Abstract
A key role for ‘lipid-sensing’ CD1-restricted natural killer T (NKT) cells in the pathogenesis of atherosclerosis has been suggested. However, the biology of NKT cells remains poorly characterized, as in different experimental settings their activation was reported to both stimulate and suppress innate and adaptive immune responses. Most of the data from experimental models suggest that NKT cells are proatherogenic; however, it is debated whether the increase in atherosclerosis observed following NKT cell stimulation is a consequence of the inability to induce functional NKT cells rather than the proatherogenic nature of NKT cells. CD1d-expressing antigen-presenting cells and NKT cells were detected in mouse and human atherosclerotic lesions. Furthermore, several lysophospholipids and glycosphingolipids, known to accumulate in atherosclerotic plaques, are antigenic for human NKT cell clones. Lipid transfer proteins, such as apolipoprotein E and microsomal triglyceride transfer protein, are central to NKT cell responses. All these data suggest a profound relation between lipid metabolism, CD1d-NKT cell axis activation and atherosclerosis. In this review, we summarize the advances and gaps in our knowledge of NKT cell biology in the context of atherosclerosis as well as the possibility of influencing NKT cell polarization toward an atheroprotective phenotype.
Key Words: Immune response, Macrophages, Scavenger receptor
Introduction
The immune system plays a key role in the pathogenesis of cardiovascular diseases, including atherosclerosis [1]. Cholesterol-rich lipoproteins, such as low-density lipoproteins (LDL), were identified as the main trigger of the immunoinflammatory response [2] and of the polarization of naïve lymphocyte T cells toward an effector memory phenotype [3], while immunoprotective functions are emerging for high-density lipoproteins [4]. Given the lipid-related ‘nature’ of atherosclerosis, the role of natural killer T (NKT) cells in atherogenesis is currently under active investigation [5]. These T cells do indeed recognize, through a nearly monospecific T cell receptor (TCR), self and foreign lipid antigens presented by the conserved nonpolymorphic MHC-class-I-like molecule receptor CD1d. Once activated, invariant NKT (iNKT) cells elicit their effector properties both by stimulating the cells of the innate immune system and by orchestrating adaptive immune responses [6]. The ability of antigen-presenting cells (APCs) to process and expose lipids through CD1d largely depends on a series of lipid transfer proteins that could influence the access, transfer, modification and loading of lipids onto CD1d. As most lipids do not circulate freely in the blood but associate with larger complexes, such as lipoproteins, it is tempting to speculate that these molecules, which are critically involved in atherosclerosis, could also influence NKT cell function. Here, we review the biology and the links between the CD1d-NKT cell axis and atherosclerosis, including the emerging role of proteins involved in lipid metabolism such as microsomal triglyceride transfer protein (MTP) and apolipoprotein E (ApoE). The possibility of influencing the polarization of NKT cells toward an atheroprotective phenotype is also discussed.
Biology of NKT Cells
What Are NKT Cells?
NKT cells are T cells with a reactivity directed against glycolipid antigens presented on the surface of APCs by MHC-like CD1d molecules [6, 7]. Based on sequence homology, human CD1 proteins are divided into group I, containing CD1a, CD1b and CD1c, and group II, represented only by CD1d [7]. Of note, CD1d is highly conserved in mammalian species, and human CD1d is highly homologous to murine CD1d [6, 8]. CD1d is expressed mainly by APCs [dendritic cells (DCs), macrophages and B cells] [9]. In addition, double-positive thymocytes use CD1d for the selection of NKT cells during their development in the thymus [6]. CD1d is also expressed by liver cells (including hepatocytes and hepatic sinusoid-lining endothelial cells) and by intestinal epithelial cells and activated T cells [7]. However, the role of CD1d expression in non-APCs is unclear; indeed, only liver DCs, but not hepatocytes, can activate the release of interferon (IFN)-γ by liver iNKT cells [10], and the expression of CD1d by cells in the liver is not required for homing of iNKT cells to the liver [11]. Interestingly, CD1d was shown to be expressed in mice by vascular smooth muscle cells (VSMCs) surrounding the blood vessels in the intestine, colon, liver, kidney, testis, thymus and other organs [12]. Whether CD1d is expressed by VSMCs in atherosclerotic plaques is unknown. CD1 isoforms are distributed to distinct intracellular compartments and follow different routes of intracellular trafficking [13]. The difference in intracellular trafficking between human and murine CD1d proteins, could, however, limit the translation of mice findings to humans [13].
A key characteristic of NKT cells is that a large proportion of these cells (defined as iNKT cells, also called type I or classical NKT cells) possess semi-invariant TCRs, which recruit Vα24-Jα18 and Vβ11 gene segments in humans and Vα14-Jα18 and Vβ8/7/2 segments in mice. Type II NKT cells, which do not express invariant TCRs, were suggested to represent the majority of NKT cells in humans [14] but not in mice [15]. However, the lack of specific markers for type II NKT cells has so far limited a clear evaluation of the frequency of these cells. Studies on type II NKT cells showed that these cells (at least partly) express TCRs which are less diverse than TCRs of traditional MHC-I/II-restricted T cells [15]. It was suggested that type II NKT cells could comprise low-frequency subsets with semi-invariant TCR sequences, and they were shown to be protective in mice models of cancer and experimental autoimmune encephalomyelitis [16]. From now on, this review will focus mainly on the CD1d-iNKT cell axis.
How Are iNKT Cells Activated?
Several ways by which iNKT cells are activated have been described. Firstly, a number of self and nonself lipid antigens presented by CD1d were reported to activate NKT cells. CD1-presented antigens are also termed ‘lipid-linked’ antigens as they (with rare exceptions, e.g. mycolic acid) present hydrophilic head groups rather than lipid moieties, which are recruited for binding to hydrophobic binding sites within binding pockets of CD1 molecules [7]. While some antigens can be presented by different types of CD1 family members (though with different affinities to corresponding TCRs), structural features of lipid-linked antigens which allow them to bind a particular type of CD1 molecule are under investigation, and some of them have been described in detail [17]. α-GalCer, the most potent iNKT cell agonist, was the first antigen reported to activate NKT cells [17]. It was initially isolated from a marine sponge (Agelas mauritianus) and later reported to originate from the bacterial wall of Sphingomonas normally present in the marine sponge [6]. Several α-GalCer analogs, which are also capable of activating NKT cells, have been synthesized, i.e. (2S, 3S, 4R)-1-O-(α-d-galactopyranosyl)-N-tetracosanoyl-2-aminononane-1,3,4-triol (OCH), C20:2 α-GalCer analog and non-glycosidic threitolceramide [7]. Other bacteria such as Borrelia burgdorferi produce diacylglycerol-based antigens which stimulate iNKT cells [7]. The fact that iNKT cells can be activated in the absence of foreign lipid antigen suggests that a physiological stimulation by self-antigens occurs. Indeed, several self-antigens have recently been reported to activate NKT cells [6]. Sphingolipids, including forms of β-D-glucopyranosylceramide (β- GlcCer) and also lysophospholipids, are responsible for NKT cell activation [6]. It was shown that β-GlcCer levels are increased in DCs following toll-like receptor 4 (TLR4) activation [6]; as this activation occurs in the atherosclerotic plaque (by minimally modified LDL or lipopolysaccharides), it is possible that β-GlcCer could act as NKT cell agonist during atherogenesis [2]. Among lysophospholipids, lysophosphatidylcholine, ether-bonded versions of plasmalogen lysophosphatidylethanolamine and lysophosphatidic acid were found to be antigenic for a subset of human iNKT cell clones [6].
Secondly, NKT cells can be activated in a CD1d-independent manner. A combination of interleukin (IL)-12 and IL-18 was reported to activate NKT cells independently of CD1d-mediated antigen presentation [18]. Whether cytokine-mediated NKT cell activation could reflect an effector response similar to that of natural killer (NK) and effector T cells should be considered. Also, T cell Ig-like mucin-like-1 engagement, which occurs in the presence of phosphatidylserine [19], a lipid exposed by apoptotic cells, was shown to promote iNKT cell activation with a polarization toward an immunosuppressive phenotype characterized by increased IL-4 and decreased IFN-γ production [20].
Thirdly, although a number of self-antigens can clearly activate NKT cells following CD1d-mediated presentation [6], there is evidence that NKT cells carrying TCRs with specific sequences of CDR3β loop can be activated simply by binding to CD1d, regardless of the nature of the lipid loaded on CD1d [21]. Moreover, a number of lipid antigens loaded onto CD1d may decrease the affinity of this binding and thus downregulate NKT cell stimulation [21].
Finally, costimulatory signals also play a role in polarizing NKT-mediated DC maturation into tolerogenic or inflammatory DCs. Indeed, CD40-CD40L interaction enhances IL-12 production by DCs, thus providing an adjuvant effect to proinflammatory NKT cell responses [22]. Homotypic interaction between signaling lymphocytic activation molecules expressed on DCs and iNKT cells also promotes the expression of T helper type 2 (Th2) cytokines by iNKT cells [23]. PD1-PD1L interactions were suggested to play a role in monocyte differentiation into regulatory APCs [24]. All these findings point to the relevance of costimulatory interactions in modulating NKT cell polarization and responses.
iNKT Cell Distribution, Subsets and Functions
Percentages of circulating iNKT cells (out of CD3+ lymphocytes) in humans are in the range from undetectable to 1%, with an average of 0.14% [25, 26], while in mice they can reach up to 3%, with an average of 0.5–1% (strain dependent) [6]. In humans, iNKT cells are most abundant in the omentum, where they represent on average 10% of T cells [27]. In mice, iNKT cells represent 20–30% of lymphocytes in the liver, 0.5–2% of lymphocytes in thymus, spleen and bone marrow, and 0.1-0.4% of TCRβ+B220- cells (approx. equivalent to T cells) in lymph nodes [28]. In contrast, the proportions of iNKT cells in human liver (averaging 0.5% of CD3+ cells) and bone marrow (averaging 0.3% of CD3+ cells) are much lower [6]. In humans, reduced levels of circulating iNKT cells have been reported in several conditions, including cancer, infectious diseases and autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis and type 1 diabetes [29]. Furthermore, even when present at normal levels, iNKT cells exhibit a ‘dysfunctional’ phenotype in cancer and autoimmune and infectious diseases, which is exhibited by reduced NKT cell proliferation and reduced IFN-γ secretion upon stimulation of peripheral blood mononuclear cells by α-GalCer [6]. More recently, a reduction in iNKT cells was also observed in obesity [27]. The reason for this is debated, and iNKT cells have been shown either to protect against obesity and improve insulin sensitivity [27] or to promote tissue inflammation and hepatic steatosis [30] in a mouse model of obesity. Further studies should investigate whether NKT cells could play a different role depending on the temporal window of obesity progression considered.
Mainly 4 iNKT cell subsets (based on the expression of CD4 and CD161) have been studied. They differ strikingly in their cytokine production and antitumor effect, in an organ-dependent manner [31]. These differences could depend on the type of lipid which is presented by CD1d; indeed, iNKT cells activated by α-GalCer rapidly produce both T helper type 1 (Th1) and Th2 cytokines, while OCH and C20:2 (both possessing the α-linked monogalactosyl identical to the α-GalCer sugar) induce an anti-inflammatory Th2-biased NKT cell response, in contrast to the inflammatory response induced by α-GalCer and threitolceramide [32].
Despite the fact that iNKT cells express TCRs, suggesting a role in adaptive immunity, iNKT cells were initially included in the innate immune arm due to their ability to rapidly (within hours) release large amounts of cytokines upon activation by α-GalCer administration [33]. Furthermore, NKT cells share common features with other participants of the innate immune system, NK cells, since both express NK 1.1 and possess antitumoral activity [34, 35]. Later, as the field developed, iNKT cells were shown to induce the activation of cells belonging to the adaptive immune arm, thus suggesting that components of innate and adaptive immune systems interact and that NKT cells may serve as a bridge between the systems [35].
A characteristic feature of iNKT cells which distinguishes them from traditional MHC-restricted T cells and group I CD1-restricted T cells is their effector memory phenotype. In particular, there are no naïve CD45RA+ NKT cells; they are all CD45RO+ in the peripheral human blood in adults [36], they are already educated (have no memory of the prime stimulation), they lack CCR7 and CD62L (homing receptors of naïve T cells) [3, 7], possess effector function (upon stimulation of TCRs with PMA and ionomycin) [36] and depend only on IL-15 for their homeostasis. CD4- iNKT cells expand after their exit from the thymus and recycle like most memory T cells, while CD4+ iNKT cells imitate naive cells in their recycling behavior [25]. More recently, CD4+ iNKT cells were shown to be biased toward a Th2 effector phenotype [37].
Once activated, the NKT cell subsets further support the immune response at several levels, as follows: (1) by providing noncognate and cognate help to B cells to boost antibody production [7]; (2) by supporting the activation, expansion and polarization (Th1/Th2) of T cells and by direct cytotoxicity [7]; (3) by promoting the differentiation of monocytes into DCs, an activity related to their ability to produce granulocyte-macrophage colony-stimulating factor, in contrast to MHC-restricted T cells [7]; (4) by favoring polarization of macrophages [38] and activation of NK cells, and (5) by alternative licensing of DCs for cross-presentation [39]. Paradoxically, iNKT cells either exhibit adjuvant function, providing antimicrobial and antitumoral responses, or promote immunosuppression [6]. However, to date, how iNKT cells are polarized in the context of cancer and autoimmune diseases is unclear. In addition to the costimulatory environment, as mentioned above, TLR4 engagement on DCs promotes the polarization of iNKT cells from a tolerogenic to a proinflammatory phenotype [22]. A similar effect is observed with lipid-enriched DCs, which are able to shift NKT cell effector function from suppressive to inflammatory, while lipid-poor DCs induce tolerogenic NKT cell responses [40].
CD1-Dependent Immunity and Lipid Metabolism Merge via Employment of the Same Lipid Transfer Proteins
Since CD1-restricted lipid antigens are integral membrane components and CD1 proteins themselves are not able to extract lipids from membranes, researchers searched for proteins which transfer lipids onto CD1 proteins. Lipid transfer proteins characterized in the context of lipid metabolism were the first suspects for accomplishing this task and were confirmed to exert this function [41, 42]. These include sphingolipid activator proteins (SAPs), MTP and ApoE. Each of these lipid transfer proteins was shown to be involved in CD1-dependent pathways, thereby linking immunity and lipid metabolism. SAPs and MTP, but not ApoE, are essential for NKT cell development [43]. SAPs, which comprise saposins A-D and GM2 activator protein, activate glycosidases which are required for sphingolipid degradation, contribute to the accessibility of endosomal/lysosomal membrane lipids to degrading enzymes [44] and promote lipid loading and unloading onto/from CD1 proteins in the endosomal/lysosomal compartments [41]. ApoE, which favors lipoprotein clearance, is involved in the delivery of exogenous lipid antigens for CD1d presentation via LDL receptor (LDLR) uptake [45]. This activity could be part of the atheroprotective activities ascribed to ApoE [42]. Recent findings show that other lipoproteins could also play an important role in lipid antigen delivery [46].
Finally, MTP, an endoplasmic reticulum-resident protein involved in lipoprotein synthesis, loads endogenous lipids onto CD1 molecules in the endoplasmic reticulum, a process required for CD1 stabilization [47, 48]. Impaired CD1-dependent immunity was recently described in abetalipoproteinemia (ABL) patients. ABL is a rare genetic disorder caused by mutations in the gene coding for MTP. DCs isolated from ABL patients are defective in lipid antigen presentation by all CD1 isoforms (to a lesser extent only for CD1b) [43]. Importantly, CD1d expression by MTP-deficient DCs was not affected, suggesting that MTP-dependent CD1d stabilization is required for endosomal lipid antigen loading [43]. These findings highlight MTP as a unique regulator of human metabolic and immune pathways and reveal that ABL is not only a disorder of lipid metabolism but also an immune disease involving CD1.
The CD1d-iNKT Cell Axis in Atherosclerosis
The relevance of the CD1d-iNKT cell axis during atherogenesis is supported by a number of experimental observations. CD1d is expressed by APCs in mouse and human atherosclerotic lesions [49, 50]. Proatherogenic factors such as lysophosphatidic acid and oxidized LDL upregulate the expression of CD1d in DCs [51, 52]. This mechanism is mediated by nuclear receptor peroxisome proliferator activated receptor (PPAR)γ activation, which indirectly regulates CD1d gene expression [51, 52]. Intriguingly, group I CD1 family proteins are also controlled by PPARγ activation, but, in contrast to CD1d, their expression is downregulated upon PPARγ activation [51]. Of note, thioglycolate-elicited macrophages pulsed with oxidized LDL showed increased CD1d levels and induced NKT cells to produce IFN-γ, a potentially proatherogenic Th1 cytokine [50]. Self lipid antigens, such as glycosphingolipids and lysophospholipids, which are known to be presented by CD1d-expressing APCs and activate NKT cells [6], have been detected in atherosclerotic plaques and were shown to contribute to atherosclerosis progression, further suggesting a link between NKT cells and vascular disorders.
The role of NKT cells in experimental atherosclerosis was addressed by two different approaches, either by manipulating NKT cell numbers (depletion or increase) or by activating the CD1d-NKT axis with NKT cell-restricted lipid antigens (table 1) [50, 53, 54, 55, 56, 57, 58, 59].
Table 1.
Effect of modulation of NKT cell numbers and activity on atherosclerotic lesions in animal models
| Depletion or increase of NKT cell numbers | ||
|---|---|---|
| CD1d–/– vs. WT (females) | 20-week HFD starting at 10 weeks of age [50] | 59%↓ (AR) |
| CD1d–/–LDLR–/– vs. LDLR–/– (both genders) | 8– or 12-week HFD starting at 5 weeks of age [53] | no change (TA) |
| CD1d–/–LDLR–/– vs. LDLR–/– (both genders) | 4-week HFD starting at 5 weeks of age [53] | males: 47%↓ (TA) |
| females: no change (TA) | ||
| CD1d–/–ApoE–/– vs. ApoE–/– (females) | 15-week-old mice on chow diet [54] | 26%↓ (AR) |
| CD1d–/–ApoE–/– vs. ApoE–/– (males) | 16-week-old mice on chow diet [55] | 68%↓ (AR) |
| [CD1d–/– → LDLR–/–] BMC mice vs. [WT → LDLR–/–] BMC mice (females) | 4 weeks after bone marrow transplantation, mice were placed on HFD for 5 weeks [50] | 39%↓ (AR) |
| Jα18–/–LDLR–/– vs. LDLR–/– (both genders) | 8-week HFD starting at 4 weeks of age [56] | approx. 20%↓ (AR) approx. 30%↓ (AA) |
| LDLR–/–RAG–/– recipients of Vα14 transgenic vs. WT B6 splenocytes (females) |
after adoptive transfer of splenocytes, 8- to 10-week-old recipient mice were placed on HFD for 12 weeks [57] | 62%↑ (AR) |
| LDLR–/–RAG–/– recipients of CD1d–/– vs. WT B6 splenocytes (females) | no change (AR) | |
| 3dTx ApoE–/– vs. 3dTx ApoE–/– recipients of CD4+ NKT or DN NKT cells (males) | 8-week HFD starting at 5 – 6 weeks of age; 1 week before switching to HFD, mice were injected with liver-derived CD4+ iNKT cells or DN iNKT cells [58] | 229%↑ (AR) for CD4+ iNKT cells no change (AR) for DN |
| NKT cell stimulation by CD1d-restricted lipid antigens | ||
| ApoE–/– (females) | α-GalCer or OCH injections (intraperitoneal) at 8, 10 and 12 weeks of age; examination at 13 weeks of age [50] | α-GalCer: 66%↑ (AR) OCH: 44%↑ (AR) |
| α-GalCer injections (intraperitoneal) from 8 to 18 weeks of age; examination 1 week after last injection [50] | no change (AR and TA) 51%↓ (collagen) |
|
| ApoE–/– (females) ApoE–/–CD1d–/– (females) |
α-GalCer injections (first intravenous followed by intraperitoneal) from 5 to 15 weeks of age; examination 48 h after last injection [54] | 50%↑ (AR) no change (AR) |
| ApoE–/– (males) | α-GalCer injections (intraperitoneal) from 4 to 14 weeks of age; examination 2 weeks after the last injection [55] | 100%↑ (AR) |
| α-GalCer injections (intraperitoneal) from 4 to 14 weeks of age; examination 10 weeks after the last injection [55] | 15%↑ (AR) 60%↑ (distal aorta) | |
| LDLR–/– (males) ApoE–/– (males) |
perivascular collars were placed around both carotid arteries of 10- to 12-week-old mice; HFD was initiated 2 weeks before the operation; 7-week α-GalCer injections (intravenous and intraperitoneal, 50%/50%, starting after operation) [59] | 84%↓ (carotid arteries) no change (carotid arteries) |
↑ = Increase in lesion areas; ↓ = decrease in lesion areas; WT = wild type; HFD = high-fat diet; AR = aortic root; TA = total aorta; AA = aortic arch; BMC = bone marrow chimeric; 3dTx = thymectomized on third day of life; DN = double-negative.
NKT Cell Number Manipulation
The absence of CD1d is associated with impaired NKT cell development, and therefore CD1d−/− animals have been extensively used to characterize the role of NKT cells in atherosclerosis. Regardless of the proatherogenic background used, both LDLR−/− and ApoE−/− mice crossed with CD1d−/− mice resulted in double knockout animals with decreased atherosclerosis development (table 1) [53, 54, 55]. Intriguingly, the proatherogenic effect appears to be more pronounced in the early stages of atherogenesis, but further studies are needed to address this issue [60]. Furthermore, selective iNKT cell deficiency (Jα18−/−) was also associated with a significant reduction in atherosclerotic lesions [56] (in both genders). NKT cell adoptive transfer experiments also confirmed the proatherogenic nature of NKT cells. Indeed, adoptive transfer of splenocytes from Vα14 transgenic mice (which have an increased number of NKT cells in the spleen) into LDLR−/−RAG−/− double knockout animals resulted in increased atherosclerosis in the aortic root compared to double knockout mice which received adoptive transfer of splenocytes from control mice or CD1d−/− mice [57]. Of note, when the effect of the adoptive transfer of specific NKT cell subsets was tested in thymectomized ApoE−/− mice, only the transfer of CD4+ NKT cells was associated with increased atherosclerosis, while that of double-negative NKT cells was not [58]. As CD4+ iNKT cells were shown to be biased toward a Th2 effector phenotype [37], these results are surprising, and a confirmation in a different experimental setting with functional thymus will be of help to fully understand the picture.
NKT Cell Stimulation by CD1d-Restricted Lipid Antigens
iNKT cell stimulation by α-GalCer in ApoE knockout mice is associated with increased atherosclerosis [50]. Furthermore, administering α-GalCer to ApoE−/− mice with established lesions did not significantly increase the atherosclerotic lesion area, but it did decrease the collagen content [50], a feature associated with a less stable plaque. Also, the α-GalCer analog OCH, which induces an anti-inflammatory Th2-biased NKT cell response [32], was reported to be proatherogenic [50].
The relevance of the proatherogenic effect of α- GalCer-mediated iNKT cell induction was confirmed in ApoE−/−CD1d−/− double knockout animals in which α-GalCer injection did not increase atherosclerosis [54].
In contrast with these findings, suggesting a proatherogenic role of NKT cell stimulation by CD1d-restricted lipid antigens [9, 61], administration of α-GalCer was associated with reduced intimal thickening and atherosclerosis in carotid arteries following perivascular injury in LDLR knockout animals but not in ApoE−/− mice [59]. Whether the approach used to induce atherosclerosis (carotid perivascular collar placement) could be responsible for the differences observed cannot be excluded. However, the same approach in CD1d−/− mice with functional LDLR resulted in decreased neointima formation [62].
Of note, 2 weeks of a high-fat diet induced a rapid and extensive increase in NKT cell percentage in the liver and spleen of LDLR−/− but not ApoE−/− mice [59]. Furthermore, splenocytes isolated from LDLR−/− mice, but not from ApoE−/− mice, were responsive to stimulation with α-GalCer [59]. This finding supports the observation by Braun et al. [63], who showed that NKT cell induction by α-GalCer is significantly dampened in ApoE−/− mice compared to C57BL6J mice, as is the CD1d function [45]. This suggests that chronic dyslipidemia could induce an iNKT cell phenotype that is unresponsive to further stimulation by exogenous glycolipid, and that sustained unresponsiveness could be iNKT cell intrinsic [63]. Therefore, the possibility that some of the findings obtained in ApoE−/− mice following the stimulation of the CD1d-iNKT cell axis are the consequence of the inability to induce functional iNKT cells rather than the induction of proatherogenic iNKT cells should be considered and could help in reconciling the opposite findings in experimental atherosclerosis.
How Do These Findings Translate into Humans?
Circulating NKT cell levels are reduced in the peripheral blood of patients with previous cardiovascular events [49], a result consistent with the observation in animal models [9], and iNKT cells were detected in atherosclerotic plaques [49]. The proportion of CD161+ T cells among the T cells in plaques has been reported to be in the range of 0.3–2% [64]. CD1d-positive cells are present mainly in neovascularized atherosclerotic lesions isolated from patients who had experienced cardiovascular events in the past (symptomatic patients), and not in the plaques from asymptomatic patients, where they are barely detected [49]. Of note, NKT cells derived from the plaques of symptomatic patients are more sensitive to induction by α-GalCer compared to NKT cells isolated from peripheral blood mononuclear cells of the same patients and are able to produce higher levels of IFN-γ upon activation [49]. Whether this might depend on the presence in the plaque of NKT cells with a CDR3β loop can not be excluded [21]. Nevertheless, nothing was reported about the sensitivity of atherosclerotic plaque-derived NKT cells compared to circulating NKT cells under different pathological conditions, including asymptomatic patients. This information will be of interest to address whether NKT cell sensitivity relates to the stage of atherosclerosis progression. In vitro, a subset of CD4-positive cells expressing CD161 (mainly represented by NKT cells) was shown to promote VSMC apoptosis via FasL/Fas activation [65]; it was suggested that this mechanism could contribute to reduced plaque stability. Of note, these cells were shown to possess a Th1-biased IL-18 receptor-positive phenotype [65].
Conclusion and Open Questions
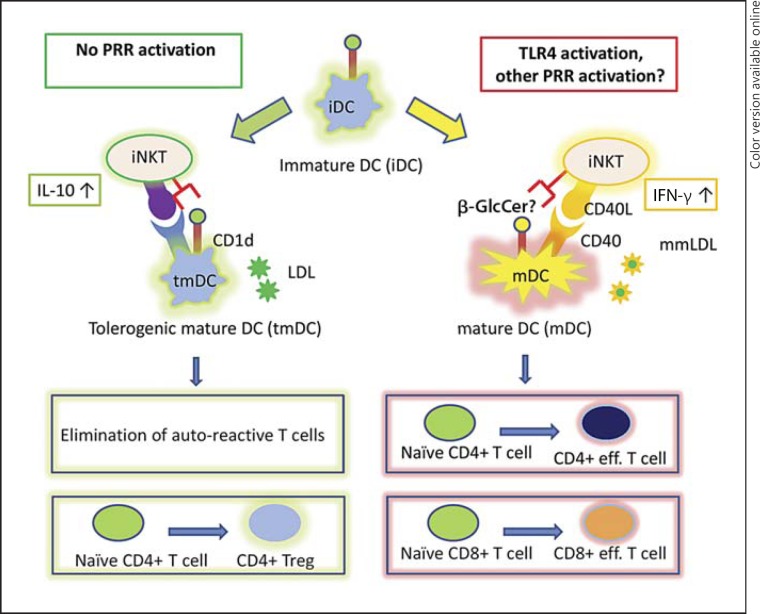
Most of the available data suggest that NKT cells are proatherogenic in animal models, while data in humans on the pathophysiological role of these cells are less clear. It is tempting to speculate that under physiological conditions, in the absence of pathogen recognition receptor stimulation, immature DCs could maintain the tolerance of the immune system to LDL by supporting the iNKT-mediated maturation of immature DCs into tolerogenic DCs (fig. 1). The presence of PPR activation (for instance TLR4 stimulation by bacterial products or by minimally modified LDL, which occurs during atherogensis), followed by the increased CD1d-mediated presentation of stimulatory NKT cell ligands and increased IL-12/IL-18 levels, may polarize NKT cell responses, leading to the NKT cell-mediated maturation of immature DCs into inflammatory mature DCs (fig. 1). These inflammatory mature DCs can promote a cellular inflammatory response and can contribute to the disruption of tolerance to LDL [2].
Fig. 1.
The CD1d-NKT cell axis in atherosclerosis: emerging concepts. During atherosclerosis, in the absence of pathogen recognition receptor (PRR) stimulation (left side), immature DCs (iDC) could maintain the tolerance of the immune system to LDL by supporting the iNKT-mediated maturation of immature DCs into tolerogenic mature DCs (tmDC). Tolerogenic DCs could then induce tolerance by elimination of a self-reactive T cell pool or by the polarization of naive CD4+ T cells into regulatory T cells. The presence of PPR activation [for instance TLR4 stimulation by bacterial products or by minimally modified LDL (mmLDL); right side] leads to an increase in the CD1d-mediated presentation of specific NKT-stimulatory lipids (such as β-GlcCer) and to an increase in IL-12 expression by DCs (which is further enhanced by CD40-CD40L interaction). These immature DC-NKT cell interactions may promote the maturation of immature DCs into inflammatory mature DCs (mDC) and, thus, result in a cellular inflammatory response against LDL. Treg = T regulatory.
In conclusion, several aspects have to be investigated to fully address the role of NKT cells in atherosclerosis. Firstly, in all studies with α-GalCer stimulation, soluble α-GalCer was injected; this could result in iNKT cell anergy. The effect on atherosclerosis of DCs pulsed with α-GalCer, which produce sustained iNKT cell expansion [66], should be studied. Secondly, as chronic hyperlipidemia results in NKT cell anergy [63], the functional state of NKT cells in a particular experimental setting has to be analyzed in relation to the temporal window in which these cells become anergic. Thirdly, the possibility of turning the NKT cell response toward an atheroprotective one should be considered. In this context, the ability of DCs pulsed with α-GalCer and LDL, and/or tolerized by IL-10, to limit the immunoinflammatory-related responses during atherosclerosis in the presence of TLR antagonists should be explored. Fourthly, the possibility of using the adjuvant properties of α-GalCer in DC-based vaccines designed to treat atherosclerosis should be explored [67]. Finally, characterizing the role of type II NKT cells in atherosclerosis will be of interest.
Addressing all these aspects will improve our understanding of the biological role of NKT cells in atherosclerosis and may provide novel therapeutic targets for the immune-related aspects of atherosclerosis.
Disclosure Statement
There are no conflicts of interest.
Acknowledgements
A.L.C. is supported by PUR 2009 University of Milan, Fondazione Cariplo (2009-2582) and Fondazione Società Italiana Studio Aterosclerosi. G.D.N. is supported by grants from Fondazione Cariplo (2010-0768), Società Italiana Studio Aterosclerosi Lombardia Chapter and PUR 2009 University of Milan.
The authors are grateful to Dr. Denis V. Baev, PhD (Virostatics s.r.l.), for valuable comments and suggestions.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 3.Ammirati E, Cianflone D, Vecchio V, Banfi M, Vermi AC, De Metrio M, Grigore L, Pellegatta F, Pirillo A, Garlaschelli K, Manfredi AA, Catapano AL, Maseri A, Palini AG, Norata GD. Effector memory T cells are associated with atherosclerosis in humans and animal models. J Am Heart Assoc. 2012;1:27–41. doi: 10.1161/JAHA.111.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norata GD, Pirillo A, Ammirati E, Catapano AL. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis. 2012;220:11–21. doi: 10.1016/j.atherosclerosis.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 5.Braun NA, Covarrubias R, Major AS. Natural killer T cells and atherosclerosis: form and function meet pathogenesis. J Innate Immun. 2010;2:316–324. doi: 10.1159/000296915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 7.Cohen NR, Garg S, Brenner MB. Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 8.Calabi F, Jarvis JM, Martin L, Milstein C. Two classes of CD1 genes. Eur J Immunol. 1989;19:285–292. doi: 10.1002/eji.1830190211. [DOI] [PubMed] [Google Scholar]
- 9.Getz GS, Vanderlaan PA, Reardon CA. Natural killer T cells in lipoprotein metabolism and atherosclerosis. Thromb Haemost. 2011;106:814–819. doi: 10.1160/TH11-05-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trobonjaca Z, Leithauser F, Moller P, Schirmbeck R, Reimann J. Activating immunity in the liver. I. Liver dendritic cells (but not hepatocytes) are potent activators of IFN-gamma release by liver NKT cells. J Immunol. 2001;167:1413–1422. doi: 10.4049/jimmunol.167.3.1413. [DOI] [PubMed] [Google Scholar]
- 11.Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canchis PW, Bhan AK, Landau SB, Yang L, Balk SP, Blumberg RS. Tissue distribution of the non-polymorphic major histocompatibility complex class I-like molecule, CD1d. Immunology. 1993;80:561–565. [PMC free article] [PubMed] [Google Scholar]
- 13.Gumperz JE. The ins and outs of CD1 molecules: bringing lipids under immunological surveillance. Traffic. 2006;7:2–13. doi: 10.1111/j.1600-0854.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 14.Exley MA, He Q, Cheng O, Wang RJ, Cheney CP, Balk SP, Koziel MJ. Cutting edge: compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J Immunol. 2002;168:1519–1523. doi: 10.4049/jimmunol.168.4.1519. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Weiss A, Benlagha K, Kyin T, Teyton L, Bendelac A. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J Exp Med. 2001;193:893–904. doi: 10.1084/jem.193.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadri N, Blomqvist M, Cardell SL. Type II natural killer T cells: a new target for immunomodulation? Exp Rev Clin Immunol. 2008;4:615–627. doi: 10.1586/1744666X.4.5.615. [DOI] [PubMed] [Google Scholar]
- 17.De Libero G, Mori L. Novel insights into lipid antigen presentation. Trends Immunol. 2012;33:103–111. doi: 10.1016/j.it.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 19.Lee HH, Meyer EH, Goya S, Pichavant M, Kim HY, Bu X, Umetsu SE, Jones JC, Savage PB, Iwakura Y, Casasnovas JM, Kaplan G, Freeman GJ, DeKruyff RH, Umetsu DT. Apoptotic cells activate NKT cells through T cell Ig-like mucin-like-1 resulting in airway hyperreactivity. J Immunol. 2010;185:5225–5235. doi: 10.4049/jimmunol.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HS, Lee CW, Chung DH. T cell Ig domain and mucin domain 1 engagement on invariant NKT cells in the presence of TCR stimulation enhances IL-4 production but inhibits IGN-gamma production. J Immunol. 2010;184:4095–4106. doi: 10.4049/jimmunol.0901991. [DOI] [PubMed] [Google Scholar]
- 21.Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, Patel O, Vivian JP, Matsuda JL, McCluskey J, Godfrey DI, Marrack P, Rossjohn J, Gapin L. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011;34:315–326. doi: 10.1016/j.immuni.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caielli S, Conforti-Andreoni C, Di Pietro C, Usuelli V, Badami E, Malosio ML, Falcone M. On/off TLR signaling decides proinflammatory or tolerogenic dendritic cell maturation upon CD1d-mediated interaction with invariant NKT cells. J Immunol. 2010;185:7317–7329. doi: 10.4049/jimmunol.1000400. [DOI] [PubMed] [Google Scholar]
- 23.Baev DV, Caielli S, Ronchi F, Coccia M, Facciotti F, Nichols KE, Falcone M. Impaired slam-slam homotypic interaction between invariant NKT cells and dendritic cells affects differentiation of IL-4/IL-10-secreting NKT2 cells in nonobese diabetic mice. J Immunol. 2008;181:869–877. doi: 10.4049/jimmunol.181.2.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegde S, Lockridge JL, Becker YA, Ma S, Kenney SC, Gumperz JE. Human NKT cells direct the differentiation of myeloid APCs that regulate T cell responses via expression of programmed cell death ligands. J Autoimmun. 2011;37:28–38. doi: 10.1016/j.jaut.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI, Metelitsa LS. Distinct homeostatic requirements of Cd4+ and CD4- subsets of Valpha24-invariant natural killer T cells in humans. Blood. 2004;104:4150–4156. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]
- 26.Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, Rugeles MT, Atkinson MA, Landay AL, Wilson SB. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6b11. Immunology. 2007;122:1–14. doi: 10.1111/j.1365-2567.2007.02647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O'Shea D, O'Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barral P, Polzella P, Bruckbauer A, van Rooijen N, Besra GS, Cerundolo V, Batista FD. CD169(+) macrophages present lipid antigens to mediate early activation of iNKT cells in lymph nodes. Nat Immunol. 2010;11:303–312. doi: 10.1038/ni.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caielli S, Sorini C, Falcone M. The dangerous liaison between iNKT cells and dendritic cells: does it prevent or promote autoimmune diseases? Autoimmunity. 2011;44:11–22. doi: 10.3109/08916931003782130. [DOI] [PubMed] [Google Scholar]
- 30.Wu L, Parekh VV, Gabriel CL, Bracy DP, Marks-Shulman PA, Tamboli RA, Kim S, Mendez-Fernandez YV, Besra GS, Lomenick JP, Williams B, Wasserman DH, van Kaer L. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc Natl Acad Sci USA. 2012;109:E1143–E1152. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bricard G, Venkataswamy MM, Yu KOA, Im JS, Ndonye RM, Howell AR, Veerapen N, Illarionov PA, Besra GS, Li Q, Chang Y-T, Porcelli SA. Α-galactosylceramide analogs with weak agonist activity for human iNKT cells define new candidate anti-inflammatory agents. PLoS One. 2010;5:e14374. doi: 10.1371/journal.pone.0014374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d- restricted and TCR-mediated activation of Valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 34.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y, Taniguchi M. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura T, Kitamura H, Iwakabe K, Yahata T, Ohta A, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, Nakui M, Sekimoto M, Koda T. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int Immunol. 2000;12:987–994. doi: 10.1093/intimm/12.7.987. [DOI] [PubMed] [Google Scholar]
- 36.D'Andrea A, Goux D, De Lalla C, Koezuka Y, Montagna D, Moretta A, Dellabona P, Casorati G, Abrignani S. Neonatal invariant Valpha24+ NKT lymphocytes are activated memory cells. Eur J Immunol. 2000;30:1544–1550. doi: 10.1002/1521-4141(200006)30:6<1544::AID-IMMU1544>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 37.O'Reilly V, Zeng SG, Bricard G, Atzberger A, Hogan AE, Jackson J, Feighery C, Porcelli SA, Doherty DG. Distinct and overlapping effector functions of expanded human CD4+, CD8alpha+ and CD4-CD8alpha- invariant natural killer T cells. PLoS One. 2011;6:12. doi: 10.1371/journal.pone.0028648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji Y, Sun S, Xia S, Yang L, Li X, Qi L. Short-term high-fat-diet challenge promotes alternative macrophage polarization in adipose tissue via natural killer T cells and interleukin-4. J Biol Chem. 2012;287:24378–24386. doi: 10.1074/jbc.M112.371807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, Rossjohn J, Perlmutter P, Cao J, Godfrey DI, Savage PB, Knolle PA, Kolanus W, Forster I, Kurts C. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol. 2010;11:313–320. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- 40.Ibrahim J, Nguyen AH, Rehman A, Ochi A, Jamal M, Graffeo CS, Henning JR, Zambirinis CP, Fallon NC, Barilla R, Badar S, Mitchell A, Rao RS, Acehan D, Frey AB, Miller G. Dendritic cell populations with different concentrations of lipid regulate tolerance and immunity in mouse and human liver. Gastroenterology. 2012;143:1061–1072. doi: 10.1053/j.gastro.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou D, Cantu C, 3rd, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, Mahuran DJ, Morales CR, Grabowski GA, Benlagha K, Savage P, Bendelac A, Teyton L. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 2004;303:523–527. doi: 10.1126/science.1092009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Champagne E, Martinez LO, Vantourout P, Collet X, Barbaras R. Role of apolipoproteins in gammadelta and NKT cell-mediated innate immunity. Immunol Res. 2005;33:241–255. doi: 10.1385/ir:33:3:241. [DOI] [PubMed] [Google Scholar]
- 43.Zeissig S, Dougan SK, Barral DC, Junker Y, Chen Z, Kaser A, Ho M, Mandel H, McIntyre A, Kennedy SM, Painter GF, Veerapen N, Besra GS, Cerundolo V, Yue S, Beladi S, Behar SM, Chen X, Gumperz JE, Breckpot K, Raper A, Baer A, Exley MA, Hegele RA, Cuchel M, Rader DJ, Davidson NO, Blumberg RS. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J Clin Invest. 2010;120:2889–2899. doi: 10.1172/JCI42703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darmoise A, Maschmeyer P, Winau F. The immunological functions of saposins. Adv Immunol. 2010;105:25–62. doi: 10.1016/S0065-2776(10)05002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van den Elzen P, Garg S, Leon L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng TY, Sacks FM, Illarionov PA, Besra GS, Kent SC, Moody DB, Brenner MB. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- 46.Freigang S, Landais E, Zadorozhny V, Kain L, Yoshida K, Liu Y, Deng S, Palinski W, Savage PB, Bendelac A, Teyton L. Scavenger receptors target glycolipids for natural killer T cell activation. J Clin Invest. 2012;122:3943–3954. doi: 10.1172/JCI62267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, Khurana A, Kronenberg M, Johnson C, Exley M, Hussain MM, Blumberg RS. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes EA, Hammond C, Cresswell P. Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Natl Acad Sci USA. 1997;94:1896–1901. doi: 10.1073/pnas.94.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kyriakakis E, Cavallari M, Andert J, Philippova M, Koella C, Bochkov V, Erne P, Wilson SB, Mori L, Biedermann BC, Resink TJ, de Libero G. Invariant natural killer T cells: linking inflammation and neovascularization in human atherosclerosis. Eur J Immunol. 2010;40:3268–3279. doi: 10.1002/eji.201040619. [DOI] [PubMed] [Google Scholar]
- 50.Nakai Y, Iwabuchi K, Fujii S, Ishimori N, Dashtsoodol N, Watano K, Mishima T, Iwabuchi C, Tanaka S, Bezbradica JS, Nakayama T, Taniguchi M, Miyake S, Yamamura T, Kitabatake A, Joyce S, Van Kaer L, Onoe K. Natural killer T cells accelerate atherogenesis in mice. Blood. 2004;104:2051–2059. doi: 10.1182/blood-2003-10-3485. [DOI] [PubMed] [Google Scholar]
- 51.Leslie DS, Dascher CC, Cembrola K, Townes MA, Hava DL, Hugendubler LC, Mueller E, Fox L, Roura-Mir C, Moody DB, Vincent MS, Gumperz JE, Illarionov PA, Besra GS, Reynolds CG, Brenner MB. Serum lipids regulate dendritic cell CD1 expression and function. Immunology. 2008;125:289–301. doi: 10.1111/j.1365-2567.2008.02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szatmari I, Pap A, Ruhl R, Ma JX, Illarionov PA, Besra GS, Rajnavolgyi E, Dezso B, Nagy L. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aslanian AM, Chapman HA, Charo IF. Transient role for CD1d-restricted natural killer T cells in the formation of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2005;25:628–632. doi: 10.1161/01.ATV.0000153046.59370.13. [DOI] [PubMed] [Google Scholar]
- 54.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004;199:417–422. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Major AS, Wilson MT, McCaleb JL, Ru Su Y, Stanic AK, Joyce S, Van Kaer L, Fazio S, Linton MF. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2351–2357. doi: 10.1161/01.ATV.0000147112.84168.87. [DOI] [PubMed] [Google Scholar]
- 56.Rogers L, Burchat S, Gage J, Hasu M, Thabet M, Willcox L, Ramsamy TA, Whitman SC. Deficiency of invariant V alpha 14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc Res. 2008;78:167–174. doi: 10.1093/cvr/cvn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.VanderLaan PA, Reardon CA, Sagiv Y, Blachowicz L, Lukens J, Nissenbaum M, Wang CR, Getz GS. Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am J Pathol. 2007;170:1100–1107. doi: 10.2353/ajpath.2007.060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.To K, Agrotis A, Besra G, Bobik A, Toh BH. NKT cell subsets mediate differential proatherogenic effects in ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2009;29:671–677. doi: 10.1161/ATVBAHA.108.182592. [DOI] [PubMed] [Google Scholar]
- 59.van Puijvelde GH, van Wanrooij EJ, Hauer AD, de Vos P, van Berkel TJ, Kuiper J. Effect of natural killer T cell activation on the initiation of atherosclerosis. Thromb Haemost. 2009;102:223–230. doi: 10.1160/TH09-01-0020. [DOI] [PubMed] [Google Scholar]
- 60.Bobryshev YV. Natural killer T cells in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:e40. doi: 10.1161/01.ATV.0000161317.01678.75. author reply e40. [DOI] [PubMed] [Google Scholar]
- 61.Whitman SC, Ramsamy TA. Participatory role of natural killer and natural killer T cells in atherosclerosis: lessons learned from in vivo mouse studies. Can J Physiol Pharmacol. 2006;84:67–75. doi: 10.1139/y05-159. [DOI] [PubMed] [Google Scholar]
- 62.Strom A, Wigren M, Hultgardh-Nilsson A, Saxena A, Gomez MF, Cardell S, Fredrikson GN, Nilsson J. Involvement of the CD1d-natural killer T cell pathway in neointima formation after vascular injury. Circ Res. 2007;101:e83–e89. doi: 10.1161/CIRCRESAHA.107.160705. [DOI] [PubMed] [Google Scholar]
- 63.Braun NA, Mendez-Fernandez YV, Covarrubias R, Porcelli SA, Savage PB, Yagita H, Van Kaer L, Major AS. Development of spontaneous anergy in invariant natural killer T cells in a mouse model of dyslipidemia. Arterioscler Thromb Vasc Biol. 2010;30:1758–1765. doi: 10.1161/ATVBAHA.110.206045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bobryshev YV, Lord RS. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J Histochem Cytochem. 2005;53:781–785. doi: 10.1369/jhc.4B6570.2005. [DOI] [PubMed] [Google Scholar]
- 65.Chan WL, Pejnovic N, Hamilton H, Liew TV, Popadic D, Poggi A, Khan SM. Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and helper T cells. Circ Res. 2005;96:675–683. doi: 10.1161/01.RES.0000160543.84254.f1. [DOI] [PubMed] [Google Scholar]
- 66.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Habets KL, van Puijvelde GH, van Duivenvoorde LM, van Wanrooij EJ, de Vos P, Tervaert JW, van Berkel TJ, Toes RE, Kuiper J. Vaccination using oxidized low-density lipoprotein-pulsed dendritic cells reduces atherosclerosis in LDL receptor-deficient mice. Cardiovasc Res. 2010;85:622–630. doi: 10.1093/cvr/cvp338. [DOI] [PubMed] [Google Scholar]