Abstract
The ideal immune response is rapid, proportionate and effective. Crucially, it must also be finite. An inflammatory response which is disproportionate or lasts too long risks injury to the host; chronic un-regulated inflammation in autoimmune diseases is one example of this. Thus, mechanisms to regulate and ultimately terminate immune responses are central to a healthy immune system. Despite extensive knowledge of what drives immune responses, our understanding of mechanisms of immune termination remains relatively sparse. It is clear that such processes are more complex than a one-dimensional homeostatic balance. Recent discoveries have revealed ever more nuanced mechanisms of signal termination, such as intrinsically self-limiting signals, multiple inhibitory mechanisms acting in tandem and activating proteins behaving differently in a variety of contexts. This review will summarise some important mechanisms, including termination by immunoreceptor tyrosine-based inhibitory motifs (ITIM), inhibition by soluble antagonists, receptor endocytosis or ubiquitination, and auto-inhibition by newly synthesised intracellular inhibitory molecules. Several recent discoveries showing immunoreceptor tyrosine-based activation motifs transducing inhibitory signals, ITIM mediating activating responses and the possible roles of immunoreceptor tyrosine-based switch motifs will also be explored.
Key Words: Endocytosis, Immunoreceptor tyrosine-based activation motifs, Immunoreceptor tyrosine-based inhibitory motifs, Intracellular signalling, Leucocyte immunoglobulin-like receptors, Ubiquitination
Introduction
The immune system constantly deals with foreign antigens appropriately with minimal host ‘collateral damage’ despite the complex immune response involved. A multitude of activating immune signals generate a potent inflammatory reaction, which is regulated in space and time by continued signalling within and between responding immune cells, eventually leading to its timely termination. Advances in the understanding of immune activation have been greatly instructive, though mechanisms of immune regulation and termination remain poorly defined. Poorly regulated immune responses risk host tissue damage and induction of autoimmunity, thus the regulation is tight, multiple and acts at different levels. These include the regulation at cellular level through signals from receptors and co-receptors leading to the down-regulation of receptor/co-receptor expression, growth inhibition, induction of apoptosis and induction of anergy. At a sub-cellular level, activating signals delivered by receptors are terminated by recruitment of tyrosine phosphatases or through de novo production of counter-regulatory signalling molecules, for instance termination of Toll-like receptor (TLR)-4-mediated responses through synthesis of IκB. Additionally, negative regulation of the immune system could occur through production of suppressive cytokines, such as interleukin (IL)-10 and transforming growth factor (TGF)-β, via activation of cytolysis and/or through physiological signalling switch-off processes. Negative immune regulation may be mediated by pairs of activating and inhibitory receptors that share common or similar ligands, by specialised inhibitory receptors that block signalling initiated by separate activation receptors, by negative feedback loops from activating receptors and/or termination by intracellular signal switching molecules.
This review aims to outline major mechanisms of immune termination and their synergistic, overlapping and/or concurrent effects. Recent findings that challenge some existing paradigms, including the role of immunoreceptor tyrosine-based activation motifs (ITAM) transducing inhibitory signals (ITAMi) and immunoreceptor tyrosine-based inhibitory motifs (ITIM) leading to immune activation (ITIMa) will be discussed.
Striking a Delicate Balance between Immune Activation and Inhibition with ITAMs and ITIMs
ITAMs were first identified by Reth [1] in the amino acid sequence encoding the cytoplasmic tails of the T cell receptor, B cell receptor (BCR) and FcεR1. These motifs have the consensus structure YxxI/Lx(6-12)YxxI/L (x represents any amino acid) spanning 14-18 amino acids (table 1). Ligand binding to an ITAM-bearing receptor leads to receptor aggregation, followed by the phosphorylation of both tyrosine residues in ITAM by proximal Src family protein tyrosine kinases [2]. A dually phosphorylated ITAM then provides a binding site for the two SH2 domains of Syk family non-receptor protein tyrosine kinases, typically Zap70 in T cells and Syk in B cells and monocytes [3]. This leads to activation of a cascade of downstream signalling molecules [2] (fig. 1a).
Table 1.
Consensus sequences for ITAMs, ITIMs and ITSMs
| ITIM | S/I/V/LxYxxI/V/L | 6 amino acids |
|---|---|---|
| ITAM | YxxI/Lx(6 – 12)YxxI/L | 14 – 20 amino acids |
| ITSM | TxYxxI/V | 6 amino acids |
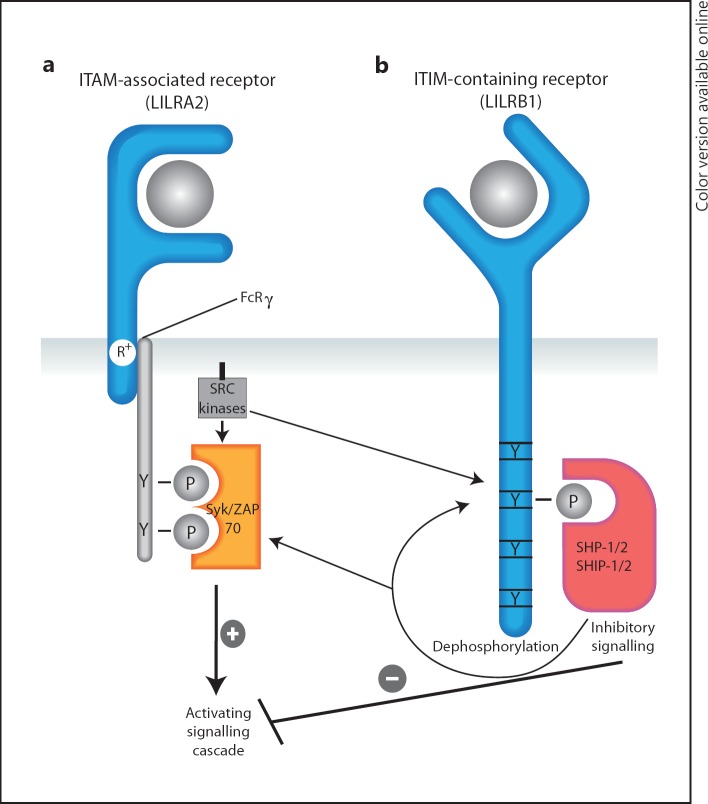
Fig. 1.
Activating LILRA2 and inhibitory LILRB1 are examples of conventional ITAM- and ITIM-containing receptors, respectively. a Following ligand binding, LILRA2 complexes with the common γ chain of the Fc receptor, altering its conformation and permitting phosphorylation of both tyrosine residues in the ITAM by Src protein tyrosine kinases. Syk family kinases are then able to bind via their dual SH2 domains and phosphorylate downstream proteins that transduce cellular activation signals. b Ligand binding to inhibitory LILRB1 leads to conformational changes in its intracellular region, facilitating phosphorylation of single tyrosine residues in the ITIM motif. These phosphorylated tyrosine residues recruit tyrosine phosphatases SHP-1, SHP-2 and/or SHIP initiating a cascade of inhibitory signalling.
ITIMs are a series of 6 amino acids (consensus sequence S/I/V/LxYxxI/V/L) found on the intracellular domain of transmembrane proteins which can transduce inhibitory signals (table 1). Ligand binding and receptor clustering lead to phosphorylation of the ITIM tyrosine residue by Src tyrosine kinases that recruit SH2-domain-containing tyrosine phosphatases, usually SHP-1 or SHP-2, or the inositol phosphatases SHIP-1 or SHIP-2. These phosphatases then dephosphorylate up- and downstream signalling molecules leading to the termination of cellular activation [4] (fig. 1b). ITIM-containing inhibitory receptors mediate this function with activating molecules via shared or different ligands [5].
There are numerous examples of ITAM-containing activating receptors [6] (table 2) and over 100 ITIM-containing inhibitory receptors potentially involved in immune regulation [7]. Of particular interest are several closely related ITAM-containing activating and ITIM-containing inhibitory receptors that upon co-clustering through similar or shared ligands regulate the threshold for and amplitude of cellular activation [5]. One family of such molecules is the leucocyte immunoglobulin (Ig)-like receptors (LILRs), also known as Ig-like transcripts or CD85a-m [8]. LILRs have two or four highly homologous Ig-like extracellular domains and are classified as inhibitory, activating or soluble receptors based on their transmembrane and cytoplasmic domains [8] (fig. 2c). Inhibitory LILRs (LILRBs) have long cytoplasmic tails containing ITIMs that transmit negative regulatory signals [9, 10]. Activating LILRs (LILRAs) have short cytoplasmic domains lacking signalling motifs; the charged arginine residue in their transmembrane domain links with ITAMs of the FcRγ chain to transduce activation [9, 10, 11]. LILRAs and LILRBs, which are widely co-expressed on the surface of leucocytes [9, 10, 11, 12], have structural homology of >80% on their ligand binding extracellular domains [9] and may fine-tune signalling through co-engagement by shared ligands. Although ligands for most LILRs are unknown, recent evidence showing shared binding of activating LILRA1, LILRA2 and LILRA3, and inhibitory LILRB1 and LILRB2 to a number of MHC class I molecules supports this [8, 13]. LILRs represent a complex system in which pairing of closely related activating and inhibitory receptors may regulate responses in innate immune cells, lymphocytes and antigen-presenting cells. This is supported by overwhelming in vitro evidence and strong associations of abnormal LILR expression in diseases characterised by excessive and/or perturbed chronic inflammation [14, 15, 16, 17, 18]. Other common examples of paired activating and inhibitory receptors that may regulate cellular activation include killer cell-activating and -inhibitory receptors [19], FcγRI and FcγRIIb IgG receptors, paired Ig-like receptors and triggering receptor expressed on myeloid cells (TREMs) [20, 21].
Table 2.
ITAM sequences in adaptor proteins involved in immune signalling
| FcRγ | DGVYTGLSTRNQETYETL |
| DAP-12 | ESPYOELOGORSDVYSDL |
| Igα | ENLYEGLNLDDCSMYEDI |
| CD3γ chain | DQLYQPLKDREDDQYSHL |
Consensus ITIM sequences are underlined, whilst canonical ITAM residues are highlighted in bold. Adapted from [2].
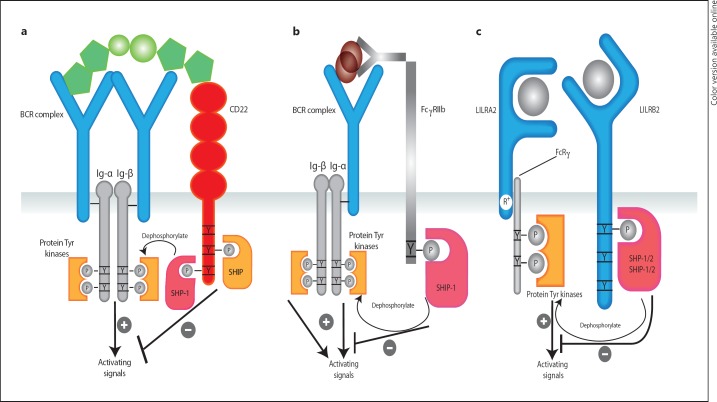
Fig. 2.
ITAM and ITIM crosstalk regulating cellular activation. a Engagement of ITAM-containing BCRs by foreign and/or self-antigens causes cellular activation. This is counter-regulated by ITIM-containing CD22 likely via shared ligands such as sialic acid motif-bearing glycans, widely expressed on native cells and foreign organisms. When the sialic acid motifs are ‘self’, CD22 exerts a net inhibitory signal by counteracting BCR-mediated activation. When the antigen is foreign, the inhibitory effects of CD22 are lower permitting a robust BCR-mediated response. b Alternatively, binding of BCR to immune complexes causes phosphorylation of the ITAM sequences on the intracellular Ig-α and Ig-β chains leading to cellular activation. This activation can be counter-regulated by shared binding of these immune complexes to the ITIM-containing FcγRIIb. c Activating LILRA2 and inhibitory LILRB2 are co-expressed on the surface of mono-myeloid cells and share 82% homology on their ligand-binding domains. They represent an ideal pair that may fine-tune cellular activation via co-engagement by similar or shared ligands.
On the other hand, B cell signalling provides a well-established model of ITAMs and ITIMs as complementary mechanisms wherein co-engagement of unrelated activating and inhibitory receptors by their respective ligands determines the net outcome. BCR Ig-α and Ig-β subunits each contain an ITAM sequence (fig. 2a). Upon binding to foreign antigens, BCRs aggregate forming an ITAM-driven signalosome that generates potent activating signals [22, 23] (fig. 2a). This is in part counteracted by an ITIM-containing cell surface receptor (CD22) expressed on B and plasma cells [23] (fig. 2a). CD22, which is excluded from the activating signalosome, simultaneously binds sialic acid-bearing ligands leading to tyrosine phosphorylation of its ITIM residues and recruitment of SHP-1 and SHIP-1 to produce inhibitory signals that prevent B cell hyperstimulation [22]. Interestingly, engagement of BCR by a self-antigen leads to formation of a signalosome that includes CD22 and other inhibitory receptors providing a strong negative signal encouraging tolerance [22]. Similarly, cross-linking of BCR with antigen and ligation of the inhibitory FcγRIIb with Ig have been shown to fine-tune BCR-mediated downstream activating signalling [20] (fig. 2b).
The Plot Thickens - Inhibitory ITAMs, Activating ITIMs and Immunoreceptor Tyrosine-Based Switch Motifs
Whilst the ITAM-mediated activation and ITIM-mediated counter-regulation is an attractive model for immune homeostasis, gathering evidence suggests that immune signalling can be much more complex. ITAMs and ITIMs may paradoxically be able to transduce negative (coined as ITAMi) and positive signalling (ITIMa), respectively. Recent studies by our group and others show that pre-stimulation of leucocytes through cross-linking of activating LILRA2 or LILRA4 profoundly suppressed pro-inflammatory mediator production in response to TLR ligation on monocytes [24] and dendritic cells [25], respectively. Importantly, the profound inhibitory effects of LILRA2 on monocytes appear to be highly selective to pro-inflammatory cytokines and not restricted to the regulation of soluble mediators [24]. For instance, LILRA2 cross-linking significantly suppresses lipopolysaccharide (LPS)-mediated tumour necrosis factor (TNF) production, Fc receptor-dependent phagocytosis [24] and GM-CSF-mediated dendritic cell maturation [25]. These results suggest that LILR-mediated immune regulation is much more complex than simple transduction of activating or inhibitory signals. We and others have proposed that the combined selective activating and inhibitory functions of activating LILRs are an important mechanism of LILR-mediated polarisation of the immune system [8, 18, 25]. This is supported by selective overexpression of LILRA2 observed in patients with Th-2-dominant lepromatous leprosy but not in Th-1-dominant tuberculoid leprosy in vivo [26], and the production of Th-2 cytokines upon LILRA2 cross-linking in monocytes [24] and macrophages infected with Mycobacterium tuberculosis[25].
Despite the overwhelming evidence showing activating LILRs transducing inhibitory signals, the underlying mechanisms are poorly understood. Some evidence from other similar ITAM-containing receptors indicate the nature of a ligand and its binding avidity/affinity may determine the net outcome. A typical example is ligation of FcαR1 by monomeric IgA eliciting anti-inflammatory effects, while IgA oligomers mediate strong pro-inflammatory effects [27]. Monomeric IgA induces partial ‘weaker’ phosphorylation of ITAMs that simultaneously recruit Syk and SHP-1 resulting in net inhibition [28]. By contrast, multimeric IgA induces greater phosphorylation with enhanced recruitment of Syk family kinases but not SHP-1 leading to net cell activation [28]. Given that both FcαR1 and activating LILRs lack cytoplasmic domains but share the ITAM-bearing FcRγ chain adaptor protein, the opposing effects observed in activating LILRs may be mediated by similar processes.
The ability of ITAMs to generate inhibitory signalling has been described in a variety of other ITAM-containing receptors [29, 30]. O'Neill et al. [29] discovered that constitutive phosphorylation of SHIP-1 and its adaptor protein Dok-1 in anergic B cells is due to BCR ITAM monophosphorylation. By contrast, engagement with foreign antigens causes dual phosphorylation of BCR ITAMs leading to potent B cell activation [29]. This phenomenon has recently been postulated as a therapeutic mechanism following the administration of intravenous Ig for inflammatory disorders [31]. Another well-established example of an ITAM-containing adaptor protein with dual activating and inhibitory functions is DAP-12 [32]. DAP-12 transduces activating signals in a heterodimeric receptor complex with KIR, Ly49 and TREM receptors [32], but macrophages with genetic deletion of DAP-12 were shown to produce higher levels of pro-inflammatory cytokines suggesting DAP-12-mediated inhibitory signalling [33]. Moreover, association of DAP-12 with natural cytotoxicity receptor NKp44 [34] or sialic-acid Ig-binding lectin-H inhibited interferon (IFN)-α production in response to CpG oligonucleotides in IFN-producing effector cells [35]. Although not fully elucidated, one proposed molecular mechanism for DAP-12-mediated inhibition is a consensus ITIM ‘SPYQEL’ within its proximal ITAM [32] (table 2). Interestingly, sequestration of proximal activating signalling molecules such as Syk by ITAM containing EBV LMP2A has been shown as an effective immune evasion strategy leading to latent infection [36]. Whether such mechanisms contribute to ITAM-mediated inhibition by native receptors remains to be elucidated.
In parallel to the effect of ITAMi, there have been several, albeit fewer, reports suggesting that ITIMs have the capacity to generate activating signalling under certain circumstances (ITIMa). In the first such report, published in 2004, TREM-like transcript 1 was considered to have ITIMa properties [37]. This surface receptor expressed on platelets has two ITIM sequences on its intracellular domain [38]. Barrow et al. [37] found that one of the two ITIMs (Y281) of TREM-like transcript 1 enhanced FcεRI-mediated intracellular calcium flux through recruitment of SHP-2. Recently, our research group has used site-targeted mutagenesis to study the role of tyrosine residues in each of three ITIMs of LILRB4 and found that the position of phosphorylated tyrosine residues dictates whether the net effect is inhibitory or activating [unpubl. data]. Although the mechanisms involved are not well defined, several other ITIM-containing receptors have been shown to transduce activating signals. These include induction of nitric oxide production in macrophages upon ligation of signal-regulatory protein-α [39], activation of transfected COS-7 cells upon ligation of ITIM-containing chemokine receptor CCK2R [40, 41, 42] and dual activating and inhibitory roles of the paired Ig-like receptor B on murine eosinophils treated with eotaxin and leukotriene B4, respectively [43].
Immunoreceptor tyrosine-based switch motifs (ITSMs) further confound the one-dimensional ITAM- versus ITIM-mediated immune-homeostasis model. The ITSM contains 6 amino acid residues with the consensus sequence TxYxxI/V [44] (table 1). The roles of these motifs are less well understood than those of ITAMs and ITIMs. It becomes immediately obvious, however, that there are similarities between the consensus sequence for ITSMs and those of ITAMs and ITIMs (tables 1, 2) [2, 44]. Indeed, an ITSM could share its tyrosine residue with the first tyrosine residue in an ITAM or ITIM. ITSMs have been identified on a number of molecules involved in mediating immune responses, including inhibitory KIRs [45], CD150 [44], sialic acid-binding Ig-like lectins [46] and programmed cell death protein 1 [47]. As yet, there are few papers providing insight into the function of ITSMs, but it is likely that they moderate ITAM/ITIM effects due to their sequence similarities and often close proximity [47]. This is supported by a recent study showing phosphorylation of both ITIMs and ITSMs found in close proximity in the orexin receptor OX1R was necessary to provide the two SH2 domains needed for functional SHP-2 binding [48].
Regulation of Immune Responses by Antagonist/Agonist Molecules
Production of soluble molecules that competitively antagonise their membrane-bound counterparts is one of the most common and effective mechanisms of regulating cellular responses. Some soluble receptors represent splice variants of their membrane-bound counterparts [49], some are cleaved products of surface receptors [50, 51] and some are molecules encoded by separate genes [52]. They act as decoy proteins by binding to the membrane-bound receptor or competitively binding ligand(s). Many such examples exist within the immune system and other biological processes. One such example is IL-1 and its receptors. In brief, interaction of IL-1α and IL-1β with the IL-1 receptor (IL-1R1) initiates a cascade of activation signalling via the upstream Toll/IL-1R (TIR) domain and the downstream MAP kinase-nuclear factor (NF)κB pathway [53]. This pathway is counter-regulated through several key mechanisms. First, through production of soluble IL-1R antagonist that terminates response by competitively binding IL-1R1 [54]. Crucially, IL-1R antagonist production is up-regulated by a broad range of inflammatory stimuli, including LPS, immune complexes and pro-inflammatory cytokines such as IL-1 itself [52] providing a negative feedback loop. Secondly, via up-regulation of a true decoy receptor, IL-1R2, which possesses an IL-1-binding extracellular domain but lacks an intracellular signalling domain [55] and, finally, inhibition through single Ig IL-1R-related molecule that is shown to down-regulate IL-1α or IL-1β signalling via competitive interaction with MyD (myeloid differentiation primary-response protein) 88 [56]. Other typical examples include a promiscuous chemokine decoy receptor D6, which is shown to bind at least 12 distinct chemokines causing their internalisation and proteasomal degradation [57], regulation of apoptosis signalling via Fas-Fas ligand and decoy receptor 3 [58], regulation of bone remodelling by myeloid lineage cells via RANK (receptor activator of NFκB), RANK ligand and osteoprotegerin [59], and control of angiogenesis involving vascular endothelial growth factor with the dummy receptor VEGFR-1 [60].
Immune Termination by Receptor De-/Ubiquitination
Ubiquitin is a highly conserved 8.5-kDa peptide that may mediate termination of immune responses by degradation of key regulatory proteins through its binding to lysine residues [61]. The ubiquitination reaction is catalysed by the sequential and cooperative actions of three enzymes: the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2) and the ubiquitin ligase (E3) [62]. Ubiquitin possesses seven lysine residues that can result in poly-ubiquitinated chains. Poly-ubiquitination formed via Lys48 marks target proteins for enzymatic degradation while poly-ubiquitination via Lys63 generally influences cell signalling by altering protein-protein interactions or by influencing cellular localisation [61]. Generally, a chain of at least four ubiquitin residues is required for proteasomal degradation [63]. Ubiquitination plays a vital role in many steps in innate immunity and is extensively reviewed by Bhoj and Chen [64].
A typical example regulated by several ubiquitination-related pathways is NFκB-mediated activation [64, 65] (fig. 3). In brief, constitutive binding of NFκB to IκB prevents its nuclear translocation, effectively keeping it in its inactive form in the cytoplasm. Activated IκB kinase (IKK) degrades IκB, allowing NFκB nuclear translocation and the induction of transcripts for pro-inflammatory mediators [65]. Ligation of TLRs [66], NOD (nucleotide-binding oligomerisation domain-containing protein)-like receptors [67, 68] or RIG-1-like receptors [69] leads to the NFκB-mediated activation and concomitant induction of the E3 ubiquitin ligases that mediate poly-ubiquitination of key intracellular regulatory molecules such as TNF receptor-associated factor (TRAF)2, TRAF3, TRAF6 or TRIM25 that act as both adaptors and E3 ubiquitin ligases catalysing Lys63-linked self-ubiquitination and the ubiquitination of other downstream signalling molecules [62]. In TLR4 responses, TRAF6 causes poly-ubiquitination of the IKK-regulatory subunit NEMO at Lys63 [63, 64, 65] (fig. 3). The IKK complex phosphorylates IκB, triggering its Lys48-linked ubiquitination, proteosomal degradation and its dissociation from p50 and p65 components of NFκB thus allowing nuclear translocation and DNA binding of NFκB thereby up-regulating the transcription of pro-inflammatory genes [63, 64, 65] (fig. 3). This is counter-regulated by concomitant up-regulation of the de-ubiquitinating enzymes A20 and CYLD and rapid de novo synthesis of IκB [63, 64, 65]. A20 forms a ubiquitin-editing complex that prevents Lys63 poly-ubiquitin chain formation by TRAF6 and IKK, leading to inhibition of NFκB signalling [63, 64, 65] (fig. 3). Newly synthesised and Lys48-deubiquitinated IκB provide another negative regulation of NFκB signalling [62].
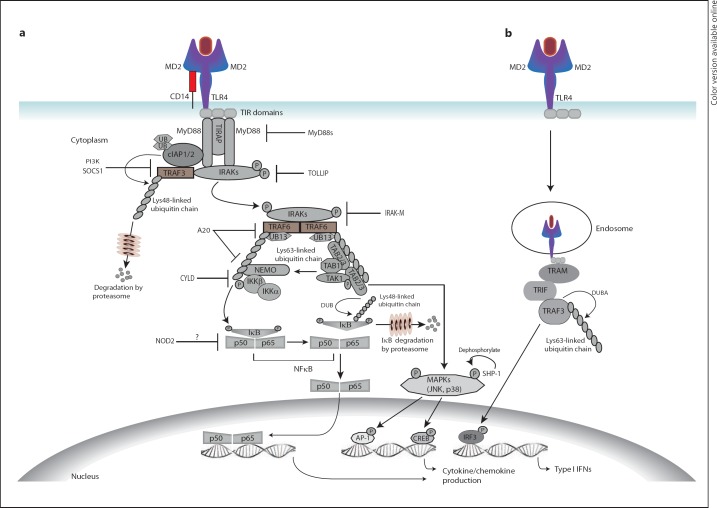
Fig. 3.
Termination of innate immune signalling by intracellular regulators. a In MyD88-dependent TLR signalling, Lys63 ubiquitination of TRAF6 causes its oligomerisation and triggers its E3 ligase activity. TRAF6 then synthesises poly-ubiquitin chains that bind the NEMO subunit of the IKK complex and the TGF-activating kinase (TAK)-binding protein (TAB2 and TAB3) subunits of the TAK1 complex with subsequent activation of kinases. Phosphorylated IκB (inhibitor of NFκB) is Lys48-linked poly-ubiquitinated and undergoes proteosomal degradation, releasing the NFκB dimer p50/p65 into the nucleus to switch on target genes including IKK inhibitor A20. Phosphorylated TAK1 may also induce target genes via downstream MAPK pathways. NFκB signalling is selectively terminated by A20- and probably CYLD-mediated Lys63 de-ubiquitination of TRAF6 and IKK leading to inhibition of IKK. Moreover, IκB is rapidly de novo synthesised and its Lys48 de-ubiquitination prevents its proteosomal degradation. b In MyD88-independent TLR signalling, de-ubiquitination of TRAF3 by DUBA is a specific and critical negative regulator of type 1 IFN production. Moreover, TLR signalling is auto-inhibited by several molecules that are induced by activation of TLRs. These include MyD88s, which competitively blocks association of IRAK4 with MyD88, IL-1R-associated kinase M (IRAK-M), which inhibits dissociation of IRAK1-IRAK4 complexes from the receptor, and other inhibitory proteins such as TOLLIP, SOCS1, PI3K, NOD2 and SHP-1. TIRAP = TIR domain-containing adaptor protein; cIAP = cellular inhibitor of apoptosis; TRIF = TIR-domain-containing adapter-inducing IFN-β; TRAM = TRIF-related adaptor molecule; IRF3 = IFN-regulatory factor 3; AP-1 = activator protein-1; CREB = cyclic AMP-responsive element-binding protein.
Auto-Inhibition of Immune Responses by Intrinsic Inhibitory Molecules
Auto-inhibition is another example whereby a stimulus initiates not only an appropriate positive response, but also begins the process of curtailing further responses to the same stimulus. In the innate immune system, the regulation of TLR signalling through the synthesis of new intracellular inhibitory molecules is one of the major pathways that fine-tune cellular responses. TLR-induced signalling follows two major pathways, MyD88-dependent and MyD88-independent pathways [70] (fig. 3). In brief, TLR (primarily TLR4) ligation recruits MyD88 and activates IL-1R-associated kinases (IRAKs) and allows propagation of downstream MAP kinase and/or NFκB activation pathways [71] (fig. 3). To counteract this, monocytes and macrophages may rapidly up-regulate intracellular negative regulators (fig. 3). These include MyD88s (a short form of MyD88), IRAK-M, SOCS1 (suppressor of cytokine signalling 1), NOD2, PI3K (phosphatidylinositol 3-kinase), TOLLIP (Toll-interacting protein), A20 and CYLD [72] and SHP-1 [73] (fig. 3).
MyD88s is a truncated alternatively spliced protein of the most important adaptor in TLR signalling. It is up-regulated in response to LPS and pro-inflammatory cytokines, thus competitively preventing IRAK-mediated activation [74]. Interestingly, induction of MyD88s can inhibit NFκB signalling but permits continued JNK and activator protein-1 activation [75], indicating selective regulation that ensures proportionate response. IRAK-M is an inactive IRAK family member without kinase activity that competitively abrogates the IRAK-mediated activation cascade [76]. In MyD88-independent TLR signalling, the intracellular counter-regulatory pathways are less well understood [77, 78] although there is evidence that de-ubiquitination plays an important role [79, 80] (fig. 3).
Other mechanisms that regulate TLR responses include down-modulation of expression/function of TLRs and their accessory molecules [81] by TRIAD3A-mediated ubiquitination [72] or by anti-inflammatory mediators such as IL-10 [82] and TGF-β [83]; through expression of a soluble TLR4/CD14; via up-regulation of a true TLR4 decoy receptor, RP105 or CD180, and its accessory molecule MD-1 [84] (fig. 4); through transmembrane protein regulators such as LILRs [24], SIRGIRR, TNF-related apoptosis-inducing ligand receptor and ST2L, and through induction of apoptosis [72] (fig. 4). Interestingly, treatment of cells with LPS temporarily down-regulates TLR4 while promoting the decoy receptor RP105 [85], further enhancing the counter-regulation [85].
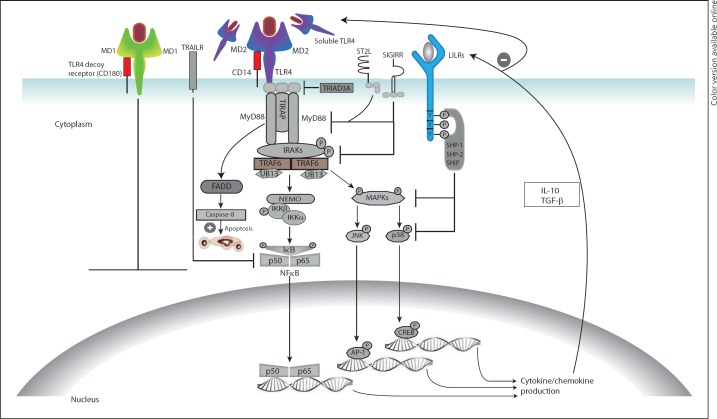
Fig. 4.
Negative regulation of innate immune responses by down-regulation of membrane receptors, induction of soluble receptors, up-regulation of decoy receptors, activation of transmembrane inhibitory molecules and/or by induction of apoptotic signals. TLR signalling can be controlled by reducing surface receptor expression through E3 ubiquitin ligase (TRIAD3A)-mediated ubiquitination and degradation or inhibition of expression by anti-inflammatory cytokines. Soluble TLRs could compete with their membrane-bound counterparts for microbial ligands or interact with MD2 and/or CD14 co-receptors preventing the formation of functional MD2-TLR complexes. Up-regulation of TLR4 decoy receptor (CD180) and its co-receptor MD1 by pro-inflammatory mediators can induce negative regulation via competitive ligand binding. Another mechanism by which TLR signalling can be abrogated is by transmembrane inhibitory receptors such as TIR domain containing receptors [single Ig IL-1R-related molecule (SIGIRR) and ST2] and ITIM-containing receptors [LILRs and TNF-related apoptosis-inducing ligand receptor (TRAILR)]. Under certain circumstances, TLR ligation may cause recruitment of FAS-associated death domain (FADD) to MyD88 and trigger caspase-dependent apoptosis of hyper-activated cells.
Termination of Immune Responses by Receptor Endocytosis
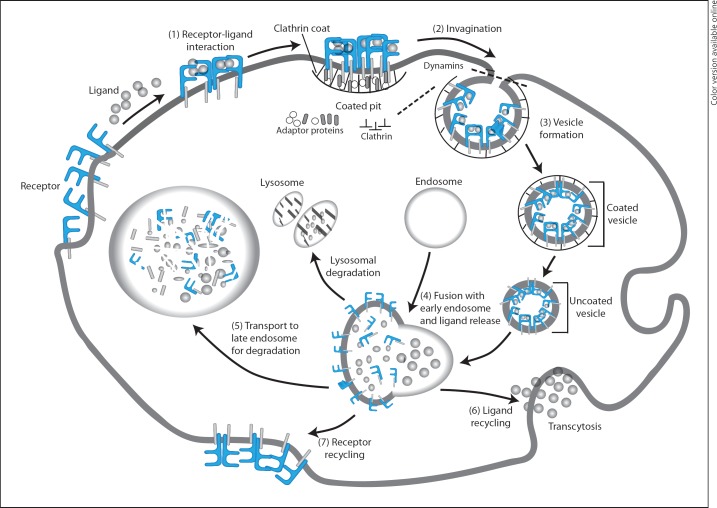
Physically removing a receptor from the cell surface is another effective way of regulating/terminating activation signals. A classic example of this is ligand binding-dependent internalisation of G-protein-coupled receptors, a broad family of receptors that play a key role in various biological processes [86]. In brief, binding of a ligand to these complex receptors induces phosphorylation and conformational changes of the different subunits of the receptor leading to transduction of activation signals [87]. This is followed by internalisation of ligand-receptor complexes and their transport into the endosomes [88] (fig. 5). In the endosomes, the ligand is removed and undergoes rapid degradation, while the receptor is either degraded or is recycled back to the cell surface [89]. Ligands for G-protein-coupled receptors relevant in inflammation include chemokines, prostaglandins, bradykinins and platelet-activating factors [86]. Other receptors that can undergo similar processes of endocytosis and degradation include epidermal and vascular endothelial growth factor receptors [90] and platelet-derived growth factor receptors [91].
Fig. 5.
Termination of signal transduction by receptor endocytosis. Ligand binding to surface receptors transduces activating signals (1) and simultaneously initiates signals that cause lateral diffusion of receptor-ligand complexes to clathrin-coated pits, membrane invagination (2) and generation of coated vesicles (3). The vesicles then undergo a process of uncoating (a precondition for vesicles to join to other membranes), fuse with early endosomes and release ligands (4). The ligand is transported either to late endosome and/or lysosome for proteosomal and/or lysosomal degradation (5) or is recycled and secreted by transcytosis (6). Similarly, receptors may undergo proteosomal degradation or recycle to the cell surface (7). Adaptor proteins including α and β adaptins, μ chain and σ chain form complexes that mediate formation of clathrin pits through interaction with membrane-bound receptors. Dynamin is a large GTPase implicated in budding and cleavage of vesicles (clathrin coated) from the parent membrane.
Mediators Involved in the Resolution of Inflammation
In recent years, a number of mediators that may bridge the immune termination events and subsequent resolution and/or repair of inflammation have been identified [92, 93, 94, 95, 96, 97, 98, 99]. These include a new class of pro-resolution lipid mediators, such as arachidonic acid-derived lipoxins, ω-3 polyunsaturated fatty acid-derived resolvins, protectins, and maresins [93, 94]. Pro-resolution lipids typically produced in the later stages of inflammation promote resolution through regulation of inflammatory cell recruitment, polarisation and death [94]. Pro-resolutions lipids may exhibit both pro-inflammatory and pro-resolution properties [95] allowing effective but well-regulated inflammatory responses and measured tissue repair that limits extensive fibrosis. Although the list is far from comprehensive, other pro-resolution or dual-effect mediators that regulate pro-resolving molecular and cellular circuits are several micro-RNAs [96], annexins [97], nitric oxide [98] and members of the S100 proteins [99].
Host Response to Foreign Antigens - A High Stakes Game
We have outlined some important mechanisms by which immune responses are regulated. As an organism, we must respond aggressively to foreign antigens, but an over-exuberant reaction is damaging and potentially fatal. Immune termination is too important to fail, thus, it is multilayered, complex and tight. We have seen how immune signalling can be self-limiting through several intracellular and extracellular mechanisms that ensure effective termination. Moreover, multiple independent processes might collaboratively act to tightly regulate excessive inflammatory responses. These processes may occur simultaneously and/or sequentially and are highly dynamic. Identification and functional characterisation of key molecules involved in immune regulation/termination and understanding the underlying mechanisms would have significant clinical implications, though, much remains to be explained with regard to the relative contribution of each process.
Acknowledgements
Barry Kane is supported by a research scholarship from Arthritis Australia.
References
- 1.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 2.Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 3.Latour S, Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr Opin Immunol. 2001;13:299–306. doi: 10.1016/s0952-7915(00)00219-3. [DOI] [PubMed] [Google Scholar]
- 4.Bounab Y, Getahun A, Cambier JC, Daëron M. Phosphatase regulation of immunoreceptor signaling in T cells, B cells and mast cells. Curr Opin Immunol. 2013;25:313–320. doi: 10.1016/j.coi.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbrugge A, Meyaard L. Signaling by ITIM-bearing receptors. Curr Immunol Rev. 2005;1:201–212. [Google Scholar]
- 6.Foddor S, Jakus Z, Mocsai A. ITAM-based signalling beyond the adaptive immune response. Immunol Lett. 2006;104:29–37. doi: 10.1016/j.imlet.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Staub E, Rosenthal A, Hinzmann B. Systematic identification of immunoreceptor tyrosine-based inhibitory motifs in the human proteome. Cell Signal. 2004;16:435–456. doi: 10.1016/j.cellsig.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens. 2004;64:215–225. doi: 10.1111/j.0001-2815.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 9.Borges L, Cosman D. LIRs/ILTs/MIRs, inhibitory and stimulatory Ig-superfamily receptors expressed in myeloid and lymphoid cells. Cytokine Growth Factor Rev. 2000;11:209–217. doi: 10.1016/s1359-6101(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 10.Fanger N, Borges L, Cosman D. The leukocyte immunoglobulin-like receptors (LIRs): a new family of immune regulators. J Leukoc Biol. 1999;66:231–236. doi: 10.1002/jlb.66.2.231. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima H, Samaridis J, Angman L, Colonna M. Cutting edge: human myeloid cells express an activating ILT receptor (ILT1) that associates with Fc receptor γ-chain. J Immunol. 1999;162:5–8. [PubMed] [Google Scholar]
- 12.Tedla N, Bandeira-Melo C, Tassinari P, Sloane D, Samplaski M, Cosman D, et al. Activation of human eosinophils through leukocyte immunoglobulin-like receptor 7. Proc Natl Acad Sci USA. 2003;100:1174–1179. doi: 10.1073/pnas.0337567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosman D, Fanger N, Borges L, Kubin M, Chin W, Peterson L, et al. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 14.An H, Chandra V, Piraino B, Borges L, Geczy C, McNeil H, et al. Soluble LILRA3, a potential natural antiinflammatory protein, is increased in patients with rheumatoid arthritis and is tightly regulated by interleukin 10, tumor necrosis factor-alpha, and interferon-gamma. J Rheumatol. 2010;37:1596–1606. doi: 10.3899/jrheum.091119. [DOI] [PubMed] [Google Scholar]
- 15.Kuroki K, Tsuchiya N, Shiroishi M, Rasubala L, Yamashita Y, Matsuta K, et al. Extensive polymorphisms of LILRB1 (ILT2, LIR1) and their association with HLA-DRB1 shared epitope negative rheumatoid arthritis. Hum Mol Genet. 2005;14:2469–2480. doi: 10.1093/hmg/ddi247. [DOI] [PubMed] [Google Scholar]
- 16.Monsivais-Urenda A, Nino-Moreno P, Abud-Mendoza C, Baranda L, Layseca-Espinosa E, Lopez-Botet M, et al. Analysis of expression and function of the inhibitory receptor ILT2 (CD85j/LILRB1/LIR-1) in peripheral blood mononuclear cells from patients with systemic lupus erythematosus (SLE) J Autoimmun. 2007;29:97–105. doi: 10.1016/j.jaut.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Ordonez D, Sanchez AJ, Martinez-Rodriguez JE, Cisneros E, Ramil E, Romo N, et al. Multiple sclerosis associates with LILRA3 deletion in Spanish patients. Genes Immun. 2009;10:579–585. doi: 10.1038/gene.2009.34. [DOI] [PubMed] [Google Scholar]
- 18.Tedla N, An H, Borges L, Vollmer-Conna U, Bryant K, Geczy C, et al. Expression of activating and inhibitory leukocyte immunoglobulin-like receptors in rheumatoid synovium: correlations to disease activity. Tissue Antigens. 2011;77:305–316. doi: 10.1111/j.1399-0039.2011.01633.x. [DOI] [PubMed] [Google Scholar]
- 19.Stanietsky N, Mandelboim O. Paired NK cell receptors controlling NK cytotoxicity. FEBS Lett. 2010;584:4895–4900. doi: 10.1016/j.febslet.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 20.Nimmerjahn F, Ravetch JV. Fc γ receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 21.Van Montfoort N, t Hoen PAC, Mangsbo SM, Camps MGM, Boross P, Melief CJM, et al. Fcγ receptor IIb strongly regulates Fcγ receptor-facilitated T cell activation by dendritic cells. J Immunol. 2012;189:92–101. doi: 10.4049/jimmunol.1103703. [DOI] [PubMed] [Google Scholar]
- 22.Poe JC, Tedder TF. CD22 and Siglec-G in B cell function and tolerance. Trends Immunol. 2012;33:413–420. doi: 10.1016/j.it.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker JA, Smith KGC. CD22: an inhibitory enigma. Immunology. 2008;123:314–325. doi: 10.1111/j.1365-2567.2007.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu HK, Mitchell A, Endoh Y, Hampartzoumian T, Huynh O, Borges L, et al. LILRA2 selectively modulates LPS-mediated cytokine production and inhibits phagocytosis by monocytes. PLoS One. 2012;7:e33478. doi: 10.1371/journal.pone.0033478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DJ, Sieling PA, Ochoa MT, Krutzik SR, Guo B, Hernandez M, et al. LILRA2 activation inhibits dendritic cell differentiation and antigen presentation to T cells. J Immunol. 2007;179:8128–8136. doi: 10.4049/jimmunol.179.12.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleharski JR, Li H, Meinken C, Graeber TG, Ochoa MT, Yamamura M, et al. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science. 2003;301:1527–1530. doi: 10.1126/science.1087785. [DOI] [PubMed] [Google Scholar]
- 27.van Egmond M, Damen CA, van Spriel AB, Vidarsson G, van Garderen E, van de Winkel JGJ. IgA and the IgA Fc receptor. Trends Immunol. 2001;22:205–211. doi: 10.1016/s1471-4906(01)01873-7. [DOI] [PubMed] [Google Scholar]
- 28.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, Ruffié C, et al. Identification of FcαRI as an inhibitory receptor that controls inflammation: dual role of FcRγ ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill SK, Getahun A, Gauld SB, Merrell KT, Tamir I, Smith MJ, et al. Monophosphorylation of CD79a and CD79b ITAM motifs initiates a SHIP-1 phosphatase-mediated inhibitory signaling cascade required for B cell anergy. Immunity. 2011;35:746–756. doi: 10.1016/j.immuni.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kersh EN, Kersh GJ, Allen PM. Partially phosphorylated T cell receptor ζ molecules can inhibit T cell activation. J Exp Med. 1999;190:1627–1636. doi: 10.1084/jem.190.11.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aloulou M, Ben Mkaddem S, Biarnes-Pelicot M, Boussetta T, Souchet H, Rossato E, et al. IgG1 and IVIg induce inhibitory ITAM signaling through FcγRIII controlling inflammatory responses. Blood. 2012;119:3084–3096. doi: 10.1182/blood-2011-08-376046. [DOI] [PubMed] [Google Scholar]
- 32.Barrow AD, Trowsdale J. You say ITAM and I say ITIM, let's call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol. 2006;36:1646–1653. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 33.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–586. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuchs A, Cella M, Kondo T, Colonna M. Paradoxic inhibition of human natural interferon-producing cells by the activating receptor NKp44. Blood. 2005;106:2076–2082. doi: 10.1182/blood-2004-12-4802. [DOI] [PubMed] [Google Scholar]
- 35.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–2476. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 37.Barrow AD, Astoul E, Floto A, Brooke G, Relou IAM, Jennings NS, et al. Cutting edge: TREM-like transcript-1, a platelet immunoreceptor tyrosine-based inhibition motif encoding costimulatory immunoreceptor that enhances, rather than inhibits, calcium signaling via SHP-2. J Immunol. 2004;172:5838–5842. doi: 10.4049/jimmunol.172.10.5838. [DOI] [PubMed] [Google Scholar]
- 38.Allcock RJN, Barrow AD, Forbes S, Beck S, Trowsdale J. The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol. 2003;33:567–577. doi: 10.1002/immu.200310033. [DOI] [PubMed] [Google Scholar]
- 39.Alblas J, Honing H, Renardel de Lavalette C, Brown MH, Dijkstra CD, van den Berg TK. Signal regulatory protein α ligation induces macrophage nitric oxide production through JAK/STAT- and phosphatidylinositol 3-kinase/Rac1/NAPDH oxidase/H2O2-dependent pathways. Mol Cell Biol. 2005;25:7181–7192. doi: 10.1128/MCB.25.16.7181-7192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrand A, Kowalski-Chauvel A, Bertrand C, Escrieut C, Mathieu A, Portolan G, et al. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R: implication of this signaling pathway in pancreatic tumor models. J Biol Chem. 2005;280:10710–10715. doi: 10.1074/jbc.M413309200. [DOI] [PubMed] [Google Scholar]
- 41.Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657–20663. doi: 10.1074/jbc.274.29.20657. [DOI] [PubMed] [Google Scholar]
- 42.Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Gastrin stimulates tyrosine phosphorylation of insulin receptor substrate 1 and its association with Grb2 and the phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26356–26361. doi: 10.1074/jbc.271.42.26356. [DOI] [PubMed] [Google Scholar]
- 43.Munitz A, McBride ML, Bernstein JS, Rothenberg ME. A dual activation and inhibition role for the paired immunoglobulin-like receptor B in eosinophils. Blood. 2008;111:5694–5703. doi: 10.1182/blood-2007-12-126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, et al. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- 45.Yusa S, Catina TL, Campbell KS. KIR2DL5 can inhibit human NK cell activation via recruitment of Src homology region 2-containing protein tyrosine phosphatase-2 (SHP-2) J Immunol. 2004;172:7385–7392. doi: 10.4049/jimmunol.172.12.7385. [DOI] [PubMed] [Google Scholar]
- 46.Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and −9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841–6849. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- 47.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 48.El Firar A, Voisin T, Rouyer-Fessard C, Ostuni MA, Couvineau A, Laburthe M. Discovery of a functional immunoreceptor tyrosine-based switch motif in a 7-transmembrane-spanning receptor: role in the orexin receptor OX1R-driven apoptosis. FASEB J. 2009;23:4069–4080. doi: 10.1096/fj.09-131367. [DOI] [PubMed] [Google Scholar]
- 49.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 50.Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173:5343–5348. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]
- 51.Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64:135–146. doi: 10.1002/jlb.64.2.135. [DOI] [PubMed] [Google Scholar]
- 52.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 53.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–1615. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 54.Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, Ju G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem. 1995;270:13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 55.Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 56.Qin J, Qian Y, Yao J, Grace C, Li X. SIGIRR inhibits interleukin-1 receptor- and toll-like receptor 4-mediated signaling through different mechanisms. J Biol Chem. 2005;280:25233–25241. doi: 10.1074/jbc.M501363200. [DOI] [PubMed] [Google Scholar]
- 57.Lee KM, Nibbs RJB, Graham GJ. D6: the ‘crowd controller’ at the immune gateway. Trends Immunol. 2013;34:7–12. doi: 10.1016/j.it.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 59.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9((suppl 1)):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 61.Malynn BA, Ma A. Ubiquitin makes its mark on immune regulation. Immunity. 2010;33:843–852. doi: 10.1016/j.immuni.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun SC. Deubiquitylation and regulation of the immune response. Nat Rev Immunol. 2008;8:501–511. doi: 10.1038/nri2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 64.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 65.Renner F, Schmitz ML. Autoregulatory feedback loops terminating the NF-κB response. Trends Biochem Sci. 2009;34:128–135. doi: 10.1016/j.tibs.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Conze DB, Wu CJ, Thomas JA, Landstrom A, Ashwell JD. Lys63-linked polyubiquitination of IRAK-1 is required for interleukin-1 receptor- and toll-like receptor-mediated NF-kappaB activation. Mol Cell Biol. 2008;28:3538–3547. doi: 10.1128/MCB.02098-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider M, Zimmermann AG, Roberts RA, Zhang L, Swanson KV, Wen H, et al. The innate immune sensor NLRC3 attenuates Toll-like receptor signaling via modification of the signaling adaptor TRAF6 and transcription factor NF-kappaB. Nat Immunol. 2012;13:823–831. doi: 10.1038/ni.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castanier C, Zemirli N, Portier A, Garcin D, Bidere N, Vazquez A, et al. MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors. BMC Biol. 2012;10:44. doi: 10.1186/1741-7007-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 71.O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 72.Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 73.Yuk JM, Shin DM, Lee HM, Kim JJ, Kim SW, Jin HS, et al. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat Immunol. 2011;12:742–751. doi: 10.1038/ni.2064. [DOI] [PubMed] [Google Scholar]
- 74.Janssens S, Burns K, Tschopp J, Beyaert R. Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol. 2002;12:467–471. doi: 10.1016/s0960-9822(02)00712-1. [DOI] [PubMed] [Google Scholar]
- 75.Janssens S, Burns K, Vercammen E, Tschopp J, Beyaert R. MyD88S, a splice variant of MyD88, differentially modulates NF-κB- and AP-1-dependent gene expression. FEBS Lett. 2003;548:103–107. doi: 10.1016/s0014-5793(03)00747-6. [DOI] [PubMed] [Google Scholar]
- 76.Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 77.Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7:1074–1081. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 78.Ahmed S, Maratha A, Butt AQ, Shevlin E, Miggin SM. TRIF-mediated TLR3 and TLR4 signaling is negatively regulated by ADAM15. J Immunol. 2013;190:2217–2228. doi: 10.4049/jimmunol.1201630. [DOI] [PubMed] [Google Scholar]
- 79.Yang Y, Liao B, Wang S, Yan B, Jin Y, Shu HB, et al. E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation. Proc Natl Acad Sci USA. 2013;110:5115–5120. doi: 10.1073/pnas.1220271110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue Q, Zhou Z, Lei X, Liu X, He B, Wang J, et al. TRIM38 negatively regulates TLR3-mediated IFN-β signaling by targeting TRIF for degradation. PLoS One. 2012;7:e46825. doi: 10.1371/journal.pone.0046825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang J, Kunkel SL, Chang CH. Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc Natl Acad Sci USA. 2009;106:18327–18332. doi: 10.1073/pnas.0905815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsumura T, Hayashi H, Takii T, Thorn CF, Whitehead AS, Inoue J, et al. TGF-beta down-regulates IL-1alpha-induced TLR2 expression in murine hepatocytes. J Leukoc Biol. 2004;75:1056–1061. doi: 10.1189/jlb.0104108. [DOI] [PubMed] [Google Scholar]
- 84.Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, et al. Inhibition of TLR-4/MD-2 signaling by RP105/MD-1. J Endotoxin Res. 2005;11:363–368. doi: 10.1179/096805105X67300. [DOI] [PubMed] [Google Scholar]
- 85.Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, et al. Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol. 2005;6:571–578. doi: 10.1038/ni1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin. 2012;33:342–350. doi: 10.1038/aps.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Magalhaes AC, Dunn H, Ferguson SSG. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol. 2012;165:1717–1736. doi: 10.1111/j.1476-5381.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferguson SSG. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 89.Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84:489–539. doi: 10.1152/physrev.00030.2003. [DOI] [PubMed] [Google Scholar]
- 90.Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HCA, et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol. 2013;15:249–260. doi: 10.1038/ncb2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takayama Y, May P, Anderson RGW, Herz J. Low density lipoprotein receptor-related protein 1 (LRP1) controls endocytosis and c-CBL-mediated ubiquitination of the platelet-derived growth factor receptor β (PDGFRβ) J Biol Chem. 2005;280:18504–18510. doi: 10.1074/jbc.M410265200. [DOI] [PubMed] [Google Scholar]
- 92.Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:1001–1006. doi: 10.1161/ATVBAHA.110.213850. [DOI] [PubMed] [Google Scholar]
- 93.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 94.Serhan CN. The resolution of inflammation: the devil in the flask and in the details. FASEB J. 2011;25:1441–1448. doi: 10.1096/fj.11-0502ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circ Res. 2010;107:1170–1184. doi: 10.1161/CIRCRESAHA.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fredman G, Li Y, Dalli J, Chiang N, Serhan CN. Self-limited versus delayed resolution of acute inflammation: temporal regulation of pro-resolving mediators and microRNA. Sci Rep. 2012;2:639. doi: 10.1038/srep00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 98.Kobayashi Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J Leukoc Biol. 2010;88:1157–1162. doi: 10.1189/jlb.0310149. [DOI] [PubMed] [Google Scholar]
- 99.Hiroshima Y, Hsu K, Tedla N, Chung YM, Chow S, Herbert C, Geczy CL. S100A8 induces IL-10 and protects against acute lung injury. J Immunol. 2014;192:2800–2811. doi: 10.4049/jimmunol.1302556. [DOI] [PubMed] [Google Scholar]