Abstract
Human immunodeficiency virus-1 (HIV-1) infection and the acquired immune deficiency syndrome (AIDS) pandemic remain global threats in the absence of a protective or a therapeutic vaccine. HIV-1 replication is reportedly inhibited by some cellular factors, including APOBEC3G (A3G) and APOBEC3F (A3F), which are well known inhibitors of HIV-1. Recently, HIV-1 Gag-virus-like particles (Gag-VLPs) have been shown to be safe and potent HIV-1 vaccine candidates that can elicit strong cellular and humoral immunity without need of any adjuvant. In this report, we stimulated human monocyte-derived dendritic cells (DCs) with Gag-VLPs and we demonstrated that Gag-VLP-treated DCs (VLP-DCs) produced interferon alpha (IFN-α), along with an increase in mRNA and protein expression of A3G and A3F. Gag-VLPs inhibited HIV-1 replication not only in DCs themselves, but also in cocultured T cells in an IFN-α-dependent manner. In addition, A3G/3F content in HIV virions released from VLP-DCs increased. Both the increase in A3G/3F expression and the inhibition of HIV-1 replication were reversed by anti-IFN-α or anti-IFNAR antibodies. Our findings in this study provide insight into the mechanism of Gag-VLP-induced inhibition of HIV-1 replication in DCs and T cells.
Key Words: HIV-1, Gag-virus-like particles, Dendritic cells, Interferon-α, APOBEC3
Introduction
Human immunodeficiency virus-1 (HIV-1) is a devastating human pathogen responsible for a worldwide pandemic of acquired immunodeficiency syndrome (AIDS). Despite extensive research on HIV, we still lack an effective prophylactic or therapeutic vaccine. Highly active antiretroviral therapy (HAART) has led to a major reduction in HIV-related mortality and morbidity; however, HIV-1 still cannot be eradicated from the human body. Therefore, the development of a safe and efficient AIDS vaccine remains a major concern, and the need to find a cure for HIV is even more urgent now than ever before [1,2].
Virus-like particles (VLPs) have been reported to be promising candidates for the development of effective vaccines against viral infections. VLPs represent a specific class of subunit vaccine that mimics the structure of authentic virus particles with better efficiency in inducing cellular and humoral immune responses than recombinant protein immunogens [3,4]. HIV-1 Gag-virus-like particles (Gag-VLPs) are also appealing as attractive AIDS vaccine candidates because of their ability to prime strong cytolytic CD8+ T cell (CTL) responses, CD4+ T cell proliferation and B cell-mediated humoral immunity in vivo [5,6,7,8,9]. We have recently reported that Gag-VLPs efficiently stimulate dendritic cells (DCs), eliciting their maturation and production of proinflammatory cytokines, which subsequently induce NK cell immune responses [10]. Gag-VLPs bind to and are taken up by DCs and processed for presentation on both MHC class I and MHC class II molecules by cross presentation without causing infection or intracellular replication [11,12].
DCs are the most potent antigen-presenting cells of the immune system. They play a major role in the initiation and regulation of immune responses with their unique properties to process and transport various infectious materials from the periphery to T cell areas in lymphoid organs, inducing activation of primary T cells and NK cells [13,14,15]. DCs are also involved in the transmission and pathogenesis of HIV-1 by capturing virions at mucosal surfaces and disseminating the virus directly to CD4+ T cells through infectious synapses (trans-infection pathway) [16,17]. Alternatively, HIV-1 can preferentially infect and replicate in DCs (cis-infection pathway) by exploiting innate immune signaling through TLR8 and DC-SIGN [18,19,20,21]. Increasing evidence indicates that HIV-infected DCs act as viral reservoirs for long-term HIV transmission in vivo [22,23,24]. Although DCs are susceptible to both R5 and X4 HIV-1 strains, HIV-1 replication is generally less productive in DCs than in CD4+ T cells, presumably because of the expression of restriction factors in DCs that block HIV-1 replication.
Among the host restriction factors, the most important and well known are the APOBEC3 (apolipoprotein B mRNA editing catalytic polypeptide 3; A3) proteins, a group of cellular cytidine deaminases with powerful antiretroviral activity that have been shown to block post-entry HIV-1 replication. Among A3 family members, A3G and A3F (A3G/3F) are reported to be the most potent inhibitors of HIV-1 replication. They are coordinately expressed in a wide range of human tissues, including DCs, and they independently inhibit retroviral infections [27,28]. A3G/3F are incorporated into HIV-1 virions and deaminate deoxycytidine to deoxyuridine in minus strand viral DNA during reverse transcription through their cytidine deaminase activity, causing the degradation of viral DNA and generation of nonfunctional proviral genomes [29]. Moreover, nonenzymatic antiretroviral activity of A3 proteins has also been implicated in inhibiting the accumulation of HIV-1 reverse transcription products, plus-strand DNA transfer and provirus integration [30,31]. In addition to inhibition of HIV infection by A3 proteins packaged into virions, cytoplasmic A3 in resting CD4+ T cells, monocytes and mature DCs can also function as a post-entry restriction to incoming HIV-1 virions [32,33]. A3G has also been shown to stimulate recognition of HIV-infected T cells by CTLs through the generation of defective translation products [34]. In addition, Norman et al. [35] provide several lines of evidence in support of a role of A3G in upregulating NKG2D ligand expression in HIV-infected cells sensitizing these cells to recognition and lysis by NK cells. They show that the amount of A3G present in HIV-infected cells is positively correlated with the amount of NKG2D ligand expression.
This antiretroviral activity of A3 is suppressed by HIV-1 virion infectivity factor (Vif), which binds and degrades human A3 via polyubiquitination and proteasomal degradation [36,37]. Vif also suppresses the antiviral role of APOBEC proteins by inhibiting their incorporation into HIV virions [38]. Furthermore, Vif inhibits the expression of NKG2D-ligands in HIV-infected cells and diminishes lysis by NK cells [35].
The expression of A3 proteins is upregulated in some cell types by treatment with interferon alpha (IFN-α). IFN-α significantly enhances the expression of A3G/3F at the transcriptional level and promotes anti-HIV-1 activity [39,40,41]. A3 levels in human DCs increase and restrict HIV-1 replication during DC maturation or after treatment with IFN-α [42].
In this report, we demonstrate for the first time that human DCs treated with Gag-VLPs (VLP-DCs) substantially upregulate cellular A3G/3F expression in an IFN-α-dependent manner. Gag-VLPs inhibit HIV-1 replication in DCs as well as in cocultured CD4+ T cells, and HIV-1 virions released from VLP-DCs contain increased amounts of A3G/3F as compared to virions released from control DCs. Neutralizing IFN-α with anti-IFN-α or blocking type I IFN receptors (IFNAR) with anti-IFNAR antibodies reversed Gag-VLP-induced upregulation of A3G/3F expression in both mRNA and protein levels, and also reversed the inhibition of HIV-1 replication in VLP-DCs and cocultured T cells.
Materials and Methods
Cell Culture
Spodoptera frugiperda (Sf-9) insect cells were grown at 27°C in BD Gold serum-free medium (BD Gold) containing 100 µg/ml kanamycin sulfate. HeLa and 293T cells were maintained in DMEM culture medium (Sigma) supplemented with 10% fetal bovine serum (Life Technologies), penicillin (100 U/ml) and streptomycin (100 µg/ml; Sigma).
Preparation of Human Monocyte-Derived DCs
Human monocyte-derived DCs were prepared from leukocyte-rich buffy coats from healthy donors as previously reported [10]. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare Bio-Sciences), and plastic-adhered monocytes were cultured for 6 days in DC culture medium in the presence of 20 ng/ml concentrations of each recombinant GM-CSF and interleukin (IL)-4 (Pepro Tech). At day 6, DCs were purified using a CD1c+ (BDCA-1) human DC isolation kit (Miltenyi Biotec Inc).
Generation of Recombinant Baculovirus AcCAG-Gag
The baculovirus transfer vector pAcCAG-gag containing the HIV-1 gag gene was generated as previously described [10]. Recombinant baculovirus (AcCAG-gag) was generated by cotransfection of Sf-9 cells with the baculovirus transfer vector pAcCAG-gag and AcMNPV-DNA using the BD Gold Baculovirus transfection kit (BD Biosciences). The recombinant baculovirus AcCAG-gag was expanded in Sf-9 cells and the titer was determined using a plaque assay.
Generation of X4 and R5 HIV-1 Viruses
T-tropic X4-HIV-1NL4–3 (HIV-1NL4–3) and M-tropic R5-HIV-1JR-CSF (HIV-1JR-CSF) strains were generated by transfection of HEK-293T cells with plasmid DNA (pNL4–3 or pJR-CSF) containing sequences of HIV-1 NL4–3 or JR-CSF. HIV-1NL-E containing the green fluorescent protein (GFP) gene in place of nef was generated from pNL-E. Culture supernatants of HEK-293T cells were collected 48 h post-transfection and filtered through 0.45 µm filters. Viral p24Gag contents were measured by ELISA using the Lumipulse automated chemiluminescent enzyme immunoassay (CLEIA) analyzer.
Production of Gag-VLPs in HeLa Cells
HIV-1 Gag-VLPs were produced as previously described [10]. Briefly, HeLa cells were infected with recombinant baculovirus AcCAG-gag at a multiplicity of infection of 100 for 1 h at 37°C washed twice with phosphate-buffered saline (PBS) and incubated at 37°C for 3 days. The culture supernatant was centrifuged at 2,500 rpm for 20 min and filtered through a 0.45-µm filter to remove cell debris. Gag-VLPs were pelleted by ultracentrifugation at 25,000 g for 2 h at 4°C and purified by sucrose gradient centrifugation through 20–60% continuous sucrose layers. VLP preparations were tested to be free of endotoxin (<0.01 endotoxin units/ml) using the Pyrodick endotoxin kit (Seikagaku Co., Tokyo, Japan).
DC Activation and Detection of IFN-α
DCs were incubated with Gag-VLPs (10 µg/ml), lipopolysaccharide (LPS; 1 µg/ml; Sigma) or medium alone for 24 h. IFN-α in the culture supernatants was quantified using an ELISA kit (BD Biosciences, San Diego, Calif., USA).
Antibody Blocking Assays
IFN-α in the DC culture medium was neutralized by adding anti-IFN-α antibody (5 µg/ml; PBL Biomedical Laboratories). To block IFN-α/β receptor signaling in DCs, anti-IFN-α/β receptor antibody (anti-IFNAR; 5 µg/ml; Santa Cruz) was used. DCs were preincubated with anti-IFN-α or anti-IFNAR for 30 min before treatment with Gag-VLPs or LPS. Blocking assays in the infection experiments were performed in the presence of anti-IFN-α or anti-IFNAR.
Western Blot Analysis
Cell lysates were prepared from DC cultures. For IFN-α blocking experiments, DCs were preincubated with anti-IFN-α (5 µg/ml) or anti-IFNAR (5 µg/ml) antibodies for 30 min before treatment with Gag-VLPs or LPS. The HIV virion fraction was prepared from the culture supernatants of HIV-infected DCs. The supernatants were first centrifuged for 20 min at 3,000 rpm to remove debris and then for 2 h at 19,000 rpm in a Beckman NVT-100 rotor to pellet viral particles. Cell or viral pellets were lysed in lysis buffer [1% Nonidet P-40, 50 mM Tris-HCl (pH 7.5), 1 mM EDTA and 1% protease inhibitor cocktail]. Lysates were then resolved by SDS-PAGE and electroblotted onto a PVDF membrane (Roche). The membrane was incubated with rabbit polyclonal anti-A3G (ProSci Inc.) or goat polyclonal anti-A3F (Santa Cruz) antibody (1:1000 dilution), followed by washing and incubation with anti-mouse or anti-rabbit IgGs conjugated with horseradish peroxidase (1:5,000; GE Healthcare Bio-Sciences) and visualization using the ECL plus Western blotting detection system (Amersham Pharmasia Biotech Inc.). β-Actin was used as a cell lysate control, and HIV-1 p24Gag was used as a viral lysate control.
RT-PCR Analysis
DCs (1 × 106) were cultured in the presence or absence of Gag-VLPs (10 µg/ml) or LPS (1 µg/ml) for 24 h. Control DCs were left untreated. After 24 h, total RNA was extracted from the cells using a GenElute™ Mammalian Total RNA Miniprep Kit (Sigma) according to the manufacturer's instructions. cDNA was synthesized using ReverTra Ace-α-™ (TOYOBO). The PCRs for A3G, A3F and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were performed using TaKaRa Ex Taq™ Hot Start Version (TaKaRa Bio Inc., Otsu, Japan), and the following primers were used: for A3G, forward primer: 5′-TCATCTAGTCCATCCCAGGG-3′ and reverse primer: 5′-TTACCTGCTTCACCTCCTGG-3′; for A3F, forward primer: 5′-TACGCAAAGCCTATGGTCGG-3′ and reverse primer: 5′-GCTCCAAGATGTGTACCAGG-3′; for GAPDH, forward primer: 5′-CCTTGGAGAAGGCTGGGG-3′ and reverse primer: 5′-CAAAGTTGTCATGGATGACC-3′. The PCR conditions consisted of an initial denaturation step (94°C for 3 min), followed by 30 cycles of denaturation (94°C for 30 s), annealing (64°C for 30 s) and extension (72°C for 12 s).
Immunofluorescence Staining and Confocal Microscopy
Control DCs, VLP-DCs and LPS-DCs were washed with PBS and fixed with 2% paraformaldehyde for 5 min. DCs were then permeabilized with 0.1% Triton X-100 in PBS for 10 min and blocked with 3% BSA for 30 min. Cells were then incubated for 1 h with rabbit polyclonal anti-A3G (ProSci) or goat polyclonal anti-A3F (Santa Cruz) antibodies (1:500 dilution), followed by FITC-conjugated anti-rabbit IgG or anti-goat IgG (Santa Cruz), respectively. Nuclei were stained with DAPI (Sigma). A3G/3F expression was visualized by confocal microscopy using a Zeiss (LSM-510, V-2.5) laser imaging confocal microscope.
HIV-1 Infection of DCs and Analysis
Control DCs, VLP-DCs and LPS-DCs (5 × 105) were infected with 100 ng of p24Gag content of HIV-1JR-CSF or 300 ng of HIV- 1NL-E for 2 h at 37°C. Cells were washed twice with PBS and cultured at 37°C for 72 h. Viral replication was estimated by measuring p24Gag contents in culture supernatants using Lumipulse and by measuring viral RNA using real-time quantitative RT-PCR analysis. HIV-1NL-E-infected DCs were nuclear stained with DAPI, and GFP fluorescence was examined under a KEYENCE (BZ-8000) immunofluorescent microscope.
Real-Time RT-PCR Analysis
Control DCs, LPS-DCs and VLP-DCs were infected with 100 ng of p24Gag content of R5-HIV-1JR-CSF for 2 h at 37°C and cultured for 72 h. Total RNA was extracted from the cells using a GenElute™ Mammalian Total RNA Miniprep Kit (Sigma). For quantification of HIV-1 RNA (R-U5 region of HIV-1JR-CSF), real-time RT-PCR was performed using ABI 7500 Real-Time PCR system and TaqMan One-Step RT-PCR master mix reagent kit from Applied Biosystems (Foster City, Calif., USA). Each qPCR mixture consisted of 12.5 µl of 2x master mix, 0.625 µl of 40x multiscribe and RNase inhibitor mix, 1.5 µl of forward primer (5 µM), 1.5 µl of reverse primer (5 µM), 0.2 µl of probe (25 µM), 4 µl (for HIV-1) or 1 µl (for 18S) of sample RNA, and RNase-free water for a final volume of 25 µl. As a loading control for normalization, 18S ribosomal RNA was quantified. The sequences of the primers and probes used in the analysis are as follows: HIV-1 forward: 5′-CAATAAAGCTTGCCTTGAGTGCT-3′; HIV-1 reverse: 5′-GGGTCTGAGGGATCTCTAGTTACC-3′; HIV-1 probe: 5′-FAM-AGTGTGTGCCCGTCTGTTGTGTGACTC-TAMRA-3′; 18S-F: 5′-GTAACCCGTTGAACCCCATT-3; 18S-R: 5′-CCATCCAATCGGTAGTAGCG-3′; 18S probe: 5′-FAM-TGCGTTGATTAAGTCCCTGCCCTTTGTA-TAMRA-3′. The real-time RT-PCR began with a reverse transcription step (48°C for 30 min), followed by a DNA denaturation and polymerase activation step (95°C for 10 min) and 45 cycles of amplification (95°C for 15 s, 60°C for 60 s).
DC/CD4+ T Cell Coculture
Autologous CD4+ T cells were isolated from human PBMCs using a CD4+ T cell isolation kit (Miltenyi Biotec Inc.) and stimulated with phytohemagglutinin A (5 µg/ml; Sigma) and IL-2 (30 U/ml; Roche) for 24 h. Control DCs, VLP-DCs and LPS-DCs (1 × 105) were infected with 300 ng of p24Gag content of HIV-1NL4–3 for 2 h and then washed with PBS. Activated CD4+ T cells were then added at a DC-to-T cell ratio of 1:5 and cultured for 5 days. An additional set of DCs, LPS-DCs and VLP-DCs infected with HIV-1NL4–3 were included as controls. The p24Gag in the culture supernatants was measured by Lumipulse. For the blocking experiment, DCs were preincubated with anti-IFN-α antibody (5 µg/ml) for 30 min before treatment with Gag-VLPs or LPS. DC/T cells were cocultured in the presence of anti-IFN-α (5 µg/ml). To further analyze the kinetics of HIV infection from DC to T cells, cocultures were continued for up to 9 days. One third of culture supernatants were collected for the measurement of p24Gag at the indicated time points and replaced with fresh medium.
Statistical Analysis
All data are presented as the mean ± SD. Statistical significance was evaluated by using Student's t test based on triplicate samples unless otherwise stated. p values <0.05 were considered statistically significant.
Results
Gag-VLPs Induce Production of IFN-α and Expression of A3G/3F in DCs
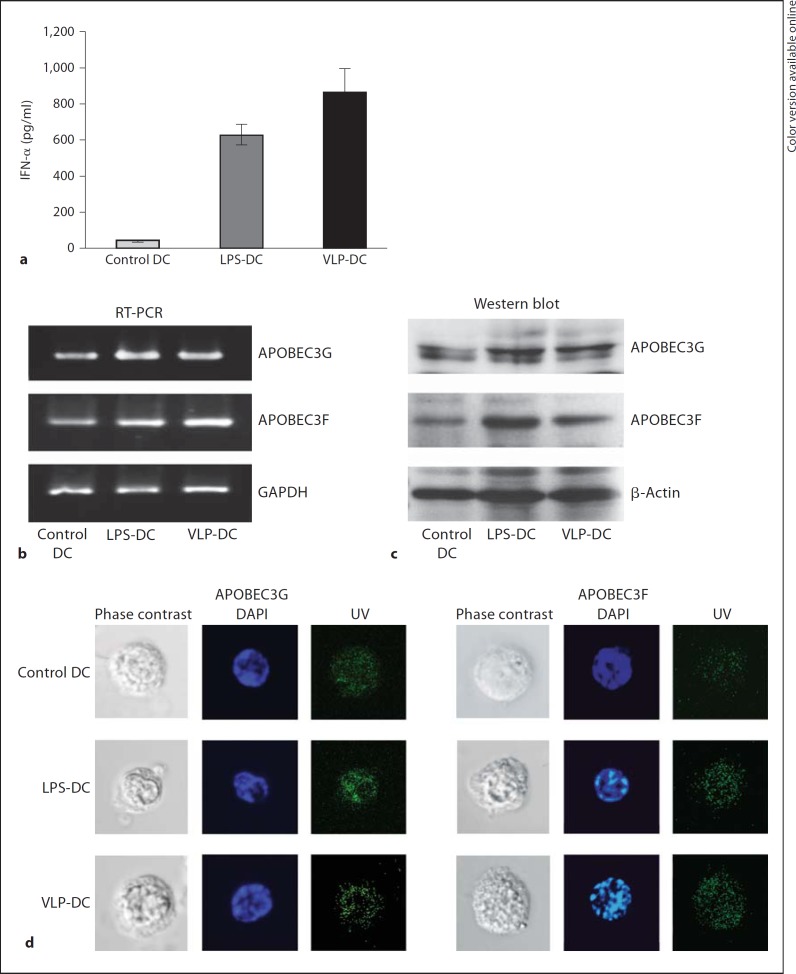
We have previously shown that treatment of human DCs with Gag-VLPs results in DC maturation and production of proinflammatory cytokines [10]. Here, we investigated whether Gag-VLP treatment could induce production of IFN-α and upregulate the expression of A3G/3F. The IFN-α levels in the culture supernatants were measured by ELISA and found to be elevated in VLP-DCs and LPS-DCs compared to control DCs (fig. 1a). Next, we examined the mRNA levels of A3G/3F in DCs by RT-PCR and A3 protein expression by Western blot analysis. The expression of both A3G/3F mRNA (fig. 1b) and protein (fig. 1c) was increased in VLP-DCs to levels comparable to those measured in LPS-DCs. Immunofluorescence staining and confocal microscopy revealed an increase in A3G/3F expression in VLP-DCs and LPS-DCs (fig. 1d). These results indicate that Gag-VLPs induce production of IFN-α and enhance A3G/3F expression in DCs.
Fig. 1.
Gag-VLPs induce production of IFN-α and expression of A3G/3F. a Production of IFN-α in the culture supernatants of DCs treated with Gag-VLPs (10 µg/ml), LPS (1 µg/ml) or medium only for 24 h was measured by ELISA. The results are expressed as the mean ± SD of triplicate cultures. b mRNA expression of A3G/3F in control DCs, LPS-DCs and VLP-DCs was measured by RT-PCR. GAPDH mRNA was used as a control. c A3G/3F protein expression was evaluated by Western blot analysis. DCs were lysed, and the total cell lysates were run on an SDS gel before being transferred to a PVDF membrane and probed with antibodies against A3G/3F. The membrane was stripped and reprobed for β-actin to ensure that comparable amounts of lysates were loaded in each lane. A representative image of 3 independent experiments is shown. d Cellular A3G/3F expression in DCs was analyzed by confocal immunofluorescence microscopy. One representative image of DCs is depicted here with the corresponding cellular markers (green: A3G or A3F, blue: nucleus; colors in the online version only).
Gag-VLPs Inhibit HIV-1 Replication in DCs and Increase Incorporation of A3G/3F into Nascent Virions
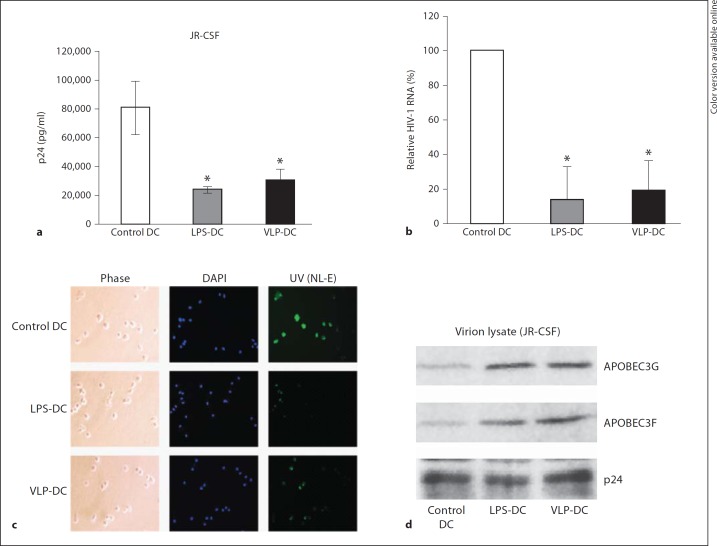
We next tried to determine whether Gag-VLPs could inhibit HIV-1 replication in DCs. The ability of Gag-VLPs to affect HIV infection in DCs was investigated using two HIV-1 strains (HIV-1JR-CSF and HIV-1NL-E). HIV-1JR-CSF-infected DCs showed an abrogation of progeny virion production (p24Gag content in culture supernatants) in VLP-DCs and LPS-DCs (fig. 2a). The p24Gag level in VLP-DCs was nearly one third of that in control DCs. Inhibition of HIV-1 in VLP-DCs was further confirmed by real-time RT-PCR. The result showed that after 72 h postinfection, HIV-1 in VLP-DCs was only about 20% of control DCs (fig. 2b). In HIV-1NL-E infection, a reduced number of HIV-1NL-E-infected, GFP-fluorescent cells were observed in VLP-DCs (fig. 2c). We next examined the incorporation of A3G/3F in nascent HIV virions released from the HIV-1JR-CSF-infected DCs by Western blot analysis. Incorporation of A3G/3F was increased in HIV virions released into culture supernatants from VLP-DCs and LPS-DCs (fig. 2d).
Fig. 2.
Gag-VLPs inhibit HIV-1 replication in DCs and increase incorporation of A3G/3F into nascent virions. DCs were treated with Gag-VLPs (10 µg/ml), LPS (1 µg/ml) or medium only for 24 h. Cells were then pulsed with HIV-1JR-CSF and cultured for 72 h. a Virus production was estimated by measuring p24Gag content in culture supernatants of DCs. b Infection was analyzed by real-time RT-PCR and normalized with 18S ribosomal RNA. The relative infection levels are shown as the percentage of control DCs. Each bar represents the average of 3 independent experiments. Statistical analysis was performed using the Student t test (* p < 0.05). c Immunofluorescent analysis of DCs infected with HIV-1NL-E by confocal microscopy. One representative image of DCs is depicted here with the corresponding cellular markers (green: GFP, blue: nucleus; colors in the online version only). d Virion incorporation of A3G and A3F was examined by Western blot analysis using viral lysates from DC culture supernatants. The p24Gag content was included as a viral lysate control. A representative image of 3 independent experiments is shown.
IFN-α-Dependent Upregulation of A3G/3F in VLP-DCs
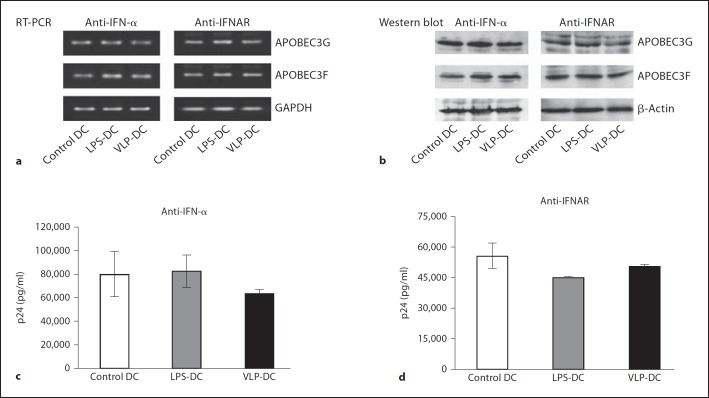
To further explore the potential role of IFN-α in A3 expression in DCs, we used an anti-IFN-α antibody to neutralize IFN-α released from VLP- or LPS-activated DCs and anti-IFNAR to block IFN-α receptors on DCs. The mRNA level of A3G/3F in DCs was examined by RT-PCR and A3 protein expression was assessed by Western blot analysis. The mRNA levels of A3G/3F were found to be the same in control DCs, LPS-DCs and VLP-DCs in the presence of anti-IFN-α or the IFN-α receptor inhibitor anti-IFNAR (fig. 3a). At the same time, anti-IFN-α and anti-IFNAR suppressed Gag-VLP- or LPS-induced A3G/3F protein expression to basal levels found in control DCs (fig. 3b). Altogether, these data demonstrate that Gag-VLP-induced upregulation of A3G/3F in DCs is IFN-α dependent and reversible by neutralizing IFN-α or blocking the IFN-α receptor on DCs.
Fig. 3.
Gag-VLPs upregulate A3G/3F expression in DCs and inhibit HIV-1 replication in an IFN-α-dependent manner. a DCs were preincubated with anti-IFN-α (5 µg/ml) or anti-IFNAR (5 µg/ml) antibodies for 30 min before treatment with Gag-VLPs or LPS for 24 h. mRNA expression of A3G/3F was measured by RT-PCR as mentioned above. GAPDH mRNA was used as a control. b A3G/3F protein expression was evaluated by Western blot analysis. A representative image of 3 independent experiments is shown. DCs were preincubated with anti-IFN-α (c) or anti-IFNAR (d) for 30 min before treatment with Gag-VLPs or LPS. Cells were then pulsed with HIV-1JR-CSF for 2 h and washed and cultured for 72 h. Virus production was estimated by measuring p24Gag content in culture supernatants.
IFN-α-Dependent Inhibition of HIV-1 Replication in VLP-DCs
We next examined the influence of IFN-α on Gag-VLP-induced restriction of HIV-1 replication. DCs were treated with anti-IFN-α or anti-IFNAR prior to stimulation with Gag-VLPs or LPS, and infected with HIV- 1JR-CSF. Restriction of HIV-1 replication in VLP-DCs or LPS-DCs was found to be reversed in the presence of anti-IFN-α (fig. 3c) or anti-IFNAR (fig 3d), increasing p24Gag levels to almost the same level observed in control DCs. Altogether, the above findings demonstrate that Gag-VLP-induced inhibition of HIV-1 replication in VLP-DCs is due to the upregulation of A3G/3F expression, which is dependent upon IFN-α.
Gag-VLPs Inhibit HIV-1 Replication in Cocultured CD4+ T Cells
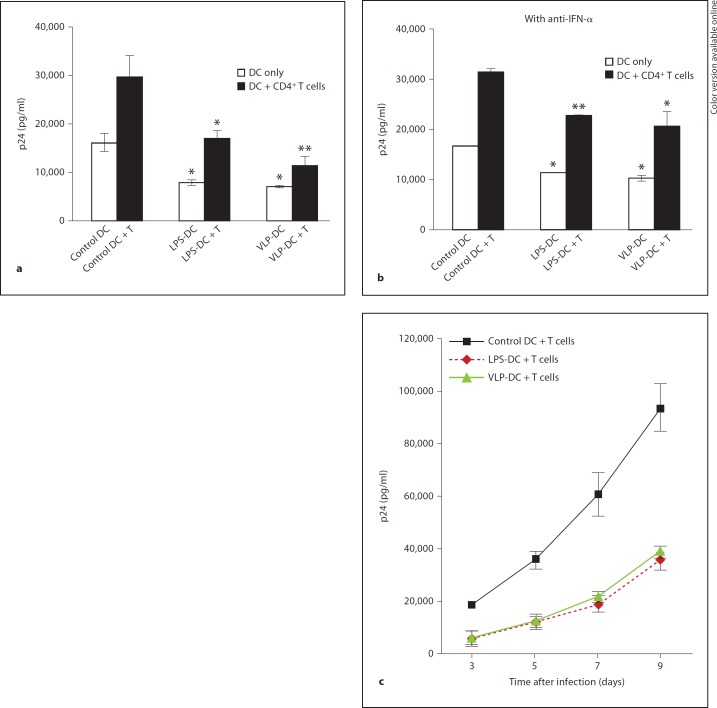
DCs have been known to transmit HIV-1 upon contact with CD4+ T cells. Despite relatively low efficiency, viral replication in DCs plays an important role in HIV-1 transmission from DCs to CD4+ T cells, which results in efficient HIV-1 replication and extensive spread of infection. To determine whether Gag-VLPs inhibit HIV-1 replication in cocultured T cells, we infected control DCs, VLP-DCs and LPS-DCs with HIV-1NL4–3 and cocultured with activated CD4+ T cells at a DC-to-T cell ratio of 1:5. After 5 days, the p24Gag content in culture supernatants was evaluated by Lumipulse. HIV-1 replication was inhibited in VLP-DC/T cells and LPS-DC/T cell cocultures (fig. 4a). Gag-VLPs inhibited HIV-1 replication in VLP-DC/T cell cocultures to nearly 30% of the p24Gag levels observed in the control DC/T cell cocultures. To differentiate viral replication in DCs from that in cocultured T cells, DC-only wells infected with HIV-1NL4–3 were also included as controls. In control DC/T cell cocultures, HIV-1 replication was increased 2-fold compared to that in control DC cultures. In VLP-DC/T cells, HIV-1 replication was increased only about 1.5-fold compared to levels in VLP-DC cultures (fig. 4a, b). This indicates that the reduction of p24Gag in VLP-DC/T cell cocultures was due to decreased replication of HIV-1 in T cells, not due to decreased HIV-1 production in DCs because of the negligible low efficiency replication of X4-HIV-1 in DCs. To assess the role of IFN-α in HIV-1 replication in DC/T cell cocultures, we added anti-IFN-α to DCs before coculturing with T cells. HIV-1 replication was measured by Lumipulse after 5 days. Inhibition of HIV-1 replication in VLP-DC/T cell cocultures was found to be only about 40% of that observed in control DC/T cell cocultures (fig. 4b). Because of the possibility that we could not completely neutralize the IFN-α released from DCs during the coculture period, VLP-induced inhibition of HIV-1 replication may partially remain even in the presence of anti-IFN-α. HIV-1 replication was increased 2-fold in VLP-DC/T cell cocultures compared to that observed in VLP-DC cultures, which is the same ratio found in control DC/T cell cocultures versus control DC cultures (fig. 4b). For the analysis of the kinetics of viral replication, DCs were infected with HIV-1NL4–3 and cocultured with CD4+ T cells for 9 days. The kinetics of the HIV-1 replication in DC/T cell cocultures was correlated with the culture period as infected T cells released newly formed, replication-competent virus into culture supernatants. Viral replication in T cells was strongly suppressed in cocultures with VLP-DCs and LPS-DCs (fig. 4c).
Fig. 4.
Gag-VLPs inhibit HIV-1 replication in DC/T cell cocultures in an IFN-α-dependent manner. a Control DCs, LPS-DCs and VLP-DCs were pulsed with HIV-1NL4–3 for 2 h. After washing, activated CD4+ T cells were added at a DC-to-T cell ratio of 1:5. Viral production was determined 5 days postinfection by measuring p24Gag in culture supernatants. b Anti-IFN-α antibody was added to DC cultures before treatment with Gag-VLPs or LPS. DCs were then infected with HIV-1NL4–3 for 2 h and CD4+ T cells were added at a DC-to-T cell ratio of 1:5. P24Gag in culture supernatants was measured 5 days postinfection. c For the analysis of the kinetics of HIV infection, DCs and T cells were cocultured for 9 days and viral replication was measured by p24Gag content at the indicated time points. Mean ± SD values of p24Gag levels in 3 independent experiments using cells from different donors. Statistical analysis was performed using the Student t test (* p < 0.05, ** p < 0.005).
In summary, we showed that Gag-VLPs efficiently stimulate human DCs to produce IFN-α and increase the expression of A3G/3F, inducing the suppression of HIV-1 replication in DCs as well as in cocultured T cells.
Discussion
The global HIV-infected population is still increasing every year despite the extensive efforts that have been made to combat AIDS. Highly active antiretroviral therapy significantly suppresses the HIV viral load but is unable to eradicate the virus from the body, leaving the possibility of the persistence of viral reservoirs. HIV-infected DCs act as viral reservoirs for long-term HIV transmission in vivo [22,23,24]. Identifying ways to trigger innate and adaptive immune responses to inhibit DC-endorsed HIV replication represents a potential strategy to limit HIV infection. DCs are essential for antigen presentation and activation of both innate and adaptive immune responses. DCs also play a critical role in the onset of HIV infection, providing one of the primary sites of HIV replication in vivo before the virus is transferred to CD4+ T cells, where robust viral amplification occurs [16,17,18]. Viruses that are transmitted from DCs to T cells are newly synthesized progeny viruses generated during de novo replication in DCs. However, DCs can preferentially replicate R5 HIV-1 strains to a greater extent than X4 strains [43]. The preferential replication of R5-HIV-1 over X4-HIV-1 in DCs appears to result from differential levels of coreceptor expression on the surface of DCs.
Previous reports have demonstrated Gag-VLP-mediated induction of CD4+ T cell activation, CTL responses and B cell-mediated humoral immunity [5,6,7,8,9]. We have previously reported the induction of DC and NK cell immune responses by Gag-VLPs. Binding and internalization of VLPs by DCs occur through macropinocytosis and receptor-mediated endocytosis. Mannose receptors, including the DC-SIGN receptor, which belongs to the C-type lectin family, are reportedly involved in VLP-uptake. The DC-SIGN receptor, present on the DC surface, recognizes VLPs and enhances internalization. Internalized VLPs induce maturation and activation of DCs and production of IFN-α, which is partially mediated through Toll-like receptors [10].
In this study, we developed a candidate HIV-1 therapeutic vaccine model based on Gag-VLPs produced in mammalian HeLa cells. The purpose of production of Gag VLPs in mammalian HeLa cells is to rule out contamination of baculovirus in VLPs during purification, which may distort the results when analyzing immunogenicity of VLPs. We first demonstrated that DCs treated with Gag-VLPs secreted increased amounts of IFN-α (fig. 1a) and induced upregulation of A3G/3F mRNA (fig. 1b) and protein (fig. 1c, d) expressions. Bakri et al. [44] indicated that the reduced replication of HIV in mature DCs is mainly due to postintegration regulatory events occurring at the transcriptional level. Their finding supports the possibility of A3-induced inhibition of HIV-1 in mature DCs, and our results are also consistent with this finding.
We used LPS as a positive control in analyzing the effects of Gag-VLP on DCs. LPS signals through the innate immune receptor TLR4 and causes both DC maturation and the release of cytokines, notably of IFN-α, which reportedly increases hA3G expression and reduces susceptibility to HIV-1 infection [45,46].
To examine whether increased expression of A3G/3F in VLP-DCs could control HIV-1 replication, we infected DCs with replication competent R5 (HIV-1JR-CSF) and X4 (HIV-1NL-E) strains of HIV-1, which have different efficiencies of infection and replication in DCs. In spite of their different replication efficiencies, both strains of HIV-1 showed low levels of viral replication in VLP-DCs (fig. 2a–c). Several groups reported different mechanisms of A3 other than postintegration inhibition by cytidine deamination, including inhibition of the accumulation of HIV-1 reverse transcription products, plus-strand DNA transfer and provirus integration [30,31]. Moreover, since we infected DCs with the replication-competent HIV-1JR-CSF virus, our results probably reflect the overall effects in inhibition of HIV-1 by Gag-VLPs (fig. 2a, b).
We next examined the incorporation of A3G/3F into nascent virions released from infected DCs, which restricts viral replication in newly infected cells. Incorporation of A3G/3F was increased in HIV virions released into culture supernatants from VLP-DCs and LPS-DCs (fig. 2d).
We further investigated the relationship between IFN-α-induced expression of A3G/3F and inhibition of HIV-1 replication. Neutralizing IFN-α and blocking IFNAR by adding relative antibodies to DC cultures before treatment with Gag-VLPs or LPS reduced the A3G/3F mRNA and protein expressions to the same levels expressed in control DCs (fig. 3a, b). At the same time, neutralizing IFN-α or blocking IFNAR resulted in an increase in HIV-1 replication in VLP-DCs and LPS-DCs, comparable to that in control DCs (fig. 3c, d). Our results implicate that Gag-VLP-induced expression of A3G/3F and the inhibition of HIV-1 replication were mainly dependent upon IFN-α. IFN-α exerts its antiviral activity through multiple mechanisms besides A3 induction, such as the RNA-dependent protein kinase/eukaryotic initiation factor 2α pathway, oligoadenylate synthetase/RNase L pathway and RNA deaminases [47]. However, Peng et al. [41] showed that depletion of A3G by small interfering RNA (siRNA) resulted in loss of the ability of IFN-α to completely inhibit HIV replication, confirming that A3 is a key downstream anti-HIV mechanism induced by IFN-α.
DCs are an important source of HIV-1 transmission to CD4+ T cells, where dissemination of infection starts by robust replication of HIV-1 [16,17]. To obtain effective control of HIV-1 replication, the initial viral replication in DCs as well as replication in T cells must be contained. We next examined the Gag-VLP-induced inhibition of HIV-1 in cocultured CD4+ T cells. To exclude the effects of A3-induced lysis of HIV-infected T cells by CTLs or NK cells, we purified CD4+ T cells from PBMCs for coculture assays. Our findings reveal that Gag-VLPs efficiently inhibit HIV-1 replication in VLP-DC/T cell cocultures (fig. 4a). To verify the inhibitory effect of IFN-α on cocultured T cells, we added anti-IFN-α to DC/T cell cocultures and evaluated HIV-1 replication. As expected, neutralizing IFN-α reversed the VLP-or LPS-induced inhibition of HIV-1 in DC/T cell coculture assays (fig. 4b). Because HIV-1 remains in endosomes of infected DCs for only 3 days [48], we measured p24Gag 5 days postinfection to exclude the effects of transinfection of HIV-1 from DCs to T cells in all coculture experiments. Then, viral replication in cocultures was examined up to day 9 to assess the kinetics of VLP-induced inhibition in cocultured T cells.
Based on our findings, we can define a mechanism of IFN-α-dependent upregulation of A3G/3F, mediated by Gag-VLPs, in association with inhibition of HIV-1, offering insight into the prevention of HIV-1 by strengthening A3G/3F-mediated intracellular innate immunity. Augmentation of this innate immune barrier could prevent HIV-1 replication and transmission in DCs, the native reservoir of HIV. Collectively, our results demonstrate that Gag-VLP-induced A3G/3F is incorporated into nascent virions and potently interferes with virus replication in both DCs and cocultured T cells. Our present findings may, therefore, have important implications for the understanding of the mechanism of Gag-VLP-induced inhibition of HIV-1 replication and open perspectives in the development of an effective immunotherapy against HIV.
Disclosure Statement
The authors declare that they have no competing interests.
Acknowledgements
We thank Dr. Hitoshi Suzuki for his excellent technical assistance in confocal microscopy and Mr. Yuuta Kasai for the helpful work he contributed to this research. This work was supported in part by a Grant-in-Aid for High Technology Research (No. 09309011) from the Ministry of Education, Science, Sports and Culture of Japan; by a Grant-in-Aid for AIDS research from the Ministry of Health, Labor and Welfare of Japan; by a grant from the Supporting Program for Creating University Ventures from Japan Science and Technology Agency, Japan, and by a grant from the Research and Development Program for New Bio-industry Initiatives from the Ministry of Agriculture, Forestry and Fisheries of Japan.
References
- 1.Kallings LO. The first postmodern pandemic: 25 years of HIV/AIDS. J Intern Med. 2008;263:218–243. doi: 10.1111/j.1365-2796.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 2.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 3.Noad R, Roy P. Virus-like particles as immunogens. Trends Microbiol. 2003;11:438–444. doi: 10.1016/s0966-842x(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 4.Grgacic EV, Anderson DA. Virus-like particles: passport to immune recognition. Methods. 2006;40:60–65. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner R, Deml L, Schirmbeck R, Niedrig M, Reimann J, Wolf H. Construction, expression, and immunogenicity of chimeric HIV-1 virus-like particles. Virology. 1996;220:128–140. doi: 10.1006/viro.1996.0293. [DOI] [PubMed] [Google Scholar]
- 6.Paliard X, Liu Y, Wagner R, Wolf H, Baenziger J, Walker CM. Priming of strong, broad, and long-lived HIV type 1 p55gag-specific CD8+ cytotoxic T cells after administration of a virus-like particle vaccine in rhesus macaques. AIDS Res Hum Retrovir. 2000;16:273–282. doi: 10.1089/088922200309368. [DOI] [PubMed] [Google Scholar]
- 7.Tsunetsugu-Yokota Y, Morikawa Y, Isogai M, Kawana-Tachikawa A, Odawara T, Nakamura T, Grassi F, Autran B, Iwamoto A. Yeast-derived human immunodeficiency virus type 1 p55(gag) virus-like particles activate dendritic cells (DCs) and induce perforin expression in Gag-specific CD8(+) T cells by cross-presentation of DCs. J Virol. 2003;77:10250–10259. doi: 10.1128/JVI.77.19.10250-10259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buonaguro L, Visciano ML, Tornesello ML, Tagliamonte M, Biryahwaho B, Buonaguro FM. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 clade A virus-like particles administered by different routes of inoculation. J Virol. 2005;79:7059–7067. doi: 10.1128/JVI.79.11.7059-7067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buonaguro L, Tornesello ML, Tagliamonte M, Gallo RC, Wang LX. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T cell responses. J Virol. 2006;80:9134–9143. doi: 10.1128/JVI.00050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang MO, Suzuki T, Suzuki H, Takaku H. HIV-1 Gag-virus-like particles induce natural killer cell immune responses via activation and maturation of dendritic cells. J Innate Immun. 2012;4:187–200. doi: 10.1159/000329226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmann MF, Lutz MB, Layton GT, Harris SJ, Fehr T, Rescigno M, Ricciardi-Castagnoli P. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996;26:2595–2600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 12.Morón VG, Rueda P, Sedlik C, Leclerc C. In vivo, dendritic cells can cross-present virus-like particles using an endosome-to-cytosol pathway. J Immunol. 2003;171:2242–2250. doi: 10.4049/jimmunol.171.5.2242. [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 14.Inaba K. Dendritic cells as antigen-presenting cells in vivo. Immunol Cell Biol. 1997;75:206–208. doi: 10.1038/icb.1997.31. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A. Division of labor by dendritic cells. Cell. 2007;128:435–436. doi: 10.1016/j.cell.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Arrighi JF, Pion M, Garcia E, Escola JM, van Kooyk Y, Geijtenbeek TB, Piguet V. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J Exp Med. 2004;200:1279–1288. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lekkerkerker AN, van Kooyk Y, Geijtenbeek TB. Viral piracy: HIV-1 targets dendritic cells for transmission. Curr HIV Res. 2006;4:169–176. doi: 10.2174/157016206776055020. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piguet V, Steinman RM. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 2007;28:503–510. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia E, Nikolic DS, Piguet V. HIV-1 replication in dendritic cells occurs through a tetraspanin-containing compartment enriched in AP-3. Traffic. 2008;9:200–214. doi: 10.1111/j.1600-0854.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- 21.Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010;11:419–426. doi: 10.1038/ni.1858. [DOI] [PubMed] [Google Scholar]
- 22.Nobile C, Petit C, Moris A, Skrabal K, Abastado JP, Mammano F, Schwartz O. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN expressing cells promotes long-term transmission to lymphocytes. J Virol. 2005;79:5386–5399. doi: 10.1128/JVI.79.9.5386-5399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popov S, Chenine AL, Gruber A, Li PL, Ruprecht RM. Long-term productive human immunodeficiency virus infection of CD1a-sorted myeloid dendritic cells. J Virol. 2005;79:602–608. doi: 10.1128/JVI.79.1.602-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology. 2009;6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franca R, Spadari S, Maga G. APOBEC deaminases as cellular antiviral factors: a novel natural host defense mechanism. Med Sci Monit. 2006;12:RA92–98. [PubMed] [Google Scholar]
- 26.Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364:675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pion M, Granelli-Piperno A, Mangeat B, Stalder R, Correa R, Steinman RM, Piguet V. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J Exp Med. 2006;203:2887–2893. doi: 10.1084/jem.20061519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defense by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- 29.Gandhi SK, Siliciano JD, Bailey JR, Siliciano RF, Blankson JN. Role of APOBEC3G/ F-mediated hypermutation in the control of human immunodeficiency virus type 1 in elite suppressors. J Virol. 2008;82:3125–3130. doi: 10.1128/JVI.01533-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holmes RK, Malim MH, Bishop KN. APOBEC-mediated viral restriction: not simply editing? Trends Biochem Sci. 2007;32:118–128. doi: 10.1016/j.tibs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Stenglein MD, Burns MB, Li M, Lengyel J, Harris RS. APOBEC3 proteins mediate the clearance of foreign DNA from human cells. Nat Struct Mol Biol. 2010;17:222–2229. doi: 10.1038/nsmb.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–114. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JL, Hope TJ. APOBEC3G restricts early HIV-1 replication in the cytoplasm of target cells. Virology. 2008;375:1–12. doi: 10.1016/j.virol.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casartelli N, Guivel-Benhassine F, Bouziat R, Brandler S, Schwartz O, Moris A. The antiviral factor APOBEC3G improves CTL recognition of cultured HIV-infected T cells. J Exp Med. 2010;207:39–49. doi: 10.1084/jem.20091933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W, Collins KL. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nat Immunol. 2011;12:975–983. doi: 10.1038/ni.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conticello SG, Harris RS, Neuberger MS. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr Biol. 2003;13:2009–2013. doi: 10.1016/j.cub.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 37.Romani B, Engelbrecht S, Glashoff RH. Antiviral roles of APOBEC proteins against HIV-1 and suppression by Vif. Arch Virol. 2009;154:1579–1588. doi: 10.1007/s00705-009-0481-y. [DOI] [PubMed] [Google Scholar]
- 38.Liu B, Yu X, Luo K, Yu Y, Yu XF. Influence of primate lentiviral Vif and proteasome inhibitors on human immunodeficiency virus type 1 virion packaging of APOBEC3G. J Virol. 2004;78:2072–2081. doi: 10.1128/JVI.78.4.2072-2081.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen K, Huang J, Zhang C, Huang S, Nunnari G, Wang FX, Tong X, Gao L, Nikisher K, Zhang H. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J Virol. 2006;80:7645–7657. doi: 10.1128/JVI.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka Y, Marusawa H, Seno H, Matsumoto Y, Ueda Y, Kodama Y, Endo Y, Yamauchi J, Matsumoto T, Takaori-Kondo A, Ikai I, Chiba T. Anti-viral protein APOBEC3G is induced by interferon-alpha stimulation in human hepatocytes. Biochem Biophys Res Commun. 2006;341:314–319. doi: 10.1016/j.bbrc.2005.12.192. [DOI] [PubMed] [Google Scholar]
- 41.Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J Exp Med. 2006;203:41–46. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang FX, Huang J, Zhang H, Ma X, Zhang H. APOBEC3G upregulation by alpha interferon restricts human immunodeficiency virus type 1 infection in human peripheral plasmacytoid dendritic cells. J Gen Virol. 2008;89:722–730. doi: 10.1099/vir.0.83530-0. [DOI] [PubMed] [Google Scholar]
- 43.Vanham G, Davis D, Willems B, Penne L, Kestens L, Janssens W, van der Groen G. Dendritic cells, exposed to primary, mixed phenotype HIV-1 isolates preferentially, but not exclusively, replicate CCR5-using clones. AIDS. 2000;14:1874–1876. doi: 10.1097/00002030-200008180-00034. [DOI] [PubMed] [Google Scholar]
- 44.Bakri Y, Schiffer C, Zennou V, Charneau P, Kahn E, Benjouad A, Gluckman JC, Canque B. The maturation of dendritic cells results in postintegration inhibition of HIV-1 replication. J Immunol. 2001;166:3780–3788. doi: 10.4049/jimmunol.166.6.3780. [DOI] [PubMed] [Google Scholar]
- 45.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 46.Simard S, Maurais E, Gilbert C, Tremblay MJ. LPS reduces HIV-1 replication in primary human macrophages partly through an endogenous production of type I interferons. Clin Immunol. 2008;127:198–205. doi: 10.1016/j.clim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turville SG, Santos JJ, Frank I, Cameron PU, Wilkinson J, Miranda-Saksena M, Dable J, Stössel H, Romani N, Piatak M, Jr, Lifson JD, Pope M, Cunningham AL. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–2179. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]