Abstract
The globally significant human pathogen group A Streptococcus (GAS) sequesters the host protease plasmin to the cell surface during invasive disease initiation. Recent evidence has shown that localized plasmin activity prevents opsonization of several bacterial species by key components of the innate immune system in vitro. Here we demonstrate that plasmin at the GAS cell surface resulted in degradation of complement factor C3b, and that plasminogen acquisition is associated with a decrease in C3b opsonization and neutrophil-mediated killing in vitro. Furthermore, the ability to acquire cell surface plasmin(ogen) correlates directly with a decrease in C3b opsonization, neutrophil phagocytosis, and increased bacterial survival in a humanized plasminogen mouse model of infection. These findings demonstrate that localized plasmin(ogen) plays an important role in facilitating GAS escape from the host innate immune response and increases bacterial virulence in the early stages of infection.
Key Words: Complement, Innate immunity, M protein, Phagocytosis, Plasmin(ogen), Streptococcus pyogenes
Introduction
The Gram-positive bacterium group A Streptococcus (GAS; Streptococcus pyogenes) is responsible for over 600 million infections each year [1]. Superficial infections including pharyngitis and impetigo follow from GAS colonizing epithelial surfaces of the upper respiratory tract and skin, while further dissemination into sterile tissue sites can lead to life-threatening conditions such as necrotizing fasciitis and streptococcal toxic shock syndrome [2]. The high disease burden of GAS underlies the need to identify key mechanisms of pathogenesis.
Subversion of the host plasminogen activation system is central to the initiation of invasive infection by GAS [3, 4, 5]. Plasminogen circulates as a 92-kDa zymogen in blood and extracellular fluids at an approximate concentration of 2 μM[6]. Plasminogen can be converted to plasmin by the host activators urokinase and tissue plasminogen activator, or bacterial activators including the secreted GAS protein streptokinase. GAS expresses multiple cell surface receptors for plasmin(ogen), including glyceraldehyde-3-phosphate dehydrogenase, surface α-enolase, and the plasminogen binding M-like proteins (PAM, Prp) [7]. Once localized to the bacterial surface, the broad-spectrum protease plasmin is thought to facilitate degradation of fibrin clots and extracellular matrix components, leading to bacterial dissemination. More recently, plasmin-mediated evasion of host immunity has emerged as a key mechanism in bacterial pathogenesis [7, 8].
The complement system is a critical component of the innate immune response against bacterial pathogens and involves the coordinated action of over 30 proteolytic plasma enzymes [9]. Activation of the complement cascade occurs via three distinct routes, comprising the classical, alternate, and lectin pathways. Central to each of these pathways is the cleavage product of C3 convertase, C3b, a key opsonin that binds to microbial surfaces. Phagocytes such as macrophages and neutrophils recognize opsonized bacteria via specific complement receptors, leading to phagocytosis and microbial killing.
Plasmin has been to shown to degrade C3b, and plasmin-mediated C3b degradation by bacteria has been observed in vitro [10, 11]. In the present study, we demonstrate for the first time that GAS-localized plasmin degrades human C3b, and that cell surface plasminogen acquisition correlates with decreased C3b deposition and resistance to complement-mediated neutrophil killing in vitro. Furthermore, we show that surface-associated plasmin(ogen) correlates with reduced C3b deposition, C3b-mediated neutrophil killing, and increased bacterial survival in vivo.
Materials and Methods
Bacterial Strains and Culture Conditions
The clinical GAS strain NS88.2 was isolated from a case of invasive blood infection and has been described previously [12]. The isogenic derivative NS88.2prp, which expresses a mutated M protein attenuated for plasminogen binding, has been described elsewhere [5]. A precise, in-frame allelic replacement of the streptokinase (ska) gene with cat encoding chloramphenicol transferase was created in GAS wild-type strain NS88.2 using established methods [13], and the resulting strain was designated NS88.2Δska. Phenotypic properties of this isogenic mutant strain (i.e. growth rate, capsule production, and streptokinase secretion) were confirmed as described [13]. To facilitate detection of GAS using flow cytometry, the green fluorescence protein (GFP) expression vector pDCermGFP, containing the e-gfpgene placed behind the constitutive tet, cat and erm promoters [14], was transformed into GAS strains NS88.2, NS88.2prp and NS88.2Δska using standard GAS electroporation techniques [15]. Streptococci were routinely cultured under static conditions at 37°C in Todd-Hewitt broth (Difco, North Ryde, N.S.W., Australia) supplemented with 1% (w/v) yeast extract or grown on horse blood agar (Oxoid, Adelaide, S.A., Australia). In all assays, GAS cultures were grown to an optical density of 0.5 at 600nm. Escherichia coli were propagated in Luria Bertani broth or agar at 37°C. For antibiotic selection, erythromycin was supplemented at 2 µg/ml for GAS and 500 µg/ml for E. coli.
Streptokinase-Mediated Cell Surface Plasmin Acquisition
To accumulate plasmin at the GAS cell surface, GAS were cultured in the presence of 100 nM human Glu-plasminogen (Haemotologic Technologies Inc., Essex Junction, Vt., USA) for 2 h at 37°C. Under these conditions, activation of plasminogen to plasmin is dependent on the secreted bacterial plasminogen activator, streptokinase. Glu-plasminogen was omitted from negative controls. Bacteria were pelleted by centrifugation for 10 min at 14,000 g and washed twice in phosphate-buffered saline (PBS) to remove unbound plasmin(ogen). Cells were then resuspended in 45 µl PBS prior to the addition of the plasmin-specific substrate Spectrozyme PL (American Diagnostica, Stamford, Conn., USA) to a final concentration of 0.5 mM. Cell surface plasmin activity was measured at 405 nm using a SpectraMax® plate reader (Molecular Devices, Sunnyvale, Calif., USA).
Cell Surface Plasmin Acquisition in Plasma
Cell surface plasmin acquisition in human or mouse plasma was performed essentially as described previously [4] in fresh plasma containing 10 mM sodium citrate. Briefly, bacteria were resuspended in plasma or PBS for 10 min at 37°C. After two washes with PBS, plasmin bound to the GAS surface was measured as described above.
Cell Surface Plasminogen Acquisition in Plasma
Binding of plasminogen to the GAS cell surface was detected as described previously [5]. Briefly, following incubation of GAS in either human or mouse plasma from mice expressing the human plasminogen transgene, plasminogen was eluted from the bacterial cell surface using 100 mM glycine-HCl (pH 2.0). The eluent was screened for the presence of plasminogen by Western blot analysis using rabbit anti-human plasminogen (Calbiochem, San Diego, Calif., USA), goat anti-rabbit IgG horseradish peroxidase (HRP) conjugate (Invitrogen, Carlsbad, Calif., USA), and enhanced chemiluminescence detection.
Human C3b Degradation
The ability of plasmin to degrade C3b was performed according to the methods described by Rooijakkers et al. [11]. Prior to an overnight incubation at 37°C, 1 µg human C3b (Calbiochem) was incubated in PBS, or PBS containing either 100 nM Glu-plasminogen, 100 nM streptokinase, 100 nM human plasmin (Haemotologic Technologies Inc.), or 100 nM Glu-plasminogen and 100 nM streptokinase. Following centrifugation, supernatant samples were collected and separated on a 10% (w/v) SDS-PAGE gel under reducing conditions. Proteins were transferred onto a nitrocellulose membrane and, after transfer, the membrane was blocked overnight in 10% (w/v) skim milk (Difco). C3b was detected using polyclonal goat antisera raised against human C3 (Calbiochem) followed by HRP-conjugated rabbit anti-goat IgG (Invitrogen) for 1 h at room temperature. Protein bands were visualized using enhanced chemiluminescence reagents (Pierce Biotechnology, Rockford, Ill., USA) following the manufacturer's instructions. To assess the impact of GAS cell surface plasmin activity on C3b, GAS with or without cell-associated plasmin was incubated overnight with 0.5 µg human C3b at 37°C in a total volume of 10 µl and C3b degradation visualized as above.
Characterization of C3b Deposition in vitro
The influence of fluid-phase plasmin on C3b deposition was analyzed by whole-cell ELISA according to the methods of Rooijakkers et al. [11]. Bacteria were resuspended in PBS and coated onto a 96-well microtiter plate and incubated for 2 h at 37°C followed by incubation overnight at 4°C. Following each incubation step, wells were washed three times with PBS containing 0.05% (v/v) Tween-20. Blocking was performed with 100 µl of 1% (w/v) bovine serum albumin (BSA) for 1 h at 37°C. Fresh human plasma was then serially diluted 1:3 across the plate in 1% BSA and incubated at 37°C for 20 min. Negative controls did not receive aliquots of human plasma. Following washing, 50 µl of 100 nM human plasmin or 1% BSA were added to each well, and the plate incubated for 1 h at 37°C. Overnight incubation at 37°C was conducted after the addition of 50 µl of goat anti-human C3 serum, followed by HRP-conjugated rabbit anti-goat IgG for 1 h at 37°C. After the addition of 50 µl substrate solution [8 mM Na2HPO4 adjusted to pH 5.0 with solid citric acid containing 0.4% (w/v) o-phenylenediamine and 4% (v/v) H2O2], reactions were stopped with 1 M HCl and C3b deposition was visualized spectrophotometrically at 490 nm. Coating of GAS to the microtiter plate was confirmed using rabbit anti-M antisera [16] followed by detection with HRP-conjugated goat anti-rabbit IgG.
To examine the effects of GAS cell surface plasmin acquisition on C3b deposition at the bacterial surface, fluorescent GAS were incubated in 100 µl of 50% human or mouse plasma diluted in PBS for 10 min at 37°C. Following two washes with PBS, nonspecific binding sites were blocked by incubating bacteria with human IgG (Invitrogen) diluted 1:500 in PBS for 30 min on ice. Bacteria were washed twice and resuspended in 200 µl of PBS, which was subsequently divided into two 100 µl samples. To detect surface-bound C3b, samples were then incubated with 0.2 µg of phycoerythrin (PE)-conjugated anti-human C3 monoclonal antibody (Cederlane Laboratories Ltd., Burlington, Ont., Canada) or 0.2 µg of PE-labeled mouse IgG1 isotype control (Cederlane Laboratories Ltd.) and light protected for 1 h on ice. For flow-cytometry analysis, bacterial cells were resuspended in 100 µl of PBS, fixed with 100 µl 4% (w/v) paraformaldehyde and made up to a final volume of 400 µl PBS. Alternatively, opsonized bacteria were incubated with Spectrozyme PL to confirm the presence or absence of cell surface plasmin activity. Gating parameters for GFP-expressing GAS were determined using a scatter plot, and the amount of C3 attached to the bacterial surface was expressed as a percentage of fluorescent GAS. Ten thousand cells were collected for each sample using a BD LSRII flow cytometer (Franklin Lakes, N.J., USA) set to detect GFP and PE fluorescence. Data were analyzed with FlowJo7 8.4.6 (TreeStar Inc., Ashland, Oreg., USA).
Generation and Characterization of Homozygous Humanized Plasminogen Mice
Male and female, heterozygous, transgenic humanized plasminogen mice (AlbPLG1+/-) were crossed to generate a homozygous line. Resultant litters were genotyped following a simple yet efficient DNA extraction from ear cuttings. Briefly, the tissue samples were incubated in 100 µl of lysis buffer [100 mM Tris, pH 7.5, 5 mM EDTA, 200 mM NaCl, 0.5% (v/v) Tween-20, 15 µl proteinase K (1 mg/ml)] at 56°C for 4 h. The DNA samples were then used directly as templates for the detection of the transgene by PCR. Heterozygous and homozygous mice are indistinguishable by standard PCR analysis, thus we performed quantitative RT-PCR to identify progeny from the cross with higher levels of the human plasminogen transgene with the primers plasminogen A (5′-CAGCTCCCTGTGATTGAGAA-3′) and plasminogen B (5′-GAAGTGACTCCTTGTAAAATG-3′). Two male and 2 female individual mice displayed elevated transgene levels for human plasminogen and were subsequently backcrossed to wild-type C57BL/6J mice for confirmation of the homozygous genotype. Transgenic parents that gave rise to 100% heterozygous offspring (n ≥6 per litter), determined by standard PCR [3], were confirmed to be homozygous (AlbPLG1+/+) for the human plasminogen transgene and retained to establish the breeding colony.
Mouse plasma was assayed for mouse plasminogen using a mouse plasminogen-specific ELISA kit (CSB-EL018188MO) according to the manufacturer's instructions (Cusabio, Wuhan, China). To assess the level of activatable human plasminogen, human and mouse plasma samples were diluted 1:10 in assay buffer (10 mM HEPES, 150 mM NaCl, 0.01% Tween-20, pH 7.4). Human plasminogen present in the plasma samples was converted to plasmin by the addition of 5 nM streptokinase from group C streptococcal strain H46A [13] and incubation at room temperature for 15 min. Plasmin activity was then measured by adding the plasmin-specific substrate S2251 (500 µM) and monitoring the change in absorbance at 405 nm over time. Plasmin activity, expressed as a percentage of human plasma, was calculated by dividing the A405/min of mouse plasma by the A405/min of human plasma and then multiplying by 100. All reactions were linear over the initial 20 min of the reaction. To confirm GAS virulence in this model, cohorts of 10 age- and sex-matched mice (AlbPLG1+/- or AlbPLG1+/+) were infected subcutaneously with GAS as described previously [5]. The ability of GAS to acquire cell surface plasmin in plasma from different mouse lines was determined as described above.
C3b Deposition, Neutrophil Phagocytosis and GAS Survival in vivo
Sex-matched transgenic homozygous mice (aged between 8 and 10 weeks) expressing human plasminogen were intradermally inoculated with 1 × 108 CFU of eGFP-expressing NS88.2 (n = 8), NS88.2prp (n = 4) or NS88.2Δska (n = 4) into the right and left shaved flanks. Mice were sacrificed by CO2 asphyxiation 4 h thereafter, and the intradermal layer lavaged twice with 500 µl of sterile saline. C3b deposition on recovered GAS was assessed by flow cytometry as described above. Supernatants collected from the right and left flanks of each mouse were pooled to increase the yield of bacterial cells. To determine the level of GAS uptake by neutrophils, cells recovered from the flank were blocked with Fc Block (anti-mouse CD16/32; Biolegend, San Diego, Calif., USA). A cocktail of appropriately diluted Ly6C, Ly6G, and CD45 (Biolegend) was added in FACS buffer (Dulbecco's PBS, 0.2% BSA, 1 mM NaN3) followed by incubation on ice for 20 min. The cells were then washed and resuspended in 0.5 ml of FACS buffer, and acquired using flow cytometry flow cytometer.
Alternatively, the ability of NS88.2 (n = 6), NS88.2prp (n = 5) and NS88.2Δska (n = 5) to survive in vivo was determined by pelleting the collected bacteria at 14,000 g for 10 min. Following serial dilutions performed in sterile Milli-Q water, bacteria were subsequently plated onto horse blood agar overnight at 37°C and the number of bacterial colonies enumerated.
C3b-Mediated Polymorphonuclear Leukocyte Killing Assay
GAS strains were opsonized with autologous human plasma for 10 min at 37°C. Following two washes with PBS, bacteria (2 × 104 CFU) were diluted in RPMI media (Invitrogen) supplemented with 2% heat-inactivated plasma and incubated with polymorphonuclear leukocytes (PMN; 2 × 105) isolated from fresh human blood as described by Hollands et al. [8]. The reaction mixture was then plated onto horse blood agar and the CFU enumerated via colony counting following an overnight incubation at 37°C. Growth controls consisted of GAS grown under identical conditions in the absence of PMNs. GAS survival was calculated as a percentage of GAS surviving following PMN incubation compared with growth controls. To confirm that GAS killing was complement-mediated, bacteria were alternatively opsonized with 3% (w/v) C3-depleted human plasma (Calbiochem). GAS cell surface plasmin acquisition assays were also performed in parallel to confirm levels of cell surface plasmin.
Statistical Analysis
Differences in the level of plasmin acquisition at the GAS cell surface were determined using Student's t-test. All other data were analyzed using one-way analysis of variance with Tukey's multiple comparison test. Data sets were considered statistically significant at p < 0.05. All analysis was performed using GraphPad Prism version 4.02 (GraphPad Software Inc.).
Ethical Approval
Animal studies complied with the Guidelines for the Care and Use of Laboratory Animals (National Health and Medical Research Council, Australia) and were approved by the University of Wollongong Animal Ethics Committee (AE11/04, AE12/05 and AE11/09). Collection of blood was performed with the approval of the University of Wollongong Human Ethics Committee (HE08/250). Volunteers provided informed consent prior to donating blood samples.
Results
Plasmin at the GAS Cell Surface Degrades C3b
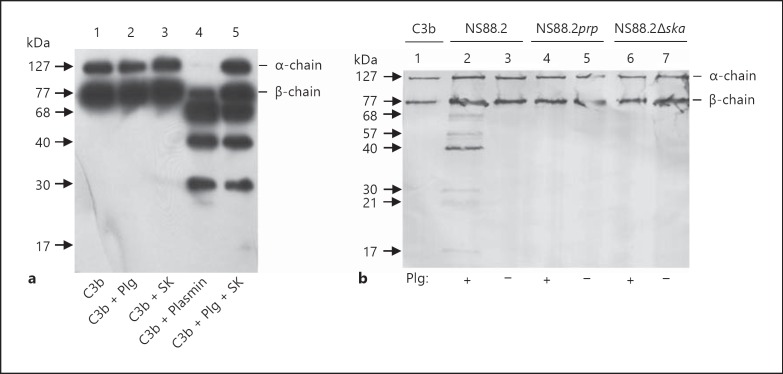
GAS streptokinase mediates plasminogen activation by the formation of a 1:1 stoichiometric complex with plasminogen, resulting in a complex with plasmin and plasminogen activator activity [17]. To confirm that plasmin generated by streptokinase could degrade C3b, as reported for plasmin generated using host activators, we employed a solution phase degradation assay followed by Western blot analysis. Streptokinase-generated plasmin degraded C3b, whereas no degradation was seen in the presence of either streptokinase or plasminogen alone (fig. 1a). To further assess the ability of plasmin sequestered to the GAS cell surface to degrade C3b, we employed a wild-type parent GAS strain (NS88.2) and its isogenic mutants abrogated for either cell surface plasmin acquisition (NS88.2prp) [5] or streptokinase expression (NS88.2∆ska; online suppl. fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000353754). C3b degradation by GAS was dependent on the presence of cell surface plasmin. Following preincubation with plasminogen, wild-type NS88.2 degraded C3b; however, incubation of C3b with NS88.2prp or NS88.2∆ska, which do not accumulate cell surface plasmin activity under these conditions, did not result in detectable C3b degradation (fig. 1b). Likewise, GAS strains were not able to degrade C3b without a source of cell surface plasmin activity (fig. 1b). These data clearly demonstrate that streptokinase-generated plasmin, and plasmin localized to the GAS cell surface mediated complement C3b degradation.
Fig. 1.
Streptokinase-generated plasmin and GAS cell surface plasmin degrade C3b. In both panels, protein samples were resolved by SDS-PAGE under reducing conditions and transferred onto a nitrocellulose membrane. Cleavage of C3b was analyzed via Western blotting using goat anti-C3 IgG and HRP-conjugated secondary antibody. a Human C3b was incubated overnight singly (lane 1) or together with plasminogen (Plg; lane 2); streptokinase (SK; lane 3); human plasmin (lane 4), or a combination of plasminogen and streptokinase (lane 5). b GAS were preincubated with (+) or without (-) 100 nM Glu-plasminogen for 2 h at 37°C, followed by incubation with C3b overnight. Intact C3b is visible as the α- and β-chain (lane 1). Plasmin at the cell surface of NS88.2 partially cleaved C3b into smaller degradation products (lane 2). C3b remained intact and was not cleaved in the absence of plasmin at the cell surface of NS88.2 (lane 3), or when NS88.2prp or NS88.2∆ska was incubated with C3b (lanes 4-7).
Plasmin(ogen) Reduces C3b Deposition on GAS in vitro
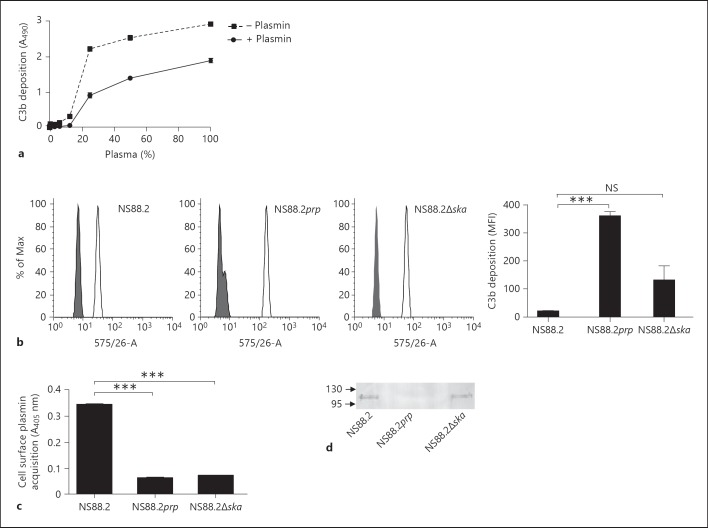
To determine if fluid-phase plasmin was able to deplete C3b from the bacterial surface, C3b deposition on the GAS cell surface in the presence and absence of plasmin was measured via whole-cell ELISA. C3b deposition was found to be dose dependent, with saturation occurring in 33% human plasma. The addition of exogenous plasmin after opsonization resulted in a significant decrease in the amount of C3b detected at the GAS cell surface (p < 0.001; fig. 2a). No signal was detected when GAS was incubated with 1% BSA alone (data not shown). To assess the impact of cell surface plasmin activity on C3b deposition, each isogenic GAS strain set was transformed with an eGFP-expressing plasmid, opsonized with human plasma, and C3b deposition measured via flow cytometry. Significantly less C3b was detected at the surface of wild-type strain NS88.2, which accumulates plasmin(ogen) on its cell surface, than NS88.2prp (p < 0.01), which does not accumulate plasmin(ogen) at the cell surface. However, no significant difference was seen in C3b deposition between NS88.2 and NS88.2∆ska (fig. 2b). No significant fluorescence was detected using an isotype-matched control antibody (data not shown). Cell surface plasmin acquisition assays confirmed that neither NS88.2prp nor NS88.2∆ska had significant levels of plasmin at the cell surface (fig. 2c), suggesting that plasminogen binding rather than plasmin activation or acquisition is responsible for the decrease in C3b deposition. Western blot analysis of proteins eluted from the GAS cell surface confirmed that both NS88.2 and NS88.2∆ska, which showed decreased levels of C3b at the cell surface, bound plasminogen in human plasma, whilst NS88.2 prp, which was readily opsonized by C3b, did not (fig. 2d).
Fig. 2.
Plasmin(ogen) decreases C3b deposition on GAS in vitro. a The impact of fluid-phase plasmin on C3b deposition on the GAS cell surface was assessed using whole-cell ELISA. The addition of exogenous plasmin after bacterial opsonization led to a significant decrease in C3b attachment to the bacterial surface (p < 0.001). b In order to assess the impact of cell surface plasmin on C3b deposition, bacteria were opsonized at 37°C for 10 min in 50% human plasma as a source of complement and plasmin(ogen). Nonspecific binding sites were blocked by incubating bacteria with human IgG and surface-bound C3b was probed using PE-conjugated monoclonal antibody raised against human C3. Alternatively, cells were stained with PE-labeled mouse IgG isotype control. C3b deposition on the bacterial surface was analyzed using flow cytometry. C3b deposition was significantly reduced on the surface of NS88.2 compared to NS88.2prp (p < 0.001), but not NS88.2Δska. Histograms show the shift in mean fluorescence intensity (MFI) between GAS stained with the isotype control (grey) and an anti-C3 antibody (white). Bars indicate means ± SE of triplicates expressed in MFI, representative of two independent experiments. c GAS cell surface plasmin activity was confirmed by incubating bacteria with the plasmin-specific substrate Spectrozyme PL overnight. d GAS cell surface plasminogen acquisition was confirmed by Western blot analysis of proteins eluted from the GAS cell surface following incubation in human plasma. Data are representative of two independent experiments performed in triplicate. Asterisks indicate statistical significance, *** p < 0.001.
Plasminogen at the GAS Cell Surface Impairs Complement-Mediated Neutrophil Killing
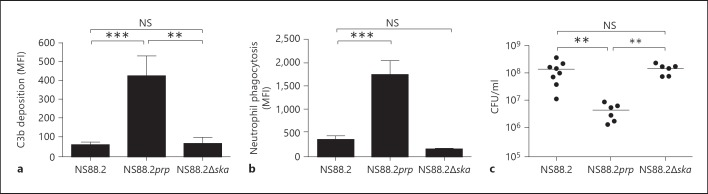
One consequence of C3b deposition on microbial surfaces is phagocytosis and killing by neutrophils. Therefore, to further examine the functional significance of observed decreases in C3b deposition, bacteria were opsonized with human plasma and survival in the presence of human neutrophils was measured. Wild-type strain NS88.2 was significantly more resistant to neutrophil killing compared to NS88.2prp (p < 0.05). There was no significant difference between the survival of NS88.2 and NS88.2∆ska in the presence of human neutrophils (fig. 3a). To confirm that this observation was complement dependent, the assay was repeated using complement-depleted plasma. No significant differences in survival of wild-type and mutant GAS strains were observed in the absence of complement (fig. 3b). Incubation of bacteria with Spectrozyme PL after opsonization confirmed that wild-type NS88.2 sequestered significantly higher levels of cell surface plasmin than either the NS88.2prp (p < 0.001) or NS88.2∆ska (p < 0.01) mutants (fig. 3c), supporting the hypothesis that increased survival does not correlate with plasmin acquisition.
Fig. 3.
Plasminogen at the GAS cell surface confers protection to C3b-mediated killing. Bacteria were opsonized with 50% human plasma for 10 min at 37°C as a source of plasminogen and complement. Washed bacteria were cocultured with purified human neutrophils for 30 min at 37°C to allow for phagocytic uptake, with phagocytosis disrupted by hypotonic lysis of neutrophils. The numbers of viable bacterial colonies were enumerated after overnight incubation at 37°C. Negative controls consisted of bacteria incubated under the same conditions without neutrophils. a NS88.2 was significantly more resistant to complement-mediated neutrophil killing than NS88.2prp (p < 0.05), but not NS88.2∆ska. b Bacteria were alternatively incubated with C3-depleted plasma. GAS strains showed no differences in neutrophil killing. c Opsonized bacteria were also incubated with the plasmin-specific substrate Spectrozyme PL overnight to confirm levels of cell surface plasmin activity. Bars indicate means ± SE of triplicate determinations from three independent experiments with different plasma donors. Asterisks indicate statistical significance, ** p < 0.01
Plasmin(ogen) Acquisition Is Associated with Immune Evasion in vivo
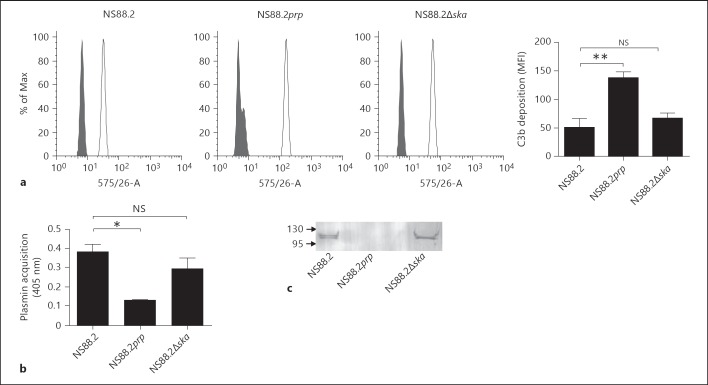
To extend our investigation of the effect of GAS cell surface plasminogen acquisition on C3b deposition in vivo, an animal model of infection was established in mice expressing the human plasminogen transgene. Our previously published work indicated that mice heterozygous for the AlbPLG1 transgene are more susceptible to infection with GAS strain NS88.2 [5]. Here, we employed a homozygous mouse line, which expresses two copies of the AlbPLG1 gene. Both mouse lines expressed equivalent levels of mouse plasminogen (197.46 ± 19.37 and 169.55 ± 28.22 µg/ml for heterozygous and homozygous mice, respectively). Mice heterozygous for the AlbPLG1 transgene express approximately 17% the level of human plasminogen found in human plasma [2]. The level of activatable human plasminogen in the homozygous mouse line was found to be 34% (±2.3%) that of the level in human plasma. Plasma from the homozygous mouse line displayed higher levels of streptokinase-mediated plasminogen activation and resulted in increased cell surface plasmin acquisition by GAS compared with plasma from heterozygous mice (online suppl. fig. 2). Overall virulence of GAS strain NS88.2 was equivalent in the two mouse lines (online suppl. fig. 2). Cohorts of 4 sex-matched mice aged between 8 and 10 weeks were intradermally inoculated with 1 × 108 CFU of eGFP-expressing NS88.2, NS88.2prp or NS88.2∆ska GAS strains. The site of infection was lavaged 4 h after inoculation, and C3b deposition analyzed by flow cytometry. Significantly lower levels of C3b were detected at the surface of wild-type strain NS88.2 compared to mutant NS88.2prp (p < 0.001; fig. 4a). The significance of decreased C3b deposition was further assessed by comparing the number of eGFP-positive neutrophils isolated from the flank 4 h after infection with eGFP-expressing NS88.2, NS88.2prp or NS88.2∆ska. NS88.2prp showed significantly higher levels of association with neutrophils than NS88.2 or NS88.2Δska (p < 0.0001; fig. 4b), suggesting that increased C3b deposition at the surface of this strain results in an increase in uptake by neutrophils. Finally, we compared bacterial load following infection with wild-type and mutant GAS strains. A significantly higher bacterial load at the site of infection was seen for NS88.2 compared with NS88.2prp (p < 0.01), however there was no significant difference between the number of bacteria recovered for NS88.2 and NS88.2∆ska after infection, as expected (p > 0.05: notsignificant, NS; fig. 4c). The propensity to acquire cell surface plasmin(ogen) therefore correlates with reduced complement deposition and increased bacterial survival in vivo. To further investigate the mechanism behind our in vivo findings, we compared C3b deposition, cell surface plasmin acquisition, and cell surface plasminogen acquisition in vitro using humanized mouse plasma. Consistent with the findings of both the in vitro studies using human plasma and the in vivo findings, C3b deposition in mouse plasma was significantly lower for NS88.2 compared with NS88.2prp but not NS88.2∆ska (fig. 5a). However, subsequent analysis of cell surface plasmin acquisition by GAS in mouse plasma revealed that NS88.2∆ska accumulated significantly higher levels of plasmin compared to NS88.2prp (p < 0.01; fig. 5b). Western blot analysis of proteins eluted from the GAS cell surface confirmed that both NS88.2 and NS88.2∆ska, which showed decreased levels of C3b at the cell surface, bound plasminogen in mouse plasma, whilst NS88.2prp, which was readily opsonized by C3b, did not (fig. 5c). The observed similarity in C3b deposition in vivo between NS88.2∆ska and the wild-type parent strain may therefore be due to either cell surface plasmin or cell surface plasminogen acquisition.
Fig. 4.
Plasmin(ogen) at the GAS cell surface is associated with immune evasion in an intradermal infection model. Transgenic humanized plasminogen mice were inoculated with 1 × 108 CFU of eGFP-expressing GAS strains NS88.2, NS88.2prp or NS88.2∆ska via intradermal injection into the right and left flanks. Four hours after infection, wound sites were lavaged with saline and the recovered supernatants collected. a C3b deposition on the surface of NS88.2 was significantly reduced compared to NS88.2prp (p < 0.05), while C3b deposition levels were comparable between NS88.2 and NS88.2∆ska. Bars indicate means ± SE of duplicates in three independent experiments (n = 6) expressed as MFI for C3b deposition. b GAS strain NS88.2prp was phagocytosed by mouse neutrophils 4 h after infection when compared to NS88.2 or NS88.2∆ska (p < 0.0001). c A significantly lower bacterial load was obtained for NS88.2prp compared to NS88.2 and NS88.2∆ska (p < 0.01). Data are combined from four (NS88.2prp and NS88.2∆ska) and five (NS88.2) independent experiments performed in duplicate. Asterisks indicate statistical significance, *** p < 0.001; ** p < 0.01.
Fig. 5.
Plasmin(ogen) decreases C3b deposition on GAS in mouse plasma. a In order to assess the impact of cell surface plasmin(ogen) on C3b deposition, bacteria were opsonized at 37°C for 10 min in 50% mouse plasma as a source of complement and plasmin(ogen). Nonspecific binding sites were blocked by incubating bacteria with human IgG and surface-bound C3b was probed using PE-conjugated monoclonal antibody raised against human C3. Alternatively, cells were stained with PE-labeled mouse IgG isotype control. C3b deposition on the bacterial surface was analyzed using flow cytometry. C3b deposition was significantly reduced on the surface of NS88.2 compared to NS88.2prp (p < 0.01), but not NS88.2Δska. Histograms show the shift in MFI between GAS stained with the isotype control (grey) and an anti-C3 antibody (white). Bars indicate means ± SE of triplicates expressed in MFI, representative of two independent experiments. b GAS cell surface plasmin activity was confirmed by incubating bacteria with the plasmin-specific substrate Spectrozyme PL overnight. c GAS cell surface plasminogen acquisition was confirmed by Western blot analysis of proteins eluted from the GAS cell surface following incubation in mouse plasma. Data are representative of two independent experiments performed in triplicate. Asterisks indicate statistical significance, ** p < 0.01; * p < 0.05.
Discussion
The ability of GAS to activate and sequester the host protease plasmin has been linked to bacterial dissemination and degradation of host tissue barriers during invasive disease initiation [3, 7]. Here we demonstrate for the first time that plasmin(ogen) acquisition protects GAS from complement-mediated phagocytosis and results in increased bacterial load during early infection.
During the initial stages of infection, invading pathogens must overcome innate immune barriers, including the bactericidal effects of complement. C3b plays a central role in the innate immune response to bacterial infection, and opsonization of bacteria with C3b facilitates neutrophil phagocytosis. GAS have evolved numerous strategies to minimize complement deposition at the bacterial cell surface, including expression of M protein [18]. The presence of M6 and M49 at the GAS cell surface has been linked to protection from C3b-mediated phagocytosis [19], while fibrinogen and factor H binding by certain M serotypes has been shown to block C3b deposition at the bacterial cell surface [18]. To date, over 200 different M protein types have been identified, and M protein function is highly diverse [20]. This study defines a new role for plasminogen-binding M proteins in GAS resistance to phagocytosis.
Plasmin exhibits broad-spectrum protease activity, which is central to its involvement in inflammation, fibrinolysis, and tissue remodeling [21]. However, these properties necessitate strict regulation of proteolysis under normal conditions. Secretion of streptokinase by GAS facilitates plasmin generation independent of host activators, while attachment of plasmin to the GAS cell surface protects plasmin from circulating inhibitors such as α2-antiplasmin. In this way, GAS are able to acquire a source of unregulated surface proteolytic activity [7]. This study shows that the ability of GAS strain NS88.2 to degrade C3b is dependent on the presence of cell surface plasmin activity. However, our data suggest it is the ability of NS88.2 to accumulate plasminogen at the cell surface, rather than plasmin, that is protective against C3b-mediated phagocytosis. Minimal C3b degradation was seen following overnight incubation of GAS with C3b. This suggests that the activity of surface-localized plasmin for soluble C3b is low. Interestingly, a number of studies looking at C3b degradation by surface-localized plasmin show similar results, with intact C3b still visible after 24 h [22, 23]. This may explain the findings in this study that there is limited correlation between cell surface plasmin acquisition and C3b deposition. Subsequent in vitro analyses using human plasma indicated no correlation between cell surface plasmin accumulation and C3b deposition at the GAS cell surface. Rather, decreased C3b deposition correlated with the ability of GAS to sequester plasminogen to the cell surface. However, assays performed using plasma from humanized plasminogen mice revealed that reduced C3b deposition correlates with both cell surface plasmin and cell surface plasminogen acquisition. This may be indicative of differences between mouse plasma and human plasma, even in this humanized model. It is therefore difficult to rule out a role for plasmin acquisition in the in vivo model, and it may be that in this context, both plasminogen and plasmin at the GAS cell surface provide protection against C3b deposition. Given the low level of soluble C3b degradation detected by surface-localized plasmin, it may be that surface-localized plasmin is more efficient at cleaving surface-deposited C3b, and our data clearly show that soluble plasmin can facilitate the removal of C3b from the GAS cell surface. Alternatively, it has been shown that degradation of C3b by plasmin inhibits complement activation by preventing the formation of the C3 convertase C3bBb [22]. It is therefore possible that plasmin-mediated removal of C3b from the GAS cell surface blocks this pathway and is responsible for the reduced levels of C3b deposition seen in vivo.
Recently, it has been demonstrated that plasminogen binds to complement components C3, C3b and C3d, and when activated to plasmin by the host urokinase plasminogen activator, plasmin cleaves C3b, inhibits complement activation, and prevents complement-mediated hemolysis of rabbit erythrocytes [22]. The cleavage fragments of C3b generated in this study using streptokinase-activated plasminogen or commercially obtained plasmin were found to be of similar but not identical in size to those reported by Barthel et al. [22]. Differences in electrophoretic mobility may reflect different commercial sources of C3b, or differences in the length of time C3b was incubated with a plasmin source between the two studies. However, both the previous study and the current study indicate that surface-localized plasmin degrades C3b.
GAS is equipped with an array of virulence factors that together exert anti-phagocytic properties. Both M and M-like proteins have been linked to the survival of certain GAS strains inside human neutrophils and blood [24, 25], while pore-forming streptolysin S and O exert cytotoxic activity on host neutrophils and impair their recruitment to the site of bacterial infection [26, 27]. Furthermore, loss of the GAS surface hyaluronic acid capsule is also associated with decreased resistance to neutrophil phagocytosis and reduced virulence in mice [28]. Thus, GAS is protected from different stages of the innate immune response via coordinated expression of multiple virulence factors. This is reflected in the finding that even in the presence of cell surface plasmin(ogen), GAS strain NS88.2prp retains approximately 80% survival compared with the wild-type parent strain in our in vitro neutrophil killing assays. Nevertheless, an ability of GAS to coopt plasmin(ogen) correlated to a significant increase in bacterial survival and a decrease in association with neutrophils during the early stages of infection in a well-characterized murine infection model. Inactivation of C3b via cleavage into smaller, nonfunctional products as a result of plasmin activation has been described for several additional human pathogens including Staphylococcus aureus[11], Bacillus anthracis[29], and Borrelia burgdorferi[30]. However, to our knowledge, this is the first time this potential virulence mechanism has been linked to bacterial survival in vivo. The ability of GAS to initiate invasive disease has been shown to be dependent on cell surface plasmin acquisition [4, 5], however, few studies of the role of plasminogen in GAS infection have looked at the early stages of disease, where the innate immune response is critical. Recently, it was shown that GAS-mediated plasminogen activation facilitates protection from the antimicrobial peptide LL-37 [8]. The current study clearly demonstrates that, in addition to facilitating tissue degradation and bacterial dissemination, localization of plasmin at the GAS cell surface via the M protein is critical for bacterial survival in the early stages of infection. These data provide new insight into the antiphagocytic role of the GAS M protein and add to our understanding of how plasmin(ogen) acquisition contributes to virulence of a significant human pathogen.
Supplementary Material
Supplementary data
Acknowledgments
D.L. and J.M.T. are recipients of an Australian Postgraduate Award. M.L.S.-S. is the recipient of an NHMRC (National Health and Medical Research Council) Career Development Fellowship. This work was funded by NHMRC Project Grant 635218.
References
- 1.Carapetis JR, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun H, et al. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004;305:1283–1286. doi: 10.1126/science.1101245. [DOI] [PubMed] [Google Scholar]
- 4.Cole JN, et al. Trigger for group A streptococcal M1T1 invasive disease. FASEB J. 2006;20:1745–1747. doi: 10.1096/fj.06-5804fje. [DOI] [PubMed] [Google Scholar]
- 5.Sanderson-Smith ML, et al. M protein-mediated plasminogen binding is essential for the virulence of an invasive Streptococcus pyogenes isolate. FASEB J. 2008;22:2715–2722. doi: 10.1096/fj.07-105643. [DOI] [PubMed] [Google Scholar]
- 6.Ogston D. Biochemistry of the plasmin system. J Clin Pathol Suppl. 1980;14:5–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Sanderson-Smith ML, De Oliveira DM, Ranson M, McArthur JD. Bacterial plasminogen receptors: mediators of a multifaceted relationship. J Biomed Biotechnol. 2012;2012:272148. doi: 10.1155/2012/272148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollands A, et al. A bacterial pathogen co-opts host plasmin to resist killing by cathelicidin antimicrobial peptides. J Biol Chem. 2012;49:40891–40897. doi: 10.1074/jbc.M112.404582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Seya T, Nagasawa S, Matsukura M, Hasegawa H, Atkinson JP. Generation of C3d,g and C3d by urokinase-treated plasma in association with fibrinolysis. Complement. 1985;2:165–174. doi: 10.1159/000467857. [DOI] [PubMed] [Google Scholar]
- 11.Rooijakkers SHM, et al. Anti-opsonic properties of staphylokinase. Microbes Infect. 2005;7:476–484. doi: 10.1016/j.micinf.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 12.McKay FC, et al. Plasminogen binding by group A streptococcal isolates from a region of hyperendemicity for streptococcal skin infection and a high incidence of invasive infection. Infect Immun. 2004;72:364–370. doi: 10.1128/IAI.72.1.364-370.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook SM, et al. Streptokinase variants from Streptococcus pyogenes isolates display altered plasminogen activation characteristics - implications for pathogenesis. Mol Microbiol. 2012;86:1052–1062. doi: 10.1111/mmi.12037. [DOI] [PubMed] [Google Scholar]
- 14.Jeng A, Sakota V, Li Z, Datta V, Beall B, Nizet V. Molecular genetic analysis of group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J Bacteriol. 2003;185:1208–1217. doi: 10.1128/JB.185.4.1208-1217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin RE, Ferretti JJ. Electrotransformation of streptococci. Methods Mol Biol. 1995;47:185–193. doi: 10.1385/0-89603-310-4:185. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson-Smith ML, Walker MJ, Ranson M. The maintenance of high affinity plasminogen binding by group A streptococcal plasminogen-binding M-like protein is mediated by arginine and histidine residues within the a1 and a2 repeat domains. J Biol Chem. 2006;281:25965–25971. doi: 10.1074/jbc.M603846200. [DOI] [PubMed] [Google Scholar]
- 17.Reddy KNN, Markus G. Mechanism of activation of human plasminogen by streptokinase. Presence of active center in streptokinase-plasminogen complex. J Biol Chem. 1972;247:1683–1691. [PubMed] [Google Scholar]
- 18.Kwinn LA, Nizet V. How group A Streptococcus circumvents host phagocyte defenses. Future Microbiol. 2007;2:75–84. doi: 10.2217/17460913.2.1.75. [DOI] [PubMed] [Google Scholar]
- 19.Jacks-Weis J, Kim Y, Cleary PP. Restricted deposition of C3 on M+ group A streptococci: correlation with resistance to phagocytosis. J Immunol. 1982;128:1897–1902. [PubMed] [Google Scholar]
- 20.Smeesters PR, et al. Genetic diversity of group A Streptococcus M protein: implications for typing and vaccine development. Vaccine. 2008;26:5835–5842. doi: 10.1016/j.vaccine.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 21.Saksela O, Rifkin DB. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- 22.Barthel D, Schindler S, Zipfel PF. Plasminogen is a complement inhibitor. J Biol Chem. 2012;287:18831–18842. doi: 10.1074/jbc.M111.323287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barthel D, et al. Haemophilus influenzae uses the surface protein E to acquire human plasminogen and to evade innate immunity. J Immunol. 2012;188:379–385. doi: 10.4049/jimmunol.1101927. [DOI] [PubMed] [Google Scholar]
- 24.Staali L, et al. Streptococcus pyogenes expressing M and M-like surface proteins are phagocytosed but survive inside human neutrophils. Cell Microbiol. 2003;5:253–265. doi: 10.1046/j.1462-5822.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 25.Kihlberg BM, et al. Biological properties of a Streptococcus pyogenes mutant generated by Tn916 insertion in mga. Microb Pathog. 1995;19:299–315. doi: 10.1016/s0882-4010(96)80003-9. [DOI] [PubMed] [Google Scholar]
- 26.Lin A, et al. Streptolysin S inhibits neutrophil recruitment during the early stages of Streptococcus pyogenes infection. Infect Immun. 2009;77:5190–5201. doi: 10.1128/IAI.00420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen BR, Duncan JL. Activation of human neutrophil metabolism by streptolysin O. J Infect Dis. 1980;141:680–685. doi: 10.1093/infdis/141.5.680. [DOI] [PubMed] [Google Scholar]
- 28.Moses AE, et al. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Infect Immun. 1997;65:64–71. doi: 10.1128/iai.65.1.64-71.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung M, et al. Bacillus anthracis interacts with plasmin(ogen) to evade C3b-dependent innate immunity. PLoS ONE. 2011;6:e18119. doi: 10.1371/journal.pone.0018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grosskinsky S, et al. Borrelia recurrentis employs a novel multifunctional surface protein with anti-complement, anti-opsonic and invasive potential to escape innate immunity. PLoS ONE. 2009;4:e4858. doi: 10.1371/journal.pone.0004858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data