ABSTRACT
Patients with epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer can benefit significantly from EGFR tyrosine-kinase inhibitors (TKIs) treatment, but almost every patient will inevitably develop resistance. The transformation to small cell lung cancer (SCLC) has been described as an EGFR-TKI resistance often associated with aggressive clinical course and poor prognosis. In this study, we report an unexpected favorable response to etoposide and cisplatin (EP) from an EGFR-mutant patient who developed SCLC transformation at disease progression after the administration of erlotinib with a progression-free survivalof 7.7 months. At disease progression (PD) after erlotinib, rebiopsy showed typical SCLC histology accompanied by positive expressions of CD56, TTF-1, CK7, and synaptophysin. Subsequently, he was switched to standard SCLC treatment regimen EP in combination with erlotinib due to the retention of EGFR 19 del and achieved PR four cycles after the treatment. His disease progressed again 7.7 months after the initiation of EP treatment, with an enlargement of both primary and metastatic lesions. Collectively, this case illustrated the transformation from adenocarcinoma to SCLC and the subsequent durable benefit from standard treatment for SCLC.
KEYWORDS: EGFR mutation, non-small-cell lung cancer, small-cell lung cancer transformation, etoposide and cisplatin, resistance mechanism
Introduction
The epidermal growth factor receptor (EGFR) -tyrosine kinase inhibitors (TKIs) have revolutionized the treatment for EGFR-mutant non-small-cell lung cancer (NSCLC).1–4 Despite initial efficacy, almost all patients will ultimately develop resistance, resulting in median PFS of approximately 12 months.5 Multiple molecular mechanisms underlying acquired resistance to EGFR-TKIs have been identified.6–8 The transformation to small-cell lung cancer (SCLC) has been described as a mechanism, occurring in 3–15% of patients.9–11 Patients developed SCLC transformation often present an aggressive clinical course with poor prognosis with a median overall survival of 6 months since the diagnosis of SCLC transformation.12 Several studies have demonstrated an initial response to etoposide and cisplatin (EP) regiment but often develop resistance within a short time.13,14 Here, we report a 53-year-old male with metastatic EGFR-mutant lung adenocarcinoma (ADC) who developed SCLC transformation as resistance and subsequently benefited from etoposide and cisplatin with a PFS of 7.7 months.
Case report
A 53-year-old Chinese man with a smoking history of 20 years presented to our clinic with bloody sputum, fever, and back pain lasting for 20 days. He was found to have stage IV ADC(T4N3M1b) with multiple bone and bilateral adrenal metastasis. Positron emission tomography-computed tomography revealed fluoro-2-deoxy-d-glucose-positive lesion measuring 3.3 × 5.8 × 5.0 cm in the left lower lobe (Figure 1(a)). He also had bronchial stenosis and occlusion and mediastinal lymphadenopathies. Emission computed tomography and magnetic resonance imaging revealed disseminated bone metastases and small nodules ischemia sites on the bilateral frontal and parietal lobes. Laboratory examinations revealed positive neuron-specific enolase (NSE) at 26.36 ng/mL, carcinoembryonic antigen at 3.99 ng/mL, and cytokeratin 19 fragment antigen 21-1 (CYFRA21-1) at 11.47 ng/mL in the serum (Figure 2). Mutation analysis by amplification refractory mutation system polymerase chain reaction showed EGFR exon 19 deletion. Immunohistochemistry staining showed an overexpression for hepatocyte growth factor receptor and lack of expression for anaplastic lymphoma kinase (ALK) and ROS proto-oncogene 1 receptor tyrosine kinase (ROS1).
Figure 1.
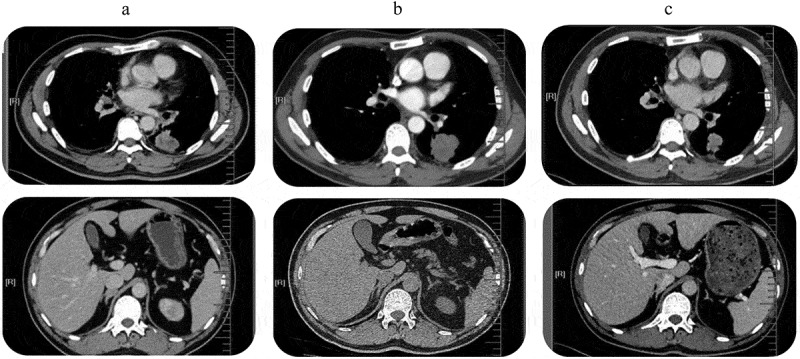
Tumor lesions detected by PET-CT. (a). Before the start of TKI treatment: central lung tumor in the left lower lobe mass infiltrating the hilum and mediastinum and bilateral adrenal metastases; (b). Progression after 6 months of erlotinib: enlarged pleural mass that showed transformation to SCLC. (c) Partial response after four cycles of EP chemotherapy and erlotinib continuation: Dramatic shrinkage of the primary lung tumor.
Figure 2.
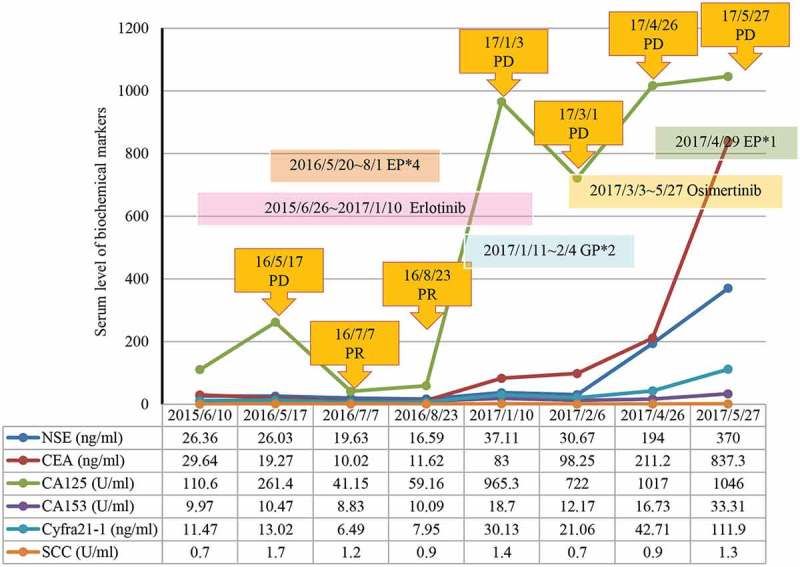
Variation curve of NSE, CEA, CA125, CA153, CYFRA21–1 and SCC. NSE, neuron‑specific enolase; CEA, carcinoembryonic antigen; CA125, carcinoma antigen 125; CA153, carcinoma antigen 153; CYFRA21-1, cytokeratin 19 fragment antigen 21-1; SCC, squamous cell carcinoma antigen.
The patient was administered erlotinib at 150 mg once daily orally and achieved partial response (PR) after 1.5 months based on Response Evaluation Criteria In Solid Tumors 1.1. On 16 May 2016, the patient returned to our clinic due to sever ostalgia. CT scan revealed an enlarged pleural mass measuring 3.8 × 4.0 × 5.0 cm (Figure 1(b)). Re-biopsy of the primary tumor revealed EGFR 19 del accompanied by SCLC transformation evident by positive expressions of CD56, TTF-1, CK7, and synaptophysin (Figure 3). H&E staining revealed typical SCLC histology consisting of nests of small cells (Figure 3). In addition, levels of NSE (26.03 ng/mL) and CYFRA21-1 (13.02 ng/mL) were also elevated (Figure2). He was subsequently switched to standard etoposide (100 mg i.v. on days 1–5) and cisplatin (40 mg i.v. on days 1–3, once every 3 weeks) (EP regime) in combination with erlotinib. He achieved PR after four cycles (Figure 1(c)) and developed disease progression (PD) with an enlargement of primary and metastatic lesions after a PFS of 7.7 months. At PD, capture-based targeted sequencing of plasma sample revealed EGFR 19 del, T790M, RB1, and TP53 mutations, and he was subsequently switched to osimertinib (80 mg, qd) and had a PFS of 1.8 months. After disease progression, he only underwent palliative care.
Figure 3.
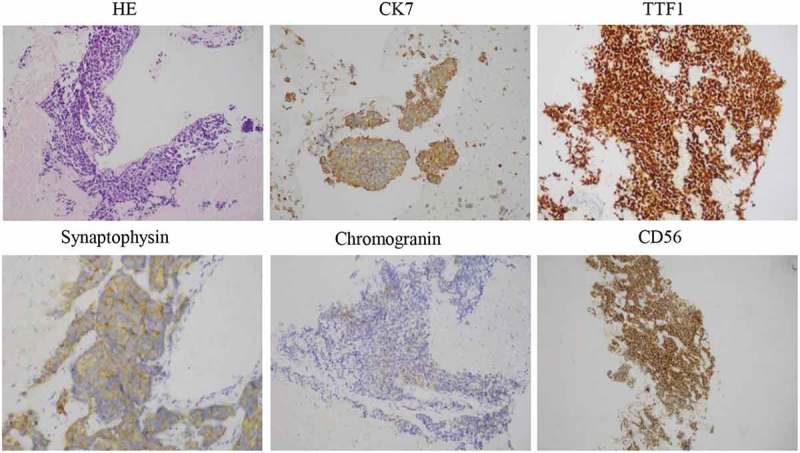
HE and IHC staining was performed on primary tumor biopsies after 6 months of erlotinib treatment. The cells displayed an SCLC phenotype with hyperchromatic nuclei, abundant cytoplasm, and inconspicuous nucleoli. Typical for SCLC, IHC was strongly positive for CD56 and TTF1, and focally for CK7 and synaptophysin (all 400×).
Discussion
Numerous EGFR-TKI resistance mechanisms have been identified. Transformation of EGFR-mutant ADC to SCLC was recently proposed as a mechanism of acquired resistance to EGFR-TKIs.9,10 Numerous studies have documented such cases.15–17 Most patients who developed SCLC transformation are often associated with aggressive clinical behaviors and poor prognosis with a median overall survival (OS) of 6 months. Several studies have reported transient response to EP regiments after the development of SCLC transformation.17,18 Here, we reported an unexpected case of a favorable response to EP of an EGFR-mutant patient who developed SCLC transformation as resistance to erlotinib with a PFS of 7.7 months.
At PD after the exposure to erlotinib, the primary mass showed typical SCLC histology consisting of nests of small cells, which were positive for CD56, TTF-1, and focally positive for CK7 and synaptophysin (Figure 2), suggesting SCLC transformation. Capture-based targeted sequencing revealed concomitant loss-of-function mutations in retinoblastoma protein (RB1) and TP53, a typically molecular feature for SCLC, providing additional evidence for SCLC transformation. The dual mutations were rarely observed in NSCLC patients.10,19–21 The significant increase of NSE level, an important marker for SCLC, also indicated SCLC transformation.22,23 The mechanism underlying SCLC transformation remains elusive. Researchers have proposed two hypotheses: (1) the primary tumor is composed of both SDC and SCLC, and SCLC becomes dominant after the EGFR-TKI treatment. (2) After the exposure to EGFR-TKI, SCLC transdifferentiates from ADC.24,25 Molecular testing revealed that both the original and the transformed tumor at the time of resistance shared the EGFR 19 del, which suggests that SCLC transformation represents an evolution from the initial ADCs rather than a second coincident event.9,11 Historically, ADCs and SCLC share a common precursor with alveolar type II cells and that EGFR mutation could be a factor promoting SCLC transformation under the selective pressure of TKI therapy.10,19
In summary, this case reported an unexpected favorable clinical response to EP with a PFS of 7.7 months of an EGFR-mutant lung ADC patient who underwent SCLC transformation as resistance to EGFR-TKI.
Funding Statement
There was no source of funding for this research.
Acknowledgments
The authors would like to thank our patient for sharing his presentation for this manuscript.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The patient provided written informed consent and gave permission for the use of biopsies and publication of case details.
This study was approved by the Ethical Committee of the First Affiliated Hospital of Guangzhou Medical University.
Availability of data and materials
The data and materials in the current study are not available to any readers since they contain the patient’s personal particulars.
Authors’ contributions
Xinqing Lin, Ming Ouyang, Yinyin Qin, Jiexia Zhang and Chengzhi Zhou were involved in diagnostic flow and patient follow-up. Junjun Liu and Suiyi Mai contributed to the interpretation of published data and were involved in drafting of the manuscript. Yingying Gu is a licensed pathologist. All the authors read and gave their final approval of the version to be published.
References
- 1.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. J Evidence-Based Med. 2011;361:947. [DOI] [PubMed] [Google Scholar]
- 2.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T., Satouchi M., Tada H., Hirashima T., et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (wjtog3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:104–105. doi: 10.1016/S1470-2045(09)70390-0. [DOI] [PubMed] [Google Scholar]
- 3.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated egfr | nejm. N Engl J Med. 2010;362:2380. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Erlotinib versus standard chemotherapy as first-line treatment for european patients with advanced egfr mutation-positive non-small-cell lung cancer (eurtac): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Engelman JA, Jänne PA.. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non–small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S M.D, PhD, Boggon TJ PhD, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B.. Mutation and resistance of non–small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 7.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale C-M, Zhao X, Christensen J, et al. Met amplification leads to gefitinib resistance in lung cancer by activating erbb3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 8.Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–1161. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- 9.Sequist LV, Waltman BA, Diassantagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, et al. Genotypic and histological evolution of lung cancers acquiring resistance to egfr inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16:e165–e172. doi: 10.1016/S1470-2045(14)71180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norkowski E, Ghigna MR, Lacroix L, Le CT, Ã F, Dartevelle P, Dorfmuller P, Thomas de Montpréville V. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol. 2013;8:1265–1271. doi: 10.1097/JTO.0b013e3182a407fa. [DOI] [PubMed] [Google Scholar]
- 12.Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: A systematic review and pooled analysis. Cancer Treat Rev. 2017;59:117–122. doi: 10.1016/j.ctrv.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Hwang KE, Jung JW, Oh SJ, Park MJ, Shon YJ, Choi KH, Jeong E-T, Kim H-R. Transformation to small cell lung cancer as an acquired resistance mechanism in egfr-mutant lung adenocarcinoma: A case report of complete response to etoposide and cisplatin. Tumori. 2015;101:e96–8. doi: 10.5301/tj.5000276. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, Chen B, Qin J, Xie F, Han N, Huang Z. Transformation to small-cell lung cancer following treatment with icotinib in a patient with lung adenocarcinoma. Oncol Lett. 2018;15:5799–5802. doi: 10.3892/ol.2018.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y. Small cell lung cancer transformation from egfr-mutated lung adenocarcinoma: A case report and literatures review. Cancer Biol Ther. 2018;19:445–449. doi: 10.1080/15384047.2018.1435222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa S, Tambo Y, Ninomiya H, Oguri T, Kawashima Y, Takano N, Kitazono S, Ohyanagi F, Horiike A, Yanagitani N, et al. A case treated with nivolumab after small cell lung cancer transformation of mutant egfr non-small cell lung cancer. Annals Oncol. 2016;27(12):2300–2303. doi:10.1093/annonc/mdw431. [DOI] [PubMed] [Google Scholar]
- 17.Santoni-Rugiu E, Grauslund M, Melchior LC, Costa JC, Sørensen JB, Urbanska EM. Heterogeneous resistance mechanisms in an egfr exon 19-mutated non-small cell lung cancer patient treated with erlotinib: persistent fgfr3-mutation, localized transformation to egfr-mutated sclc, and acquired t790m egfr-mutation. Lung Cancer. 2017;113:14–17. doi: 10.1016/j.lungcan.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe S, Sone T, Matsui T, Yamamura K, Tani M, Okazaki A, Kurokawa K, Tambo Y, Takato H, Ohkura N, et al. Transformation to small-cell lung cancer following treatment with egfr tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer. 2013;82:370–372. doi: 10.1016/j.lungcan.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Dorantesheredia R, Ruizmorales JM, Canogarcía F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl Lung Cancer Res. 2016;5:401–412. doi: 10.21037/tlcr.2016.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN, Moran T, et al. Rb loss in resistant egfr mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. 2015;6:6377. doi: 10.1038/ncomms7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JK, Lee J, Kim S, Kim S, Youk J, Park S, An Y, Keam B, Kim D-W, Heo DS, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin Oncol. 2017;35:3065–3074. doi: 10.1200/JCO.2016.71.9096. [DOI] [PubMed] [Google Scholar]
- 22.Isgrò MA, Bottoni P, Scatena R. Neuron-specific enolase as a biomarker: biochemical and clinical aspects. In. Adv Cancer Biomarkers. 2015;867:125–143. doi:10.1007/978-94-017-7215-0_9. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Li XY, Tang Y, Xu Y, Guo WH, Li YC, Liu X-K, Huang C-Y, Wang Y-S, Wei Y-Q. Rapid increase of serum neuron specific enolase level and tachyphylaxis of egfr-tyrosine kinase inhibitor indicate small cell lung cancer transformation from egfr positive lung adenocarcinoma? Lung Cancer. 2013;81:302–305. doi: 10.1016/j.lungcan.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Gazdar AF, Minna JD. Deregulated egfr signaling during lung cancer progression: mutations, amplicons, and autocrine loops. Cancer Prev Res. 2008;1:156–160. doi: 10.1158/1940-6207.CAPR-08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant egf receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 2006;20:1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and materials in the current study are not available to any readers since they contain the patient’s personal particulars.


