Abstract
The barrier epithelia of multicellular organisms frequently come into direct contact with microorganisms and thus need to fulfill the important task of preventing the penetration of pathogens that could cause systemic infections. A functional immune defence in the epithelial linings of the digestive, respiratory and reproductive organs as well as the epidermis/skin of animals is therefore of crucial importance for survival. Epithelial defence reactions are likely to be evolutionarily ancient, and the use of invertebrate animal models, such as insects and nematodes, has been crucial in unravelling the mechanisms underlying epithelial immunity. This review addresses basic questions of epithelial immunity in animals and humans. It focuses on recent developments in the understanding of the immune responses in the fruit fly Drosophila melanogaster and how the innate immune system acts locally in the epidermis and cuticle, tracheae, gut and genital organs. Both basal immune activities in epithelia that are constantly exposed to microbes as well as positive and negative regulation in response to pathogenic organisms are covered. Important immuno-physiological aspects of epithelial defence mechanisms are also discussed, such as wound healing, re-epithelialization and intestinal homeostasis.
Key Words: Drosophila melanogaster, Barrier epithelia, Innate immunity, Antimicrobial peptides
Introduction
A functional immune system is of paramount importance to all multicellular organisms. Vertebrates rely on the combined actions of the nonspecific, immediately triggered innate immune response and specific, but slower-acting, adaptive immunity. Invertebrates rely solely on a robust innate immune response for protection. Great strides have been made in recent years in the understanding of innate immunity, especially in model organisms like Drosophila melanogaster and Caenorhabditis elegans. Much attention has been given to the inducible expression and large-scale release of antimicrobial peptides (AMPs) into extracellular fluids. More recently, local immune responses in barrier epithelia and the host-pathogen interactions that occur at the site of infection have come into focus. Barrier epithelia consist of tissues that are normally exposed to and prevent invasion of potentially pathogenic organisms which could cause a systemic infection. The protective role of the epithelium of multicellular animals is therefore crucial, and epithelial immunity is likely evolutionarily ancient. Recent findings in simple multicellular animals, such as Hydra and other Cnidaria, indicate that inducible expression of AMPs in epithelial cells constitutes an ancestral defence mechanism [1]. The protective role of the barrier epithelia is twofold. Firstly, it provides an impenetrable physical barrier, and secondly, it provides a chemical barrier in the form of constitutively expressed AMPs. Upon infection, the epithelia also activate a large battery of immune-regulated genes. Furthermore, the barrier epithelia elicit different immune responses which depend on whether a tissue is transiently (e.g. infected wound) or continuously (e.g. commensal organisms in the gut) exposed to microbial elicitors. The role of AMPs and other immune defences in barrier epithelia will be the focus of this review article, and especially what has been learned in recent years from the wealth of studies in the fruit fly D. melanogaster.
The Role of AMPs in Innate Immunity
In mammals, the production of a wide variety of AMPs provides a first line of defence against microbial invaders and includes AMPs such as cathelicidin and defensin (Def), which have been shown to directly kill microbes [2]. AMP expression can be stimulated by a variety of triggers, including Toll-like receptor signalling, the release of pro-inflammatory cytokines, tumour necrosis factor, interleukin-1β, interferon-γ, phorbol myristate acetate, histone acetylation and vitamin D [3]. In addition, pro-inflammatory genes that play a role in activating the immune response, including chemokines, cytokines and adhesion molecules, and also enzymes and molecules with microbicidal activity are known to be the targets of nuclear factor (NF)-κB signalling [4].
Interestingly, several human diseases have been linked to inappropriate regulation of the immune response in barrier epithelia. These include: (1) Crohn's disease, which may be caused by a defect in barrier function of the intestinal mucosa [5] and is associated with decreased AMP expression [6]; (2) psoriasis and atopic dermatitis, which are correlated with an increased [7] and decreased [8] level of AMP expression in the skin, respectively; (3) chronic wounds, which display decreased AMP expression [9], and (4) proposed roles in the development of colorectal cancer [10] and in altered AMP effectiveness in cystic fibrosis [11].
Invertebrates rely heavily on the action of AMPs for a robust innate immune response. The Drosophila immune response involves the release of over 20 AMPs, which fall into seven gene families, into the haemolymph following septic injury, as reviewed elsewhere [12, 13, 14]. Individual AMPs have varied actions against different pathogens. Gram-negative bacteria are combated by Diptericin (Dpt), Drosocin (Dro) and Attacin (Att). Def is important for killing Gram-positive bacteria. Drosomycin (Drs) and Metchnikowin (Metch) are induced following fungal infection. Finally, Cecropins (Cec) have both antibacterial and antifungal properties. An additional AMP, andropin, which is not immune-inducible, is expressed constitutively in male reproductive organs.
The systemic Drosophila immune response is regulated primarily by the actions of two signalling pathways, the Toll pathway, from which the mammalian Toll-like receptor was named [15], and the IMD pathway, which has homology to the tumour necrosis factor-α pathway in mammals [16]. The Toll pathway relies on cleavage of the extracellular ligand, Spätzle, followed by signalling through the Toll receptor and its intracellular adaptor protein complex, which contains MyD88, Tube and the Pelle kinase. In the absence of infection, negative regulation is conferred by IκB/Cactus, while in response to infection by Gram-positive bacteria and fungi, the NF-κB/Relish (Rel) transcription factors Dorsal-related immunity factor and Dorsal are activated [14]. The IMD pathway, activated by its receptor peptidoglycan recognition protein (PGRP)-LC and the Imd protein for which it is named, acts through TAK1, signalling the IκB kinase complex to activate the NF-κB transcription factor Rel, which responds to infection by Gram-negative bacteria [14]. In addition, the IMD pathway is linked to Jun kinase (JNK) signalling via bifurcation of the signal at TAK1 to activate the mitogen-activated protein kinase (MAPK) Basket, causing activation of the heterodimeric transcription factor activator protein 1 (AP1), which is composed of a Jun and a Fos subunit [17]. The systemic immune response in Drosophila has been reviewed extensively elsewhere and will not be the subject of this article.
This review will focus on the barrier epithelia of the epidermis and cuticle, the respiratory tract, the gastrointestinal tract and the genital organs in Drosophila. Several questions will be addressed, as follows: (1) what responses occur at the site of transient infection to return the organism to its pre-infection condition?; (2) in cases where exposure to potentially infectious organisms is continuous, what mechanisms exist to maintain homeostasis?, and (3) what conclusions can we draw about the global regulation of the immune response of the barrier epithelia?
AMP Expression in the Barrier Epithelia of Drosophila
The constitutive and inducible expression of AMPs provides an early chemical defence against invading microbes. Barrier epithelial tissues are all competent to express at least a subset of the AMPs (fig. 1). In a comprehensive study examining the local expression of green fluorescent protein (GFP) reporter genes for representative members of all of the AMP families, it was shown that each AMP, with the exception of Dro, was expressed in some parts of the digestive system in response to local infection [18]. This includes Def and Metch in the oral/pharynx region and Att in the digestive tract of larvae, and Def and Metch in labellar glands, Drs in salivary glands, Dpt, Att, Drs, Metch and Def in the cardia and midgut, and Dpt, Cec and Metch in Malpighian tubules in adults. Unlike the systemic release of AMPs from the fat body, the tissue-specific AMP production in response to local infections was independent of the Toll pathway and relied on the IMD pathway for induction [18].
Fig. 1.
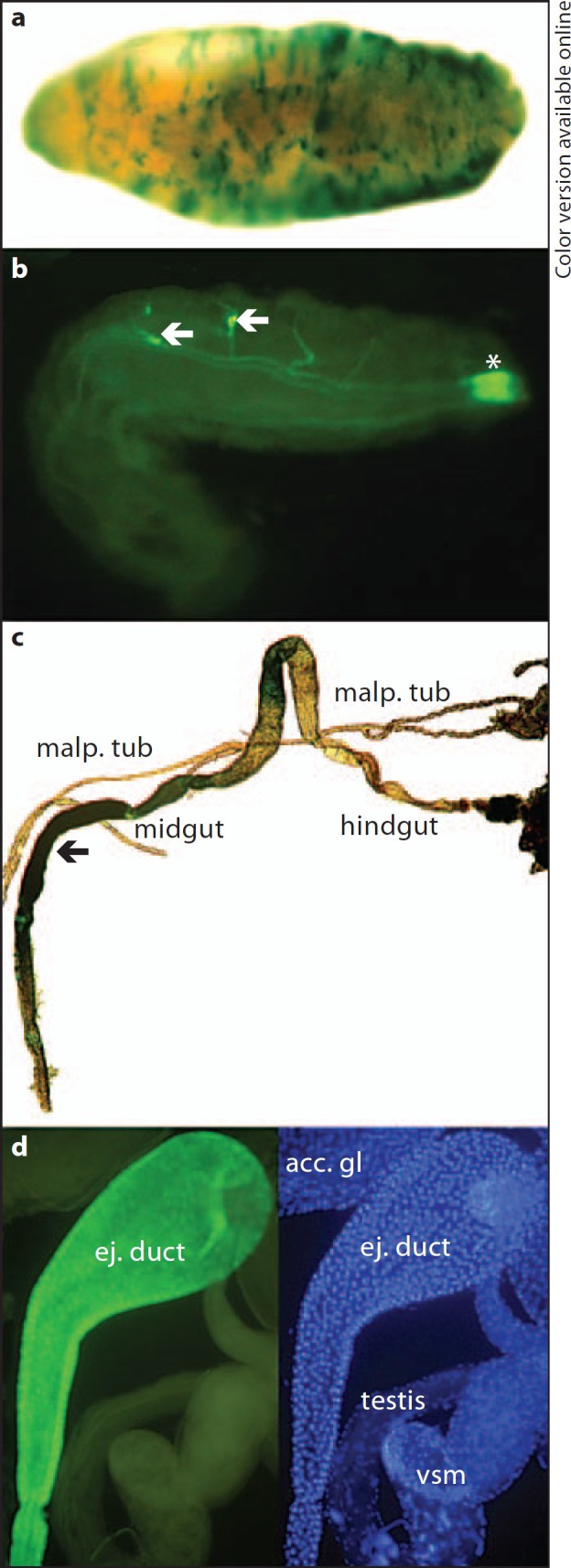
AMP expression in barrier epithelia. Transgenic Drosophila embryos, larvae and flies carrying AMP promoter-reporter fusion constructs were infected or treated with microbial products, followed by staining and visualization of the respective reporter gene (LacZ or GFP). a Epidermal β-galactosidase staining of Cec-LacZ embryos treated with microbial fragments (sonicated bacteria) after permeabilization of the vitelline membrane [76]. b Drs-GFP expression in the trachea (arrows) and posterior spi racles (asterisk) of a larva exposed to Micrococcus luteus and Enterobacter cloacae in the surrounding medium [21]. c Epithelial β-galactosidase staining in dissected gut (arrow) from a Dpt-LacZ fly after feeding with M. luteus and E. cloacae. Malpighian tubules (malp. tub), midgut and hindgut are labelled [60]. d Constitutive Cec-GFP expression in the ejaculatory duct of an uninfected male [26]. acc. gl = Accessory gland; ej. duct = ejaculatory duct; vsm = seminal vesicle.
The above analysis was performed with GFP expression constructs. GFP expression is less sensitive than reporters that employ the use of enzymes such as β-galactosidase for visualization. As such, reading too much into the absence of local AMP expression should be avoided, since it could simply be a reflection of the strength of the reporter gene used. Figure 1c shows expression of a Dpt-β-galactosidase reporter construct in the gut of orally infected flies. Using CecA1-β-galactosidase reporter constructs, it was shown that the epidermis has the ability to induce the synthesis of AMPs in response to infected wounds during all three larval instars [19]. In fact, the epidermis is competent to mount an AMP response following lipopolysaccharide/peptidoglycan (PGN) exposure as early as 9–12 h after egg laying (fig. 1a) [19]. Both embryonic and larval epidermal AMP expression required the IMD pathway.
Some issues with the sensitivity of reporter constructs can be limited by using transcriptional profiling, which has been used to show that there is constitutive expression of Def, Metch, Drs, Att and Dpt in uninfected tracheae [20]. The entirety of the tracheal system is competent to respond to infection, as was shown with a Drs-GFP reporter construct (fig. 1b) [20, 21]. Dro expression is also immune-inducible in tracheal cells [18]. The expression of Drs (and Dro) relies on the IMD pathway and not the Toll pathway [18, 21], despite Drs being a Toll-responsive gene in the systemic immune response.
Inducible expression of several AMPs in the larval midgut epithelium also required GATA transcription factor activity [22]. This mimics the situation in the larval fat body, where the tissue specificity of AMP expression is regulated by dGATAb/Serpent [23]. In fact, mechanistically there seems to be little difference in the activation of AMPs in response to infection if we compare the barrier epithelia (local response) and fat body (systemic response), except that the local response in epithelia relies specifically on the IMD signalling pathway while the fat body can respond to both Toll and IMD signalling. In both conditions, AMP activation depends on NF-κB/Rel transcription factor activity downstream of infection-induced signalling, in combination with tissue-specific GATA factors [13], and in both cases, the transcription factors bind to nested NF-κB/Rel and GATA binding sites in the proximal promoters of the AMP genes [24]. Thus, there are no strong data to support a primary difference in the mechanism of transcriptional activation of the so-called systemic and local immune responses. The difference lies rather in whether the AMPs are secreted from the fat body into the circulating haemolymph, in which they spread throughout the organism, or whether the AMPs are secreted by the epithelial cells into the lumen of an organ, such as the male ejaculatory duct or the trachea, or onto the surface of the epithelium, for example at a wound site or in an inflamed gut.
Constitutive expression of AMPs in barrier epithelia, which most likely creates a hostile local environment and prevents microbes from attaching to and penetrating the epithelium, has been shown to be regulated in a different manner than that described above. This expression does not involve NF-κB/Rel and GATA transcription factors. Instead, the homeobox transcription factor Caudal (Cad) and the POU domain transcription factor Ventral Veinless (Vvl) were shown to bind to tissue-specific enhancers and promote the expression of Drs and Cec in salivary glands and reproductive organs in uninfected, healthy flies [25, 26]. Sex-based differences in AMP expression are apparent within the genital organs of Drosophila. Females are known to constitutively express several AMPs, including Dro, Drs, Cec and Def [18, 21]. Furthermore, upregulation of Metch and Drs expression has been shown to occur in response to accessory gland proteins that are present in the male seminal fluid [21, 27]. In addition to constitutive and inducible expression of AMPs in females, males have also been shown to constitutively express AMPs in their genitalia. These include Cec, Att, Drs and Andropin, primarily in the ejaculatory duct (fig. 1d) [18, 25, 26, 28]. In addition to the widespread constitutive and inducible expression of AMPs in barrier epithelial tissues, each tissue also has its own distinctive immune response, which we will attempt to address below.
Wound Healing and Epidermal Immunity
Although it could be argued that wound healing is not part of the immune response, apart from in highly controlled laboratory experiments, it is nearly impossible to achieve wounding without exposure to potentially infectious organisms. Furthermore, insects in the wild are often injured by parasites, during mating or by predators, increasing the potential for systemic infections arising from wounding in nature. Much effort has gone into understanding the Drosophila wound response, in embryos, larvae and adults.
In recent years, many components of the signalling cascades that control epithelial wound healing have been uncovered. In particular, involvement of the transcription factors Grainy-head (GRH) and AP1 appears to be important [29, 30]. The Rho family of small GTPases including Rho, Rac and Cdc42 is known to be important for the rapid cytoskeletal movements that control cell shape changes of wound margin cells [31]. Pvr, a platelet-derived growth factor/vascular endothelial growth factor-like receptor tyrosine kinase, and one of its ligands, Pvf1, regulate margin cell actin-based cellular extension processes, while the JNK pathway regulates the dedifferentiation of cells around the wound edge [32]. Pvf1 is present in the haemolymph and signals to Pvr upon wounding, thereby sensing tissue damage and initiating wound healing. Furthermore, it has recently been shown that Karst, the Drosophila homolog of βHeavy-spectrin, accumulates around wound edges in a cable-like manner, potentially playing an important role in wound healing [33]. The exact signalling pathway required to regulate the response of cells at the wound edge is unknown, but several observations have suggested that the extracellular signal response kinase (ERK) is involved. Firstly, ERK phosphorylation following wounding has been shown to be required for a robust wound response [29]. Secondly, both GRH and FOS proteins are known targets of ERK phosphorylation in vitro [34, 35]. Finally, the receptor tyrosine kinase Stitcher, which induces ERK phosphorylation and is a target of GRH regulation, is essential for wound healing [36]. Stitcher has been shown to be both a target of GRH and an activator of signalling via GRH, suggesting that it is a critical member of a positive feedback loop that may act to amplify the cellular healing response, thus ensuring appropriate wound repair [36].
Epidermal activity of both Dopa decarboxylase (Ddc) and tyrosine hydroxylase, encoded by pale, is essential for the production of melanin and sclerotin during development for maturation of the cuticle of larvae and adults [37, 38]. Ddc and pale have also been shown to be responsive to wounding in the embryo [29], and along with misshapen (encoding an upstream activating kinase in the JNK pathway), have recently been shown to require both GRH and AP1 binding sites in their upstream enhancers for proper expression [30]. Interestingly, the same AP1 binding site had previously been shown to play a role in the activation of Ddc transcription in the epidermis of larvae and adults following septic infection [39]. While the activation of Ddc transcription in the wound response in Drosophila embryos appears to occur through Fos binding in the absence of Jun [30], it appears that neither component is necessary for activation of Ddc in the larval and adult immune response [39]. In fact, the JNK pathway is dispensable for Ddc transcriptional activation in the immune response and instead requires a member of the p38 MAPK pathway, p38c. These observations show the multiple levels of regulation that allow pleiotrophy in the Drosophila wound and immune responses. GRH and AP1 binding sites are important for the function of the Ddc wound response enhancer in embryos; however, these alone are insufficient to regulate the whole wound response, as mutation of these sites in another wound-induced gene, krotzkopf verkehrt (encoding chitin synthase), failed to eliminate its transcriptional induction following wounding [30].
An additional level of complexity is apparent during wound healing in Drosophila larvae and adults since clotting and melanization at the wound site is essential. In these later stages, it is nearly impossible to separate the response at the wound site from the immune response. This is because the clot is situated such that it both prevents blood loss and acts to sequester invading organisms at the wound site [40], performing an immune function during the initial stages of infection [41]. Many proteins have been shown to play a role in clot formation in Drosophila, including the humoral procoagulants apolipophorin, hexamerins and their receptor (fat body protein 1), fondue and the blood cell-derived proteins hemolectin and tiggrin [42]. In addition, it has been shown that the enzyme transglutaminase (TG), a highly conserved enzyme that cross-links glutamine and lysine residues in clot proteins, plays a role in sequestering bacteria following septic injury in Drosophila [41]. TG mediates the incorporation of humoral procoagulants into localized small aggregates on microbial surfaces [41]. In fact, knock-down of TG by RNAi leads to a brittle clot that entraps fewer microbes than wild-type controls, indicating an important role for TG, not only in the wound response, but also in the earliest stages of the immune response.
Tracheal Immunity
The tracheal system of Drosophila is another potential route of pathogen entry. This is true not only for airborne but also food-borne pathogens, since the spiracles of larvae are in contact with potentially infectious organisms growing in their food. Therefore, at least during the larval stages, it could be argued that the region of the tracheae nearest the spiracles is constantly exposed to infecting organisms, while the remainder of the tracheae are only transiently exposed.
The tracheae consist of an epithelial monolayer around a gas-transporting lumen containing primary, secondary and terminal branches [20]. In addition to AMP expression, the Drosophila tracheae have been shown by transcriptional profiling to express three lysozyme genes. While lysozymes play a role in immunity in vertebrates, thus far, in Drosophila, lysozymes only have a proven role in digestion [43]. It remains to be elucidated if they also play a role in tracheal immunity. Genes encoding enzymes that play a role in the oxidative stress response have been shown to be up-regulated in the tracheae [20]. These include superoxide dismutases, peroxiredoxins, glutathione-S-transferases and dual oxidase (DUOX), an enzyme that plays an important role in gut immunity (see below) [44, 45, 46]. Finally, the gene encoding transferrin was also specifically expressed in the airway epithelial cells, suggesting that its protein product may play a role in iron depletion on the epithelial surface to prevent the growth of most bacteria [20]. While functional analysis of the role of all of these constitutively expressed genes is needed to confirm an immune function, this suggests that multiple levels of control exist to prevent pathogen growth in airway epithelia.
It appears that the Toll pathway components tube and pelle are not expressed in tracheal cells, which explains the observation that Drs expression was dependent on the IMD and not the Toll pathway [20]. Direct exposure to Pseudomonas aeruginosa led to expression of some IMD-, JNK- and Janus kinase (JAK)/signal transducer and activator of transcription (STAT)-responsive genes, suggesting a role for all of these pathways in tracheal immunity. Among the genes that were induced in tracheal tissues upon infection is vvl, a transcription factor that is present in many immune-competent tissues and was recently shown to play a role in the regulation of CecA1 expression [26]. Vvl most likely acts with other regulators to control expression of AMP genes in a tissue-specific manner.
Since the tracheae are constantly exposed to potentially invasive organisms, tight control is necessary to prevent precocious expression of immune response genes but still allow a rapid response in the event of a breach of this epithelial barrier. Two serpins, Spn28D and Spn77Ba, are essential for the prevention of phenol oxidase activity in the tracheae [47, 48]. Spn28D plays a role in other tissues, but Spn77Ba appears to be trachea-specific Spn77Ba acts to inhibit the serine protease cascade that activates phenol oxidase through MP1 and Sp7 [48]. Interestingly, the melanization of the tracheae caused local and systemic Drs expression, suggesting that melanization plays an important role in rapid signalling in the event that the barrier epithelium of the tracheae are penetrated by microorganisms.
Gastrointestinal Immunity
In recent years, the combined efforts of many laboratories have shed light on Drosophila immune homeostasis, particularly in the midgut. Complexity lies in the need for Drosophila to be able to ward off food-borne pathogens while maintaining a commensal community of 3.5 × 105 cells that constitute a range of 5–20 different microbial species [49]. This is of particular importance given that Drosophila lives on a niche of rotting food and debris and faces a constant onslaught of potentially pathogenic organisms in the wild.
The architecture of the Drosophila gut is important for understanding the immune response mechanisms it employs. The tube structure of the digestive tract can be divided into the foregut, midgut and hindgut. The salivary gland, which is attached to the mouthparts, secretes saliva to aid in digestion. The midgut is the location of food absorption and thus is exposed to more immune threats than the cuticle-lined foregut and hindgut. Between the midgut and the hindgut, the Malpighian tubules are attached, and these constitute the main renal organ of insects, performing analogous functions to the human kidneys.
Of particular interest is the finely tuned regulation of multiple signalling networks that produce both AMPs and reactive oxygen species (ROS) and also regulate the re-epithelialization of damaged cells of the midgut wall. To prevent constitutive activation of AMP expression in response to the presence of large numbers of commensal bacteria, multiple levels of control exist. Without such controls, PGN fragments that are naturally released by commensal bacteria in the gut would bind to and activate the IMD pathway receptor PGRP-LC [50]. This would lead to activation of the NF-κB transcription factor Rel, causing its translocation into the nucleus and activation of AMP gene transcription. In fact, Rel is constitutively activated in the intestine, as is evident from its persistent nuclear localization in midgut epithelial cells [49]. This localization is absent in axenically reared flies, demonstrating the dependence of Rel activation on the presence of commensal bacteria. Unrestricted AMP expression leads to a reduced life span, epithelial cell apoptosis and alterations in the commensal bacterial population [49]. At the transcriptional level, this is prevented by binding of the repressor Cad to AMP promoters, and mutation of Cad-binding sites leads to precocious expression of AMPs [25, 49]. In addition, within the intestinal lumen, prevention of IMD pathway activation is achieved by the amidase PGRPs PGRP-SC and PGRP-LB, which scavenge PGN molecules that are released by commensal bacteria [51, 52]. Also, PIRK (also known as PIMS and RUDRA) acts to repress IMD pathway activation by interacting with and inhibiting the activity of PGRP-LC [53, 54, 55]. The membrane-bound PGRP-LF was also reported to prevent constitutive activation of PGRP-LC [56]. In the absence of PGRP-LF, both the IMD and the JNK pathways were precociously activated in the absence of bacterial infection. Inappropriate activation of the JNK pathway led to developmental defects, demonstrating the importance of PGRP-LF in regulation of the IMD pathway. As mutations in a single gene encoding Cad, PIRK, PGRP-SC, PGRP-LB or PGRP-LF result in high levels of AMP expression, each of these genes is individually necessary but not sufficient for proper repression of IMD pathway activity [49, 51, 52, 55, 56]. Under circumstances where pathogenic bacteria invade the gut tissues and establish an infection, the large amount of PGN that is present will overwhelm these multiple levels of negative regulation, thereby activating the IMD pathway and expressing AMPs that can act in consort with ROS to combat the infection. Since the negative regulators are also IMD responsive, this immune stimulation of AMP production will be rapidly attenuated upon disappearance of an active infection.
ROS production adds another level of microbicidal protection in the gut. Interestingly, there is very little overlap in the regulation of ROS and AMP production. Production of ROS is achieved through the action of DUOX, an NADPH oxidase family member [44]. The production of ROS is necessary, since knock-down of duox expression results in sensitivity of these flies to infection due to over-proliferation of ingested bacteria, leading to death [44, 45]. There are multiple levels of regulation to control DUOX activity, including PGN-independent activation of enzyme activity and PGN-dependent and -independent control of duox transcription [45, 46]. The non-PGN regulatory branch appears to be regulated by a still unidentified G protein-coupled receptor that acts through signal transduction via Gαq and phospholipase Cβ (PLCβ) [46]. Signalling through these proteins leads to generation of inositol 1,4,5-phosphate, which causes mobilization of intracellular calcium. The increased calcium concentration is sufficient for DUOX activation and bactericidal ROS production. Under normal conditions, low-level ROS production is constant due to the presence of commensal organisms in the gut. Upon active infection, PLCβ is robustly activated, thus increasing ROS production; however, this increased production alone is insufficient to combat the infection. Removal of functional copies of Gαq or PLCβ results in flies that are extremely susceptible to infection, which is lost in axenically raised flies. Together, these results demonstrate the importance of DUOX production stimulated by the commensal flora.
In addition to PLCβ regulation of DUOX activity, this protein is also involved in regulation of duox transcription through its activation of the MAPK kinase kinase (MEKK) 1-MAPK kinase (MKK) 3-p38 MAPK pathway [45]. Transcription of duox is not restricted to activation through PLCβ but also occurs in a PGN-dependent manner through PGRP-LC activation of the IMD pathway. In this case, Rel is not required for duox transcription; rather, there is a bifurcation of signalling downstream of the Imd protein that results in activation of the MEKK1-MKK3-p38 MAPK pathway, which leads to transcriptional activation of duox via the activating transcription factor 2 [45]. Together, PGN-independent and -dependent pathways ensure strong activation of duox transcription upon infection. In fact, this cross-talk ensures that there will be an appropriate level of duox expression and activity depending on whether the organism comes into contact with pathogenic infections or just commensal organisms. In the absence of active infection, basal DUOX activity is maintained by non-PGN activation of low PLCβ activity. This is likely a reflection of repressive measures that remove PGN and reduce PGRP-LC activity in the gut lumen, thereby preventing transcriptional activation of duox expression via the IMD pathway. Another layer of down-regulation occurs due to PLCβ induction of calcineurin B transcription. Calcineurin B then activates expression of MAPK phosphatase 3, which reduces the activity of p38 MAPK [45]. These negative regulators are essential to fly survival, since knockdown of MAPK phosphatase 3 results in a reduced life span. This effect is eliminated by down-regulation of duox expression. This suggests that tight regulation of DUOX activity is essential to prevent extensive ROS-induced oxidative damage that may reduce viability.
It is easy to imagine that even under ideal conditions, the interplay between Drosophila gut tissues and commensal organisms can lead to significant tissue damage. Indeed, newly produced cells derived from evenly spaced intestinal stem cells (ISCs) replace damaged cells on a weekly basis [57]. The division of an ISC results in the production of two daughter cells [58], one a replacement ISC and the other an immature gut epithelial cell called an enteroblast (EB), which will eventually mature into a mature enterocyte (EC) or an enteroendocrine cell. The Delta-Notch signalling pathway is crucial to the determination of the fate of the two initially similar cells [57, 58]. The ISC and EB cells retain high and low levels of Delta, respectively, soon after division. Wingless production by the underlying visceral muscle cells suppresses differentiation and is important for ISC survival at the basement membrane. Not surprisingly, EB cell differentiation correlates with diminishing contact with the basement membrane.
Ingestion of several compounds that lead to intestinal tissue damage, including DNA-damaging agents and invasive bacteria [like Erwinia carotovora (Ecc15) or Pseudomonas entomophila], or expression of apoptotic genes in the gut has been shown to stimulate ISC division and renewal of intestinal cells [59, 60, 61]. These experiments show that ECs relay damage signals to ISCs requiring cell division to produce EBs, which restore intestinal integrity. Components of the IMD pathway, including PGRP-LC, are dispensable for the stimulation of ISC division following infection-induced intestinal damage by Ecc15 [60]. In fact, cell damage as a result of excessive ROS production seems to be the major stimulator of ISC division resulting in tissue removal [61]. Accordingly, duox gene silencing and antioxidant feeding eliminate ISC division stimulation in Ecc15-infected flies.
The genetic control of activation of ISC division by the cell damage caused by infection has been addressed by transcriptional profiling after Ecc15 or Serratia marcescens oral infection [60, 63] and by more targeted studies [61, 62, 64, 65]. It is clear from these studies that the JAK/STAT pathway is a major player in triggering ISC proliferation [60, 61, 62, 63], while the JNK pathway is important for maintaining overall ISC number [60, 61, 64]. Production of the JAK/STAT pathway ligands, Unpaired (Upd) proteins, is strongly induced in ECs after bacterial ingestion [61, 62, 63] in a DUOX-dependent manner [61]. Mechanical injury is sufficient to induce Upd3 expression in ECs, suggesting that tissue damage is a major inducer of Upd3 expression [61]. It was recently shown that JNK signalling, which is triggered by cell damage, activates Yorkie, which is normally repressed by Warts in ECs [65]. Yorkie activates the JAK/STAT pathway in ISCs, through expression of Upd3, thereby stimulating cell division. This demonstrates a potential role for the Hippo/Warts pathway in epithelial renewal via production of the cytokine Upd3. Upd3 likely acts through activation of the JAK/STAT pathway receptor Domeless expressed on the surface of ISCs [61, 62, 63]. In fact, loss of JAK/STAT signalling in ISCs reduces infection-induced intestinal regeneration [61, 63].
The JNK pathway is activated in both ISCs and ECs [61]. Expression in ISCs is essential for maintenance of ISC number, and inhibition of this pathway in ISC cells reduces the ability of the intestine to regenerate due to loss of these cells. The importance of Upd3 expression in ECs and JAK/STAT and JNK signalling in ISCs is apparent upon their selective removal, which increases sensitivity to Ecc15 infection [61]. Furthermore, some activation of both the JAK/STAT and JNK pathways is required for proper intestinal epithelial renewal in the absence of active infection, by regulating the cell division and appropriate differentiation of ISCs and their progeny [61]. Axenically reared flies show reduced intestinal cell renewal, demonstrating that commensal bacteria also play a role in stimulation of ISC division. In fact, it seems that commensal bacterial interaction with the gut tissues leads to activation of the IMD, PLCβ, JAK/STAT and JNK pathways, thereby regulating AMP production, DUOX expression and activity, and intestinal epithelial renewal.
While most digestive tract-related research has focussed on midgut homeostasis, some work has been done to examine the role of the Malpighian tubules in Drosophila immunity. It is apparent that the Malpighian tubules are competent to express many AMPs, and since this is the site of waste processing in the fly, they will be exposed to many potential immune elicitors [18, 66]. A role for nitric oxide (NO) signalling in activation of AMP expression has been proposed for the Malpighian tubules [66, 67]. Tissue-specific expression of NO synthase in Malpighian tubules is associated with increased Dpt expression following systemic Escherichia coli infection, and this increase in expression can be prevented by inhibition of the NO-induced pathway. Furthermore, increased production of NO in the Malpighian tubules is associated with increased survival of flies following E. coli injection. Undoubtedly, future research will help elucidate the roles of all parts of the gastrointestinal system in the Drosophila immune response.
Genital Immunity
The genitalia constitute another organ complex that is, at least transiently, exposed to infectious organisms. Accordingly, many AMP genes have been shown to be either constitutively or inducibly expressed in male and female genitalia (see above). It has been shown that male Drosophila inflict wounds on the female genitalia during copulation [68]. In fact, mating has been postulated to be a significant cause of infection in the wild [69], and male to female transfer of bacteria has been demonstrated in the lab [70]. Thus, females would be at higher risk of infection than males due to traumatic mating. It has recently been shown that the genital plate is a route of pathogen entry in males [71]. In fact, systemic expression of all inducible AMP genes was demonstrated following application of a bacteria solution to the genital plate. This was not dependent on live bacteria; rather, it required PGN components to enter the haemolymph. Females, on the other hand, failed to respond to deposition of bacteria on their genitals [71], although they did respond to the presence of sex peptide provided by the males [27]. The sex-based difference in immune response at the genital plate may be due to the reduced risk of infection during mating for males and the fact that males maintain clean genitalia to avoid transmission of bacteria to mated females [71].
Concluding Remarks
The importance of the role of the barrier epithelia in prevention and survival of infection is evident. The intricate regulation of the immune response that allows the organism to both tolerate the presence of commensal bacteria and react to an onslaught of infecting organisms is impressive. The rapid responses elicited upon transient infection of barrier epithelia, including the rapid clotting of the wound, re-epithelialization, induction of local AMP production and expression of other immune-regulated genes, are clearly important. The multiple levels of regulation required to control the immune response in the gut, which is constantly exposed to immune elicitors, constitute added complexity to each of these processes, due to the constant replacement of damaged cells in the epithelial barrier. It is also impossible to overlook the importance of communication between local immune responses in the barrier epithelia and the triggering of systemic responses. One such example is how tracheal melanization causes systemic drs induction [72]. However, little is known about the molecular mechanisms involved in signalling from the site of a local infection to generate a systemic response. Undoubtedly, such local responses are often the initial triggers for the large-scale systemic responses that rely on the activation of the Toll and IMD pathways.
While there seems to be some overlap in the transcriptional regulation of immune-inducible genes in the barrier epithelia, such as the role of the IMD pathway in induction of epithelial AMP expression, each tissue seems to have its own mechanism for dealing with infections. This is particularly evident in the well-studied gut immune response, but recent insights into the transcriptome of the tracheae may implicate new genes in its local immune response. Definitely, future research will uncover more levels of complexity in both the systemic and local immune responses.
The evolutionary origin of the innate immune system is still not well understood. Recent findings suggest that inducible expression of AMPs in epithelial cells constitutes one of the earliest immune mechanisms we can trace today [1], since many simple organisms lack specialized immune cells or dedicated phagocytes/macrophage-like cells. Future studies of immune defence reactions provided by epithelial cells in model animals and in humans will provide a basis for deeper understanding of epithelial immunity in general. Examples of parallels in epithelial immunity are apparent in the involvement of homologous signalling pathways in different organisms. One such example is the conservation of the Toll-like receptor family from the most simple metazoans to mammals [1]. In addition, p38 MAPK has been shown to be important for intestinal immunity in Drosophila (see above), where it regulates the oxidative stress response through the transcriptional activation of duox [45], in C. elegans, where it regulates the transcription factor SKN-1 in the oxidative stress response [73, 74], and in humans, where inhibition of p38 MAPK has been shown to block differentiation of colonic cells following application of butyrate, a short-chain fatty acid that is derived from bacterial fermentation in the colon [75]. The use of the p38 signalling pathway for regulation of intestinal epithelial immunity in these three organisms suggests that there has been a conservation of immune responses in higher organisms. Furthermore, this observation gives weight to the suggestion that insight into human immunity can be gained by the study of the immune system of model organisms. Finally, there is hope that future discoveries in model organisms can aid in the understanding of human diseases, such as Crohn's disease, psoriasis, atopic dermatitis, cystic fibrosis and possibly even cancer.
Acknowledgement
We would like to apologise to all those researchers whose work we were unable to cite due to space restrictions. The continuous support of Drosophila immunity projects by The Swedish Research Council and The Swedish Cancer Society to Y.E. is gratefully acknowledged.
References
- 1.Bosch TC, Augustin R, Anton-Erxleben F, Fraune S, Hemmrich G, Zill H, Rosenstiel P, Jacobs G, Schreiber S, Leippe M, Stanisak M, Grotzinger J, Jung S, Podschun R, Bartels J, Harder J, Schroder JM. Uncovering the evolutionary history of innate immunity: the simple metazoan hydra uses epithelial cells for host defence. Dev Comp Immunol. 2009;33:559–569. doi: 10.1016/j.dci.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 3.Lai Y, Gallo RL. Amped up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 6.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, Schroeder JM, Stange EF. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Harder J, Schroder JM. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol. 2005;77:476–486. doi: 10.1189/jlb.0704409. [DOI] [PubMed] [Google Scholar]
- 8.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 9.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide ll-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 10.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 11.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 12.Hultmark D. Drosophila immunity: paths and patterns. Curr Opin Immunol. 2003;15:12–19. doi: 10.1016/s0952-7915(02)00005-5. [DOI] [PubMed] [Google Scholar]
- 13.Uvell H, Engstrom Y. A multilayered defense against infection: combinatorial control of insect immune genes. Trends Genet. 2007;23:342–349. doi: 10.1016/j.tig.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 15.Kimbrell DA, Beutler B. The evolution and genetics of innate immunity. Nat Rev Genet. 2001;2:256–267. doi: 10.1038/35066006. [DOI] [PubMed] [Google Scholar]
- 16.Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, Kopczynski C, Duyk G, Reichhart JM, Hoffmann JA. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001;1:503–514. doi: 10.1016/s1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 17.Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002;3:711–722. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 18.Tzou P, Ohresser S, Ferrandon D, Capovilla M, Reichhart JM, Lemaitre B, Hoffmann JA, Imler JL. Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity. 2000;13:737–748. doi: 10.1016/s1074-7613(00)00072-8. [DOI] [PubMed] [Google Scholar]
- 19.Onfelt Tingvall T, Roos E, Engstrom Y. The imd gene is required for local cecropin expression in Drosophila barrier epithelia. EMBO Rep. 2001;2:239–243. doi: 10.1093/embo-reports/kve048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner C, Isermann K, Fehrenbach H, Roeder T. Molecular architecture of the fruit fly's airway epithelial immune system. BMC Genomics. 2008;9:446. doi: 10.1186/1471-2164-9-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrandon D, Jung AC, Criqui M, Lemaitre B, Uttenweiler-Joseph S, Michaut L, Reichhart J, Hoffmann JA. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J. 1998;17:1217–1227. doi: 10.1093/emboj/17.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senger K, Harris K, Levine M. GATA factors participate in tissue-specific immune responses in Drosophila larvae. Proc Natl Acad Sci USA. 2006;103:15957–15962. doi: 10.1073/pnas.0607608103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen UM, Kadalayil L, Rehorn KP, Hoshizaki DK, Reuter R, Engstrom Y. Serpent regulates Drosophila immunity genes in the larval fat body through an essential GATA motif. EMBO J. 1999;18:4013–4022. doi: 10.1093/emboj/18.14.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadalayil L, Petersen UM, Engstrom Y. Adjacent GATA and kappa B-like motifs regulate the expression of a Drosophila immune gene. Nucleic Acids Res. 1997;25:1233–1239. doi: 10.1093/nar/25.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryu JH, Nam KB, Oh CT, Nam HJ, Kim SH, Yoon JH, Seong JK, Yoo MA, Jang IH, Brey PT, Lee WJ. The homeobox gene Caudal regulates constitutive local expression of antimicrobial peptide genes in Drosophila epithelia. Mol Cell Biol. 2004;24:172–185. doi: 10.1128/MCB.24.1.172-185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Junell A, Uvell H, Davis MM, Edlundh-Rose E, Antonsson A, Pick L, Engstrom Y. The POU transcription factor Drifter/Ventral veinless regulates expression of Drosophila immune defense genes. Mol Cell Biol. 2010;30:3672–3684. doi: 10.1128/MCB.00223-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng J, Zipperlen P, Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr Biol. 2005;15:1690–1694. doi: 10.1016/j.cub.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 28.Samakovlis C, Kylsten P, Kimbrell DA, Engstrom A, Hultmark D. The andropin gene and its product, a male-specific antibacterial peptide in Drosophila melanogaster. EMBO J. 1991;10:163–169. doi: 10.1002/j.1460-2075.1991.tb07932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–385. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- 30.Pearson JC, Juarez MT, Kim M, Drivenes O, McGinnis W. Multiple transcription factor codes activate epidermal wound-response genes in Drosophila. Proc Natl Acad Sci USA. 2009;106:2224–2229. doi: 10.1073/pnas.0810219106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, Parkhurst SM, Martin P. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005;168:567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Brock AR, Wang Y, Fujitani K, Ueda R, Galko MJ. A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae. Curr Biol. 2009;19:1473–1477. doi: 10.1016/j.cub.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campos I, Geiger JA, Santos AC, Carlos V, Jacinto A. Genetic screen in Drosophila melanogaster uncovers a novel set of genes required for embryonic epithelial repair. Genetics. 2010;184:129–140. doi: 10.1534/genetics.109.110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uv AE, Harrison EJ, Bray SJ. Tissue-specific splicing and functions of the Drosophila transcription factor Grainyhead. Mol Cell Biol. 1997;17:6727–6735. doi: 10.1128/mcb.17.11.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciapponi L, Jackson DB, Mlodzik M, Bohmann D. Drosophila Fos mediates ERK and JNK signals via distinct phosphorylation sites. Genes Dev. 2001;15:1540–1553. doi: 10.1101/gad.886301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Tsarouhas V, Xylourgidis N, Sabri N, Tiklova K, Nautiyal N, Gallio M, Samakovlis C. The tyrosine kinase Stitcher activates Grainy head and epidermal wound healing in Drosophila. Nat Cell Biol. 2009;11:890–895. doi: 10.1038/ncb1898. [DOI] [PubMed] [Google Scholar]
- 37.Morgan BA, Johnson WA, Hirsh J. Regulated splicing produces different forms of dopa decarboxylase in the central nervous system and hypoderm of Drosophila melanogaster. EMBO J. 1986;5:3335–3342. doi: 10.1002/j.1460-2075.1986.tb04648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friggi-Grelin F, Iche M, Birman S. Tissue-specific developmental requirements of Drosophila tyrosine hydroxylase isoforms. Genesis. 2003;35:260–269. doi: 10.1002/gene.1082. [DOI] [PubMed] [Google Scholar]
- 39.Davis MM, Primrose DA, Hodgetts RB. A member of the p38 mitogen-activated protein kinase family is responsible for transcriptional induction of dopa decarboxylase in the epidermis of Drosophila melanogaster during the innate immune response. Mol Cell Biol. 2008;28:4883–4895. doi: 10.1128/MCB.02074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun H. The interaction between pathogens and the host coagulation system. Physiology (Bethesda) 2006;21:281–288. doi: 10.1152/physiol.00059.2005. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Wilhelmsson C, Hyrsl P, Loof TG, Dobes P, Klupp M, Loseva O, Morgelin M, Ikle J, Cripps RM, Herwald H, Theopold U. Pathogen entrapment by transglutaminase – a conserved early innate immune mechanism. PLoS Pathog. 2010;6:e1000763. doi: 10.1371/journal.ppat.1000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dushay MS. Insect hemolymph clotting. Cell Mol Life Sci. 2009;66:2643–2650. doi: 10.1007/s00018-009-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Daffre S, Kylsten P, Samakovlis C, Hultmark D. The lysozyme locus in Drosophila melanogaster: an expanded gene family adapted for expression in the digestive tract. Mol Gen Genet. 1994;242:152–162. doi: 10.1007/BF00391008. [DOI] [PubMed] [Google Scholar]
- 44.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 45.Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat Immunol. 2009;10:949–957. doi: 10.1038/ni.1765. [DOI] [PubMed] [Google Scholar]
- 46.Ha EM, Lee KA, Park SH, Kim SH, Nam HJ, Lee HY, Kang D, Lee WJ. Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell. 2009;16:386–397. doi: 10.1016/j.devcel.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 47.Scherfer C, Tang H, Kambris Z, Lhocine N, Hashimoto C, Lemaitre B. Drosophila Serpin-28D regulates hemolymph phenoloxidase activity and adult pigmentation. Dev Biol. 2008;323:189–196. doi: 10.1016/j.ydbio.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 48.Tang H, Kambris Z, Lemaitre B, Hashimoto C. A serpin that regulates immune melanization in the respiratory system of Drosophila. Dev Cell. 2008;15:617–626. doi: 10.1016/j.devcel.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319:777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- 50.Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci USA. 2000;97:13772–13777. doi: 10.1073/pnas.97.25.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bischoff V, Vignal C, Duvic B, Boneca IG, Hoffmann JA, Royet J. Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2. PLoS Pathog. 2006;2:e14. doi: 10.1371/journal.ppat.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaidman-Remy A, Herve M, Poidevin M, Pili-Floury S, Kim MS, Blanot D, Oh BH, Ueda R, Mengin-Lecreulx D, Lemaitre B. The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity. 2006;24:463–473. doi: 10.1016/j.immuni.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 53.Aggarwal K, Rus F, Vriesema-Magnuson C, Erturk-Hasdemir D, Paquette N, Silverman N. Rudra interrupts receptor signalling complexes to negatively regulate the IMD pathway. PLoS Pathog. 2008;4:e1000120. doi: 10.1371/journal.ppat.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kleino A, Myllymaki H, Kallio J, Vanha-aho LM, Oksanen K, Ulvila J, Hultmark D, Valanne S, Ramet M. Pirk is a negative regulator of the Drosophila Imd pathway. J Immunol. 2008;180:5413–5422. doi: 10.4049/jimmunol.180.8.5413. [DOI] [PubMed] [Google Scholar]
- 55.Lhocine N, Ribeiro PS, Buchon N, Wepf A, Wilson R, Tenev T, Lemaitre B, Gstaiger M, Meier P, Leulier F. PIMS modulates immune tolerance by negatively regulating Drosophila innate immune signalling. Cell Host Microbe. 2008;4:147–158. doi: 10.1016/j.chom.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Maillet F, Bischoff V, Vignal C, Hoffmann J, Royet J. The Drosophila peptidoglycan recognition protein PGRP-LF blocks PGFP-LC and IMD/JNK pathway activation. Cell Host Microbe. 2008;3:293–303. doi: 10.1016/j.chom.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 58.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signalling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 59.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signalling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, Simoes Rde M, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, Penninger JM. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGettigan J, McLennan RK, Broderick KE, Kean L, Allan AK, Cabrero P, Regulski MR, Pollock VP, Gould GW, Davies SA, Dow JA. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem Mol Biol. 2005;35:741–754. doi: 10.1016/j.ibmb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 67.Davies SA, Dow JA. Modulation of epithelial innate immunity by autocrine production of nitric oxide. Gen Comp Endocrinol. 2009;162:113–121. doi: 10.1016/j.ygcen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Kamimura Y. Twin intromittent organs of Drosophila for traumatic insemination. Biol Lett. 2007;3:401–404. doi: 10.1098/rsbl.2007.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siva-Jothy M, Reproductive immunity . Insect Infection and Immunity. In: Rolff J, Reynolds S, editors. Oxford: Oxford University Press; 2009. [Google Scholar]
- 70.Miest TS, Bloch-Qazi M. Sick of mating: sexual transmission of a pathogenic bacterium in Drosophila melanogaster. Fly (Austin) 2008;2:215–219. doi: 10.4161/fly.6726. [DOI] [PubMed] [Google Scholar]
- 71.Gendrin M, Welchman DP, Poidevin M, Herve M, Lemaitre B. Long-range activation of systemic immunity through peptidoglycan diffusion in Drosophila. PLoS Pathog. 2009;5:e1000694. doi: 10.1371/journal.ppat.1000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang H, Kambris Z, Lemaitre B, Hashimoto C. Two proteases defining a melanization cascade in the immune system of Drosophila. J Biol Chem. 2006;281:28097–28104. doi: 10.1074/jbc.M601642200. [DOI] [PubMed] [Google Scholar]
- 73.An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, Blackwell TK. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci USA. 2005;102:16275–16280. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Luhrs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–741. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Esfahani SS, Engstrom Y. Activation of an innate immune response in large numbers of permeabilized Drosophila embryos. Dev Comp Immunol. 2010;35:263–266. doi: 10.1016/j.dci.2010.11.002. [DOI] [PubMed] [Google Scholar]


