ABSTRACT
Thyroid carcinoma is the most widespread malignancy in endocrine system with the increasing incidence. Despite of the advanced approaches to the management of thyroid carcinoma, the therapeutic effects remain unpleasant largely due to the radiosensitivity of thyroid carcinoma cells. LncRNAs play important part in the tumorigenesis and development, especially in the radiosensitivity of tumor cells. However, their roles in thyroid carcinoma still needed to be explored deeply. The purpose of our research is to inspect the possible biological role and regulation mechanism of LINC00511 desirable for therapies of thyroid carcinoma patients. In the present study, LINC00511 was significantly overexpressed in thyroid carcinoma and its silencing boosted radiosensitivity of thyroid carcinoma cells. Then we unveiled that LINC00511 regulated JAK2/STAT3 signaling pathway which was resistant to radiation treatment. Besides, TAF1 modulated JAK2 at transcriptional level. Moreover, LINC00511 bound to TAF1 and further promoted JAK2 expression. In conclusion, rescue experiments verified that the radiosensitivity of thyroid carcinoma cells was attributed to LINC00511/TAF1/JAK2/STAT3 axis. The current paper investigated the underlying mechanism of LINC00511 and set a new therapeutic direction for the therapy of thyroid carcinoma.
KEYWORDS: Thyroid carcinoma, radiosensitivity, LINC00511, TAF1, JAK2/STAT3 signaling pathway
Introduction
Thyroid carcinoma has become the most common endocrine malignancy, and its incidence rate is increasing worldwide,1 especially among women. The conventional strategies containing thyroidectomy, radioiodine ablation, and adjuvant long-term thyrotropin suppression therapy have already displayed favorable outcomes in a variety of thyroid carcinoma cases. However, due to limited therapy options and the radioresistance of thyroid carcinoma, the prognostic analysis of patients with thyroid carcinoma is not very satisfactory. Therefore, the comprehension of the molecular mechanisms in thyroid carcinoma is an extremely urgent and necessary task.
Long noncoding RNAs (lncRNAs) are of >200 nt in length and are located in both the nucleus and cytoplasm.2 They are involved in the development of various tumors. Moreover, the majority of findings have indicated that lncRNAs are related to cancer radioresistance,3 including thyroid carcinoma.4 For example, downregulation of lncRNA TUG1 enhances radiosensitivity of bladder cancer cells via suppressing HMGB1 expression.5 LncRNA NEAT1 enhances the radioresistance of cervical cancer via miR-193b-3p/CCND1 axis.6 High expression of lncRNA AFAP1-AS1 predicts chemoradioresistance and poor prognosis of patients with esophageal squamous cell carcinoma.7
LncRNA long intergenic non-protein-coding RNA 511 (LINC00511) is an oncogene that is highly expressed in multiple tumors, including bladder cancer,8 pancreatic ductal adenocarcinoma,9 breast cancer,10 tongue squamous cell carcinoma11 and non-small-cell lung cancer,12 and that also affects tumor size, metastasis, stemness and poor prognosis to a large proportion. Although emerging evidence has illustrated the pivotal role of LINC00511 in human cancer tumorigenesis and progression, its exploration in thyroid carcinoma remains unclear, not to mention radioresistance.
The general transcription factor IID (TFIID) is the first component of the preinitiation complex (PIC) binding with the core promoter of RNA polymerase II transcribed genes.13 And TATA-box binding protein-associated factor 1 (TAF1) is the main component of TFIID and ubiquitous in thyroid. TAF1 has been previously defined as potential markers in gastric cancer,14 hepatocellular carcinoma15 and triple-negative breast cancer,16 but the existence of TAF1 in thyroid carcinoma is untold.
The Janus kinase (JAK)2/signal transducer and activator of transcription (STAT)3 signaling pathway is constitutively stimulated at high level, and regulates the expression of various genes as well as physiological functions in tumors.17–19 Despite multiple reports have claimed the radioresistant effect of signaling pathways on tumors,20,21 the function of JAK3/STAT3 signaling pathway in the radioresistance of carcinomas is still controversial.
In this study, we investigated the expression pattern of LINC00511 in thyroid carcinoma and also its impact on the radiosensitivity of thyroid carcinoma cells.
Materials and methods
Cell lines and cell culture
The human thyroid cancer cell lines (TPC-1, BCPAP and IHH-4) and normal thyroid epithelial cell (Nthy-ori 3–1) were purchased from the Cell Bank, Chinese Academy of Sciences (Shanghai, China). BCPAP and Nthy-ori 3–1 were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium mixed with 10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) while other cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Life Technologies, CA) containing 10% FBS. All cells were cultured in a humidified incubator at 37°C containing 5% CO2.
Cell transfection
For cell transfection, TAF1 and JAK2 were all subcloned into the pcDNA3.1 plasmids (pcDNA3.1-TAF1 and pcDNA3.1-JAK2) to upregulate TAF1 and JAK2, respectively. And the shRNAs targeting LINC00511 (sh-LINC00511#1/2) and the shRNA targeting TAF1 (sh-TAF1) were separately constructed to downregulate LINC00511 and TAF1. All plasmids obtained from Invitrogen were transfected into TPC-1 and BCPAP cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) conforming to the guide books of the providers.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cells using TRIzol (Life Technologies, Carlsbad, USA) and cDNA was synthesized with the Prime-Script miRNA cDNA Synthesis Kit (TaKaRa, Tokyo, Japan) in accordance with the protocols of the manufacturers. qPCR analysis was performed on a ABI PRISM 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) with the SYBR® Premix Ex TaqTM reagent (TaKaRa). The 2−ΔΔCt method was adopted to quantify the data. GADPH was used as an internal control. The primer sequences were as follows: LINC00511, forward, 5′-CGCAAGGACCCTCTGTTAGG-3′ and reverse, 5′-GAAGGCGGATCGTCTCTCAG-3′; TAF1, forward, 5′-AGAGTCGGGAGAGCTTTCTG-3′ and reverse, 5′-CACAATCTCCTGGGCAGTCT-3′; JAK2, forward, 5′-CTCTTTGTCACAACCTCTTTGCC-3′ and reverse, 5′- TTGGAGCATACCAGAGCTTGG-3′; GAPDH, forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse, 5′-GGCTGTTGTCATACTTCTCATGG-3′.
Cell counting kit-8 (CCK-8) assay
The transfected cells (3 × 103 cells/well) were inoculated into 96-well plates and cultured for 24 h at 37℃ in a humid incubator with 5% CO2. The proliferation ability of the cells was detected with the Cell Counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan) after incubation for 24, 48, 72 and 96 h. After the irradiation of 4Gy, the absorbance was measured at the wavelength of 450 nm using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Colony formation assay
TPC-1 and BCPAP cells at a density of 3 × 103 cells per well were seeded into 6-well plates (corning, USA.) and maintained for 24 h. The cells were irradiated respectively with 0, 2, 4, 6 and 8Gy doses of X-ray. Cells were then fixed and stained with 4% paraformaldehyde and 0.1% Crystal Violet (Sigma, USA.) solution. The number of colonies more than 50 cells were counted under a multifunctional microplate reader (Thermo, Waltham, MA, USA).
Flow cytometry
After the transfection, TPC-1 and BCPAP cells were collected. Then the cells were treated with or without an X-ray of 4Gy dose. 24 h later, the cells were dyed with propidium iodide (PI) referring to the instructions of the manufacturers. The reaction was analyzed using the Cell Quest software (Becton Dickinson, USA.) and the PI apoptosis detection kit was used to measure the rate of cell apoptosis.
Luciferase reporter assay
In order to verify the binding between JAK2 and TAF1, we generated mutants of JAK2 (Mut-JAK2) to be co-transfected with sh-NC or sh-TAF1, in comparison with the wild type JAK2 (WT-JAK2). To verify the binding between LINC00511 and TAF1, we generated mutants of LINC00511 (Mut-LINC00511) to be co-transfected with sh-NC or sh-TAF1, in comparison with the wild-type LINC00511 (WT-LINC00511). Then, the constructed luciferase reporter vectors were transfected into cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the instructions of the manufacturers. After 48 h, the cells were collected, and luciferase activity levels were measured with a dual-luciferase reporter assay system (Promega, Madison, WI) and normalized to the luciferase activity of Renilla.
Chromatin immunoprecipitation (chip)
The EZ-ChIP™ Chromatin immunoprecipitation kit (Millipore, USA) was adopted to perform the ChIP assay. Cells (1 × 107 cells/well) were incubated using anti-IgG (Beyotime) and anti-TAF1 (Abcam) antibody. Then crosslinking reversal was performed and the associated DNA fragments are purified. The retrieved DNA was detected by qRT-PCR as above described to measure the enrichment of JAK2 or LINC00511 promoter.
Western blot
Cells were lysed in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)) supplemented with protease inhibitors (Roche, China). The protein extracts were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gels and then electrophoretically transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). The membranes were blocked with 5% skim milk dissolved in TBST (Tris-buffered saline (TBS), 0.1% Tween). Then, the membranes were incubated with primary antibodies, followed by culture with the secondary antibody. Finally, an enhanced chemiluminescence system (Beyotime Institute of Biotechnology) was adopted to visualize protein bands. The primary antibodies were: anti-PARP and anti-GAPDH (Abcam, Cambridge, UK); JAK1, p-JAK1, J AK2, p-JAK2, and STAT3, p-STAT3, STAT5, p-STAT5 (all from Cell Signaling Technology, Danvers, MA, USA). GADPH was used as a loading control.
Statistical analysis
The SPSS software package (20.0 version, Chicago, IL, USA) was adopted to analyze the result. All data were presented as the mean ± standard deviation (SD). The differences among groups were estimated by one-way analysis of variance (ANOVA) and the differences between two groups were estimated by Student’s t-test. It was considered statistically significant when P value is less than 0.05. Each assay was performed in triplicates.
Results
LINC00511 is upregulated in thyroid carcinoma and its silencing promotes radiosensitivity of thyroid carcinoma cells
To explore whether LINC00511 was involved in thyroid carcinoma, we tested its expression level in human thyroid carcinoma cell lines (TPC‐1, BCPAP and IHH‐4) and the normal thyroid epithelial cell line (Nthy‐ori 3‐1). qRT-PCR assay illustrated that LINC00511 expression was significantly higher in thyroid carcinoma cell lines than that in normal cell line (Figure 1A). As TPC‐1 and BCPAP cells showed relatively high levels of LINC00511, we chose them for subsequent experiments. As shown in Figure 1B, the expression level of LINC00511 was also upregulated under 4Gy irradiation, as measured by qRT-PCR assay. Based on these results above, we further investigated the function role of LINC00511 in thyroid carcinoma by lost-of-function experiments. The transfection efficiency of sh-LINC00511#1 or LINC00511#2 in TPC‐1 and BCPAP cells was determined through qRT-PCR assay (Figure 1C). CCK-8 assay revealed that LINC00511 knockdown significantly inhibited cell proliferation of TPC‐1 and BCPAP cells. Moreover, 4Gy irradiation also repressed cell proliferation in TPC‐1 and BCPAP cells, which was strengthened by the silencing of LINC00511 (Figure 1D). Colony formation assay demonstrated that the increasing irradiation caused an obvious decrease of survival fraction in TPC‐1 and BCPAP cells, which was enhanced through LIN00511 downregulation (Figure 1E). In flow cytometry analysis of cell apoptosis, cell apoptosis rates of TPC‐1 and BCPAP cells were improved by sh-LINC00511#1, and when cells were given 4Gy irradiation the apoptosis rate was distinctly increased (Figure 1F). Furthermore, western blot showed that PARP levels were dramatically reduced in TPC‐1 and BCPAP cells with LINC00511 downregulation, and after 4Gy irradiation, the reduction was strikingly apparent (Figure 1G). All these data elucidate that LINC00511 is overexpressed in thyroid carcinoma and its silencing improves the radiosensitivity of thyroid carcinoma cells.
Figure 1.
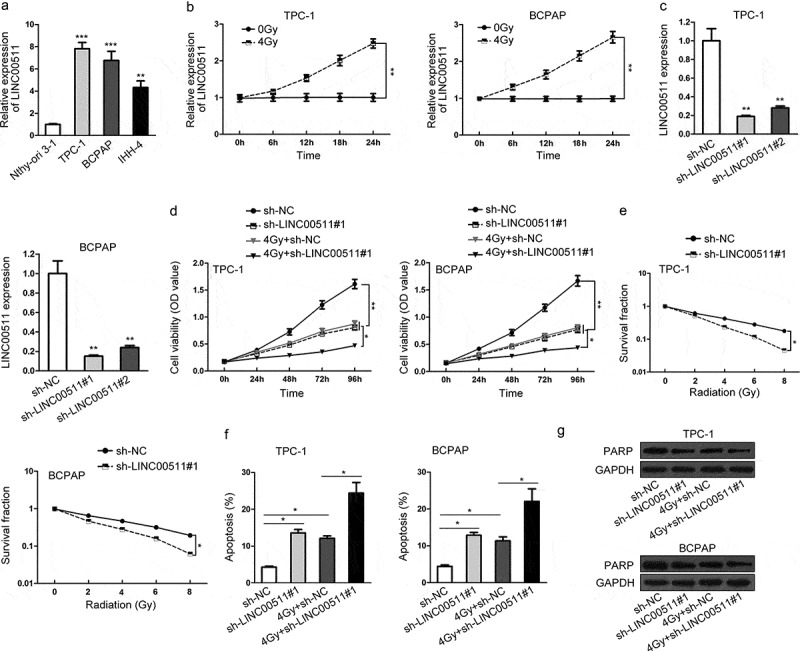
LINC00511 is upregulated in thyroid carcinoma and its silencing promotes radiosensitivity of thyroid carcinoma cells. (A) The expression level of LINC00511 in thyroid carcinoma cell lines (TPC-1, BCPAP and IHH-4) and normal thyroid epithelial cell line (Nthy-ori 3–1) was measured by qRT-PCR. (B) qRT-PCR analysis of LINC00511 expression in TPC-1 and BCPAP cells under 4Gy irradiation. (C) qRT-PCR was used again to examine the expression level of LINC00511 in TPC-1 and BCPAP cells treated with sh-NC, sh-LINC00511#1 or LINC00511#2. (D) Cell proliferation of TPC-1 and BCPAP cells with the silencing of LINC00511 under 0 or 4Gy irradiation was, respectively, tested by CCK-8 assay. (E) Colony formation result of sh-LINC00511#1-transfected TPC-1 and BCPAP cells at the indicated irradiation of 0, 2, 4, 6 or 8Gy. (F) Under 0 or 4Gy irradiation, cell apoptosis rates of TPC-1 and BCPAP cells with the treatment of sh-LINC00511#1 were individually assessed by flow cytometry. (G) Western blot analysis of PARP levels in sh-LINC00511#1-treated TPC-1 and BCPAP cells with 0 or 4Gy radiation. *P < .05, **P < .01.
LINC00511 regulates JAK2/STAT3 signaling pathway
LncRNAs have been reported to regulate gene expression in various cancers.22 JAK2, as an upstream of JAK2/STAT3 signaling pathway, was selected for its cellular function in tumors in accordance with the role of LINC00511. Besides, previous studies have showed that proteins involved in signaling pathways can affect the radioresistance of tumors.23 So we supposed that LINC00511 impacted the radioresistance of thyroid carcinoma via modulating JAK2/STAT3 signaling pathway. To verify the hypothesis, we firstly measured JAK2 expression in thyroid carcinoma cell lines and normal thyroid epithelial cell line. JAK2 expression was dramatically high in thyroid carcinoma cell lines as estimated by qRT-PCR (Figure 2A). The expression level of JAK2 was relatively downregulated in sh-LINC00511#1-treated TPC‐1 and BCPAP cells (Figure 2B). Western blotting indicated that the levels of JAK2, p-JAK2 and p-STAT3 were markedly depleted after sh-LINC00511#1 was transfected into TPC‐1 and BCPAP cells, except for STAT3 levels (Figure 2C). Similarly, the protein levels of JAK1, p-JAK1 and p-STAT5 were markedly depleted by sh-LINC00511#1, except for STAT5 protein levels (Figure 2D). Besides, we examined the expression of JAK2 in TPC‐1 and BCPAP cells under 4Gy irradiation and found the increase in JAK2 expression, hinting the possible radioresistant role of JAK2 (Figure 2E). All the results suggested that LINC00511 regulates JAK2/STAT3 signaling pathway in response to the radiosensitivity of thyroid carcinoma cells.
Figure 2.
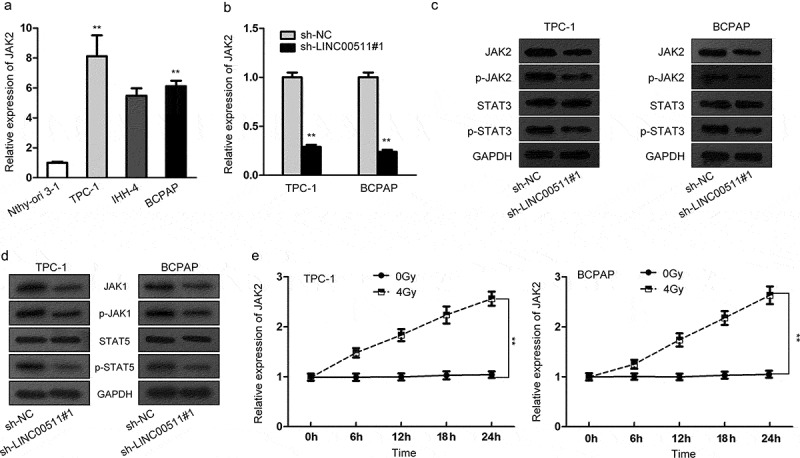
LINC00511 regulates JAK2/STAT3 signaling pathway. (A) JAK2 expression in thyroid carcinoma cells and normal thyroid epithelial cells was measured by qRT-PCR. (B) qRT-PCR assay was performed to evaluate JAK2 expression when LINC00511 was silenced. (C) The protein levels of genes participated in JAK2/STAT3 signaling pathway (JAK2, p-JAK2, STAT3 and p-STAT3) in TPC-1 and BCPAP cells with LINC00511 downregulation were detected by western blotting. (D) Besides, the protein levels of JAK1, p-JAK1, STAT5 and p-STAT5 by sh-LINC00511#1 were analyzed through western blotting. (E) JAK2 expression in TPC-1 and BCPAP cells under 0 or 4Gy irradiation was examined by qRT-PCR assay. **P < .01.
TAF1 modulates JAK2 at transcriptional level
The regulation of genes was attributed to several factors, among which transcription factors are universally known and researched.24,25 From UCSC gene browser, we found TAF1, the transcription factor of JAK2 (Figure 3A). qRT-PCR assay exhibited that TAF1 expression was overtly downregulated in sh-TAF1-transfected TPC‐1 and BCPAP cells (Figure 3B). Luciferase reporter assay proved that TAF1 knockdown significantly reduced the luciferase activity of WT-JAK2 while had no influence on that of Mut-JAK2 in H293T cells (Figure 3C). ChIP assay confirmed the enrichment of JAK2 promoter in the complex immunoprecipitated by anti-TAF1 (Figure 3D). qRT-PCR assay exhibited that JAK2 expression was dramatically silenced in TPC‐1 and BCPAP cells under sh-TAF1 treatment, confirming the transcriptional regulation of TAF1 on JAK2 (Figure 3E). In brief, TAF1 modulates JAK2 at transcriptional level.
Figure 3.
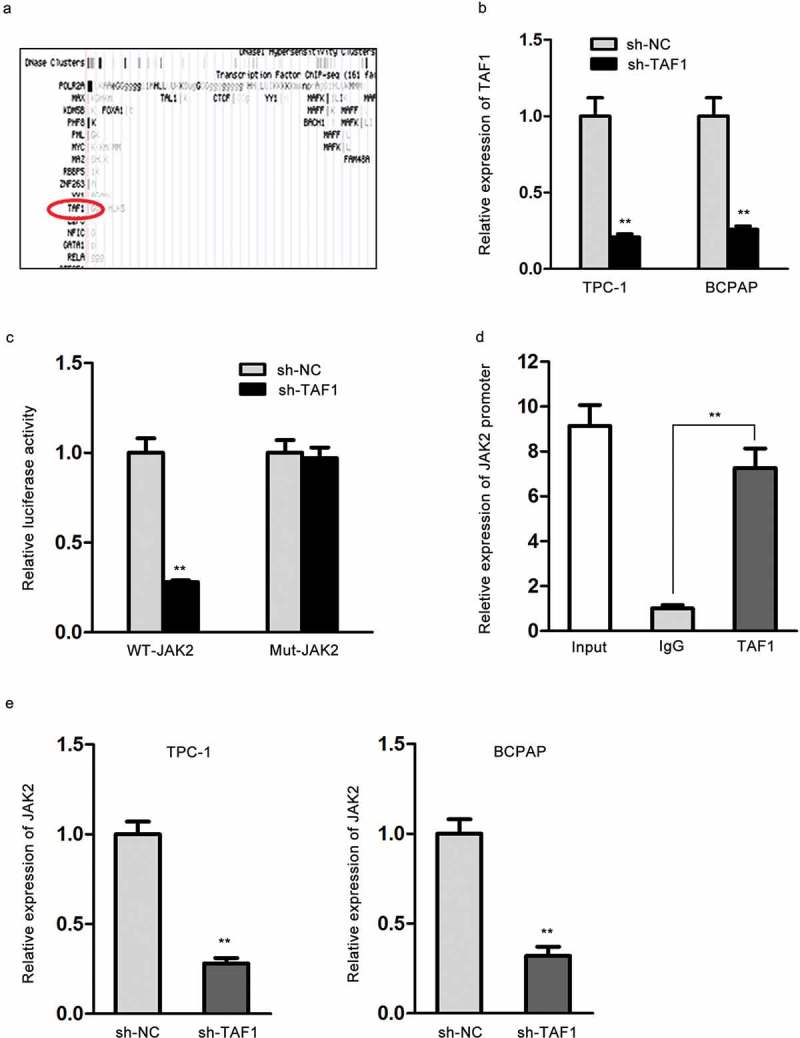
TAF1 modulates JAK2 at transcriptional level. (A) The predicted binding of TAF1 to JAK2 from UCSC gene browser. (B) TAF1 expression in sh-TAF1-transfected TPC-1 and BCPAP cells was measured by qRT-PCR. (C) The combination of TAF1 with JAK2 as estimated by luciferase reporter assay. (D) The interaction between TAF1 and JAK2 promoter was confirmed by ChIP assay. (E) The expression level of JAK2 in TPC-1 and BCPAP cells under sh-TAF1 transfection was assessed through qRT-PCR. *P < .05, **P < .01, ***P < .001.
LINC00511 binds to TAF1 and further regulates the expression level of JAK2
Previous findings have reported that lncRNAs can enhance the regulation of transcription factors on target mRNAs.26 We boldly assumed that LINC00511 might interact with TAF1 so as to promote its transcriptional regulation on JAK2. As presented in Figure 4A, we discovered the predicted combination of TAF1 with LINC00511 from UCSC gene browser. Luciferase reporter assay founded that TAF1 knockdown inhibited the luciferase activity of wild-type LINC00511, but had no effect on that of mutant LINC00511 in H293T cells (Figure 4B). In ChIP assay, LINC00511 promoter was enriched in the mixture immunoprecipitated by anti-TAF1 (Figure 4C). After affirming the interaction between LINC00511 and TAF1, we further probed the relationship among LINC00511, TAF1 and JAK2. In qRT-PCR assay, TAF1 expression had no significant alteration in either sh-LINC00511#1 group or sh-NC group (Figure 4D). The expression level of TAF1 was relatively high in TPC‐1 and BCPAP cells transfected with pcDNA3.1-TAF1 (Figure 4E). Finally, qRT-PCR assay suggested that LINC00511 downregulation silenced JAK2 expression, which was restored by the upregulation of TAF1 in TPC‐1 and BCPAP cells. To sum up, LINC00511 enhances the modulation of TAF1 on JKA2.
Figure 4.
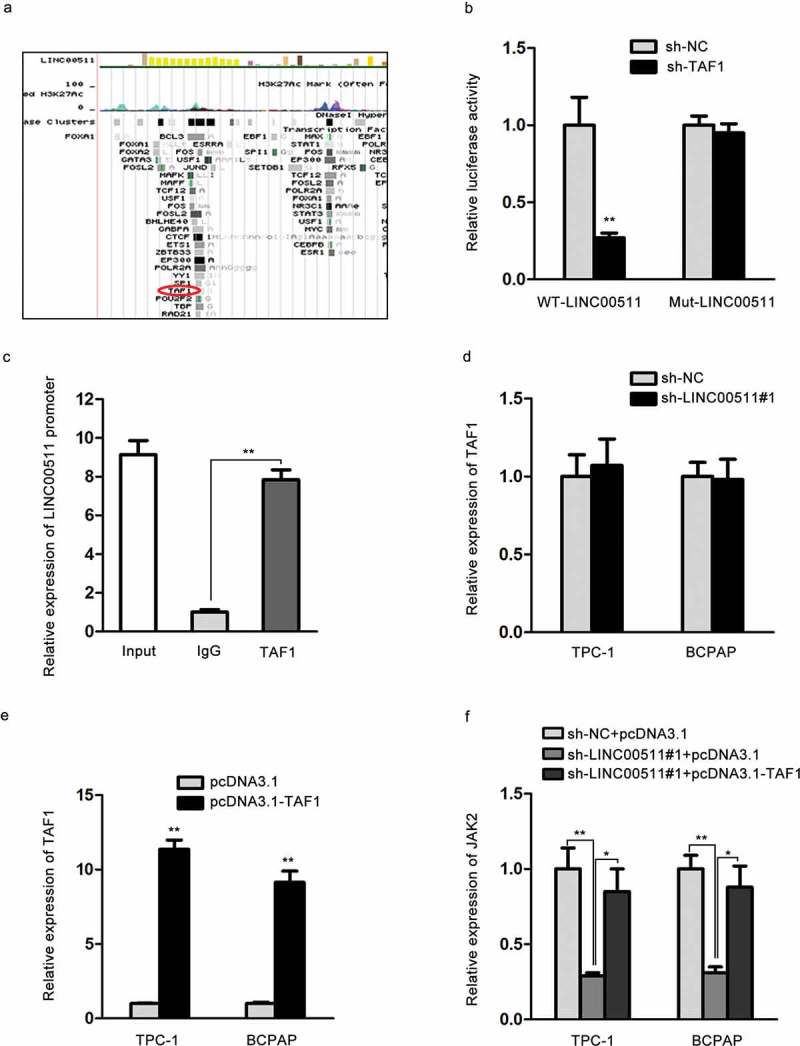
LINC00511 binds to TAF1 and further regulates the expression level of JAK2. (A) The putative combination of TAF1 and LINC00511 according to UCSC gene browser. (B-C) Luciferase reporter and ChIP assays were conducted to test the binding of TAF1 to LINC00511. (D) qRT-PCR result of TAF1 levels in sh-LINC00511#1-treated TPC-1 and BCPAP cells. (E) The transfection efficacy of pcDNA3.1-TAF1 was evaluated by qRT-PCR. (F) qRT-PCR detection of JAK2 expression in TPC-1 and BCPAP cells with the co-transfection of sh-NC and pcDNA3.1, sh-LINC00511#1 and pcDNA3.1 or sh-LINC00511#1 and pcDNA3.1-TAF1. *P < .05, **P < .01.
The radiosensitivity of thyroid carcinoma cells is attributed to LINC00511/TAF1/JAK2 axis
For the purpose of inspecting whether LINC00511 influenced the radioresistance of thyroid carcinoma through regulating JAK2/STAT3 signaling pathway, we first performed qRT-PCR assay to test the transfection efficiency of pcDNA3.1-JAK2, and then rescue experiments. JAK2 levels were evidently high in pcDNA3.1-JAK2-treated TPC‐1 and BCPAP cells, compared to control groups (Figure 5A). CCK-8 experiment disclosed that under 4Gy irradiation, the inhibitory effect of LINC00511 knockdown on cell proliferation of TPC‐1 and BCPAP cells was rescued by JAK2 overexpression (Figure 5B). Colony formation assay exposed that with the increase of irradiation, cell proliferation capacities of TPC‐1 and BCPAP cells were suppressed, which was further restrained by LINC00511 silence. Similarly, this effect was abolished via the upregulation of JAK2 (Figure 5C). In flow cytometry analysis of cell apoptosis, after the treatment of 4Gy irradiation, apoptotic cells was dramatically increased when LINC00511 was downregulated, but relatively decreased when JAK2 was upregulated in TPC‐1 and BCPAP cells (Figure 5D). In the end, western blot uncovered that PARP levels were lessened via LINC00511 knockdown, which was augmented by the overexpression of JAK2 (Figure 5E). Taken together, LINC00511 modulates the radiosensitivity of thyroid carcinoma cells via enhancing the transcriptional regulation of TAF1 on JAK2.
Figure 5.
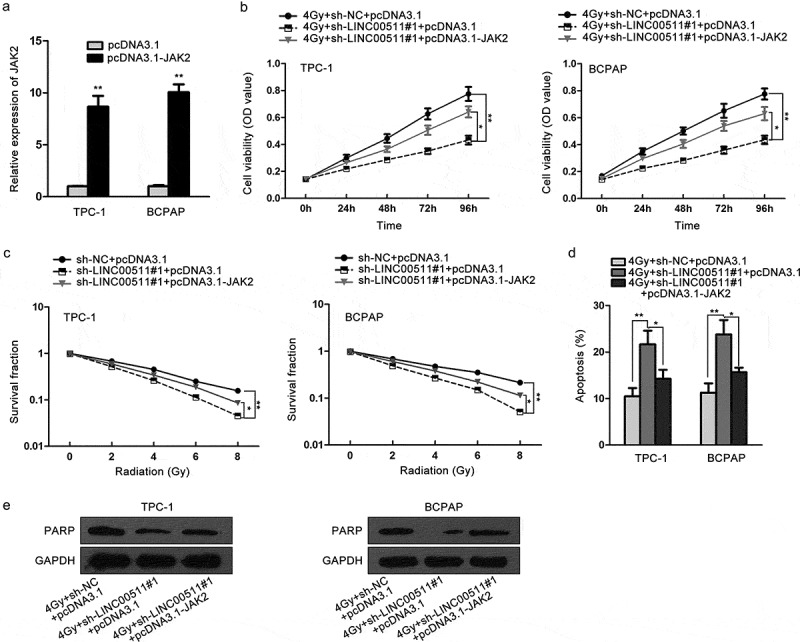
The radiosensitivity of thyroid carcinoma cells is attributed to LINC00511/TAF1/JAK2 axis. TPC-1 and BCPAP cells were co-transfected with sh-NC and pcDNA3.1, sh-LINC00511#1 and pcDNA3.1 or sh-LINC00511#1 and pcDNA3.1-JAK2. (A) The transfection efficiency of pcDNA3.1-JAK2 was examined by qRT-PCR. (B) CCK-8 analysis of cell proliferation capacities of TPC-1 and BCPAP cells after 4Gy irradiation. (C) Colony formation detection of the survival fraction of TPC-1 and BCPAP cells. (D) Cell apoptotic rates of TPC-1 and BCPAP cells as estimated by flow cytometry. (E) Western blot was implemented to detect the levels of PARP in TPC-1 and BCPAP cells. *P < .05, **P < .01.
Discussion
Thyroid carcinoma is a common endocrine cancer, with a rapidly increasing rate.27 Despite the progress we made in novel surgical and chemotherapeutic approaches to treating thyroid carcinoma, the diagnosis and therapy remain controversial.28 Hence, the inquiry of more novel treatments is needed.
In previous findings, aberrant lncRNAs have been reported to exert stimulative or suppressive functions in multiple carcinomas.29–31 Long intergenic non-protein-coding RNA 511 (LINC00511) was an oncogene in various cancers, involving bladder cancer,8 pancreatic ductal adenocarcinoma,9 breast cancer,10 tongue squamous cell carcinoma11 and non-small-cell lung cancer.12 In this work, it was the first time that LINC00511 was explored in thyroid carcinoma. Our study revealed that LINC00511 expression was higher in thyroid carcinoma cells (TPC‐1, BCPAP and IHH‐4) than that in normal thyroid epithelial cells (Nthy‐ori 3‐1). Accumulating evidence has demonstrated that LINC00511 can facilitate cellular activities of carcinomas,8,10 such as cell proliferation, migration and invasion. However, its role in the radiosensitivity of tumor cells was never researched. In the present study, we disclosed that under the indicated doses of irradiation, LINC00511 knockdown significantly inhibited cell proliferation and stimulated apoptosis of thyroid carcinoma.
Furthermore, it has been elucidated that lncRNAs modulate the radioresistance of tumors through modulating gene expression,32 and signaling pathways against radiotherapy in tumors.21,33,34 Thereupon, we attempted to explore the association between LINC00511 and JAK2/STAT3 signaling pathway. Our paper displayed that LINC00511 regulated JAK2/STAT3 signaling pathway and JAK2 expression was upregulated when given 4Gy irradiation in thyroid carcinoma, where the association of JAK2/STAT3 signaling pathway with radioresistance was probed for the first time.
Subsequently, we investigated the underlying mechanism of LINC00511 in thyroid carcinoma. Transcription factors are known to modulate its target genes at transcriptional level.24,25 Consistently, we uncovered that TAF1 transcriptionally regulated JAK2. LncRNAs have been explained to interact with transcription factors and then further affect its regulation on target genes,26 thereafter we investigated the relationship among LINC00511, TAF1 and JAK2. The current research drew a conclusion that LINC00511 modulate JAK2 through combining with TAF1, but not affecting TAF1 expression in mRNA level. Finally, rescue experiments verified that LINC00511 regulated the radioresistance of thyroid carcinoma via JAK2/STAT3 signaling pathway.
In summary, overexpression of LINC00511 improved the radiosenitivity of thyroid carcinoma cells through TAF1-mediated JAK2/STAT3 signaling pathway. This current work exposed the potential biomarker role of LINC00511 in the treatment of thyroid carcinoma. Whereas, there are some limits in this study as through western blotting analysis, the repressive impact of LINC00511 silencing on JAK1/STAT5 pathway, another downstream pathway under JAK family, was uncovered. There are some possibilities for the correlation between LINC00511 and JAK pathways in the future research.
Funding Statement
This study was supported by the Science Technology Department of Zhejiang Province of China [Grant number 2013C33171] and a program of Wenzhou Science and Technology Bureau [Grant number Y20130235].
Acknowledgments
The authors thank all participants for supporting materials.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ulitsky I, Bartel DP.. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li G, Liu Y, Liu C, Su Z, Ren S, Wang Y, Deng T, Huang D, Tian Y, Qiu Y. Genome-wide analyses of long noncoding RNA expression profiles correlated with radioresistance in nasopharyngeal carcinoma via next-generation deep sequencing. BMC Cancer. 2016;16:719. doi: 10.1186/s12885-016-2755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Cao S, Li W, Wei D, Wang Z, Li G, Pan X, Lei D. Time-course differential lncRNA and mRNA expressions in radioresistant hypopharyngeal cancer cells. Oncotarget. 2017;8:40994–41010. doi: 10.18632/oncotarget.17343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang H, Hu X, Zhang H, Li W. Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression. Radiat Oncol. 2017;12:65. doi: 10.1186/s13014-017-0802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han D, Wang J, Cheng G. LncRNA NEAT1 enhances the radio-resistance of cervical cancer via miR-193b-3p/CCND1 axis. Oncotarget. 2017;9:2395–2409. doi: 10.18632/oncotarget.23416. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Zhou X-L, Wang -W-W, Zhu W-G, Yu C-H, Tao G-Z, Wu -Q-Q, Song Y-Q, Pan P, Tong Y-S. High expression of long non-coding RNA AFAP1-AS1 predicts chemoradioresistance and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Mol Carcinog. 2016;55:2095–2105. doi: 10.1002/mc.22454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Li Y, Meng F, Fu L, Kong C. Knockdown of long non-coding RNA linc00511 suppresses proliferation and promotes apoptosis of bladder cancer cells via suppressing Wnt/β-catenin signaling pathway. Biosci Rep. 2018;38:undefined. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X, Liu Y, Li Z, Zheng S, Wang Z, Li W, Bi Z, Li L, Jiang Y, Luo Y, et al. Linc00511 acts as a competing endogenous RNA to regulate VEGFA expression through sponging hsa-miR-29b-3p in pancreatic ductal adenocarcinoma. J Cell Mol Med. 2018;22:655–667. doi: 10.1111/jcmm.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu G, Li Y, Ma Y, Lu J, Chen Y, Jiang Q, Qin Q, Zhao L, Huang Q, Luo Z, et al. Long noncoding RNA LINC00511 contributes to breast cancer tumourigenesis and stemness by inducing the miR-185-3p/E2F1/Nanog axis. J Exp Clin Cancer Res. 2018;37:289. doi: 10.1186/s13046-018-0945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding J, Yang C, Yang S. LINC00511 interacts with miR-765 and modulates tongue squamous cell carcinoma progression by targeting LAMC2. J Oral Pathol Med. 2018;47:468–476. doi: 10.1111/jop.12677. [DOI] [PubMed] [Google Scholar]
- 12.Sun CC, Li SJ, Li G, Hua RX, Zhou XH, Li DJ. Long Intergenic Noncoding RNA 00511 Acts as an Oncogene in Non-small-cell Lung Cancer by Binding to EZH2 and Suppressing p57. Mol Ther Nucleic Acids. 2016;5:e385. doi: 10.1038/mtna.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curran EC, Wang H, Hinds TR, Zheng N, Wang EH. Zinc knuckle of TAF1 is a DNA binding module critical for TFIID promoter occupancy. Sci Rep. 2018;8:4630. doi: 10.1038/s41598-018-22879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saberi Anvar M, Minuchehr Z, Shahlaei M, Kheitan S. Gastric cancer biomarkers; A systems biology approach. Biochem Biophys Rep. 2018;13:141–146. doi: 10.1016/j.bbrep.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan H, Wang Q, Shen Q, Li Z, Tian J, Jiang Q, Gao L. Identification of potential transcription factors, long noncoding RNAs, and microRNAs associated with hepatocellular carcinoma. J Cancer Res Ther. 2018;14:S622–S7. doi: 10.4103/0973-1482.204846. [DOI] [PubMed] [Google Scholar]
- 16.Brough R, Gulati A, Haider S, Kumar R, Campbell J, Knudsen E, Pettitt SJ, Ryan CJ, Lord CJ. Identification of highly penetrant Rb-related synthetic lethal interactions in triple negative breast cancer. Oncogene. 2018;37:5701–5718. doi: 10.1038/s41388-018-0368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Zhou H, Liu W, Wu J, Yue X, Wang J, Quan L, Liu H, Guo L, Wang Z, et al. Ganoderic acid A exerts antitumor activity against MDA-MB-231 human breast cancer cells by inhibiting the Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway. Oncol Lett. 2018;16:6515–6521. doi: 10.3892/ol.2018.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Qiao B, Fan J. Overexpression of miR-4443 promotes the resistance of non-small cell lung cancer cells to epirubicin by targeting INPP4A and regulating the activation of JAK2/STAT3 pathway. Pharmazie. 2018;73:386–392. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Zhang J, Wang G, Chen X, Zhang R, Liu H, Zhu J. Oxymatrine exhibits anti-tumor activity in gastric cancer through inhibition of IL-21R-mediated JAK2/STAT3 pathway. Int J Immunopathol Pharmacol. 2018;32:2058738418781634. doi: 10.1177/2058738418781634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao S, Liu M, Shen D, Zhang W, Wang T, Bai Y. TGF-β/Smads Signaling Affects Radiation Response and Prolongs Survival by Regulating DNA Repair Genes in Malignant Glioma. DNA Cell Biol. 2018;37:909–916. doi: 10.1089/dna.2018.4310. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Yuan D, Yang Y, Ren M. LincRNA-p21 enhances the sensitivity of radiotherapy for gastric cancer by targeting the β-catenin signaling pathway. J Cell Biochem. 2018;undefined:undefined. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Hang Y, Xu W, Wu J, Chen L, Chen J, Mao Y, Song J, Song J, Wang H. BLACAT1 predicts poor prognosis and serves as oncogenic lncRNA in small-cell lung cancer. J Cell Biochem. 2018;11. doi: 10.1002/jcb.27548 [DOI] [PubMed] [Google Scholar]
- 23.Thurner EM, Krenn-Pilko S, Langsenlehner U, Stojakovic T, Pichler M, Gerger A, Kapp KS, Langsenlehner T. The elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapy. Eur J Cancer. 2015;51:610–619. doi: 10.1016/j.ejca.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Celada LJ, Kropski JA, Herazo-Maya JD, Luo W, Creecy A, Abad AT, Chioma OS, Lee G, Hassell NE, Shaginurova GI, et al. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-β1 production. Sci Transl Med. 2018;10:eaar8356. doi: 10.1126/scitranslmed.aao4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurtado M, Sankpal UT, Kaba A, Mahammad S, Chhabra J, Brown DT, Gurung RK, Holder AA, Vishwanatha JK, Basha R. Novel Survivin Inhibitor for Suppressing Pancreatic Cancer Cells Growth via Downregulating Sp1 and Sp3 Transcription Factors. Cell Physiol Biochem. 2018;51:1894–1907. doi: 10.1159/000495715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo G, Liu D, Huang C, Wang M, Xiao X, Zeng F, Wang L, Jiang G. LncRNA GAS5 Inhibits Cellular Proliferation by Targeting P27. Mol Cancer Res. 2017;15:789–799. doi: 10.1158/1541-7786.MCR-16-0331. [DOI] [PubMed] [Google Scholar]
- 27.Khatami F, Tavangar SM. Liquid Biopsy in Thyroid Cancer: new Insight. Int J Hematol Oncol Stem Cell Res. 2018;12:235–248. [PMC free article] [PubMed] [Google Scholar]
- 28.Caglar O, Cayir A. Total circulating cell-free miRNA in plasma as a predictive biomarker of the thyroid diseases. J Cell Biochem. 2018;undefined:undefined. [DOI] [PubMed] [Google Scholar]
- 29.Mallardo M, Poltronieri P, D’Urso OF. Non-protein coding RNA biomarkers and differential expression in cancers: a review. J Exp Clin Cancer Res. 2008;27:19. doi: 10.1186/1756-9966-27-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponzio G, Rezzonico R, Bourget I, Allan R, Nottet N, Popa A, Magnone V, Rios G, Mari B, Barbry P. A new long noncoding RNA (lncRNA) is induced in cutaneous squamous cell carcinoma and down-regulates several anticancer and cell differentiation genes in mouse. J Biol Chem. 2017;292:12483–12495. doi: 10.1074/jbc.M117.776260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Li Y, Yang Z, Liu K, Wang D. Genome-Wide Microarray Analysis of Long Non-Coding RNAs in Eutopic Secretory Endometrium with Endometriosis. Cell Physiol Biochem. 2015;37:2231–2245. doi: 10.1159/000438579. [DOI] [PubMed] [Google Scholar]
- 32.Chi H-C, Tsai C-Y, Tsai -M-M, Yeh C-T, Lin K-H. Roles of Long Noncoding RNAs in Recurrence and Metastasis of Radiotherapy-Resistant Cancer Stem Cells. Int J Mol Sci. 2017;18:1903. doi: 10.3390/ijms18091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Chen H, Xu X, Ye M, Cao H, Xu L, Hou Y, Tang J, Zhou D, Bai Y, et al. Insulin-like growth factor-1 receptor knockdown enhances radiosensitivity via the HIF-1α pathway and attenuates ATM/H2AX/53BP1 DNA repair activation in human lung squamous carcinoma cells. Oncol Lett. 2018;16:1332–1340. doi: 10.3892/ol.2018.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardama GA, Alonso DF, Gonzalez N, Maggio J, Gomez DE, Rolfo C, Menna PL. Relevance of small GTPase Rac1 pathway in drug and radio-resistance mechanisms: opportunities in cancer therapeutics. Crit Rev Oncol Hematol. 2018;124:29–36. doi: 10.1016/j.critrevonc.2018.01.012. [DOI] [PubMed] [Google Scholar]


