ABSTRACT
Autophagy plays a complicated role in tumorigenesis, and the effects of autophagy in drug resistance have not been fully known. The aim of this study was to evaluate autophagy activity in lung cancer cells derived from different origins and explore the mechanism regulating autophagy in tyrosine kinase inhibitor (TKI) resistance. We found basal level of autophagy had no significant increase in resistant H1650 and H1975 cells compared with sensitive HCC827 and PC9 cells. After erlotinib treatment, the autophagy activity enhanced threefold in H1650 cells but a little in H1975 cells. Inhibiting autophagy with 3-MA increased apoptosis in H1650 rather than H1975 cells. Combined with transmission microscope, results showed PC9 cells tended to be apoptotic and more autophagosomes formed in H1650 cells, which may be correlated with cell viability. GATA6 expression was found markedly elevated in H1650 cells after erlotinib and knocking down GATA6 led to significantly reduced autophagy activity and cell viability. Furthermore, a significant increase of GATA6 and LC3-II expression was observed in insensitive tissues compared with sensitive ones by immunostaining in nonsmall cell lung cancer (NSCLC) patients. With chi-square test, we found GATA6 was positively correlated with LC3-II. The Kaplan-Meier curve analyses further showed patients with high GATA6 had lower overall survival and progression-free survival rates than those with low GATA6 after EGFR-TKI treatment. Our results suggest that GATA6 could enhance autophagy activity contributing to TKI resistance. Targeting GATA6 and autophagy together with TKI may be promising to overcome drug resistance in NSCLC.
KEYWORDS: Non-small cell lung cancer, GATA6, autophagy, TKI resistance, Exon 19 deletions
Abbreviations
NSCLC, nonsmall cell lung cancer; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; GATA6, GATA6-binding factor 6; 3-MA, 3-Methyladenine
Introduction
Lung cancer is the most common malignant tumors worldwide with high incidence.1 Epidermal growth factor receptor (EGFR) mutation contributes to the process of lung tumor and drugs targeting EGFR (tyrosine kinase inhibitor, TKI) has gained effectiveness.2–4 However, TKI drug resistance is emerging and to be a thorny problem patients facing all over the world. Though extensive work has been carried out, the underlying mechanisms have not been fully elucidated. Autophagy is a self-degradative process to remove damaged proteins and organelles and maintain cell viability.5 However, autophagy plays a double-edge sword impact in tumorigenesis and recent studies showed that autophagy is involved in drug resistance both in chemotherapy and targeted therapy.6–10 Fusobacterium nucleatum-modulating autophagy-promoted chemoresistance in colorectal cancer.11 Blocking autophagy improved the antitumor activity of afatinib in lung adenocarcinoma with activating EGFR mutations.12 Inhibition of EGFR can trigger autophagy, although the consequences of autophagy activation in nonsmall cell lung cancer (NSCLC) patients’ response to anti-EGFR therapies are not clear.
GATA6-binding factor 6 (GATA6), a zinc finger transcription factor, is important in the endodermal differentiation and is crucial in proper lung development.13 It is reported that GATA6 also contributes to tumorigenesis and stemness.14,15 microRNA-regulating ectopic GATA6 expression promoted invasion and metastasis in gastrointestinal cancer.16 Recently, GATA6 has been found upregulated in TKI-resistant H2170 cells in lung cancer.17 However, little is known about the role of GATA6 in TKI resistance and the advanced mechanisms need to be investigated. Zhang et al indicate that GATA6 accumulation is closely related to autophagy18 and our previous study showed that GATA6 has association with autophagy-related genes such as ATG5, ATG7, and ATG12 by ChIP-sequence test. Therefore, we speculate that GATA6 may promote TKI resistance via autophagy in NSCLC. The aim of this study is to evaluate the effects of GATA6 on autophagy and TKI sensitivity in NSCLC. To gain further insight into the prosurvival impact of autophagy, the benefit of combined TKI and autophagy inhibitor treatment was also examined.
Results
Autophagy promotes TKI resistance in NSCLC
To evaluate the efficacy of erlotinib in a panel of EGFR-mutant NSCLC cell lines and the alteration of autophagy activity, CCK-8 test were first performed. We found that 2 μM erlotinib led to cell death of more than 50% in sensitive HCC827 and PC9 cells, while most resistant H1650 and H1975 cells remained alive (Figure 1a). Thus 2 μM erlotinib was selected and used in follow-up experiments. To evaluate the autophagy activity in different cell lines, autophagy-related proteins were measured and we found the basal level of LC3-II as well ATG5 and beclin1 had no significance between sensitive and resistant cells (Figure 1b). After treatment of erlotinib, the conversion of LC3-I to LC3-II markedly increased in H1650 cells, but slightly in H1975 cells (Figure 1b,c). ATG5 and beclin1 had the same trend. In addition, erlotinib promoted apoptosis in H1299, HCC827, and PC9 cells as caspase-3 expression significantly enhanced (Figure 1c). With 3-MA inhibiting autophagy, we found a significant increase of apoptosis in H1650 cells, while only a small rise of H1975 cells underwent apoptosis (Figure 1d). However, 3-MA did not impact PI3K pathway (data not shown). Immunofluorescence (Figure 1e) and TEM (Figure 1f) images further confirmed more LC3-II and autophagosomes formed in H1650 cells compared with PC9 cells and H1975 cells. Otherwise, PC9 cells had apoptotic body formation and little alteration of morphology was observed in H1975 cells. Our results showed that resistant H1650 cells had high autophagy activity, and sensitive cells tended to undergo apoptosis with erlotinib treatment.
Figure 1.
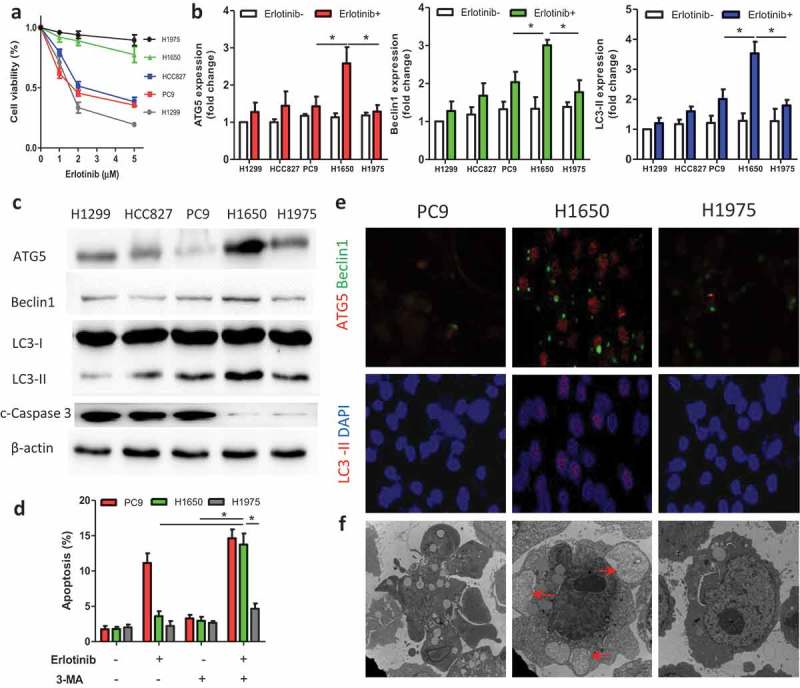
Autophagy activity in lung cancer cells with or without erlotinib.
(A) H1299 (wild type), HCC827/PC9 (sensitive), and H1650/H1975 (resistant) cells were treated with erlotinib for 24 h and the cell viability was tested by CCK-8 tests. (B, C) Cells were treated with 2 μM erlotinib or untreated, and autophagy-related proteins were evaluated by Western blot. (D) PC9, H1650, and H1975 cells were treated with erlotinib (2 μM) or autophagy inhibitor 3-MA, and the apoptotic percentage was analyzed by flow cytometry. (E) Immunofluorescence images showed the autophagy-related proteins expression in PC9, H1650, and H1975 cells treated with erlotinib (2 μM). (F) After erlotinib (2 μM) treatment, the morphology of cells were measured by transmission electron microscopy. PC9 cells had apoptotic body formation; H1650 cells formed autophagosomes (arrows); little alteration was seen in H1975 cells. Data are present as mean ± SD. * p < 0.05.
GATA6 is associated with cell viability after erlotinib treatment
In our previous ChIP-sequence test, GATA6 was found associated with autophagy-related genes such as ATG5, ATG7, and ATG12 in NSCLC (data not shown). Thus, we speculate that GATA6 may regulate autophagy activity and is selected for further study. First, GATA6 expression was tested with or without erlotinib. It was found that H1650 cells had increased GATA6 after erlotinib treatment, which was higher than that in PC9 and H1975 cells (Figure 2a,b). Similarly, more GATA6 staining (green) was observed in H1650 cells by immunofluorescence (Figure 2c). Furthermore, knocking down GATA6 with siGATA6 in H1650 cells markedly reduced the cell viability while upregulating GATA6 in PC9 cells rescued cells from death to a certain extent (Figure 2d). Thus, we speculate that enhanced GATA6 may have prosurvival role in lung cancer after erlotinib treatment.
Figure 2.
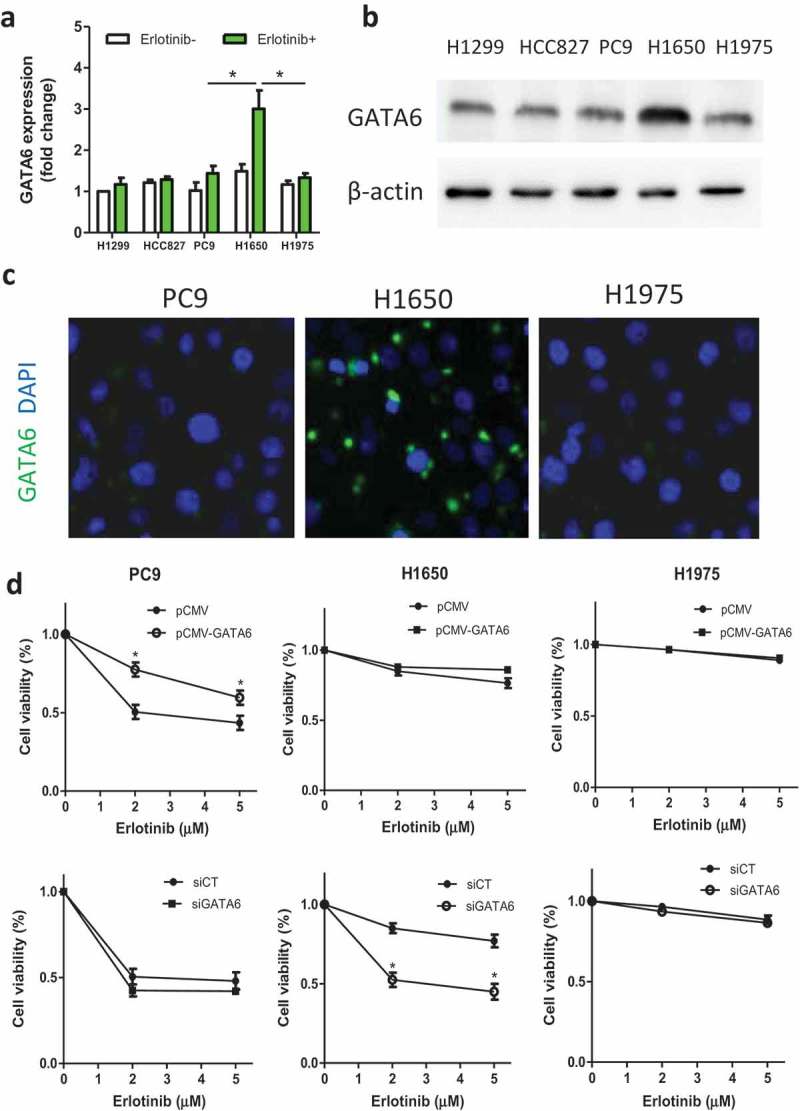
GATA6 is associated with cell viability after erlotinib treatment.
(A, B) Cells were untreated or treated with 2 μM erlotinib for 24 h and the GATA6 expression was evaluated by Western blot. (C) GATA6 expression in PC9, H1650, and H1975 cells treated with erlotinib (2 μM) by immunofluorescence. (D) PC9 cells were treated with pCMV-GATA6 or control (pCMV) and GATA6 gene were knocked down in H1650 and H1975 cells with siRNA GATA6 or control (siCT). The cell viability was then tested. Data are present as mean ± SD. * p < 0.05.
GATA6 induces autophagy in resistant H1650 cells
To investigate the association of increased GATA6 and enhanced autophagy in H1650 cells, siGATA6 was used in H1650 cells treated with 2 μM erlotinib, and we found the expression of LC3-II as well as ATG5 and beclin1 was decreased than that with control (Figure 3a). Addition of 3-MA with siGATA6 had no significant decrease in cell viability compared with siGATA6 or 3-MA alone, indicating that siGATA6 may have consistent effects like 3-MA (Figure 3b). Immunofluorescence images further showed that less staining of LC3-II, ATG5, and beclin1 was seen after siGATA6 in H1650 cells (Figure 3c). These results suggest that GATA6 may enhance autophagy in TKI resistant cells.
Figure 3.
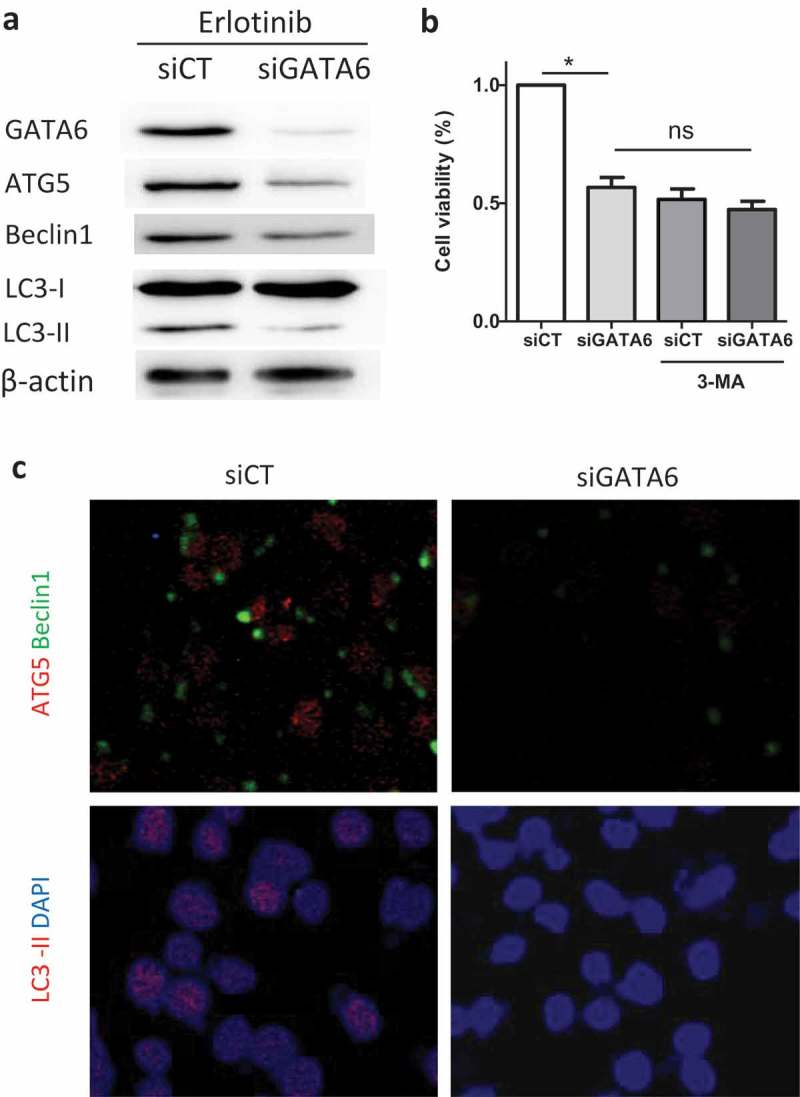
Increased GATA6 enhanced autophagy in H1650 cells.
H1650 cells were treated with 2 μM erlotinib for 24 h and siRNA GATA6 (siGATA6) or control (siCT) were added. (A) The expression of autophagy-related proteins was measured by Western blot. (B) To inhibit autophagy activity, 3-MA was used and the cell viability of H1650 cells with siGATA6 or siCT was analyzed. (C) Autophagy-related proteins were stained and imaged by immunofluorescence. Data are present as mean ± SD. * p < 0.05.
GATA6 and autophagy-related protein expression in NSCLC patients
The characteristics of patients (n = 60) were shown in Table 1. Immunostaining images showed GATA6 and LC3-II expression in sensitive tissues (PFS ≥ 6 months) and insensitive ones (PFS < 6 months) (Figure 4a). With chi-square test, GATA6 was found positively correlated with LC3-II (p < 0.001) (Figure 4b). GATA6 was significantly higher in insensitive tissues than that in sensitive ones (Figure 4c).
Table 1.
Characteristics of the patients.
| Characteristic | Patients, n (%) |
|---|---|
| Age, median (range, yr) | 54 (29–79) |
| Sex | |
| Male | 28 (46.7) |
| Female | 32 (53.3) |
| Smoking status | |
| Never | 43 (71.7) |
| Former or current | 17 (28.3) |
| ECOG PS | |
| 0–1 | 54 (90.0) |
| ≥2 | 6 (10.0) |
| EGFR mutation (sensitive/resistant) | |
| Exon 19 deletions | 35 (15/20) (58.3) |
| Exon 21 L858R | 23 (10/13) (41.6) |
| Other | 2 (2/0) (0.03) |
| TKIs type | |
| Gefitinib | 32 (53.4) |
| Erlotinib | 26 (43.3) |
| Icotinib | 2 (3.3) |
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor
Figure 4.
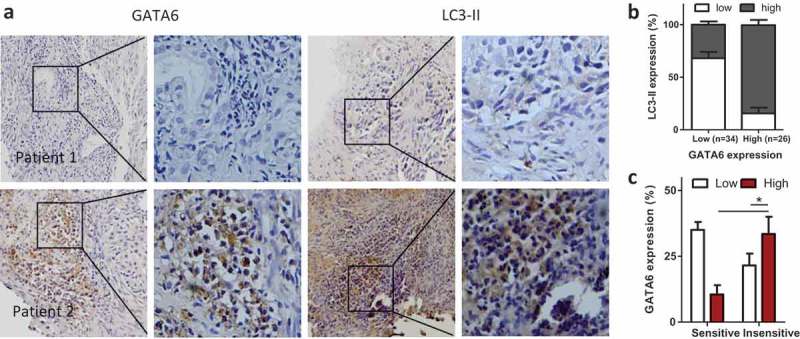
GATA6 and autophagy activity in patients of NSCLC.
(A) Representative immunostaining of GATA6 and LC3-II expression in sensitive tissues (patient 1) and insensitive tissues (patient 2). Magnification, ×100 (left) and ×400 (right). (B) The significant correlation between GATA6 and LC3-II in 60 cases of NSCLC. (C) GATA6 expression was analyzed in sensitive and insensitive tissues. Data are present as mean ± SD. * p < 0.05.
Clinical relevance of GATA6 in NSCLC
The Kaplan-Meier curve analyses showed that the median overall survival (OS) for the high and low GATA6 groups was 22.3 and 33.5 months, respectively (p = 0.012, Figure 5a), and the median progression-free survival (PFS) of patients with high and low GATA6 expression was 5.6 and 12.8 months, respectively (p = 0.009, Figure 5b). These results indicated that patients with high GATA6 had lower OS and PFS rates than those with low GATA6 after EGFR-TKI treatment. Furthermore, we analyzed the GATA6 expression in patients with Exon 19 deletions or Exon 21 L858R mutation and found GATA6 had a positive relation with Exon 19 deletions (Table 2). Thus, we speculate that GATA6 may be closely related to TKI resistance in patients with Exon 19 deletions.
Figure 5.
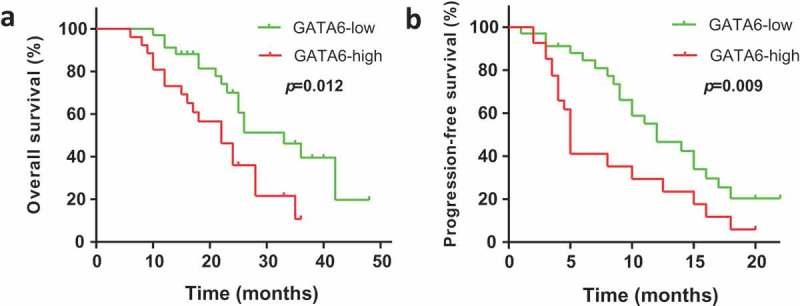
Kaplan-Meier curve analyses of overall survival (OS) and progression-free survival (PFS) of patients after EGFR-TKI.
Table 2.
GATA6 expression in patients with different resistance mutation.
| Patients (n, %) | Exon 19 deletions | Exon 21 L858R | p |
|---|---|---|---|
| GATA6 low | 18.2 ± 2.3 | 30.3 ± 3.8 | 0.004 |
| GATA6 high | 42.4 ± 6.5 | 9.1 ± 2.2 |
Discussion
TKI resistance is an urgent problem to be solved in clinic, which could effectively improve the outcome of lung cancer patients. Though the two and three generations of TKIs have been developed in succession, the first generation of TKIs such as erlotinib and gefitinib are still widely used due to their relatively less side effects and lower price. In this study, erlotinib was used for the purpose to explore the fundamental role of autophagy in TKI resistance in lung cancer cell lines with different origins. We found that drug-resistant H1650 cells (with Exon 19 deletions) but not 1975 cells (T790M mutant) had a burst of autophagy compared to the sensitive cells after erlotinib treatment. GATA6 expression significantly increased in H1650 cells and knocking down GATA6 resulted in a markedly reduction of autophagy-related proteins and resistance ability to erlotinib.
Autophagy has been reported to promote cancer development and chemoresistance, and recently it is considered to be associated with TKI resistance.19,20 Our results showed that after erlotinib treatment, high caspase-3 expression was found in HCC827 and PC9 cells that tended to be apoptotic while autophagy enhanced in H1650 cells which may help to maintain the cell viability. Wang et al have measured the basal autophagy level without TKI treatment and shown that resistant cells express considerable level of LC3-II, indicating an active basal autophagy activity.21 Autophagy is a self-protective behavior and considered to maintain with low activity when cells are in a preferable environment. In our study, both sensitive and resistant cells had low level of autophagy before erlotinib treatment. With the increasing concentration of erlotinib, more conversion of LC3-I to LC3-II as well as ATG5 and belcin1 were found in H1650 cells, compared to which H1975 cells had slight autophagy activity. In addition, autophagy inhibitor 3-MA significantly reduced the autophagy-related proteins and cell viability in H1650 cells but little in H1975 cells. Given L858R/T790M mutation occurs in H1975 cells, we speculate that the resistance ability of H1975 cells may largely rely on the alteration of EGFR structure thereby impeding erlotinib combination and little is associated with autophagy when treated with first-generation TKIs. Though the PTEN deletion may be the primary cause of resistance to EGFR inhibitors in H1650 cells, results in our study indicate that autophagy indeed contributes to erlotinib resistance in H1650 cells to a certain extent.
Previous studies showed GATA6 promotes differentiation and self-renewal of lung and colon cancer stem cells contributing to tumorigenesis.22,23 However, Fantini et al reported a increase of miR-196b correlates with a reduced GATA6 protein expression in colon cancer and Zito et al found retinoic acid-mediated GATA6 activation is necessary for EGFR and Wnt inhibition leading to loss of proliferation in NSCLC.24,25 The undefined and contrary roles of GATA6 in tumor prompts us to explore what the effects of GATA6 are on TKI resistance in lung cancer. First transcriptional proteins regulated by GATA6 were predicted in this study and autophagy-related proteins such as ATG5 and beclin1 were involved. Furthermore, we found GATA6 expression significantly increased in H1650 cells compared with HCC827, PC9 as well as H1975 cells after erlotinib treatment and downregulating GATA6 resulted in a markedly reduction of LC3-II as well as the loss of resistance to erlotinib. Hence, GATA6 is supposed to be a key promoter for autophagy in lung cancer to keep cells alive during TKI treatment.
TKIs combined with autophagy inhibitor have been proposed for cancer therapy,26,27 and our study provides further evidence that active autophagy maintains cell viability in resistant cells with an exon 19 deletion but not T790M mutant after treatment of erlotinib and resistant cells with an exon 19 deletion may be more sensitive to the combined effects of TKI and autophagy inhibitor. TKI-induced GATA6 upregulation in resistant cells with an exon 19 deletion plays an important role in drug resistance and targeting GATA6 may be effective in reversion and prevention of TKI resistance that warrants further investigation.
Materials and methods
Reagents
RPMI 1640 medium and fetal bovine serum (FBS) were obtained from Gibco (Grand Island, NY, USA). Bovine serum albumin (BSA), 4’,6-diamidino-2-phenylindole (DAPI), erlotinib, 3-methyladenine (3-MA) were from Sigma-Aldrich (St. Louis, MO, USA). Annexin V and PI was from Shanghai Dobio Co., Ltd (Shanghai, China). CCK-8 kit was from Yiyuan Biocechnologies. (Guangzhou, China). Rabbit anti-GATA6 (ab22600), rabbit anti-LC3II (ab51520), rabbit anti-ATG5 (ab108327), mouse anti-beclin1 (ab114071), and rabbit anti-caspase3 (ab13847) antibody were from Abcam (Cambridge, MA, USA). Rabbit anti-LC3I/II polyclonal Ab (sc-292354) was obtained from Santa Cruz Biotechanology Inc. (Santa Cruz, CA, USA). Goat antirabbit FITC or Cy3, goat antimouse FITC or goat antirabbit/mouse antibody (Invitrogen, Carlsbad, CA, USA) was utilized as a secondary antibody.
Cell lines and culture
The NSCLC cell lines H1299, PC9, HCC827, H1650, and H1975 were obtained from Heilongjiang Cancer Institute (Harbin, China). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin at 37°C in a humidified atmosphere of 5% CO2. In certain cases, erlotinib with different concentrations was incubated with cells for 24 h and then cells were washed for further tests.
Cell proliferation assay
CCK-8 kit was used for cell proliferation test. The reagents contain WST-8, which is dehydrogenated by cell dehydrogenase under the action of electron carrier 1-Methoxy PMS to form a highly water-soluble yellow armor product (Formazan dye). The number of armor is proportional to the number of living cells, which represents the viability of cell proliferation. Briefly, suspensions of NSCLC cell lines (1 × 105/well) were plated in 96-well plates and grown at 37°C with different concentrations of erlotinib. After treatment, the media were removed, and RPMI1640 (90 µL) and CCK-8 (10 µL) were added. The plates were incubated for 3 h in the incubator. The absorbance at 450 nm was measured on an automated reader. The inhibition rate (IR) was calculated.
Flow cytometry
For apoptosis analysis, cells were collected, washed with PBS, resuspended in 100 µL of 1X binding buffer and stained with 5 µL of Annexin V and 5 µL of PI at room temperature for 15 min in the dark. A flow cytometer (Becton-Biosciences) was utilized to evaluate the apoptotic levels in each sample following the manufacturer’s instructions.
Transmission electron microscopy (TEM)
For TEM experiments, cells were collected and double fixed in 2.5% glutaraldehyde and 1% OsO4. After dehydration and embedding, ultrathin sections were prepared with a Reichert-Jung Ultracut Ultramicrotome (Leica, Vienna, Austria). Images were observed under a H7650 transmission electron microscope (Hitachi Ltd, Tokyo, Japan).
Immunofluorescence assays
Cells were seeded on glass coverslips with polylysine in a 24-well culture plate and fixed with 4% PFA, blocked (2% BSA) and incubated with primary anti-GATA6 (1:100), anti-LC3II (1:50), anti-ATG5 (1:100) and antibeclin1 (1:100) and were detected with secondary antibodies coupled to Cy3 or FITC (1:50). For DNA detection, DAPI (100 ng/ml) was used. Specimens were mounted and analyzed on a fluorescence microscope (Leica, DM400B, Wetzlar, Germany).
Western blotting
Whole-cell lysates were resolved on a denaturing 10% SDS-PAGE gel and subsequently transferred to polyvinylidene fluoride membranes via semidry transfer. After blocking the membrane at room temperature with 5% skim milk for 1 h, the membrane was incubated overnight at 4°C with anti-GATA6 (1:1000), anti-ATG5 (1:1000), antibeclin1 (1:1000), anti-caspase-3 (1:500), and anti-LC3I/II (1:500) antibodies. After incubation with peroxidase-conjugated secondary antibodies at a dilution of 1:2000 for 1 h and washed three times with PBS, the signals were visualized using enhanced chemiluminescence.
Overexpression and silencing of GATA6
Cells were transiently transfected with siRNA GATA6 (100 nM, GenePharma, Shanghai, China) or pCMV-GATA6 (200 nM, GenePharma, Shanghai, China) using Lipofectamine 2000 Transfection Reagent (Life Technology, Grand Island, NY, USA), according to the manufacturer’s instruction (Reverse Transfections Lipofectamine). The cells were also treated with a scrambled siRNA or pCMV (GenePharma, Shanghai, China) as a negative control. The efficiency of GATA6 silencing or overexpression was determined by Western blot.
Immunohistochemistry
A total of 60 NSCLC patients were enrolled and each patient signed an informed consent form for medical record review and tissue sample donation. This study was approved by the Ethics Committee of Harbin Medical University and conducted according to all current ethics guidelines. Tissue sections were immersed in MEDTA, bathed in a steamer at 100°C for 15 min and incubated in methanol containing 0.3% H2O2 for 15 min. The slides were incubated with GATA6 (1:200) and LC3-II (1:100) primary antibodies; stained using DAB; and counterstained using haematoxylin. The staining results were independently interpreted by two pathologists in a blinded manner. For each slide, three to five randomly selected fields were evaluated. For each field, the percentage of DAB-positive tumor cells was calculated as [(number of DAB-positive tumor cells/total number of tumor cells)×100]. The relative staining intensity was defined as low < 25% and high ≥ 25%. Cells were imaged with a light microscopy (Leica, Germany).
Statistical analysis
Results are presented as the mean ± SD of three independent experiments. Statistical differences were evaluated by Student’s t-test or ANOVA as appropriate. The correlation analysis was measured by chi-square test. Survival curves were analyzed by Kaplan-Meier method. The criterion for statistical significance was set at p < 0.05.
Ethic Statement
This study was approved by the Ethics Committee of Harbin Medical University and conducted according to all current ethics guidelines.
Acknowledgments
We thank Fan Yang, Shaohong Fang, Shouchen Zhang, and Xiaoyuan Wang for excellent technical assistance. This work was supported by grants from the National Science Foundation of China (81673024 to Y.Z.), Natural Science Foundation of Heilongjiang Province China (JJ2018LX0182 to Y.Z.), Outstanding academic leaders of Harbin technological innovation Fund (2016RAXYJ076 to Y.Z.), N10 program of Harbin Medical University Cancer Hospital (nN10PY2017-04 to Y.W.), The Haiyan Science Fund of Harbin Medical University Cancer Hospital (JJZD2017-06 to Y.W.), China Postdoctoral Science Foundation (2017M621306, R.M.), and Heilongjiang Postdoctoral Foundation (LBH-Z17168, R.M.).
Disclosure statement
The authors declare no conflict of interest.
References
- 1.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, et al. 2017. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 2.Roncato F, Rruga F, Porcù E, Casarin E, Ronca R, Maccarinelli F, Realdon N, Basso G, Alon R, Viola G, et al. 2018. Improvement and extension of anti-EGFR targeting in breast cancer therapy by integration with the avidin-nucleic-acid-nano-assemblies. Nat Commun. 9:4070. doi: 10.1038/s41467-018-06602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin Y, Shi X, Zhao J, He Q, Chen M, Yan J, Ou Q, Wu X, Shao YW, Yu X, et al. 2018. Mechanisms of primary resistance to EGFR targeted therapy in advanced lung adenocarcinomas. Lung Cancer. 124:110–116. doi: 10.1016/j.lungcan.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Li X, Xue X, Ou Q, Wu X, Liang Y, Wang X, You M, Shao YW, Zhang Z, et al. Clinical outcomes of EGFR kinase domain duplication to targeted therapies in NSCLC. Int J Cancer. 2018. September 26 doi: 10.1002/ijc.31895 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty J, Baehrecke EH.. 2018. Life, death and autophagy. Nat Cell Biol. 20:1110–1117. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagotto A, Pilotto G, Mazzoldi EL, Nicoletto MO, Frezzini S, Pastò A, Amadori A. 2017. Autophagy inhibition reduces chemoresistance and tumorigenic potential of human ovarian cancer stem cells. Cell Death Dis. 8:e2943. doi: 10.1038/cddis.2017.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang P, Liu X, Li H, Chen Z, Yao X, Jin J, Ma X. 2017. TRPC5-induced autophagy promotes drug resistance in breast carcinoma via CaMKKβ/AMPKα/mTOR pathway. Sci Rep. 7:3158. doi: 10.1038/s41598-017-03230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su Z, Li G, Liu C, Ren S, Deng T, Zhang S, Tian Y, Liu Y, Qiu Y. 2017. Autophagy inhibition impairs the epithelial-mesenchymal transition and enhances cisplatin sensitivity in nasopharyngeal carcinoma. Oncol Lett. 13:4147–4154. doi: 10.3892/ol.2017.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sobhakumari A, Schickling BM, Love-Homan L, Raeburn A, Fletcher EV, Case AJ, Domann FE, Miller FJ Jr, Simons AL. 2013. NOX4 mediates cytoprotective autophagy induced by the EGFR inhibitor erlotinib in head and neck cancer cells. Toxicol Appl Pharmacol. 272:736–745. doi: 10.1016/j.taap.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Wekken AJ, Saber A, Hiltermann TJ, Kok K, van Den Berg A, Groen HJ. 2016. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit Rev Oncol Hematol. 100:107–116. doi: 10.1016/j.critrevonc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, et al. 2017. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 170:548–563. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu X, Shi S, Wang H, Yu X, Wang Q, Jiang S, Ju D, Ye L, Feng M. 2017. Blocking autophagy improves the anti-tumor activity of afatinib in lung adenocarcinoma with activating EGFR mutations in vitro and in vivo. Sci Rep. 7:4559. doi: 10.1038/s41598-017-04258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allison TF, Smith AJH, Anastassiadis K, Sloane-Stanley J, Biga V, Stavish D, Hackland J, Sabri S, Langerman J, Jones M, et al. 2018. Identification and single-cell functional characterization of an endodermally biased pluripotent substate in human embryonic stem cells. Stem Cell Reports. 10:1895–1907. doi: 10.1016/j.stemcr.2018.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao CM, Mukherjee S, Tiyaboonchai A, Maguire JA, Cardenas-Diaz FL, French DL, Gadue P. 2018. GATA6 suppression enhances lung specification from human pluripotent stem cells. J Clin Invest. 128:2944–2950. doi: 10.1172/JCI96539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu P, Wu J, Wang Y, Zhu X, Lu T, Liu B, He L, Ye B, Wang S, Meng S, et al. 2018. LncGata6 maintains stemness of intestinal stem cells and promotes intestinal tumorigenesis. Nat Cell Biol. 20:1134–1144. doi: 10.1038/s41556-018-0194-0. [DOI] [PubMed] [Google Scholar]
- 16.Kamijo H, Miyagaki T, Shishido-Takahashi N, Nakajima R, Oka T, Suga H, M S, Sato S. 2018. Aberrant CD137 ligand expression induced by GATA6 overexpression promotes tumor progression in cutaneous T-cell lymphoma. Blood. 132:1922–1935. doi: 10.1182/blood-2018-04-845834. [DOI] [PubMed] [Google Scholar]
- 17.Botting GM, Rastogi I, Chhabra G, Nlend M, Puri N. 2015. Mechanism of resistance and novel targets mediating resistance to egfr and c-met tyrosine kinase inhibitors in non-small cell lung cancer. PLoS One. 10:e0136155. doi: 10.1371/journal.pone.0136155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Yao Z, Zhao S, Shao J, Chen A, Zhang F, Zheng S. 2017. Interaction between autophagy and senescence is required for dihydroartemisinin to alleviate liver fibrosis. Cell Death Dis. 8:e2886. doi: 10.1038/cddis.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Zhang M, Liu H, Yin W. 2019. AZD9291 promotes autophagy and inhibits PI3K/Akt pathway in NSCLC cancer cells. J Cell Biochem. 120:756–767. doi: 10.1002/jcb.27434. [DOI] [PubMed] [Google Scholar]
- 20.Ye M, Wang S, Wan T, Jiang R, Qiu Y, Pei L, Pang N, Huang Y, Huang Y, Zhang Z. 2017. Combined inhibitions of glycolysis and AKT/autophagy can overcome resistance to EGFR-targeted therapy of lung cancer. J Cancer. 8:3774–3784. doi: 10.7150/jca.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Du T, Dong X, Li Z, Wu G, Zhang R. 2016. Autophagy inhibition facilitates erlotinib cytotoxicity in lung cancer cells through modulation of endoplasmic reticulum stress. Int J Oncol. 48:2558–2566. doi: 10.3892/ijo.2016.3468. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji S, Kawasaki Y, Furukawa S, Taniue K, Hayashi T, Okuno M, Hiyoshi M, Kitayama J, Akiyama T. 2014. The miR-363-GATA6-Lgr5 pathway is critical for colorectal tumourigenesis. Nat Commun. 5:3150. doi: 10.1038/ncomms4150. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Feng C, Shi S. 2018. miR-196b promotes lung cancer cell migration and invasion through the targeting of GATA6. Oncol Lett. 16:247–252. doi: 10.3892/ol.2018.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fantini S, Salsi V, Reggiani L, Maiorana A, Zappavigna V. 2017. The miR-196b miRNA inhibits the GATA6 intestinal transcription factor and is upregulated in colon cancer patients. Oncotarget. 8:4747–4759. doi: 10.18632/oncotarget.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zito G, Naselli F, Saieva L, Raimondo S, Calabrese G, Guzzardo C, Forte S, Rolfo C, Parenti R, Alessandro R. 2017. Retinoic acid affects lung adenocarcinoma growth by inducing differentiation via GATA6 activation and EGFR and Wnt inhibition. Sci Rep. 7:4770. doi: 10.1038/s41598-017-05047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y, Su C, Zhao L, Shi Y. 2017. mAb MDR1-modified chitosan nanoparticles overcome acquired EGFR-TKI resistance through two potential therapeutic targets modulation of MDR1 and autophagy. J Nanobiotechnology. 15:66. doi: 10.1186/s12951-017-0302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng X, Feng H, Wu H, Jin Z, Shen X, Kuang J, Huo Z, Chen X, Gao H, Ye F, et al. 2018. Targeting autophagy enhances apatinib-induced apoptosis via endoplasmic reticulum stress for human colorectal cancer. Cancer Lett. 431:105–114. doi: 10.1016/j.canlet.2018.05.046. [DOI] [PubMed] [Google Scholar]


