Abstract
Listeria monocytogenes is a facultative intracellular pathogen which can infect Drosophila melanogaster. Upon infection, Drosophila mounts an immune response including antimicrobial peptide production and autophagy activation. A set of previously published results prompted us to study the role of the deubiquitinating enzyme dUSP36 in response to L. monocytogenes infections. We show in this report that flies with dUsp36-specific inactivation in hemocytes are susceptible to L. monocytogenes infections (as are flies with autophagy-deficient hemocytes) but are still able to control bacterial growth. Interestingly, flies with dUsp36-depleted hemocytes are not sensitized to infection by other pathogens. We conclude that dUsp36 plays a major role in hemocytes for tolerance to L. monocytogenes.
Key Words: Autophagy, Deubiquitinating enzymes, Intracellular pathogens, Ubiquitin-specific protease, USP36
Introduction
Listeria monocytogenes, a Gram-positive, facultative intracellular bacterium, is responsible for severe food-borne infections that primarily affect immunocompromised individuals and pregnant women [1]. It has been widely used as a model pathogen to study the molecular and cellular aspects of intracellular pathogenesis. L. monocytogenes is taken up by the host cell through pathogen-induced endocytosis and enclosed in a vacuole, from which the bacteria can evade by expressing a set of toxins [2, 3]. Following escape from the vacuole, the bacteria grow and divide in the host cytosol [4, 5]. Using host actin to form comet-like tails, L. monocytogenes propels itself through the cytosol and into neighboring cells [6, 7].
The fruit fly Drosophilamelanogaster has been established as a model host for L. monocytogenes infections [8]. The immune system of Drosophila relies on two conserved nuclear factor-κB-like signaling pathways, Toll and imd (immune deficiency), which are induced upon infection [9, 10]. The Toll pathway is activated by Lys-type peptidoglycans and results in the activation of a set of specific antimicrobial peptide genes [11, 12, 13] whereas the imd pathway is activated by diaminopimelic acid (DAP)-containing-peptidoglycans and results in the activation of another set of antibacterial peptide genes [14, 15, 16, 17]. Both Toll and imd pathways are required for Drosophila survival to L. monocytogenes infections [8]. Moreover, Drosophila survival after L. monocytogenes infections also relies on autophagy [18].
Autophagy is the major lysosomal degradation pathway in cells. It is a highly conserved cellular mechanism in which cytoplasmic components are sequestered into double-membrane structures called autophagosomes and are eventually degraded in lysosomes [19]. Autophagy is involved in diverse functions, including the removal of damaged organelles, protein turnover, supply of nutrients in nutrient-deprived conditions, and cell survival and death. In addition to L. monocytogenes, autophagy is also involved in innate immune defenses against other invading pathogens such as group A streptococci, Shigellaflexneri, Mycobacteriumtuberculosis and Toxoplasmagondii, as shown in human cells cultures [20].
Besides these resistance mechanisms (responsible for the control of pathogen growth), tolerance plays also a major role in the survival of infected organisms. Tolerance is defined as the set of physiological mechanisms that keep organisms healthy during infections or that help enduring infections [21, 22]. Although most of the work on Drosophila immunity has been focused on resistance mechanisms, a number of studies now points out the role of tolerance in particular during L. monocytogenes infections [23, 24].
We have previously demonstrated that the dUSP36 (syn. Scrawny or Emperor's thumb) deubiquitinating enzyme acts as a negative regulator of the imd pathway by deubiquitinating the IMD protein [25]. We have also shown that dUsp36 controls cell growth and selective autophagy activation by ubiquitinated proteins [26]. Interestingly, a genome-wide RNAi-based screen conducted to identify host processes that contribute to L. monocytogenes pathogenesis identified dUsp36 (referred to as CG5505) as part of a group of genes whose knockdown led to enhanced L. monocytogenes intracellular growth [27]. These results place dUsp36 at the crossroads between immune signaling, autophagy and L. monocytogenes intracellular growth containment.
In this report, we show that dUsp36 function is required in vivo in adult hemocytes for survival to L. monocytogenes infections. We also confirm the role of autophagy as a protective mechanism during L. monocytogenes infections. Interestingly, flies with dUsp36-depleted hemocytes, although being sensitive to L. monocytogenes infections, do not display increased bacterial loads, which indicates defects in tolerance mechanisms. These results are a first step towards the understanding of the role of the dUSP36 deubiquitinating enzyme during infections by the intracellular pathogen L. monocytogenes.
Materials and Methods
Fly Strains and Culture
The UAS-dUsp36-IR transgenic line was obtained from the Vienna Drosophila RNAi Center and the UAS-Atg5-IR transgenic line from Dr. Thomas P. Neufeld (University of Minnesota). The Bloomington stock center provided the hml-, hmlΔ- and Hemese-GAL4 lines (stock Nos. 6395, 30139 and 8699, respectively) and the Tub-GAL80ts line for the TARGET system (stock No. 7019).
Flies were kept in standard fly vials or bottles containing dextrose medium and raised under a 12-hour light-dark cycle at 18, 25 or 29°C prior to infections.
Bacterial Strains and Culture
L. monocytogenes strain 10403s was grown standing overnight in BHI medium at 37°C and injected at an OD600 of 0.01, 0.001 and 0.0001 for 1,000, 100 and 10 colony forming units (CFUs), respectively. Streptococcus pneumoniae was grown standing at 37°C in a 5% CO2 incubator and injected at an OD600 of 0.2. Salmonella typhimurium was grown standing in LB medium at 37°C and injected at an OD600 of 0.1.
Infection
Five- to 7-day-old males were used for injection. Flies were anesthetized with CO2 and injected with 50 nl of diluted culture using a Picospritzer (Parker Hannifin) and pulled glass needles. Flies were then placed in vials containing dextrose medium in groups of 20 and incubated at 29°C for L. monocytogenes and S. typhimurium infections or at 25°C for S. pneumoniae infections under a 12-hour light-dark cycle.
Survival Curves
After infection, the number of dead flies was counted daily. Using the GraphPad Prism software, Kaplan-Meier survival curves were generated and statistical analysis was performed using log-rank analysis. Survival was tested at least two times on more than 60 flies and gave similar results for each trial.
CFU Determination and Gentamicin Chase
Infected flies were homogenized in PBS. Appropriate dilutions of the homogenates were plated on LB agar plates using a spiral plater (QCL) and incubated overnight. The data were plotted using the GraphPad Prism software and the p values were determined according to an unpaired two-tailed t test. For the gentamicin chase experiments, flies were injected with 50 nl of 1 mg/ml gentamicin 3 h prior to homogenizing and plating.
Results
dUsp36 Is Required in Adult Hemocytes for Survival to L. monocytogenes Infections
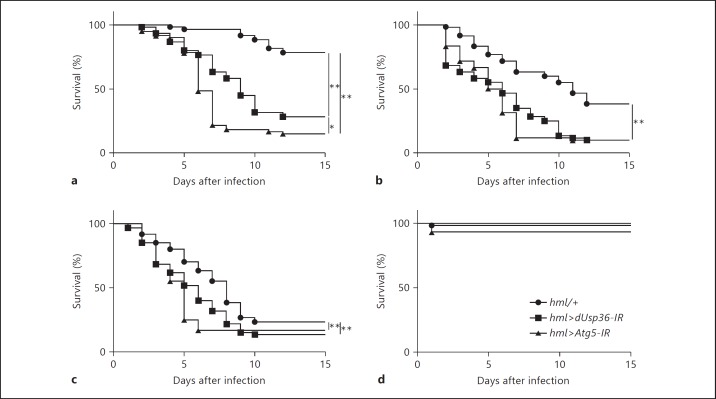
A former RNAi screen showed that L. monocytogenes intracellular growth containment in the Drosophila hemocytic-like S2 cells requires dUsp36 function [27]. To determine whether dUsp36 is also required in the hemocytic lineage for fighting L. monocytogenes infections in vivo, we have first assessed the survival of flies with dUsp36-depleted hemocytes infected with wild-type L. monocytogenes (fig. 1). dUsp36 hemocyte-specific knockdown was achieved by expressing a double-stranded RNA (dsRNA) targeting dUsp36 in hemocytes using the driver line hml-Gal4 [28]. The efficiency and specificity of the dsRNA construct used in this study have already been thoroughly characterized [26]. We observed that, with the three different doses of L. monocytogenes used for infection (10, 100 or 1,000 CFUs), flies with dUsp36-depleted hemocytes (hml>dUsp36-IR) died significantly faster than control flies (hml/+; fig. 1a-c) whereas survival of PBS-injected flies was not affected (fig. 1d). Moreover, a second dsRNA transgene targeting a different sequence in dUsp36[25] also induced a significant sensitivity to L. monocytogenes infections (online suppl. fig. 1; see www.karger.com/doi/10.1159/000360293) indicating that the observed phenotype is not due to putative off-target effects but is actually the consequence of dUsp36 loss of function.
Fig. 1.
dUsp36 inactivation in hemocytes sensitizes flies to L. monocytogenes infections. Flies were injected with 10 (a), 100 (b) or 1,000 (c) CFUs of wild-type L. monocytogenes and PBS (d), and monitored for survival. Significance was determined by log-rank analysis of the survival curves (* p < 0.05, ** p < 0.001).
Autophagy is required for Drosophila survival after L. monocytogenes infections [18]. The susceptibility of hml>dUsp36-IR individuals was thus compared to that of flies with autophagy-deficient hemocytes. First, we confirmed that flies with a hemocyte-targeted inactivation of the Atg5 gene, which encodes an obligatory component of the autophagy machinery, are indeed more sensitive to L. monocytogenes infections than control flies (fig. 1). Even if, at the 10-CFU dose, flies with autophagy-deficient hemocytes died significantly faster than flies with dUsp36-depleted hemocytes (fig. 1a), similar survival kinetics between autophagy-deficient and dUsp36-depleted flies were observed when flies were infected with 100 and 1,000 CFUs (fig. 1b, c). This indicates that flies with autophagy-deficient hemocytes might be only slightly more susceptible to L. monocytogenes infections than flies with dUsp36-depleted hemocytes.
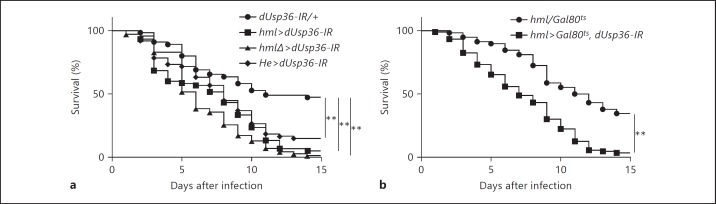
We also used two additional transgenic lines specifically expressing the GAL4 protein in hemocytes: hmlΔ-Gal4[29] and Hemese-Gal4[30]. We found that, using these Gal4 lines to express the dsRNA targeting dUsp36, flies with dUsp36-depleted hemocytes were systematically and significantly more sensitive to L. monocytogenes infections than the control flies (fig. 2a), which indicates that this sensitivity is actually the result of dUsp36 loss of function in hemocytes.
Fig. 2.
dUsp36 function is required in adult hemocytes for survival to L. monocytogenes infections. Flies were injected with 100 CFUs of wild-type L. monocytogenes and monitored for survival. dUsp36 hemocyte-specific inactivation was carried out either throughout life time with different hemocyte-specific driver lines (a) or only during the adult stage (b). Significance was determined by log-rank analysis of the survival curves (** p < 0.001).
We have previously shown that dUsp36 controls cell growth during larval development [26] and the three Gal4 lines used in this study are expressed in the hemocytic lineage during larval development [28, 29, 30]. This raises the possibility that the sensitivity of flies with dUsp36-depleted hemocytes to L. monocytogenes infections may be a consequence of developmental defects rather than a consequence of an actual role of dUsp36 in adult hemocyte functions. To investigate this question, we used the TARGET system [31] to temporally control dUsp36 inactivation. Development and the first 5 days of the adult life were achieved at restrictive temperature (18°C, no expression of the dsRNA targeting dUsp36). Five-day-old flies were then shifted to permissive temperature (29°C) 2 days before infection allowing for the expression of the dsRNA targeting dUsp36. We observed that flies with the adult-specific inactivation of dUsp36 in hemocytes are still more sensitive to L. monocytogenes infections than control flies (fig. 2b), which indicates that this sensitivity is not the result of developmental defects.
Taken altogether, these results show unambiguously that dUsp36 function is required in the adult hemocytes for survival to L. monocytogenes infections.
dUsp36 Inactivation in Hemocytes Does Not Increase L. monocytogenes Load
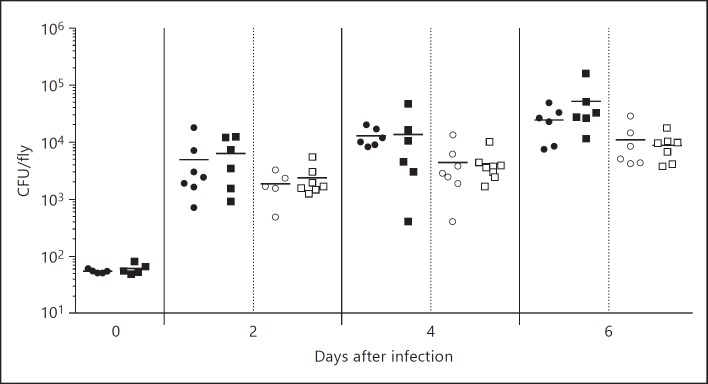
We have then quantified CFUs obtained from control and hml>dUsp36-IR flies at different time points after L. monocytogenes infection (0, 2, 4 and 6 days after infection) and, using a gentamicin chase to specifically eliminate the extracellular bacteria, we have also quantified the intracellular L. monocytogenes load (fig. 3). We observed that the total and intracellular bacterial loads increase with time, which indicates bacterial growth. However, comparison of the CFUs of control and hml>dUsp36-IR at the same time point reveals no significant differences. This indicates that the total and intracellular growth rates of L. monocytogenes are not different in dUsp36-deficient and wild-type hemocytes. These results further show that the sensitivity of flies with dUsp36-depleted hemocytes to L. monocytogenes infections is not accompanied by an increase in the total or intracellular bacterial load.
Fig. 3.
dUsp36 inactivation in hemocytes does not increase bacterial loads. Flies were injected with 100 CFUs of wild-type L. monocytogenes and bacterial loads were determined by plating at different time points after infection. Significance was determined by Student's t test. ⚫ = hml/+ total bacterial load; ◼ = hml>dUsp36-IR total bacterial load; ⚪ = hml/+ intracellular bacterial load; ◻ = hml>dUsp36-IR intracellular bacterial load.
dUsp36 Inactivation in Hemocytes Does Not Compromise Survival to Other Infections
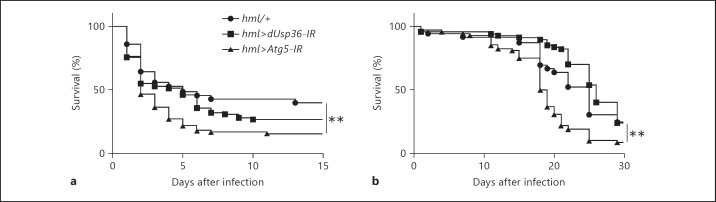
We have next infected flies with the hemocyte-specific inactivation of dUsp36 with a variety of bacteria: a DAP-type extracellular microbe such as S. pneumoniae (fig. 4a) and a Lys-type intracellular pathogen such as S. typhimurium (fig. 4b). We observed that survival rates of control and hml>dUsp36-IR flies are not significantly different when infected with these pathogens. We also observed that flies with autophagy-deficient hemocytes are more susceptible to these infections than control flies. This indicates that hemocyte-specific inactivation of dUsp36 does not result in a general sensitivity towards DAP-type or intracellular bacteria but rather in a host-pathogen interaction defect with L. monocytogenes.
Fig. 4.
dUsp36 inactivation in hemocytes does not sensitize flies to S. pneumoniae and S. typhimurium infections. Flies were injected with S. pneumoniae (a) or S. typhimurium (b) and monitored for survival. Significance was determined by log-rank analysis of the survival curves (** p < 0.001).
Discussion
We have shown that dUsp36 inactivation in hemocytes is sufficient to sensitize adult flies to L. monocytogenes infections. This is not a consequence of a putative dUsp36 requirement during hemocyte development that would reduce their number or alter their functions since dUsp36 adult-specific inactivation has the same effects. Moreover, we have observed that flies with dUsp36-depleted hemocytes are not sensitized to S. pneumoniae and S. typhimurium infections. This indicates that dUsp36 inactivation in hemocytes does not impair major immune or cellular functions. From these experiments, we conclude that dUsp36 plays an important role in hemocytes during L. monocytogenes infections.
To get further insight into the nature of this role, we have measured the total and intracellular bacterial loads of L. monocytogenes-infected flies and observed no difference between flies with dUsp36-depleted hemocytes and control flies. This observation is surprising for two reasons. First, dUsp36 had been identified in a genome-wide RNAi-based screen performed in the hemocytic-like S2 cell line as part of a group of genes whose knockdown led to enhanced L. monocytogenes intracellular growth [27]. If this function also takes place in the adult hemocytes, an increase in the intracellular bacterial load should have been observed. This discrepancy is probably due to a difference between S2 cells and adult hemocytes. S2 cells are derived from embryos, are not fully mature and are closely related to larval circulating hemocytes. Published results demonstrating substantial differences between larval and adult hemocytes support this hypothesis [32, 33, 34].
Second, as stated earlier, no significant difference in the total bacterial load has been observed in flies with dUsp36-depleted hemocytes compared to control flies, which, combined with the fact that they are not sensitive to the other pathogens tested, indicates that their immune system is functional. This raises the question as to why flies with dUsp36-depleted hemocytes succumb faster whereas they are still able to control L. monocytogenes infections. Decreased survival with no change in the associated pathogen load is the hallmark of a tolerance defect [21], which indicates that dUsp36 is required in the adult hemocyte for tolerance to L. monocytogenes infections. The mechanisms involved in this process are still unclear and may imply a role of the dUSP36 deubiquitinating enzyme in some specific aspects of stress resistance or metabolism [35] or in the degradation of specific toxins during L. monocytogenes infections.
In conclusion, we have shown that dUsp36 is required in vivo in the adult hemocyte for survival to L. monocytogenes infections by acting on tolerance mechanisms. We have also confirmed the previously demonstrated role of autophagy during L. monocytogenes infections. These results are a first step towards the understanding of the role of the dUSP36 deubiquitinating enzyme during L. monocytogenes infections in vivo.
Supplementary Material
Supplementary data
Acknowledgments
We are grateful to Dr. T. Neufeld, the Bloomington Drosophila Stock Center and the Vienna Drosophila RNAi Center for providing mutant and transgenic fly strains. This work was supported by funding from the Région Rhône Alpes (ExploraPro) and the Eurotalents program.
References
- 1.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreft J, Vazquez-Boland JA. Regulation of virulence genes in Listeria. Int J Med Microbiol. 2001;291:145–157. doi: 10.1078/1438-4221-00111. [DOI] [PubMed] [Google Scholar]
- 3.Portnoy DA, Jacks PS, Hinrichs DJ. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gedde MM, Higgins DE, Tilney LG, Portnoy DA. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun. 2000;68:999–1003. doi: 10.1128/iai.68.2.999-1003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhn M, Kathariou S, Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988;56:79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;68:521–531. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 7.Tilney LG, Portnoy DA. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansfield BE, Dionne MS, Schneider DS, Freitag NE. Exploration of host-pathogen interactions using Listeria monocytogenes and Drosophila melanogaster. Cell Microbiol. 2003;5:901–911. doi: 10.1046/j.1462-5822.2003.00329.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 10.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 11.Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414:756–759. doi: 10.1038/414756a. [DOI] [PubMed] [Google Scholar]
- 12.Gobert V, Gottar M, Matskevich AA, Rutschmann S, Royet J, Belvin M, Hoffmann JA, Ferrandon D. Dual activation of the Drosophila Toll pathway by two pattern recognition receptors. Science. 2003;302:2126–2130. doi: 10.1126/science.1085432. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff V, Vignal C, Boneca IG, Michel T, Hoffmann JA, Royet J. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat Immunol. 2004;5:1175–1180. doi: 10.1038/ni1123. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko T, Goldman WE, Mellroth P, Steiner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity. 2004;20:637–649. doi: 10.1016/s1074-7613(04)00104-9. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH, Kurata S, Silverman N. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- 16.Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann JA. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA. 1995;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidal S, Khush RS, Leulier F, Tzou P, Nakamura M, Lemaitre B. Mutations in the DrosophiladTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev. 2001;15:1900–1912. doi: 10.1101/gad.203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yano T, Mita S, Ohmori H, Oshima Y, Fujimoto Y, Ueda R, Takada H, Goldman WE, Fukase K, Silverman N, Yoshimori T, Kurata S. Autophagic control of Listeria through intracellular innate immune recognition in Drosophila. Nat Immunol. 2008;9:908–916. doi: 10.1038/ni.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 20.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayres JS, Schneider DS. Tolerance of infections. Annu Rev Immunol. 2012;30:271–294. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayres JS, Freitag N, Schneider DS. Identification of Drosophila mutants altering defense of and endurance to Listeria monocytogenes infection. Genetics. 2008;178:1807–1815. doi: 10.1534/genetics.107.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayres JS, Schneider DS. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 2009;7:e1000150. doi: 10.1371/journal.pbio.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thevenon D, Engel E, Avet-Rochex A, Gottar M, Bergeret E, Tricoire H, Benaud C, Baudier J, Taillebourg E, Fauvarque MO. The Drosophila ubiquitin-specific protease dUSP36/Scny targets IMD to prevent constitutive immune signaling. Cell Host Microbe. 2009;6:309–320. doi: 10.1016/j.chom.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Taillebourg E, Gregoire I, Viargues P, Jacomin AC, Thevenon D, Faure M, Fauvarque MO. The deubiquitinating enzyme USP36 controls selective autophagy activation by ubiquitinated proteins. Autophagy. 2012;8:767–779. doi: 10.4161/auto.19381. [DOI] [PubMed] [Google Scholar]
- 27.Cheng LW, Viala JP, Stuurman N, Wiedemann U, Vale RD, Portnoy DA. Use of RNA interference in Drosophila S2 cells to identify host pathways controlling compartmentalization of an intracellular pathogen. Proc Natl Acad Sci USA. 2005;102:13646–13651. doi: 10.1073/pnas.0506461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goto A, Kadowaki T, Kitagawa Y. Drosophilahemolectin gene is expressed in embryonic and larval hemocytes and its knock down causes bleeding defects. Dev Biol. 2003;264:582–591. doi: 10.1016/j.ydbio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Sinenko SA, Mathey-Prevot B. Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated hemocytes. Oncogene. 2004;23:9120–9128. doi: 10.1038/sj.onc.1208156. [DOI] [PubMed] [Google Scholar]
- 30.Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci USA. 2004;101:14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 32.Charroux B, Rival T, Narbonne-Reveau K, Royet J. Bacterial detection by Drosophila peptidoglycan recognition proteins. Microbes Infect. 2009;11:631–636. doi: 10.1016/j.micinf.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Defaye A, Evans I, Crozatier M, Wood W, Lemaitre B, Leulier F. Genetic ablation of Drosophila phagocytes reveals their contribution to both development and resistance to bacterial infection. J Innate Immun. 2009;1:322–334. doi: 10.1159/000210264. [DOI] [PubMed] [Google Scholar]
- 34.Shia AK, Glittenberg M, Thompson G, Weber AN, Reichhart JM, Ligoxygakis P. Toll-dependent antimicrobial responses in Drosophila larval fat body require Spätzle secreted by haemocytes. J Cell Sci. 2009;122:4505–4515. doi: 10.1242/jcs.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS. Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol. 2006;16:1977–1985. doi: 10.1016/j.cub.2006.08.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data