Abstract
Streptococcus pneumoniae is a common cause of pneumonia and sepsis. Toll-like receptors (TLRs) play a pivotal role in the host defense against infection. In this study, we sought to determine the role of single immunoglobulin interleukin-1 receptor-related molecule (SIGIRR a.k.a. TIR8), a negative regulator of TLR signaling, in pneumococcal pneumonia and sepsis. Wild-type and SIGIRR-deficient (sigirr−/−) mice were infected intranasally (to induce pneumonia) or intravenously (to induce primary sepsis) with S. pneumoniae and euthanized after 6, 24, or 48 h for analyses. Additionally, survival studies were performed. sigirr−/− mice showed delayed mortality during lethal pneumococcal pneumonia. Accordingly, sigirr−/− mice displayed lower bacterial loads in lungs and less dissemination of the infection 24 h after the induction of pneumonia. SIGIRR deficiency was associated with increased interstitial and perivascular inflammation in lung tissue early after infection, with no impact on neutrophil recruitment or cytokine production. sigirr−/− mice also demonstrated reduced bacterial burdens at multiple body sites during S. pneumoniae sepsis. sigirr−/− alveolar macrophages and neutrophils exhibited an increased capacity to phagocytose viable pneumococci. These results suggest that SIGIRR impairs the antibacterial host defense during pneumonia and sepsis caused by S. pneumoniae.
Key Words: Innate immunity, Toll-like receptors, Gram-positive bacterial infections, Pneumococcal pneumonia, Sepsis
Introduction
The Gram-positive diplococcus Streptococcus pneumoniae is the leading cause of community-acquired pneumonia and the most common cause of death from infection in developed countries today [1, 2]. In the USA alone, S. pneumoniae is responsible for more than half a million pneumonia cases and 50,000 episodes of bacteremia each year, with case fatality rates of 7 and 20%, respectively [3]; similar figures have been reported for Europe [4]. Globally, the annual pneumococcal related death toll has been estimated at approximately 2 million [2]. As such, S. pneumoniae represents a major health burden despite vaccination programs and effective antibiotic treatments.
Toll-like receptors (TLRs) are an important part of the innate defense against infection [5, 6]. TLRs recognize conserved motifs expressed by microbes (pathogen/microbe-associated molecular patterns) and host-derived damage-associated molecular patterns, resulting in the recruitment of intracellular adaptor molecules, the activation of nuclear factor (NF)-κB and other signaling pathways, and the production of proinflammatory cytokines. Multiple TLRs are involved in the detection of pneumococci. TLR2 is mainly responsible for recognition of S. pneumoniae cell wall components [7, 8, 9], while TLR4 induces cytokine release in response to pneumolysin, a toxin expressed by all virulent pneumococcal strains [10, 11]. During experimental pneumococcal pneumonia, protective roles have been reported for TLR4 [10, 11, 12] and TLR9 [13], the receptor that recognizes bacterial DNA [5, 6], while TLR2 contributes to induction of inflammation in the airways [7, 14]. All TLRs are involved in sensing S. pneumoniae signals via a common adapter, i.e. myeloid differentiation primary response gene 88 (MyD88), which also mediates the intracellular effects of the interleukin (IL)-1 receptor (R) and IL-18R [15]. Not unexpectedly, mice with a genetic deletion of the myd88 gene (myd88−/− mice) showed a strongly impaired host defense during pneumococcal pneumonia, as reflected by enhanced bacterial growth and increased mortality [16].
Unrestrained activation of TLRs can cause disproportionate inflammation and collateral tissue damage. Therefore, TLR signaling is securely regulated in order to avoid such injurious inflammatory responses [17]. Single immunoglobulin IL-1 receptor-related molecule (SIGIRR or TIR8) has been shown to inhibit NF-κB activation dependent on TLRs and IL-1R-like receptors (ILRs) [18]. SIGIRR is ubiquitously expressed in different tissues, including the lung, where the main SIGIRR-positive cell types are bronchial epithelium, leukocytes, and blood endothelial cells [19]. Recent research has implicated SIGIRR as an important regulator of inflammation in the respiratory tract. In a model of acute pneumonia caused by Pseudomonas aeruginosa, a Gram-negative pathogen primarily affecting immunocompromised hosts, sigirr−/− mice showed increased lethality and higher bacterial burdens together with exaggerated local and systemic inflammation [19]. Likewise, in chronic lung infection caused by Mycobacterium tuberculosis, SIGIRR deficiency was associated with excessive lung and systemic inflammation, and consequently increased lethality [20]. In accordance, overexpression of SIGIRR in lung epithelial cells attenuated acute lung injury elicited by airway exposure to LPS, the toxic component of Gram-negative bacteria [21]. Thus far, the contribution of SIGIRR to the host response during Gram-positive infection has not been studied. Here we sought to determine the role of SIGIRR in pneumonia and sepsis caused by S. pneumoniae.
Materials and Methods
Animals
Specific pathogen-free 9- to 11-week-old C57BL/6 wild-type (WT) mice were from Charles River (Maastricht, The Netherlands). sigirr−/− mice [22], backcrossed 6 times to a C57BL/6 background, were bred in the animal facility of the Academic Medical Center in Amsterdam (The Netherlands). Age- and sex-matched animals were used in all experiments. The Animal Care and Use Committee of the University of Amsterdam approved all of the experiments.
Experimental Infections
The models of pneumococcal pneumonia and pneumococcal sepsis have previously been described [23, 24]. In short, mice were inoculated intranasally (to induce pneumonia) with 5 × 104 CFU of S. pneumoniae (serotype 3; American Type Culture Collection, ATCC 6303, Rockville, Md., USA) or intravenously (to induce primary sepsis) with 5 × 105 CFU S. pneumoniae. Lungs, blood, spleens, and livers were harvested 6, 24, or 48 h postinfection for quantitative bacterial cultures as described (n = 8 or 16 per group at each time point) [23, 24]. Neutrophil counts in bronchoalveolar lavage (BAL) fluid were determined as described [25]. In separate studies, mice were followed for 4 days and survival was monitored at least every 12 h (n = 20 per group).
Histopathological Analysis
Lung histopathology was semiquantitatively analyzed as described [7, 26]. In short: the ‘lung inflammation score’ was expressed as the sum of 6 parameters [graded on a scale of 0 (absent) to 4 (severe)]: pleuritis, bronchitis, edema, interstitial inflammation, percentage of pneumonia, and endothelialitis. Granulocyte staining was done with an Ly-6G monoclonal antibody (BD Pharmingen, San Diego, Calif., USA) as described previously [23, 27]. The entire Ly-6G-stained lung sections were digitized with a slide scanner (Olympus, Tokyo, Japan). Immunopositive (Ly-6G+) areas were analyzed with ImageJ (version 2006.02.01; National Institutes of Health, Bethesda, Md., USA) and expressed as a percentage of the total lung surface area [26, 27]. Analyses were performed in a blinded manner, i.e. without knowledge of the genotype (n = 8 per group at each time point).
Assays
Lung homogenates were prepared as described [7]. In lung homogenates, tumor necrosis factor (TNF)-α, IL-1β, IL-6, macrophage inflammatory protein (MIP-2), and cytokine-induced neutrophil chemoattractant (KC) were measured using specific enzyme-linked immunosorbent assays (R&D Systems, Abingdon, UK) in accordance with the manufacturer's recommendations. In plasma TNF-α, IL-6 and monocyte chemoattractant protein (MCP)-1 were measured by cytometric bead array multiplex assay (BD Biosciences, San Jose, Calif., USA).
Phagocytosis
Heparinized whole blood from WT and sigirr−/− mice was collected and murine alveolar macrophages (AMs) were obtained by BAL and cultured to adhere overnight. Phagocytosis of UV-irradiated (254 nm, 30 min at 0.12 J/cm2, BLX-254; Vilber Lourmat, France) CFSE-labeled (Invitrogen) opsonized (10% autologous normal mouse serum) S. pneumoniae by AMs (MOI 100) and in whole blood by GR-1-identified neutrophils (8 × 107 CFU/ml blood) was determined with the help of flow cytometry as described previously [23]. The percentage of phagocytosing cells at 37°C was corrected for the percentage of phagocytosis at 4°C.
Statistical Analysis
Data are expressed as box and whisker diagrams depicting the smallest observation, the lower quartile, the median, the upper quartile, and the largest observation or as bar graphs depicting means ± SEM. Differences were analyzed using the Mann-Whitney U test. Survival was compared by Kaplan-Meier analysis followed by a log-rank test. p < 0.05 was considered statistically significant.
Results
sigirr−/− Mice Show Delayed Mortality and Diminished Bacterial Outgrowth during S. Pneumoniae Pneumonia
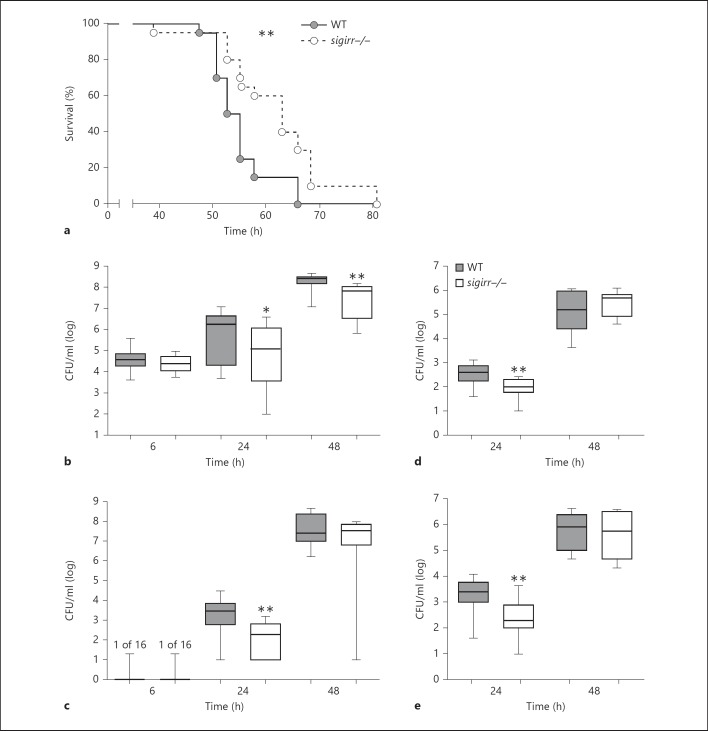
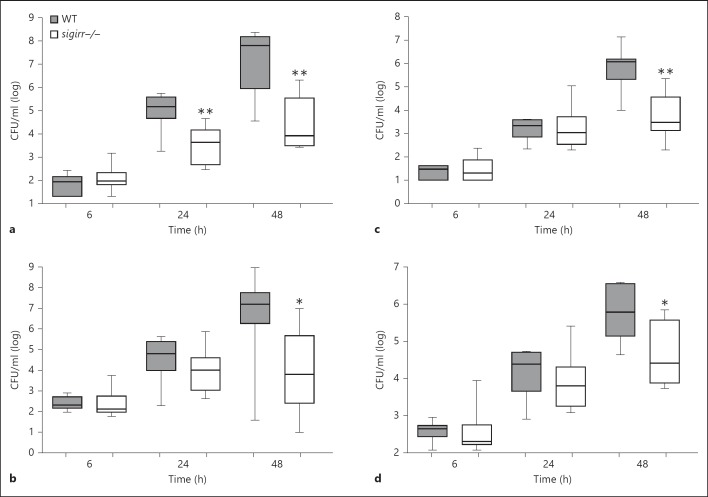
To gain insight into the potential role of SIGIRR in the outcome of pneumococcal pneumonia, WT and sigirr−/− mice were infected intranasally with S. pneumoniae and followed for 4 days (fig. 1a). sigirr−/− mice showed prolonged survival (median survival 63 h) compared to WT mice (median survival 54 h, p < 0.01). In order to determine whether the survival advantage of sigirr−/− mice corresponded with an improved antibacterial response, we next harvested lungs, blood, livers, and spleens from both mouse strains at predefined time points following the induction of pneumonia for quantitative cultures (fig. 1b-e). At 6 h postinfection, pneumococci were cultured from lungs only (with the exception of one positive blood culture in each group) and the bacterial loads were similar in sigirr−/− and WT mice. In contrast, 24 h after infection, sigirr−/− mice showed lower bacterial counts at all body sites examined compared to WT mice (lungs p < 0.05; blood, spleen, and liver all p < 0.01). At 48 h, differences in bacterial loads between mouse strains had subsided in distant organs, whereas at this late time point sigirr−/− mice still had lower pneumococcal burdens in their lungs (p < 0.01 vs. WT mice). Together these data suggest that sigirr−/− mice show prolonged survival in this lethal model of pneumococcal pneumonia resulting from a transient limitation of bacterial growth and dissemination.
Fig. 1.
Survival and bacterial loads in WT and sigirr−/− mice during pneumococcal pneumonia. WT and sigirr−/− mice were inoculated with S. pneumoniae intranasally. Lack of SIGIRR improved survival following intranasal infection (a). sigirr−/− mice displayed decreased bacterial loads after 24 and 48 h in lungs (b) and after 24 h in blood (c), liver (d), and spleen (e). CFUs are expressed as box and whisker plots showing the smallest observation, the lower quartile, the median, the upper quartile and the largest observation. Survival, n = 20 mice per group; 6 h and 24 h, n = 16 mice per group; 48 h, n = 8 mice per group. * p < 0.05, ** p < 0.01.
sigirr−/− Mice Demonstrate an Increased Pulmonary Inflammatory Response Early after Induction of Pneumonia
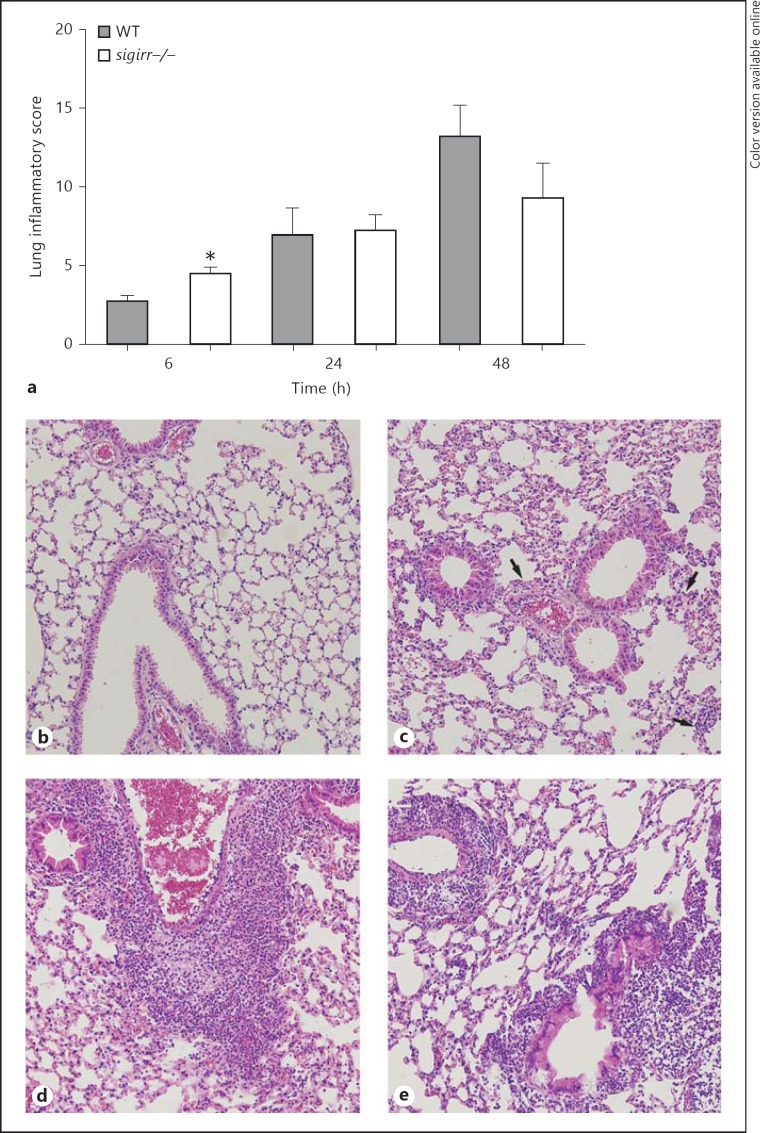
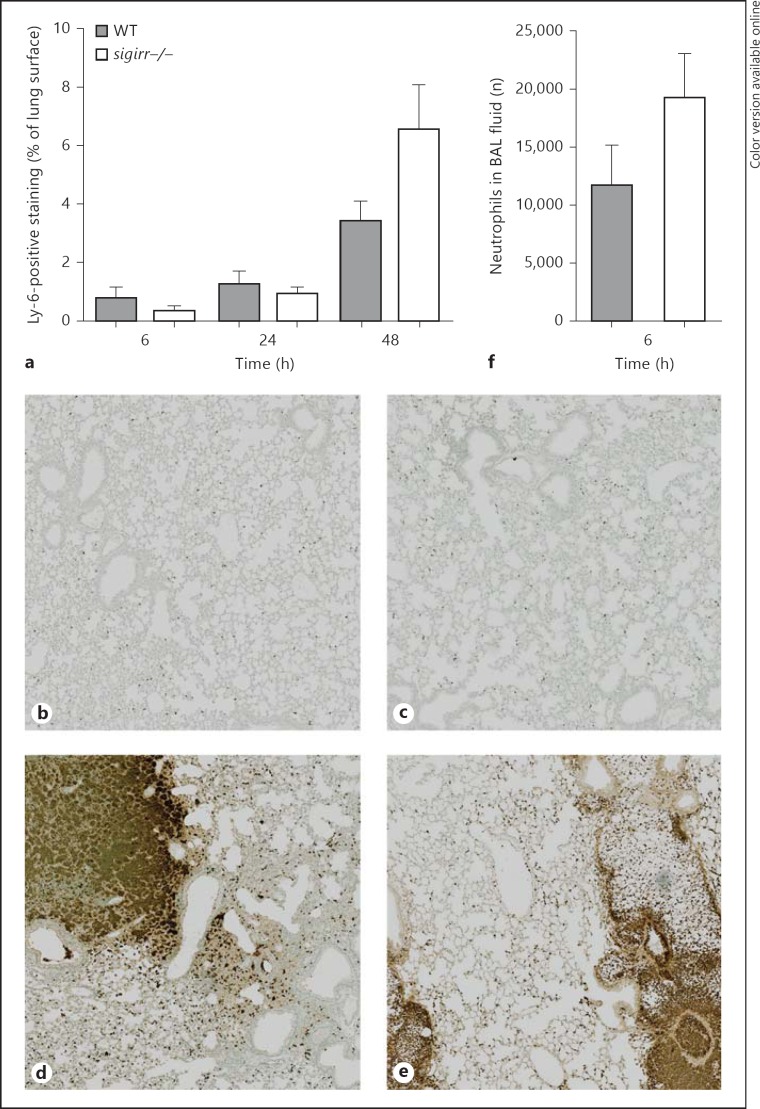
In order to gain insight into the role of SIGIRR in the regulation of lung inflammation induced by S. pneumoniae, lung tissue slides prepared 6, 24, and 48 h after infection were semiquantitatively analyzed according to the scoring system described in Methods (fig. 2a-e). As reported earlier, this model of pneumococcal pneumonia is characterized by a gradually developing inflammatory response in lung tissue with typical features of lower respiratory tract infection, including bronchitis, perivascular and interstitial inflammation, edema, and, especially during the progressed phase, accumulation of neutrophils [12, 23, 24]. At the earliest time point (6 h) sigirr−/− mice showed significantly increased lung inflammation (p < 0.05 relative to WT mice) which was caused by enhanced interstitial and perivascular inflammation. At later time points, when the extent of the lung pathology had clearly increased, pathology scores did not differ between sigirr−/− and WT mice. The recruitment of neutrophils to the primary site of infection is a prominent part of the innate immune response to invading respiratory pathogens. We determined the number of neutrophils in lung tissue by quantifying the amount of Ly-6+ cells in lung slides by digital imaging (fig. 3a-e). In both mouse strains, the number of Ly6+ cells in lung tissue gradually increased during the course of the infection; no differences between groups were observed at any time point (6 h, p = 0.5; 24 h, p = 0.9; 48 h, p = 0.13). Considering the importance of early neutrophil influx into the bronchoalveolar space, we also analyzed the number of neutrophils in BAL fluid harvested 6 h after infection; no difference between sigirr−/− and WT mice was found (p = 0.13) (fig. 3f). BAL fluid macrophage and lymphocyte numbers were also similar between groups at this early time point (data not shown). Together these data argue against an important role for SIGIRR in neutrophil recruitment during pneumococcal pneumonia; SIGIRR deficiency did have an impact on early interstitial and perivascular inflammation.
Fig. 2.
Histopathology of lungs from WT and sigirr−/− mice during pneumococcal pneumonia. Semiquantitative histology scores of lung slides as determined by the scoring system described in Methods from WT and sigirr−/− mice (a) and hematoxylin-eosin staining of the lung 6 (b, c) and 48 h (d, e) after pneumococcal infection. Representative lung slides of WT (b, d) and sigirr−/− mice (c, e); original magnification ×10. Histology scores are the means ± SEM of 8 mice per group at each time point. The arrows in c indicate several foci of increased perivascular and interstitial inflammation. * p < 0.05.
Fig. 3.
Neutrophil influx into the lungs of WT and sigirr−/− mice during pneumococcal pneumonia. Neutrophil numbers in WT and sigirr−/− lung tissue were evaluated via Ly-6 staining of lung slides (a-e) during pneumonia. Neutrophil numbers were further counted in BAL fluid 6 h after inoculation (f). Representative Ly-6-stained lung sections of WT (b, d) and sigirr−/− mice (c, e) are depicted 6 (b, c) and 48 h (d, e) after the induction of pneumonia; original magnification ×10. Data in a and f are the means ± SEM of 8 mice per group at each time point.
sigirr−/− Mice Demonstrate Unaltered Lung Cytokine and Chemokine Levels in Lungs following S. Pneumoniae Infection
In order to gain further insight into the potential role of SIGIRR in the regulation of lung inflammation during pneumococcal pneumonia, we measured proinflammatory cytokines (TNF-α, IL-1β, and IL-6) and chemokines (MIP-2 and KC) in whole-lung homogenates harvested 6, 24, or 48 h after intranasal inoculation with S. pneumoniae (table 1). Lung cytokine and chemokine levels were similar in sigirr−/− and WT mice at all time points. Moreover, as a readout for systemic inflammation, we measured the plasma concentrations of TNF-α, IL-6, and MCP-1; no differences were found between groups (table 1).
Table 1.
Cytokines measured in lung homogenates and plasma during S. pneumoniae pneumonia
| 6 h |
24 h |
48 h |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| WT | sigirr–/– | p | WT | sigirr–/– | p | WT | sigirr–/– | p | |
| Lung | |||||||||
| TNF-α, pg/ml | 45±17 | 109±58 | 0.62 | 73±34 | 124±60 | 0.46 | 138±39 | 199±43 | 0.32 |
| IL-1β, pg/ml | 90±45 | 164±79 | 0.42 | 491±260 | 677±504 | 0.60 | 488±240 | 1,828±768 | 0.18 |
| IL-6, pg/ml | 48±12 | 102±30 | 0.15 | 616±194 | 574±249 | 0.65 | 1,603±354 | 2,384±658 | 0.80 |
| MIP-2, pg/ml | 477±78 | 894±255 | 0.09 | 1,560±694 | 2,050±1,025 | 0.88 | 33,555±5,024 | 96,068±36,498 | 0.38 |
| KC, pg/ml | 1,398±290 | 942±255 | 0.19 | 8,882±3,474 | 4,318±1,563 | 0.38 | 28,174±4,591 | 21,275±7,723 | 0.23 |
| Plasma | |||||||||
| TNF-α, pg/ml | 5±1 | 5±1 | 0.83 | 11±2 | 15±3 | 0.34 | 60±5 | 45±7 | 0.13 |
| IL-6, pg/ml | 3±0.4 | 7±2 | 0.19 | 133±38 | 124±46 | 0.96 | 1,087±262 | 1,197±339 | 0.75 |
| MCP-1, pg/ml | 23±2 | 19±4 | 0.49 | 157±42 | 161±52 | 0.87 | 343±57 | 269±54 | 0.28 |
Mice were infected with S. pneumoniae at t = 0. Data are the means ± SEM of 8 mice per group. p values are for sigirr–/– vs. WT.
Enhanced Phagocytosis of S. Pneumoniae by sigirr−/− AMs and Neutrophils ex vivo
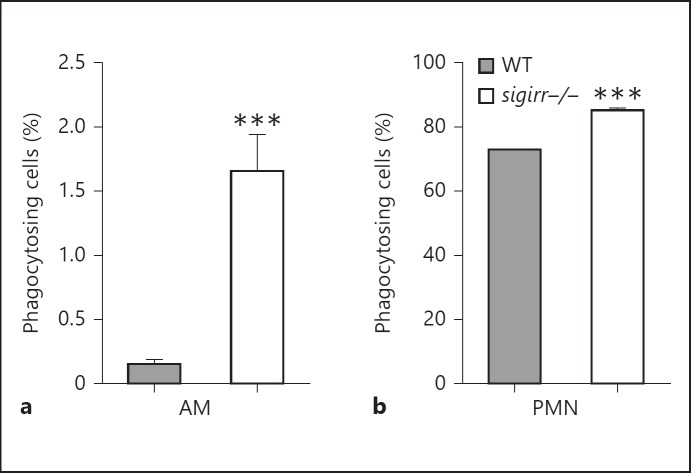
Next, we investigated the ability of WT and sigirr−/− AMs and neutrophils to phagocytose S. pneumoniae. For this, AMs and whole blood were exposed to growth-arrested CFSE-labeled bacteria for 60 min at 4 or 37°C and internalization was analyzed by flow cytometry (fig. 4). AMs showed a relatively low ability to phagocytose viable S.pneumoniae; nonetheless, sigirr−/− AMs demonstrated an enhanced capacity to internalize S. pneumoniae compared to WT macrophages (fig. 4a, p < 0.001). Neutrophils of both genotypes readily phagocytosed pneumococci; clearly sigirr−/− neutrophils showed an increased ability to phagocytose S. pneumoniae relative to WT neutrophils (fig. 4b, p < 0.001).
Fig. 4.
Phagocytosis of S. pneumoniae by AMs and neutrophils. AMs and whole blood were exposed to fluorescently labeled S. pneumoniae for 60 min at 4 or 37°C. Depicted are the percentages of phagocytosing AMs (a) and neutrophils (b) at 37°C when corrected for their 4°C controls. Data are means ± SEM (AM: n = 6 WT vs. 8 sigirr−/−; PMN: n = 8 WT vs. 8 sigirr−/−). *** p < 0.001. PMN = Polymorphonuclear neutrophils.
sigirr−/− Mice Show Diminished Bacterial Outgrowth during Primary S. Pneumoniae Sepsis
We wondered whether the improved antibacterial defense in sigirr−/− mice after the induction of pneumonia was primarily caused by limitation of bacterial growth in the lungs and, as a consequence thereof, impeded dissemination and/or by an additional reduction of pneumococcal multiplication at body sites distant from the lungs. To address this question, sigirr−/− and WT mice were infected with S. pneumoniae by intravenous injection via the tail vein, thereby bypassing the initial interaction between the host and the pathogen in the respiratory tract, and euthanized 6, 24, or 48 h later for quantitative cultures of multiple body sites. Once more, lower bacterial counts were observed in sigirr−/− mice, especially 48 h after infection when SIGIRR deficiency was associated with reduced bacterial loads in the blood (p < 0.05 vs. WT mice), spleen (p < 0.05), liver (p < 0.01) and lungs (p < 0.01; fig. 5). Notably, in these experiments SIGIRR deficiency did not significantly influence the plasma concentrations of TNF-α, IL-6, or MCP-1 (table 1).
Fig. 5.
Bacterial loads in WT and sigirr−/− mice during pneumococcal sepsis. WT and sigirr−/− mice were inoculated with S. pneumoniae intravenously. sigirr−/− mice displayed decreased bacterial loads after 24 and 48 h in lungs (a), and after 48 h in the blood (b), liver (c), and spleen (d). Data are expressed as box and whisker plots showing the smallest observation, the lower quartile, the median, the upper quartile, and the largest observation; n = 8 mice per group at each time point. * p < 0.05, ** p < 0.01.
Discussion
S. pneumoniae is a common human pathogen that can reside as a commensal in the nasopharynx, from where it is able to enter the lower respiratory tract, causing pneumonia and sepsis [1, 2]. The family of TLRs constitutes an important part of the innate defense against invading pneumococci. SIGIRR is a negative regulator of TLR and ILR signaling that is abundantly expressed in the lungs. Here we investigated the potential role of SIGIRR in pneumococcal pneumonia and sepsis. SIGIRR was found to impair the antibacterial host defense during both pneumonia and sepsis caused by S. pneumoniae, as reflected by a reduced survival accompanied by increased bacterial growth and dissemination in WT mice compared to sigirr−/− animals.
Upon recognition of S. pneumoniae by, in particular, TLR2 [7, 8], TLR4 [10, 11], and TLR9 [13], NF-κB is activated and proinflammatory cytokines are produced with the distinct purpose of eradicating the pathogen [5]. Uncontrolled stimulation of TLRs leads to disproportionate inflammation and tissue injury. Excessive TLR activation is prevented by negative regulators of TLR signaling, several of which have been identified [17], including SIGIRR [18, 28]. Specifically, the inhibitory activity of SIGIRR has been demonstrated on signaling by TLR4, TLR7, TLR9, IL-1R type I (IL-1RI), IL-18R, and ST2 [18]. The current study does not elucidate via which receptor SIGIRR exerts its detrimental effect during pneumococcal pneumonia. Potential candidates are TLR4, TLR9, IL-1RI, and IL-18R, considering that mice deficient for either one of these signaling pathways were reported to have an impaired antibacterial defense response during infection with S. pneumoniae. Indeed, mice with a mutant nonactive form of TLR4 showed enhanced pneumococcal growth and dissemination in models of nasopharyngeal colonization and lower respiratory tract infection, accompanied by increased lethality [10, 11, 12]. Similarly, tlr9−/− mice displayed accelerated growth of pneumococci upon infection of the lower airways together with enhanced mortality [13], while il-1rI−/−[29] and il-18−/−[30] mice showed a more modestly impaired immune response only reflected by higher bacterial loads. To our knowledge, the role of TLR7 has not been studied in the context of pneumococcal infections, while our laboratory recently showed that ST2 does not contribute to the host defense during S. pneumoniae pneumonia [26]. Together these data suggest that SIGIRR may impair the host defense during pneumococcal pneumonia via inhibition of TLR4, TLR9, IL-1RI, and/or IL-18R.
In contrast to the results presented here, previous studies have revealed a protective role of SIGIRR in the host defense against pulmonary infections. In a high-dose lung infection model with P. aeruginosa associated with acute inflammation, sigirr−/− mice showed reduced survival and diminished bacterial clearance relative to WT mice [19]. sigirr−/− mice had markedly elevated concentrations of proinflammatory cytokines in whole-lung homogenates, including IL-1β, and elimination of IL-1RI signaling in sigirr−/− mice partially reversed their worse outcome [19]. Similarly, sigirr−/− mice displayed exaggerated pulmonary inflammation and strongly elevated plasma levels of TNF-α and IL-1β during experimental lung tuberculosis, and combined treatment with anti-TNF-α and anti-IL-1β antibodies improved their survival in this model [20]. Hence, the protective role of SIGIRR in these previous investigations on lung infection was most likely related to its inhibitory effect on the production of proinflammatory cytokines [19, 20]. Our current data, indicating a detrimental role for SIGIRR, suggest another underlying mechanism, considering that SIGIRR deficiency did not alter the local or systemic cytokine response during S. pneumoniae pneumonia or sepsis. Nonetheless, studies in which IL-1 signaling is inhibited are warranted to address this issue. Of note, our model of Gram-positive pneumonia differs considerably from the previously reported model of Gram-negative pneumonia caused by P. aeruginosa [19]. Indeed, while S. pneumoniae (infectious dose 5 × 104 CFU) gradually grows in the lungs of normal mice resulting in a slowly building inflammatory response, airway infection by P. aeruginosa (infectious inoculum 106 CFU) is associated with a brisk inflammatory reaction while bacteria are cleared from the airways. The difference between S. pneumoniae and P. aeruginosa pneumonia models is further illustrated by the different roles of proinflammatory cytokines, which play a protective role in pneumococcal pneumonia [29, 30, 31], while they hamper bacterial clearance during Pseudomonas pneumonia [32, 33, 34].
Earlier studies have indicated that the role for SIGIRR in the immune response to infection varies depending on the infecting organism and the infected site. In accordance with the enhanced susceptibility of sigirr−/− mice during P. aeruginosa pneumonia [19], an anti-SIGIRR antibody caused increased bacterial loads in corneas during experimental Pseudomonas keratitis [35]. In addition, sigirr−/− mice were more susceptible to infection via mucosal and systemic infection by Candida albicans and to lung infection by Aspergillus fumigatus [36]. In contrast, however, sigirr−/− mice demonstrated transiently reduced bacterial burdens in kidneys during Escherichia coli pyelonephritis, possibly caused by a faster recruitment of neutrophils to the primary site of infection [37]. Although clearly the host defense against Gram-positive and Gram-negative bacteria is regulated in partially different ways [5], the present results, obtained after infection with a Gram-positive bacterium, taken together with previous reports using Gram-negative organisms [19, 35, 37], cannot be used to establish a differential role of SIGIRR in infections caused by either one of these very broad groups of pathogens. Indeed, the receptors known to be influenced by SIGIRR (see above) are not exclusively activated by either Gram-positive or Gram-negative bacteria. We consider it likely that the primary site of infection, the initial bacterial load, and whether the pathogen multiplies or is cleared, together with differential expression of pathogen-associated molecular patterns, determine the eventual role of SIGIRR in host defense.
We have provided evidence that SIGIRR attenuates S. pneumoniae phagocytosis by neutrophils and AMs, which at least in part may explain the lower bacterial loads in sigirr−/− mice. TLR stimulation induced expression of phagocytic genes (Fc and complement receptor genes, scavenger receptor and scavenger receptor pathway genes) in bone marrow-derived macrophages [38] and resulted in an enhanced uptake of both Gram-negative (E. coli) and Gram-positive (Staphylococcus aureus) bacteria by RAW cells in an MyD88- and p38 mitogen-activated kinase (MAPK)-dependent manner [38]. p38 MAPK signaling dependency was established in LPS-enhanced microglial Fc receptor-mediated phagocytosis as well [39]. In addition, IL-1β and IL-18 have been shown to enhance phagocytosis [39, 40]. Thus, in theory, SIGIRR deficiency could impact phagocytosis by inhibiting the effects of TLR on phagocytic gene expression and/or by attenuating IL-1β and/or IL-18 production and/or signaling. To the best of our knowledge, this is the first report implicating SIGIRR in phagocytosis. The fact that this highly virulent S. pneumoniae strain cannot be killed by macrophages or neutrophils ex vivo [24] (and data not shown) precludes studies on the effect of SIGIRR on bacterial killing.
Contrary to E. coli pyelonephritis [37], SIGIRR deficiency did not influence the neutrophil influx into the lungs during pneumonia caused by either P. aeruginosa[19] or S. pneumoniae (reported here). SIGIRR is expressed by both hematopoietic and parenchymal cells [19]. Bone marrow transfers, creating chimeric mice expressing SIGIRR only in the hematopoietic or parenchymal compartment, can provide insight into which cells drive the phenotype of sigirr−/− mice in pneumococcal infection.
The pneumococcus is a highly relevant human pathogen, especially in the context of community-acquired pneumonia. The immune system rapidly responds to pneumococci that try to invade the lower airways. TLRs are of paramount importance for the recognition of S. pneumoniae and for the activation of inflammatory pathways among which those that trigger and are triggered by ILRs. Here we report on the role of SIGIRR, a negative regulator of TLRs and ILRs, in S. pneumoniae pneumonia and sepsis. In contrast to earlier investigations that have studied the function of SIGIRR during lung infections caused by A. fumigatus [36], M. tuberculosis[20], or P. aeruginosa [19], in which SIGIRR improved outcomes via inhibition of excessive inflammation, our results indicate that SIGIRR impairs the host defense during pneumonia and sepsis caused by S. pneumoniae.
Disclosure Statement
The authors declare that they have no competing interests as defined by the Journal of Innate Immunity, or other interests that might be perceived to influence the results and discussion reported in this paper.
Acknowledgements
We thank Joost Daalhuisen and Marieke ten Brink for their expert technical assistance.
References
- 1.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–1556. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 2.Dockrell DH, Whyte MK, Mitchell TJ. Pneumococcal pneumonia: mechanisms of infection and resolution. Chest. 2012;142:482–491. doi: 10.1378/chest.12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevention of., pneumococcal disease recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 4.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 5.Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Knapp S, Wieland CW, van ‘t Veer C, Takeuchi O, Akira S, Florquin S, van der Poll T. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004;172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- 8.Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J Leukoc Biol. 2006;80:267–277. doi: 10.1189/jlb.1105626. [DOI] [PubMed] [Google Scholar]
- 9.Dessing MC, Schouten M, Draing C, Levi M, von Aulock S, van der Poll T. Role played by Toll-like receptors 2 and 4 in lipoteichoic acid-induced lung inflammation and coagulation. J Infect Dis. 2008;197:245–252. doi: 10.1086/524873. [DOI] [PubMed] [Google Scholar]
- 10.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, Paton JC, Wessels MR, Golenbock DT, Malley R. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun. 2005;73:6479–6487. doi: 10.1128/IAI.73.10.6479-6487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Branger J, Knapp S, Weijer S, Leemans JC, Pater JM, Speelman P, Florquin S, van der Poll T. Role of Toll-like receptor 4 in Gram-positive and Gram-negative pneumonia in mice. Infect Immun. 2004;72:788–794. doi: 10.1128/IAI.72.2.788-794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albiger B, Dahlberg S, Sandgren A, Wartha F, Beiter K, Katsuragi H, Akira S, Normark S, Henriques-Normark B. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007;9:633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 14.Dessing MC, Florquin S, Paton JC, van der Poll T. Toll-like receptor 2 contributes to antibacterial defence against pneumolysin-deficient pneumococci. Cell Microbiol. 2008;10:237–246. doi: 10.1111/j.1462-5822.2007.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 16.Albiger B, Sandgren A, Katsuragi H, Meyer-Hoffert U, Beiter K, Wartha F, Hornef M, Normark S, Normark BH. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell Microbiol. 2005;7:1603–1615. doi: 10.1111/j.1462-5822.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 17.Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33:449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Riva F, Bonavita E, Barbati E, Muzio M, Mantovani A, Garlanda C. TIR8/SIGIRR is an interleukin-1 receptor/Toll like receptor family member with regulatory functions in inflammation and immunity. Front Immunol. 2012;3:322. doi: 10.3389/fimmu.2012.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veliz Rodriguez T, Moalli F, Polentarutti N, Paroni M, Bonavita E, Anselmo A, Nebuloni M, Mantero S, Jaillon S, Bragonzi A, Mantovani A, Riva F, Garlanda C. Role of Toll interleukin-1 receptor (IL-1R) 8, a negative regulator of IL-1R/Toll-like receptor signaling, in resistance to acute Pseudomonas aeruginosa lung infection. Infect Immun. 2012;80:100–109. doi: 10.1128/IAI.05695-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garlanda C, Di Liberto D, Vecchi A, La Manna MP, Buracchi C, Caccamo N, Salerno A, Dieli F, Mantovani A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J Immunol. 2007;179:3119–3125. doi: 10.4049/jimmunol.179.5.3119. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Zhao Y, Wu X, Qian G. Enhanced expression of single immunoglobulin IL-1 receptor-related molecule ameliorates LPS-induced acute lung injury in mice. Shock. 2011;35:198–204. doi: 10.1097/SHK.0b013e3181f226f3. [DOI] [PubMed] [Google Scholar]
- 22.Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E, Vecchi A, Hirsch E, Mantovani A. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci USA. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Windt GJ, Blok DC, Hoogerwerf JJ, Lammers AJ, de Vos AF, Van't Veer C, Florquin S, Kobayashi KS, Flavell RA, van der Poll T. Interleukin 1 receptor-associated kinase m impairs host defense during pneumococcal pneumonia. J Infect Dis. 2012;205:1849–1857. doi: 10.1093/infdis/jis290. [DOI] [PubMed] [Google Scholar]
- 24.van der Windt GJ, Hoogendijk AJ, Schouten M, Hommes TJ, de Vos AF, Florquin S, van der Poll T. Osteopontin impairs host defense during pneumococcal pneumonia. J Infect Dis. 2011;203:1850–1858. doi: 10.1093/infdis/jir185. [DOI] [PubMed] [Google Scholar]
- 25.Hoogendijk AJ, Roelofs JJ, Duitman J, van Lieshout MH, Blok DC, van der Poll T, Wieland CW. R-roscovitine reduces lung inflammation induced by lipoteichoic acid and Streptococcus pneumoniae. Mol Med. 2012;18:1086–1095. doi: 10.2119/molmed.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blok DC, van der Sluijs KF, Florquin S, de Boer OJ, van ‘t Veer C, de Vos AF, van der Poll T. Limited anti-inflammatory role for interleukin-1 receptor like 1 (ST2) in the host response to murine postinfluenza pneumococcal pneumonia. PLoS One. 2013;8:e58191. doi: 10.1371/journal.pone.0058191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kager LM, Wiersinga WJ, Roelofs JJ, Meijers JC, Levi M, Van't Veer C, van der Poll T. Plasminogen activator inhibitor type I contributes to protective immunity during experimental Gram-negative sepsis (melioidosis) J Thromb Haemost. 2011;9:2020–2028. doi: 10.1111/j.1538-7836.2011.04473.x. [DOI] [PubMed] [Google Scholar]
- 28.Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 29.Rijneveld AW, Florquin S, Branger J, Speelman P, Van Deventer SJ, van der Poll T. TNF-alpha compensates for the impaired host defense of IL-1 type I receptor-deficient mice during pneumococcal pneumonia. J Immunol. 2001;167:5240–5246. doi: 10.4049/jimmunol.167.9.5240. [DOI] [PubMed] [Google Scholar]
- 30.Lauw FN, Branger J, Florquin S, Speelman P, van Deventer SJ, Akira S, van der Poll T. IL-18 improves the early antimicrobial host response to pneumococcal pneumonia. J Immunol. 2002;168:372–378. doi: 10.4049/jimmunol.168.1.372. [DOI] [PubMed] [Google Scholar]
- 31.van der Poll T, Keogh CV, Buurman WA, Lowry SF. Passive immunization against tumor necrosis factor-alpha impairs host defense during pneumococcal pneumonia in mice. Am J Respir Crit Care Med. 1997;155:603–608. doi: 10.1164/ajrccm.155.2.9032201. [DOI] [PubMed] [Google Scholar]
- 32.Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol. 1999;276:L715–L727. doi: 10.1152/ajplung.1999.276.5.L715. [DOI] [PubMed] [Google Scholar]
- 33.Schultz MJ, Knapp S, Florquin S, Pater J, Takeda K, Akira S, van der Poll T. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect Immun. 2003;71:1630–1634. doi: 10.1128/IAI.71.4.1630-1634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz MJ, Rijneveld AW, Florquin S, Edwards CK, Dinarello CA, van der Poll T. Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L285–L290. doi: 10.1152/ajplung.00461.2000. [DOI] [PubMed] [Google Scholar]
- 35.Huang X, Hazlett LD, Du W, Barrett RP. SIGIRR promotes resistance against Pseudomonas aeruginosa keratitis by down-regulating type-1 immunity and IL-1R1 and TLR4 signaling. J Immunol. 2006;177:548–556. doi: 10.4049/jimmunol.177.1.548. [DOI] [PubMed] [Google Scholar]
- 36.Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D'Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, Puccetti P, Mantovani A, Romani L. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J Immunol. 2008;180:4022–4031. doi: 10.4049/jimmunol.180.6.4022. [DOI] [PubMed] [Google Scholar]
- 37.Leemans JC, Butter LM, Teske GJ, Stroo I, Pulskens WP, Florquin S. The Toll interleukin-1 receptor (IL-1R) 8/single Ig domain IL-1R-related molecule modulates the renal response to bacterial infection. Infect Immun. 2012;80:3812–3820. doi: 10.1128/IAI.00422-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doyle SE, O'Connell RM, Miranda GA, Vaidya SA, Chow EK, Liu PT, Suzuki S, Suzuki N, Modlin RL, Yeh WC, Lane TF, Cheng G. Toll-like receptors induce a phagocytic gene program through p38. J Exp Med. 2004;199:81–90. doi: 10.1084/jem.20031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira R, Santos T, Viegas M, Cortes L, Bernardino L, Vieira OV, Malva JO. Neuropeptide Y inhibits interleukin-1beta-induced phagocytosis by microglial cells. J Neuroinflammation. 2011;8:169. doi: 10.1186/1742-2094-8-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henan X, Toyota N, Yanjiang X, Fujita Y, Zhijun H, Touma M, Qiong W, Sugimoto K. Enhancement of phagocytosis and cytotoxicity in macrophages by tumor-derived IL-18 stimulation. BMB Rep. 2013 doi: 10.5483/BMBRep.2014.47.5.152. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]