Abstract
Recruitment of neutrophils, regarded as a key mechanism in acute lung injury (ALI), is orchestrated by cell adhesion molecules and chemokines. While the importance of cell adhesion molecules has been carefully investigated, little is known about the importance of chemokines and their receptors in the recruitment of neutrophils in models of ALI. Wild-type Ccr2−/−, Ccr5−/−, Fpr1−/− or Fpr2−/− mice were exposed to aerosolized lipopolysaccharide and the number of neutrophils in the lung tissue (intravascular, interstitial) and in the bronchoalveolar lavage was quantified. Lack of CCR5 or FPR1, but not CCR2 or FPR2, significantly reduced lung neutrophil infiltration in all compartments. Similarly, blockade of CCR5 or FPR1 with specific antagonists reduced counts of alveolar, interstitial and intravascular neutrophils. Such treatments also inhibited lung edema formation and histological lung tissue alterations, thus underscoring the protective role of CCR5 and FPR1 neutralizing strategies in ALI.
Key Words: Neutrophil, Recruitment, Chemokine, Lung injury
Introduction
Although considerable progress has been made in understanding the pathophysiology of acute lung injury (ALI) and despite all innovations in intensive care medicine, the mortality rate in ALI remains high [1]. Recruitment of neutrophils is a key event in the development of ALI [1, 2], leading to plasma leakage and deterioration of oxygenation. Classically, neutrophil tissue infiltration requires a sequential involvement of selectins, chemokines and cell adhesion molecules where tissue- and stimulus-specific recruitment patterns are just beginning to emerge [3]. In lipopolysaccharide (LPS)-induced ALI, the recruitment of neutrophils was found to depend on β2-integrins [4] and P-selectin [5]. Although chemokines and their receptors have been shown to be important targets of anti-inflammatory strategies, only CXCR2 ligands such as IL8 in humans and KC or MIP2 in mice have been identified as important guiding cues of neutrophils during LPS-induced ALI [6]. Recent studies, however, point towards the importance of CC-chemokines and endogenous chemotactic ligands of formyl-peptide receptors in the recruitment of neutrophils during acute and chronic inflammation [7, 8]. Thus, we here investigated the role of CCR2, CCR5, FPR1 and FPR2 in neutrophils lung tissue infiltration upon LPS inhalation.
Methods
Animals
Male wild-type (WT) C57Bl/6J Ccr2−/− [9], Ccr5−/−[10], Fpr1−/− [11] or Fpr2−/−[12] mice, 8 weeks of age, were used for this study. C57Bl/6J were treated with antagonists to CCR5 (maraviroc; Tocris Bioscience; 10 µg/g body weight, oral gavage, 30 min before or 30 min after LPS), FPR1 (cyclosporine H; Santa Cruz Biotechnology; 5 µg/g body weight, intraperitoneally, 30 min before or 30 min after LPS) or vehicle controls. All experiments were approved by the local ethical authorities.
LPS-Induced ALI
Aerosolized LPS from Salmonella enteritidis dissolved in 0.9% saline (500 μg/ml, 4 h) was utilized to induce neutrophil infiltration in the lung. Thirty minutes before euthanasia, 5 µl of anti-Ly6G and 100 µl of FITC-dextran (30 mg/ml; 70 kDa) were applied intravenously. The lungs were removed, minced, digested with liberase, and passed through a cell strainer (online suppl. fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000353229).
Flow Cytometry
Cell pellets were labeled with PerCP-Cy5.5 anti-mouse Ly-6G, PE anti-mouse CD115, APC-Cy7 anti-mouse CD45 and APC anti-Mouse F4/80. Neutrophils were identified by their typical appearance in the forward scatter-side scatter and as CD45+ CD115- and PerCP-Gr1+ cells (online suppl. fig. S2). Within the lung, FITC-Gr1 antibody was used to distinguish between interstitial neutrophils (CD45+, CD115-, PerCP-Gr1+, FITC-Gr1-) and intravascular neutrophils (CD45+, CD115-, PerCP-Gr1+, FITC-Gr1+).
Lung Permeability
FITC-Dextran (70 kDa; Sigma-Aldrich) was used to assess vascular leakage. One hundred microliters of FITC-dextran (30 mg/ml) were administered by tail vein injection 30 min prior to euthanasia and dye extravasation was used to assess change in vascular permeability. The fluorescence of the 100 µl bronchoalveolar lavage (BAL) supernatant (FluoBAL) and of 50 µl serum (FluoSerum) was measured and permeability volume was expressed in microliters (VPerm = (FluoBAL/100 µl)/(FluoSerum/50 µl) × BAL volume).
Histology
Paraffin-embedded lung sections were stained with Mayer's hematoxylin and histologically examined. Scoring of histological sections was done in compliance with recommendations of the American Thoracic Society [13]. Criteria for scoring are detailed in online suppl. table S1.
Statistics
All data are expressed as mean ± SD. Statistical significance was tested using one-way analysis of variance with Dunnett's post hoc test. p values <0.05 were considered statistically significant.
Results
CCR5 and FPR1 Orchestrate Neutrophil Recruitment in LPS-Induced Lung Inflammation
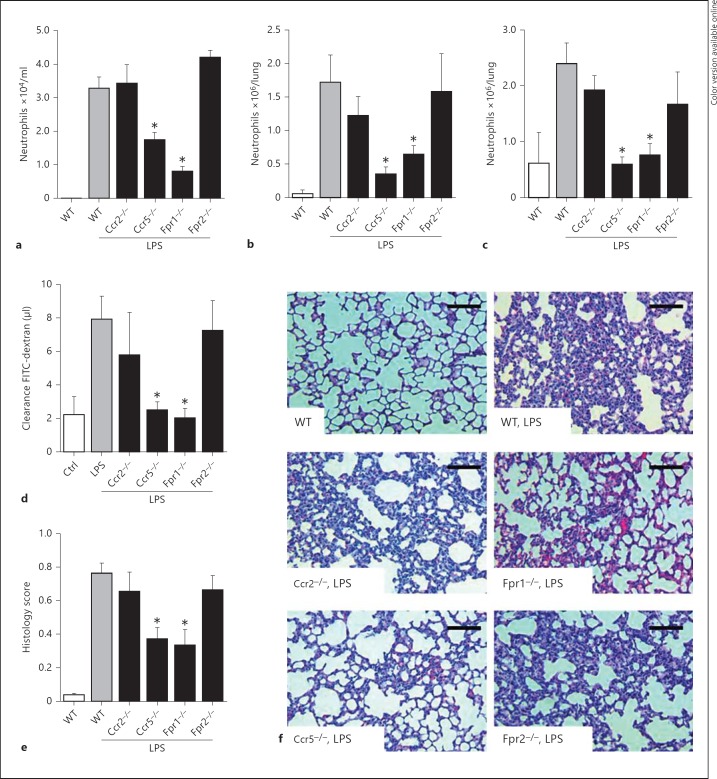
Recruitment of neutrophils, which is a key event in lung injury, is orchestrated by chemokines binding to G protein-coupled receptors during routine immune surveillance or inflammation. In order to investigate the role of different chemokine receptors in ALI, we exposed WT mice and mice lacking CCR2, CCR5, FPR1 or FPR2 to aerosolized LPS and monitored neutrophil recruitment by flow cytometry of digested lungs and BAL fluid. To discriminate between interstitial and intravascular neutrophils, an antibody to neutrophils was administered shortly before sacrifice, thus labeling adherent neutrophils. In this model neutrophil lung infiltration is a major contributor to subsequent lung damage [14]. Baseline counts of circulating white blood cells and platelets did not differ between the various strains (online suppl. table S2). LPS exposure increased the number of alveolar, interstitial and intravascular neutrophils in WT mice (fig. 1a-c). While neutrophil recruitment after LPS inhalation was not altered in Ccr2−/− mice, lung neutrophil infiltration in Ccr5−/− mice was significantly diminished in all three compartments (fig. 1a-c). Similarly, lack of FPR1 strongly reduced neutrophil accumulation in all lung compartments, while deletion of FPR2 was without effect (fig. 1a-c). With the importance of neutrophils in mediating lung damage we also investigated permeability changes and histological damages in these mouse strains. Changes in both parameters paralleled observations made for neutrophil recruitment, i.e. reduction of LPS-mediated permeability increases and structural damages in Ccr5−/− and Fpr1−/− mice, while no significant effects were observed in Ccr2−/− and Fpr2−/− mice (fig. 1d-f).
Fig. 1.
Neutrophil recruitment in response to LPS is reduced in Ccr5−/− and Fpr1−/− mice. Mice were challenged with LPS by inhalation and sacrificed 4 h later. The quantifications of alveolar (a), interstitial (b) and intravascular (c) neutrophils within the lungs of WT Ccr2−/−, Ccr5−/−, Fpr1−/− and Fpr2−/− mice are displayed. n = 8-10 for each bar. d Microvascular permeability was assessed by measurement of FITC-dextran clearance. Structural analyses of histological lung sections were made based on HE staining (e, f). Scale bar = 100 µm. Statistical significance was tested using one-way analysis of variance with Dunnett's post hoc test. Asterisk indicates significant difference compared with LPS-treated WT mice.
Inhibition of CCR5 and FPR1 Prevents Lung Neutrophil Recruitment
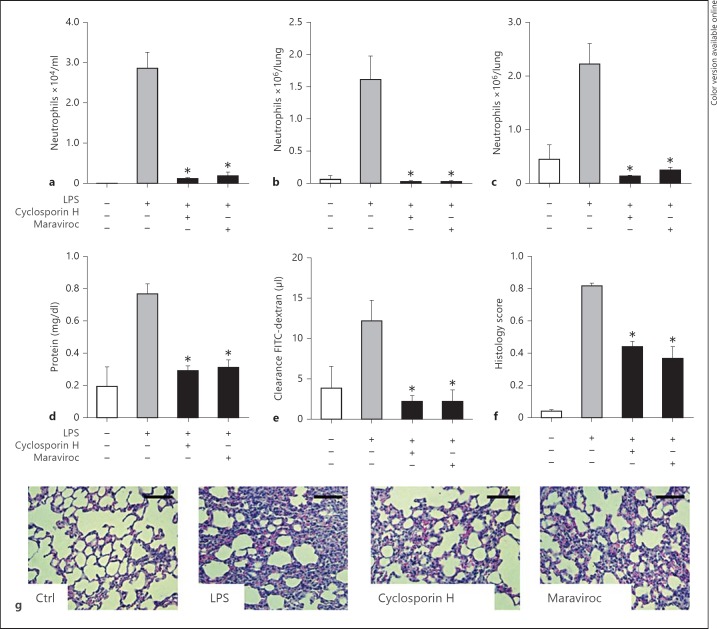
Based on the results obtain from various gene-targeted strains, we further aimed at investigating the effect of antagonists to CCR5 and FPR1 on neutrophil lung tissue infiltration upon LPS inhalation. To test the importance of these receptors, mice were treated with maraviroc, a specific CCR5 antagonist, or cyclosporine H, an antagonist to FPR1. Pretreatment of mice before challenge with LPS abolished neutrophil adhesion and interstitial tissue infiltration (online suppl. fig. S3A-C). Interestingly, similar results were obtained when mice received the antagonists after LPS inhalation (fig. 2a-c), supporting the therapeutic relevance of these approaches.
Fig. 2.
Neutralization of CCR5 and FPR1 blocks lung neutrophil recruitment and acute lung injury. WT mice were treated with antagonists to CCR5 (maraviroc, 10 µg/g body weight, oral gavage) or FPR1 (cyclosporine H, 5 µg/g body weight, i.p.) 30 min after LPS inhalation and sacrificed 210 min later. The quantifications of alveolar (a), interstitial (b) and intravascular (c) neutrophils within the lungs are displayed. Microvascular permeability was assessed by measurement of BAL protein concentration (d) and FITC-dextran clearance (e). Structural analyses of histological lung sections were made based on HE staining (f, g). Scale bar = 100 µm. n = 8-10 for each bar. Statistical significance was tested using one-way analysis of variance with Dunnett's post hoc test. Asterisk indicates significant difference compared with LPS-treated WT mice.
CCR5 and FPR1 Neutralization Attenuates Endotoxin-Induced Lung Injury
Lung injury is characterized by an increased permeability of the alveolar-capillary barrier, resulting in lung edema with protein-rich fluid. Permeability was quantified by assessment of BAL protein concentration and the clearance of fluorescent dextran. Both parameters were found to be increased upon LPS treatment indicative of elevated plasma leakage (fig. 2d, e). Treatment of mice with antagonists to CCR5 or FPR1 either before or after LPS exposure largely inhibited lung edema formation (fig. 2d, e; online suppl. fig. S3D, E).
Histological analyses of lungs following LPS exposure revealed alveolar septal thickening, accumulation of inflammatory cells in the interstitium and alveoli, and influx of protein-rich fluid into the alveolar space as compared to control mice. Antagonists to CCR5 and FPR1 administered before or after LPS inhalation abrogated histological changes indicating protective effects in ALI (fig. 2f, g; online suppl. fig. S3F).
Discussion
In the study reported here, we identify the importance of CCR5 and FPR1 in neutrophil recruitment to inflamed lungs. CCR5, a receptor known for its importance in the recruitment of monocytes [15, 16], was recently also appreciated for its importance in the recruitment of neutrophils to atherosclerotic lesions or sites of ischemia [8, 17]. In these settings as well as in models of ALI, CCL5, a major CCR5 ligand, was found to be platelet derived [8, 14]. Indeed, neutralization of CCL5 prevents LPS and acid-induced ALI, whereas overexpression of CCL5 in murine lungs increases neutrophil accumulation altogether, supporting an important role for CCL5 in lung neutrophil recruitment [14, 18]. While CCR2 has been reported to be important in the recruitment of neutrophils to atherosclerotic lesions and in ischemia [8, 17], our study, as well as previous work [19], exclude a major role for CCR2 in lung neutrophil recruitment upon LPS stimulation. In fact, the impact of CCR2 on neutrophil recruitment at later time points following LPS inhalation appear indirect, as CCR2 primarily impairs the accumulation of monocytes and macrophages which create a milieu that favors the recruitment of neutrophils [19]. In addition to the importance of CCR5, we identify a crucial role for FPR1 but not FPR2 in neutrophil lung infiltration following LPS inhalation. The high affinity receptor FPR1 recognizes formylated peptides released from bacteria or from the mitochondria of necrotic cells. Recent studies propose a hierarchical gradient of guidance cues where chemokines bring the neutrophils to the vicinity of the site of inflammation and formylated peptides from either origin direct the neutrophils the final few hundred microns into the injury [20, 21]. In contrast to FPR1, FPR2 responds to both proinflammatory and proresolving ligands [22, 23], which may at least in part explain the indifferent outcome of FPR2 deletion observed here. Specifically, the lipid metabolites lipoxin A4 and resolvin D1, as well as the peptide annexin A1 were shown to interact with FPR2, resulting in attenuated leukocyte recruitment [24, 25], and may hence neutralize the chemotactic activity exerted by ligands such as formylated peptides or cathelicidin. Taken together, our data provide novel insights into the chemokine-driven neutrophil recruitment in ALI and may allow for the design of specific neutralizing strategies.
Disclosure Statement
None.
Acknowledgements
The authors wish to acknowledge Patricia Lemnitzer and Zohreh Packsereshtmogharab for excellent technical assistance. This study was supported by the Else-Kröner-Fresenius Stiftung, the B. Braun Stiftung, and the DFG (SO876/3-1, FOR809, SFB914 TP B08). The authors would like to thank Dr. Philip M. Murphy for providing Fpr1−/− mice and Dr. Ji-Min Wang for providing Fpr2−/− mice.
References
- 1.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossaint J, Zarbock A. Tissue-specific neutrophil recruitment into the lung, liver, and kidney. J Innate Immun. 2012 doi: 10.1159/000345943. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreland JG, Fuhrman RM, Pruessner JA, Schwartz DA. CD11b and intercellular adhesion molecule-1 are involved in pulmonary neutrophil recruitment in lipopolysaccharide-induced airway disease. Am J Respir Cell Mol Biol. 2002;27:474–480. doi: 10.1165/rcmb.4694. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi H, Koike H, Kurata Y, Imanishi N, Tojo SJ. Protective effects of sialyl Lewis X and anti-P-selectin antibody against lipopolysaccharide-induced acute lung injury in rabbits. Eur J Pharmacol. 1999;370:47–56. doi: 10.1016/s0014-2999(99)00068-0. [DOI] [PubMed] [Google Scholar]
- 6.Reutershan J, Morris MA, Burcin TL, et al. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 8.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 9.Boring L, Gosling J, Chensue SW, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Kurihara T, Ryseck RP, et al. Impaired macrophage function and enhanced T cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 coreceptor. J Immunol. 1998;160:4018–4025. [PubMed] [Google Scholar]
- 11.Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen K, Le Y, Liu Y, et al. A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J Immunol. 2010;184:3331–3335. doi: 10.4049/jimmunol.0903022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grommes J, Alard JE, Drechsler M, et al. Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury. Am J Respir Crit Care Med. 2012;185:628–636. doi: 10.1164/rccm.201108-1533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soehnlein O, Drechsler M, Döring Y, et al. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber C, Weber KS, Klier C, et al. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and TH1-like/CD45RO+ T cells. Blood. 2001;97:1144–1146. doi: 10.1182/blood.v97.4.1144. [DOI] [PubMed] [Google Scholar]
- 17.Reichel CA, Khandoga A, Anders HJ, Schlöndorff D, Luckow B, Krombach F. Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tissue. J Leukoc Biol. 2006;79:114–122. doi: 10.1189/jlb.0605337. [DOI] [PubMed] [Google Scholar]
- 18.Pan ZZ, Parkyn L, Ray A, Ray P. Inducible lung-specific expression of RANTES: preferential recruitment of neutrophils. Am J Physiol Lung Cell Mol Physiol. 2000;279:L658–L666. doi: 10.1152/ajplung.2000.279.4.L658. [DOI] [PubMed] [Google Scholar]
- 19.Maus UA, Waelsch K, Kuziel WA, et al. Monocytes are potent facilitators of alveolar neutrophil emigration during lung inflammation: role of the CCL2-CCR2 axis. J Immunol. 2003;170:3273–3278. doi: 10.4049/jimmunol.170.6.3273. [DOI] [PubMed] [Google Scholar]
- 20.Pittman K, Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013 doi: 10.1159/000347132. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wantha S, Alard JE, Megens RT, et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res. 2013;112:792–801. doi: 10.1161/CIRCRESAHA.112.300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gavins FN, Yona S, Kamal AM, Flower RJ, Perretti M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood. 2003;101:4140–4147. doi: 10.1182/blood-2002-11-3411. [DOI] [PubMed] [Google Scholar]
- 25.Norling LV, Perretti M. Control of myeloid cell trafficking in resolution. J Innate Immunity. 2013;5:367–376. doi: 10.1159/000350612. [DOI] [PMC free article] [PubMed] [Google Scholar]