11) [29]. Bm11 was grown overnight in Luria broth (LB), washed, diluted 1: 20 in RPMI1640 supplemented with 10% LB and cultivated at 37°C. After reaching an optical density (OD600) of 0.6, bacteria were diluted 1:3 in RPMI. Mucosal supernatants at the indicated dilution and 10 µl of Bm11 suspension containing 104 CFU were filled with RPMI1640 to a total volume of 100 µl in triplicates. After incubation for 30 min at 37°C, the number of viable bacteria was determined by serial dilution and plating on LB agar plates. CFU are expressed as the percent of the initial bacterial inoculum (×104) recovered after incubation with the supernatant at the indicated dilution. Variation in the background antibacterial activity of individual animals required the simultaneous analysis of stimulated and nonstimulated tissue from the same animal and the measurement of antibacterial activity at different dilutions. This comparative approach allowed the characterization of the stimulatory potential of a given agent examined. Representative results from 3-12 individual experiments are shown in figure 1, 2 and 3.
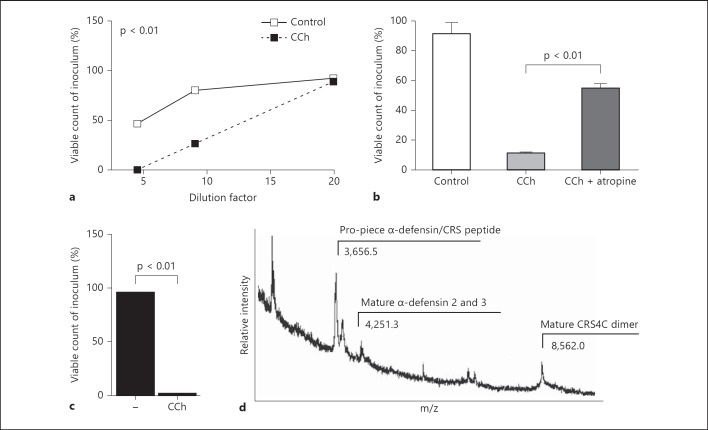
Fig. 1.
Release of antibacterial activity and antimicrobial peptides by muscarinic receptor stimulation of small intestinal tissue. a Antibacterial activity was measured at various dilutions of mucosal supernatant of small intestinal tissue left untreated or stimulated with carbachol (CCh; 100 µM) using the indicator strain Bm11. Results are representative of 10 independent experiments. b CCh-induced antibacterial activity was inhibited in the presence of the muscarinic inhibitor atropine (1 mM). Values are representative of 12 independent experiments. c Viable count of the indicator strain Bm11 after incubation in supernatant of isolated primary crypts incubated in PBS or CCh for 30 min at 37°C. d Mass spectrometric analysis of the CCh-induced crypt supernatant identified the presence of a number of Paneth cell-derived antimicrobial peptides. Values are means ± SD.
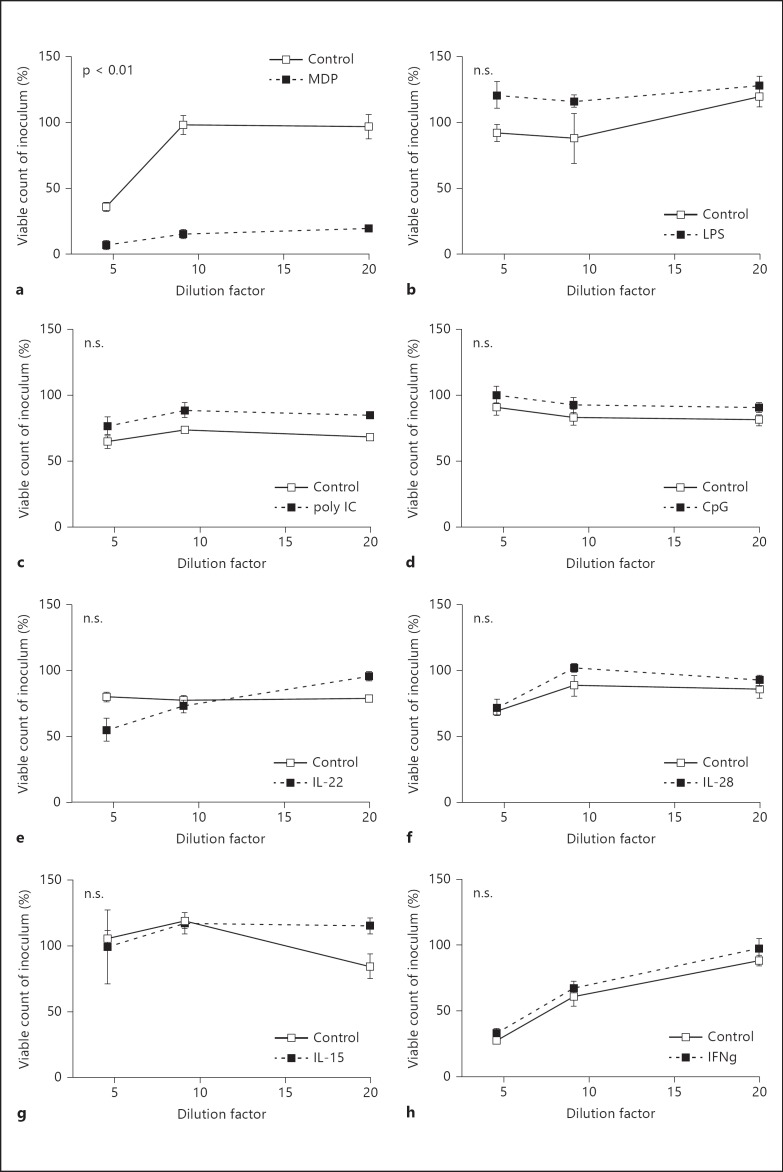
Fig. 2.
Release of mucosal antibacterial activity by microbial stimuli and endogenous immune mediators. Ileal mucosal tissue planted in Ussing chambers was left untreated or stimulated for 45 min with microbial stimuli such as MDP (100 µg/ml; a), LPS (100 ng/ml; b), poly(I:C) (10 ng/ml; c) and CpG DNA (1 µg/ml; d), as well as endogenous immune mediators such as IL-22 (10 ng/ml; e), IL-28 (10 ng/ml; f), IL-15 (10 ng/ml; g) and IFN-γ (10 ng/ml; h), and the tissue supernatant was analyzed at 5-20× dilutions for its antibacterial activity against the indicator strain Bm11. Values are means ± SD and indicate a representative dataset of 3-12 independent experiments. n.s. = Not significant.
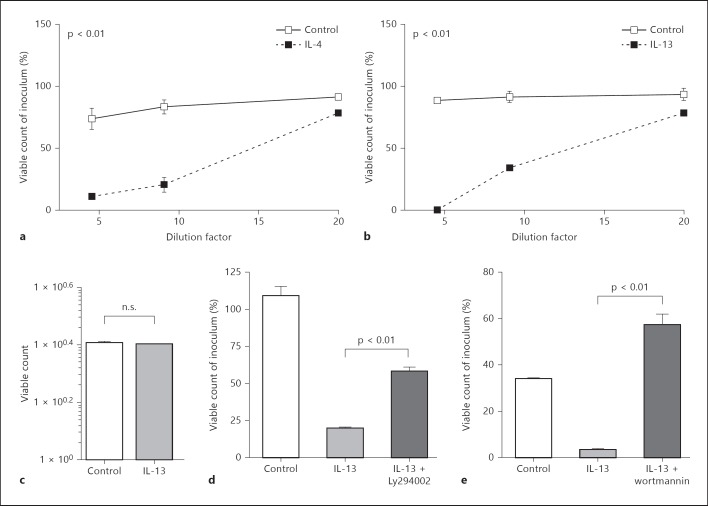
Fig. 3.
IL-4 and IL-13 promote the release of antibacterial activity in a PI3K-dependent fashion. Mucosal tissue planted in Ussing chambers was left untreated or stimulated for 45 min with IL-4 (10 ng/ml; a) or IL-13 (10 ng/ml; b), and the tissue supernatant was analyzed at various dilutions for its antibacterial activity against the indicator strain Bm11. Results are representative of at least 12 independent experiments. c Incubation of Bm11 in the absence or presence of IL-13 at 10 ng/ml. Mucosal tissue was stimulated with IL-13 in the absence or presence of Ly294002 (50 µM; d) or wortmannin (0.5 µM; e) and the tissue supernatant was analyzed for its antibacterial activity against the indicator strain Bm11. A representative dataset of at least 3 independent experiments is shown. Values are means ± SD. n.s. = Not significant.
Immunostaining and Immunoblot
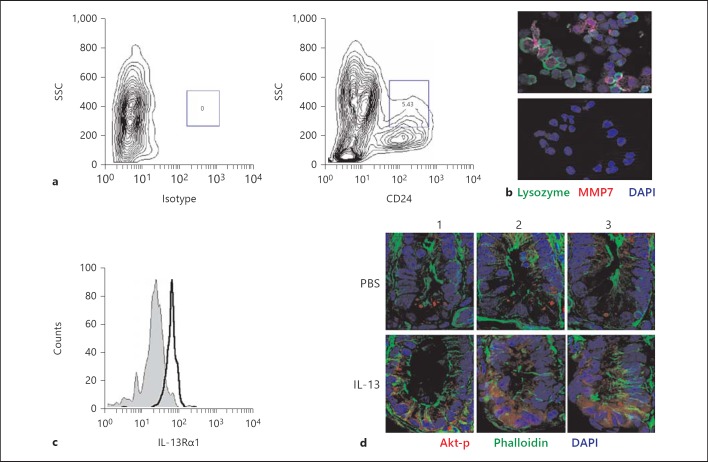
Mice were injected with PBS or IL-13 (300 ng per mouse) in 500 µl of PBS i.p. After 15 and 30 min, the animals were euthanized, ileal small intestinal tissue was removed and fixed in formaldehyde. Antigen retrieval in deparaffinized formaldehyde-fixed tissue sections was performed by boiling in 0.01 M sodium citrate buffer (pH 6.0). For cryptdin staining, slides were blocked with normal goat serum (Jackson ImmunoResearch, Newmarket, UK) and stained with anti-cryptdin 2 antiserum in combination with a Cy3-conjugated goat anti-rabbit antibody [29]. Due to the high sequence identity of most cryptdin family members, the antibody employed most probably detects several cryptdin family members. Approximately 400 Paneth cells lining the circumference of four individual ileal small intestinal tissue sections were visually analyzed for granularity. Rabbit anti-lysozyme P antiserum (1:250; DakoCytomation, Glostrup, Danmark) was incubated for 1 h at room temperature and detected using a TR-conjugated anti-rabbit secondary antibody (1:50; Jackson ImmunoResearch). Phosphorylated Akt was stained using a rabbit anti-phospho-Akt antibody (1:25; Cell Signaling Technology, Danvers, Mass., USA) overnight at 4°C in combination with a Cy3-conjugated goat anti-rabbit secondary antibody (1:500). For MMP7 staining, flow cytometrically sorted CD24-positive intestinal epithelial cells were blocked with normal donkey serum (Jackson ImmunoResearch) and stained with 1:50 diluted goat polyclonal antibodies against MMP7 (R&D Systems, Wiesbaden-Nordenstadt, Germany) in combination with a Cy3-conjugated donkey-anti-goat secondary antibody (Jackson ImmunoResearch). Sorted cells were stained for lysozyme as described above. A mouse monoclonal anti-E-cadherin antibody (BD Bioscience Pharmingen, Heidelberg, Germany) followed by the appropriate AF488-conjugated secondary antibody (Molecular Probes, Jackson ImmunoResearch), FITC-labeled wheat germ agglutinin or FITC-conjugated phalloidin was used for counterstaining. Slides were mounted in DAPI containing Vectashield (Vector) and visualized with an ApoTome-equipped Axioplan 2 microscope connected to an AxioCam MR digital Camera (Carl Zeiss MicroImaging Inc., Göttingen, Germany). m-ICcl2 cells were cultured as recently described [27]. A rabbit-anti-phospho-STAT6 (Tyr641) polyclonal antibody (Cell Signaling Technology, Beverly, Mass., USA) and an anti-β-actin antibody (N-terminal domain; Sigma-Aldrich, Taufenkirchen, Germany) were used in combination with peroxidase-labeled goat-anti-mouse or goat-anti-rabbit secondary antibodies (Jackson ImmunoResearch). Detection was performed using peroxidase-labeled goat-anti-rabbit secondary antibodies (Jackson ImmunoResearch) in combination with an ECL kit (Amersham Bioscience, Amersham, UK).
Flow Cytometry
Intestinal epithelial cells were prepared as described before [27]. In brief, small intestinal tissue was inverted and incubated in 0.3 M EDTA at 37°C for 10 min. Subsequently, the epithelium was removed in large cell aggregates by mechanical sharing and epithelial crypts were separated at 4°C from epithelium-associated leukocytes and enriched from villus epithelial cells by repeated differential sedimentation at 1 g. For flow cytometry, epithelial cells were trypsinized, filtered and stained for E-cadherin and CD45 using an APC-conjugated anti-E-cadherin (Abcam, Cambridge, UK) and a FITC-labeled anti-CD45 antibody (BD Bioscience) to demonstrate cell purity. Epithelial cells were >96% E-cadherin+ (not shown) and <2% CD45+ (data not shown). Approximately 4–6% stained SSChiCD24hi using a PE-conjugated anti-CD24 antibody from eBioscience (San Diego, Calif., USA). SSChiCD24hi cells stained positive for lysozyme P by flow cytometry. Immunofluorescence staining of sorted SSChiCD24hi cells revealed expression of the Paneth cell marker MMP7 and lysozyme following the protocol described above. Additionally, SSChiCD24hi cells were stained using a PE-conjugated anti-mouse IL-13Rα1 (CD213a1, clone 13MOKA) antibody (eBioscience) after being blocked by a mouse BD Fc Block™ (BD Bioscience Pharmingen) or the supernatant from 2.4G2 hybridoma. Flow cytometry was performed using a FACSCalibur or FACSCanto (Becton Dickinson, Franklin Lakes, N.J., USA) and analyzed with CellQuest™ or FlowJo software. FACS sorting was performed at the flow cytometry core facility of Hannover Medical School.
Statistical Analysis
Results are expressed as means ± SD, and are representative of at least three independent experiments for each of the experimental conditions tested. Differences were analyzed with the unpaired Student t test and the two-way ANOVA test. p < 0.05 or < 0.01 was considered significant or highly significant, respectively.
Results
Muscarinic Receptor Activation Stimulates the Release of Antibacterial Activity and Paneth Cell-Derived Antimicrobial Peptides from Murine Intestinal Tissue
In order to investigate the potential of endogenous and exogenous stimuli to induce the release of antimicrobial peptides by enteric Paneth cells, an ex vivo model of mucosal small intestinal tissue was established. Ileal intestinal tissue was obtained from healthy 6- to 8-week-old C3H/HeN mice. The visceral peritoneum was removed and the specimen was placed into an Ussing chamber allowing exposure of the serosal or mucosal surface with various stimuli and the analysis of luminal secretion of antibacterial activity. Medium was harvested 45 min after stimulation and analyzed for antibacterial activity using a bactericidal assay with the indicator strain Bm11 at various dilutions [28]. Although a certain variation in the degree of antibacterial activity released spontaneously or after stimulation from mucus tissue obtained from different mice was noted, this method allowed the identification of stimuli of Paneth cell release in an in situ setting. Addition of the parasympathomimetic carbachol led to a significantly increased antibacterial activity as compared to the untreated control tissue (8 out of 10, 8/10, experiments; fig. 1a). The specificity of the carbachol stimulus on the muscarinic receptor was confirmed using the muscarinic inhibitor atropine. Addition of atropine prior to carbachol stimulation significantly (p < 0.01) reduced the secreted antibacterial activity (9/12 experiments; fig. 1b). Additionally, the composition of the secreted antimicrobial peptides by carbachol stimulation was analyzed by mass spectrometry. Due to the minute quantities of supernatant obtained from Ussing chambers, 2-3 × 103 isolated small intestinal crypts were incubated in the presence of carbachol and the cell-free supernatant containing significant antibacterial activity was analyzed by mass spectrometry (fig. 1c). As expected, a number of prominent Paneth cell-derived antimicrobial peptides such as mature α-defensin 2 or 3 and dimers of the CRS peptide CRS4C were identified in tissue supernatant after carbachol exposure in accordance with induced antimicrobial peptide release (fig. 1d). Of note, MALDI-TOF spectra reveal an incomplete antimicrobial peptide spectrum due to differences in their ability to be released from the matrix.
MDP but Not LPS, poly(I:C), CpG DNA, IL-15, IL-22, IL-28 or IFN-γ Induce the Release of Antibacterial Activity
Several microbial constituents have previously been reported to induce Paneth cell-mediated antimicrobial peptide secretion. Using our ex vivo assay for the release of antibacterial activity from mucosal small intestinal tissue, we next tested the stimulatory activity of microbial stimuli such as the peptidoglycan motif MDP, the ligand of the nucleotide-binding oligomerization domain (Nod2) receptor, as well as LPS, poly(I:C) and hypomethylated CpG DNA, and ligands of the Toll-like receptor (Tlr) 4, 3 and 9, respectively. Among the microbial stimuli tested, only MDP reproducibly enhanced the mucosal antibacterial activity (8/12 experiments; fig. 2a). No significant increase was found after exposure to LPS (0/7 experiments; fig. 2b), poly(I:C) (0/3 experiments; fig. 2c) or CpG oligonucleotides (0/6 experiments; fig. 2d).
Next, endogenous immune mediators involved in intestinal mucosal host defense and homeostasis were tested. Several cytokines such as IL-15, IL-22, IL-28 (IFN-λ) and IL-4 as well as IL-13 are known to significantly contribute to epithelial barrier integrity and antiviral, antibacterial and antiparasitic host response [30, 31, 32, 33]. No increase was noted after stimulation with IL-22 (0/6 experiments; fig. 2e), IL-28 (0/3 experiments; fig. 2f), IL-15 (0/5 experiments; fig. 2g) or IFN-γ (0/3 experiments; fig. 2h).
Paneth Cell Degranulation Is Induced by IL-13 and IL-4
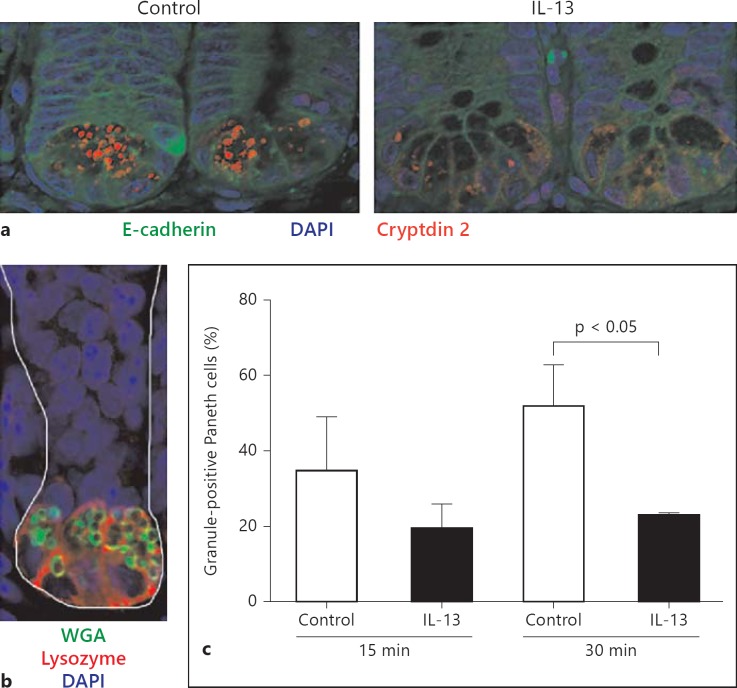
Strikingly, a significant release of antibacterial activity was noted after mucosal exposure to IL-4 (7/12 experiments; fig. 3a) or IL-13 (8/12 experiments; fig. 3b). No direct antibacterial activity of IL-13 against the antimicrobial peptide-sensitive indicator strain Bm11 was observed (fig. 3c) as previously described for chemokines [34]. The high-affinity IL-13 receptor composed of the IL-13Rα1 and IL-4Rα chain binds both IL-4 and IL-13, and transduces signals through the signal transducer and activator of transcription (STAT)6 via Jak kinases. IL-13Rα1 and IL-4Rα expression as well as IL-13-induced STAT6 phosphorylation have previously been observed in primary intestinal epithelial cells and small intestinal epithelial m-ICcl2 cells (online suppl. fig. 1D; for all online suppl. material, see www.karger.com/doi/10.1159/000357644) [35]. Additional signaling pathways induced by the IL-13 receptor include the phosphatidylinositol 3-kinase (PI3K). IL-13-induced PI3K activation has been described in gut epithelial cells and associated with degranulation in other cell types such as mast cells and pancreatic β-cells [36, 37, 38, 39]. Indeed, IL-13 stimulation in the presence of the PI3K inhibitors Ly294002 (p < 0.01; 2/3 experiments; fig. 3d) or wortmannin (3/5 experiments; p < 0.01; fig. 3e) was significantly impaired. Paneth cell granules stain positive for cryptdin 2, a prominent α-defensin in C3H/HeN mice. Cryptdin 2 immunostaining was therefore used to determine IL-13-induced Paneth cell degranulation. Following i.p. administration of 300 ng of IL-13 per animal, a marked reduction of cryptdin 2-positive granules was detected (fig. 4a). Staining of small intestinal tissue sections with antibodies against lysozyme P and the lectin wheat germ agglutinin confirmed the anatomical localization of secretory Paneth cells (fig. 4b). Quantitative analysis indicated that i.p. IL-13 administration induced a significant (p < 0.05) reduction of granulated Paneth cells 30 min after i.p. administration in accordance with an IL-13-mediated secretion of Paneth cell antimicrobial peptides (fig. 4c).
Fig. 4.
IL-13-induced Paneth cell degranulation. a Small intestinal tissue sections from C3H/HeN mice treated i.p. with 500 µl of PBS (left panel, control) or IL-13 (300 ng/animal, in 500 µl; right panel) for 30 min were stained for cryptdin 2 (red) and E-cadherin (green). Counterstained with DAPI. Original magnification ×630. b Small intestinal tissue sections were stained for lysozyme P (red) and mucus (green) to illustrate the anatomical position of granulated Paneth cells. Counterstained with DAPI. Original magnification ×630. c Quantitative determination of Paneth cell granulation (%) every 15 and 30 min after administration of PBS (control) or IL-13.
Primary Paneth Cells Express the IL-13 Receptor IL-13Rα1
The functional role of IL-13 for mucosal host response, namely for antiparasitic defense and worm expulsion, has widely been studied [40]. Its potential influence on Paneth cell function, however, has not been investigated. Likewise, it is unclear whether Paneth cells express the IL-13 receptor. Since immunohistological staining of small intestinal tissue sections with an anti-IL-13Rα1 antiserum revealed no clear result, flow cytometric analysis of Paneth cells was established following a recently reported staining strategy [41]. Crypt epithelial cells were isolated [27], trypsinized and stained for E-cadherin and CD24 following the protocol described. A significant fraction of crypt epithelial cells (5–6%) stained positive for CD24 by flow cytometry (fig. 5a; online suppl. fig. 1A). CD24hi cells stained negative for CD45 (online suppl. fig. 1B) but positive for the Paneth cell marker lysozyme (online suppl. fig. 1C). To confirm the specificity, E-cadherin+ and CD24hi cells were flow cytometrically sorted and immunohistologically stained for expression of lysozyme as well as the proteolytic enzyme and Paneth cell MMP7 (fig. 5b) [9]. Subsequent immunostaining with an anti-IL-13Rα1 antibody and flow cytometric analysis of CD24hi cells confirmed IL-13 receptor expression by Paneth cells (fig. 5c).
Fig. 5.
Paneth cells express the IL-13 receptor IL-13Rα1 and respond to stimulation. a Small intestinal crypts were isolated, trypsinized, stained for CD24 and analyzed by flow cytometry. Paneth cells are gated as CD24hiSSChi cells resulting in 4–6% of all CD45-E-cadherin+ cells. b CD24hi cells were sorted and immunostained for lysozyme P and MMP7 expression. The lower panel depicts staining with isotype controls. Counterstained with DAPI. Original magnification ×630. c Isolated cells were stained using an anti-IL-13Rα1 antibody or an isotype control and CD24hi cells were analyzed by flow cytometry for expression of the IL-13 receptor. d Immunohistological staining of phosphorylated Akt in sections of the small intestine of mice 30 min after injection of PBS or IL-13 (300 ng) i.p. Images of three individual crypts (1-3) visualized in small intestinal tissue sections from PBS- and IL-13-treated mice are shown. Counterstained with phalloidin (green) and DAPI (blue). Original magnification ×400.
IL-13Rα1 receptor-mediated signaling in intestinal epithelial cells induces phosphorylation of STAT6 (online suppl. fig. 1D) [35] and via PI3K of the downstream protein kinase B (Akt). To confirm direct IL13Rα1-mediated signaling also in primary Paneth cells, mice were treated with 300 ng of IL-13 or PBS i.p. Thirty minutes after stimulation, animals were sacrificed and the small intestinal tissue was embedded for histological analysis. Immunostaining for phosphorylated Akt confirmed enhanced staining of Paneth cells in mice after IL-13 treatment (fig. 5d). These results indicate that Paneth cells express the IL-13 receptor IL-13Rα1 and stimulate PI3K-dependent signal transduction upon IL-13 exposure in vivo in accordance with a direct functional role of IL-13 in Paneth cell degranulation.
Discussion
More than 20 years ago, Satoh [22] observed enhanced numbers of electron-dense granules in Paneth cells after administration of the muscarinic receptor antagonist atropine, suggesting a role of the parasympathetic nerve stimulation for Paneth cell degranulation. Since then, a role of parasympathetic stimulation for the release of α-defensins has been confirmed [20, 24, 25]. In addition, innate immune receptors such as Toll-like receptors (Tlr) and the nucleotide-binding oligomerization domain (Nod)2 receptor have been implicated in Paneth cell degranulation. Ayabe et al. [20] reported rapid release of cryptdin-mediated antibacterial activity from isolated crypts in response to LPS and lipid A (both ligands of Tlr4), MDP (ligand of Nod2), lipoteichoic acid (ligand of Tlr2) similar to whole Gram-positive or Gram-negative bacteria. Strikingly, Tanabe et al. [25] observed LPS and lipid A-induced antimicrobial activity release even in Tlr4 defective C3H/HeJ mice. The effect of MDP is consistent with a marked reduction in Paneth cell antimicrobial peptide expression in Nod2-deficient mice associated with enhanced susceptibility to Listeria monocytogenes infection reported by another group [42]. Interestingly, intact Nod2 signaling appears to be required to maintain the enteric microbiota [43]. Rumio et al. [23, 26] additionally demonstrated Paneth cell degranulation following Tlr9 and Tlr3 stimulation associated with protection from Salmonella infection. Although these findings have not been confirmed, Tlr9 signals via the Tlr adaptor protein MyD88, and could thus have contributed to the preserved mucosal barrier formation in Paneth cell-specific MyD88-expressing mice [4].
In the present study we identified the first endogenous immune mediators, IL-4 and IL-13, to induce Paneth cell degranulation. Although we did not formally exclude indirect stimulation, the rapid kinetic of Paneth cell degranulation and release of antibacterial activity upon cytokine administration and the expression of the IL-13Rα1 chain on Paneth cells are highly suggestive of a direct cytokine-mediated effect. Binding of both IL-4 and IL-13 to the so-called type II IL-4 receptor suggests similar downstream effects upon receptor interaction. In the lamina propria, large amounts of IL-13 are produced by adaptive CD4+ Th2 lymphocytes and the recently discovered type 2 innate lymphoid cells [44, 45, 46]. Also, other cell types such as eosinophils, basophils, mast cells, natural killer cells and natural killer T cells have the capacity to produce IL-13. IL-13 production by type 2 innate lymphoid cells was shown to be enhanced by gut epithelium-derived signals such as IL-25 (IL-17E), IL-33 and thymic stromal lymphopoietin in response to mucosal infection or inflammatory stimuli [45, 47]. Thereby, a purely innate signal loop involving epithelial immune recognition triggered IL-13 secretion by innate lymphoid cells and Paneth cell degranulation might exist and protect the epithelial barrier from microbial insult. Alternatively, adaptive Th2 lymphocytes cause enhanced IL-13 production, e.g. during helminth infection that significantly contributes to worm expulsion [30]. Helminth infection has been associated with Paneth cell hyperplasia also in the absence of IL-4, and a striking correlation between Paneth cell hyperplasia and the extent of the elicited Th2 response has been noted [48, 49, 50]. It is therefore tempting to speculate that the Paneth cell hyperplasia observed under these circumstances might arise as a consequence of sustained IL-13 stimulation [48, 49]. Indeed, Paneth cell hyperplasia was also observed after repeated IL-9 administration acting in an IL-13-dependent manner [51]. IL-13-induced enhancement of Paneth cell-derived antimicrobial peptide secretion might further explain the lack of inflammation in epithelium-specific IL-13 transgenic mice as suggested by Mannon and Reinisch [47]. The simultaneous stimulation of Paneth cell degranulation and mucus secretion is in accordance with the described enrichment of antimicrobial peptides within the mucus layer and its proposed function as a physico-chemical barrier [52]. Finally, IL-13 has been shown to control inappropriate IL-17 effects and protect from colitis injury [53]. In contrast, Th1-mediated inflammatory conditions such as Crohn's disease counteract elevated IL-13 expression levels. In accordance, a marked reduction in Paneth cells and Paneth cell antimicrobial peptide production has been noted in Crohn's disease patients [13, 14, 17, 18, 19].
Several technical difficulties are encountered when investigating stimulatory agents for the release of antibacterial activity from mucosal tissue samples and these difficulties might explain differences between published reports and the current study [20, 23, 25]. The method employed, e.g. the use of isolated crypts versus intact mucosa, might significantly influence the results obtained. We feel that the use of an intact tissue sample with an intact anatomical tissue organization in combination with ligand exposure of the mucosal surface most closely resembles the situation in vivo. However, we detected antimicrobial peptide secretion also from highly enriched crypt preparations in response to carbachol. In contrast, no release was observed after stimulation of isolated crypts (data not shown) or intact tissue with LPS [20, 25]. This questions a previous report on LPS-induced peptide secretion by crypts isolated from Tlr4-defective C3H/HeJ mice [25]. Also, stimulatory agents might act directly or indirectly, i.e. stimulate other cell types that in turn activate Paneth cells. The release of antimicrobial peptide-containing mucus from the crypt lumen might thereby contribute to an increase in antibacterial activity [52]. In addition, the transcriptional upregulation of the antimicrobial protein RegIIIγ by Paneth cells or enterocytes might account for antibacterial activity after prolonged incubation [4]. Finally, differences in the antimicrobial peptide production between mouse strains, the possibility of distinct granule types and granule-specific release mechanisms, and technical limitations to assign antibacterial activity to the presence of antimicrobial peptides make this kind of analysis technically challenging [29]. The demonstration of a rapid increase in IL-13-induced PI3K-dependent antibacterial activity, the confirmation of IL-13Rα1 expression on Paneth cells and the visualization of cryptdin 2-positive vesicle degranulation provides strong evidence that IL-13 represents a direct endogenous mediator of Paneth cell antimicrobial peptide release. In accordance, IL-13 and downstream PI3K signaling have previously been implicated in the granular release of pancreatic β-cells and mast cells [37, 38, 39]. Our own attempts to demonstrate a beneficial effect of IL-13 administration on bacterial infection failed to reveal a significant reduction in bacterial organ counts. This might, however, be explained by the stimulation of antimicrobial peptide release by additional pathways such as stimulation of Nod2 or local production of acetylcholine under inflammatory conditions. Further investigations are thus required, to characterize the functional role of IL-13-mediated Paneth cell secretion in vivo.
In conclusion, we have identified a novel role of IL-13 as a potent inducer of small intestinal Paneth cell degranulation and antimicrobial peptide release. IL-13 secretion by cells of the innate or adaptive immune system might thus contribute to provide antimicrobial protection, reinforce the mucosal barrier and maintain host-microbial homeostasis. The described effect might contribute to the clinical success of helminth therapy in patients with Crohn's disease [54]. On the other hand, reduced mucosal IL-13 levels in the absence of continuous Th2-mediated immune stimulation due to improved hygiene conditions and a lower prevalence of helminth infections in industrialized countries might lead to alterations in the enteric microbiota, particularly of the infant population, and in turn predispose to inflammatory and autoimmune diseases [55, 56].
Disclosure Statement
The authors have no conflict of interest to declare.
Supplementary Material
Supplementary data
Acknowledgments
We would like to thank Dominique Gütle for excellent technical help. M.W.H., T.A. and A.D. were supported by the Collaborative Research Center SFB621 and 900, the German Research Foundation (Ho2236/8-1), the Lower Saxony Research Network on Neuroinfectiology (N-RENNT), the International Research Training Program (IRTG)1273 and the German Israel Collaborative Initiative. C.U.D. was supported by the Canadian Institutes of Health Research and the German National Academy of Sciences Leopoldina. S.M. received postdoctoral fellowships from the French Fondation de la Recherche Médical. S.S. was supported by a long-term fellowship from the Federation of European Biochemical Societies (FEBS) and an APART postdoctoral fellowship from the Austrian Academy of Sciences.
References
- 1.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 2.Selsted ME, Miller SI, Henschen AH, Ouellette AJ. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992;118:929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouellette AJ, Lualdi JC. A novel mouse gene family coding for cationic, cysteine-rich peptides: regulation in small intestine and cells of myeloid origin. J Biol Chem. 1990;265:9831–9837. [PubMed] [Google Scholar]
- 4.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linzmeier RM, Ganz T. Human defensin gene copy number polymorphisms: comprehensive analysis of independent variation in α- and β-defensin regions at 8p22-p23. Genomics. 2005;86:423–430. doi: 10.1016/j.ygeno.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Amid C, Rehaume LM, Brown KL, Gilbert JG, Dougan G, Hancock RE, Harrow JL. Manual annotation and analysis of the defensin gene cluster in the C57BL/6J mouse reference genome. BMC Genomics. 2009;10:606. doi: 10.1186/1471-2164-10-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson ML, Karlsson-Sjöberg JM, Pütsep KL. CRS-peptides: unique defense peptides of mouse Paneth cells. Mucosal Immunol. 2012;5:367–376. doi: 10.1038/mi.2012.22. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez MI, Regnault B, Mulet C, Tanguy M, Jay P, Sansonetti PJ, Pédron T. Maturation of Paneth cells induces the refractory state of newborn mice to Shigella infection. J Immunol. 2008;180:4924–4930. doi: 10.4049/jimmunol.180.7.4924. [DOI] [PubMed] [Google Scholar]
- 9.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, López-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal α-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 10.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 11.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schäffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, Schröder JM, Bevins CL, Fellermann K, Stange EF. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal α-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H, Jr, Fellermann K, Ganz T, Stange EF, Bevins CL. Reduced Paneth cell α-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehkamp J, Wang G, Kübler I, Nuding S, Gregorieff A, Schnabel A, Kays RJ, Fellermann K, Burk O, Schwab M, Clevers H, Bevins CL, Stange EF. The Paneth cell α-defensin deficiency of ileal Crohn's disease is linked to Wnt/Tcf-4. J Immunol. 2007;179:3109–3118. doi: 10.4049/jimmunol.179.5.3109. [DOI] [PubMed] [Google Scholar]
- 16.Bevins CL, Stange EF, Wehkamp J. Decreased Paneth cell defensin expression in ileal Crohn's disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut. 2009;58:882–883. [PubMed] [Google Scholar]
- 17.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW., 4th A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000;1:113–118. doi: 10.1038/77783. [DOI] [PubMed] [Google Scholar]
- 21.Menendez A, Willing BP, Montero M, Wlodarska M, So CC, Bhinder G, Vallance BA, Finlay BB. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal Paneth cell α-defensins. J Innate Immun. 2013;5:39–49. doi: 10.1159/000341630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satoh Y. Atropine inhibits the degranulation of Paneth cells in ex-germ-free mice. Cell Tissue Res. 1988;253:397–402. doi: 10.1007/BF00222296. [DOI] [PubMed] [Google Scholar]
- 23.Rumio C, Sommariva M, Sfondrini L, Palazzo M, Morelli D, Viganò L, De Cecco L, Tagliabue E, Balsari A. Induction of Paneth cell degranulation by orally administered Toll-like receptor ligands. J Cell Physiol. 2012;227:1107–1113. doi: 10.1002/jcp.22830. [DOI] [PubMed] [Google Scholar]
- 24.Satoh Y, Habara Y, Ono K, Kanno T. Carbamylcholine- and catecholamine-induced intracellular calcium dynamics of epithelial cells in mouse ileal crypts. Gastroenterology. 1995;108:1345–1356. doi: 10.1016/0016-5085(95)90681-9. [DOI] [PubMed] [Google Scholar]
- 25.Tanabe H, Ayabe T, Bainbridge B, Guina T, Ernst RK, Darveau RP, Miller SI, Ouellette AJ. Mouse Paneth cell secretory responses to cell surface glycolipids of virulent and attenuated pathogenic bacteria. Infect Immun. 2005;73:2312–2320. doi: 10.1128/IAI.73.4.2312-2320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rumio C, Besusso D, Palazzo M, Selleri S, Sfondrini L, Dubini F, Ménard S, Balsari A. Degranulation of Paneth cells via Toll-like receptor 9. Am J Pathol. 2004;165:373–381. doi: 10.1016/S0002-9440(10)63304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotz M, Gütle D, Walther S, Ménard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putsep K, Axelsson LG, Boman A, Midtvedt T, Normark S, Boman HG, Andersson M. Germ-free and colonized mice generate the same products from enteric prodefensins. J Biol Chem. 2000;275:40478–40482. doi: 10.1074/jbc.M007816200. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson J, Pütsep K, Chu H, Kays RJ, Bevins CL, Andersson M. Regional variations in Paneth cell antimicrobial peptide expression along the mouse intestinal tract. BMC Immunol. 2008;9:37. doi: 10.1186/1471-2172-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 31.Pott J, Mahlakõiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, Hornef MW. IFN-λ determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci USA. 2011;108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulthess J, Meresse B, Ramiro-Puig E, Montcuquet N, Darche S, Bègue B, Ruemmele F, Combadière C, Di Santo JP, Buzoni-Gatel D, Cerf-Bensussan N. Interleukin-15-dependent NKp46+ innate lymphoid cells control intestinal inflammation by recruiting inflammatory monocytes. Immunity. 2012;37:108–121. doi: 10.1016/j.immuni.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 34.Linge HM, Collin M, Nordenfelt P, Mörgelin M, Malmsten M, Egesten A. The human CXC chemokine granulocyte chemotactic protein 2 (GCP-2)/CXCL6 possesses membrane-disrupting properties and is antibacterial. Antimicrob Agents Chemother. 2008;52:2599–2607. doi: 10.1128/AAC.00028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotz M, König T, Ménard S, Gütle D, Bogdan C, Hornef MW. Cytokine-mediated control of lipopolysaccharide-induced activation of small intestinal epithelial cells. Immunology. 2007;122:306–315. doi: 10.1111/j.1365-2567.2007.02639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceponis PJ, Botelho F, Richards CD, McKay DM. Interleukins 4 and 13 increase intestinal epithelial permeability by a phosphatidylinositol 3-kinase pathway. Lack of evidence for STAT 6 involvement. J Biol Chem. 2000;275:29132–29137. doi: 10.1074/jbc.M003516200. [DOI] [PubMed] [Google Scholar]
- 37.Ali K, Camps M, Pearce WP, Ji H, Rückle T, Kuehn N, Pasquali C, Chabert C, Rommel C, Vanhaesebroeck B. Isoform-specific functions of phosphoinositide 3-kinases: p110δ but not p110γ promotes optimal allergic responses in vivo. J Immunol. 2008;180:2538–2544. doi: 10.4049/jimmunol.180.4.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pigeau GM, Kolic J, Ball BJ, Hoppa MB, Wang YW, Rückle T, Woo M, Manning Fox JE, MacDonald PE. Insulin granule recruitment and exocytosis is dependent on p110γ in insulinoma and human β-cells. Diabetes. 2009;58:2084–2092. doi: 10.2337/db08-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endo D, Gon Y, Nunomura S, Yamashita K, Hashimoto S, Ra C. PI3Kγ differentially regulates FcepsilonRI-mediated degranulation and migration of mast cells by and toward antigen. Int Arch Allergy Immunol. 2009;149:66–72. doi: 10.1159/000211375. [DOI] [PubMed] [Google Scholar]
- 40.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 41.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 43.Petnicki-Ocwieja T, Hrncir T, Liu YJ, Biswas A, Hudcovic T, Tlaskalova-Hogenova H, Kobayashi KS. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc Natl Acad Sci USA. 2009;106:15813–15818. doi: 10.1073/pnas.0907722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 46.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mannon P, Reinisch W. Interleukin 13 and its role in gut defence and inflammation. Gut. 2012;61:1765–1773. doi: 10.1136/gutjnl-2012-303461. [DOI] [PubMed] [Google Scholar]
- 48.Kamal M, Wakelin D, Ouellette AJ, Smith A, Podolsky DK, Mahida YR. Mucosal T cells regulate Paneth and intermediate cell numbers in the small intestine of T. spiralis-infected mice. Clin Exp Immunol. 2001;126:117–125. doi: 10.1046/j.1365-2249.2001.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamal M, Dehlawi MS, Brunet LR, Wakelin D. Paneth and intermediate cell hyperplasia induced in mice by helminth infections. Parasitology. 2002;125:275–281. doi: 10.1017/s0031182002002068. [DOI] [PubMed] [Google Scholar]
- 50.Dehlawi MS, Mahida YR, Hughes K, Wakelin D. Effects of Trichinella spiralis infection on intestinal pathology in mice lacking interleukin-4 (IL-4) or intestinal trefoil factor (ITF/TFF3) Parasitol Int. 2006;55:207–211. doi: 10.1016/j.parint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 51.Steenwinckel V, Louahed J, Lemaire MM, Sommereyns C, Warnier G, McKenzie A, Brombacher F, Van Snick J, Renauld JC. IL-9 promotes IL-13-dependent Paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa. J Immunol. 2009;182:4737–4743. doi: 10.4049/jimmunol.0801941. [DOI] [PubMed] [Google Scholar]
- 52.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Pütsep K, Andersson M. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57:764–771. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 53.Newcomb DC, Boswell MG, Zhou W, Huckabee MM, Goleniewska K, Sevin CM, Hershey GK, Kolls JK, Peebles RS., Jr Human TH17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. J Allergy Clin Immunol. 2011;127:1006–1013. doi: 10.1016/j.jaci.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reddy A, Fried B. The use of Trichuris suis and other helminth therapies to treat Crohn's disease. Parasitol Res. 2007;100:921–927. doi: 10.1007/s00436-006-0416-4. [DOI] [PubMed] [Google Scholar]
- 55.Grześkowiak Ł, Collado MC, Mangani C, Maleta K, Laitinen K, Ashorn P, Isolauri E, Salminen S. Distinct gut microbiota in southeastern African and northern European infants. J Pediatr Gastroenterol Nutr. 2012;54:812–816. doi: 10.1097/MPG.0b013e318249039c. [DOI] [PubMed] [Google Scholar]
- 56.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data