ABSTRACT
Objective: To explore a method for culturing hepatocellular carcinoma and tumor-infiltrating lymphocytes (HCC-TIL) and investigate the mechanism of TIL in killing tumors.
Methods: The distribution of regulatory T cells (Treg) in HCC was detected by immunohistochemistry. Conventional TIL and oligoclonal TIL were isolated by the traditional method of enzyme digestion combined with mechanical treatment for whole HCC and micro HCC tissue block culturing method. MTT was used to compare the killing activity of TIL. Flow cytometry was used to analyze the proportion of CD8+ T cells and Treg cells in TIL. Tumor-bearing mice were established, and TIL adoptive immunotherapy was performed.
Results: Treg cells were mainly distributed in the stroma of HCC. In vitro experiments showed oligoclonal TIL had higher cytotoxicity to tumor cells which negatively correlated with the proportion of Treg cells. In vivo experiments showed oligoclonal TIL had a higher anti-tumor effect. IFN-γ in peripheral blood and the positive rate of intratumoral lymphocytic infiltration in oligoclonal TIL group were both higher. TGF-β and IL-10 in peripheral blood and the positive rate of intratumoral FoxP3 and IL-17 were both lower than those in conventional TIL group.
Conclusion: The oligoclonal TIL culture method could obtain TIL with higher purity, and cytotoxicity to tumor cells was associated with Treg cells. The oligoclonal TIL had cytotoxicity to autologous HCC cells and significant inhibitory effect on the growth of transplanted tumors. The mechanism might be associated with the inhibition of Treg cells proliferation, increase of IFN-γ secretion, and decrease of TGF-β, IL-10, and IL-17 secretion.
KEYWORDS: Anti-tumor activity, oligoclone, tumor infiltrating lymphocytes, hepatocellular carcinoma, mechanism
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world.1 With the rapid development of immunology and molecular biology, surgery, radiotherapy and chemotherapy, the biological treatment of tumor has become the fourth mode of cancer treatment.2 Adoptive T cell immunotherapy has attracted great attention as a promising treatment strategy for liver cancer in recent years. The immune function of patients with liver cancer was poor, especially the abnormal local immune microenvironment caused damage to the immune defense function of the body, which were important factors for the immune escape, recurrence, and metastasis of liver cancer.3 Although some progress had been made in researches on liver cancer immunity, the exact mechanism of immune tolerance and escape from immune surveillance has not been fully perceived. Because of inducing the activation of antigen-specific T cell responses, remarkable attention should be paid to the inhibitory effect of local immune microenvironment of the liver on activated T cells. Thus, the anti-tumor immunity can be effectively stimulated and superior anti-tumor effect can be produced as well.
Tumor-infiltrating lymphocytes (TIL) are a group of heterogeneous lymphocytes dominated by T cells. They mainly exist in the tumor stroma, contain highly specific cytotoxicity to autologous tumor cells, and possess the advantages of high efficiency, specificity, and small side effects.4–6 Studies showed that there were a large number of TIL in liver cancer tissues, their number and function state reflected the overall level and intensity of the anti-tumor response of the body to a certain extent.7 However, the antitumor activity of TIL extracted by conventional methods was poor. One of the important factors was that there was a certain number of regulatory T cells (Treg) in TIL. Treg has an inhibitory effect on the antitumor activity of TIL, leading to autogenic apoptosis of TIL, in addition to its immune function in an inhibitory state. Even if a physician would like to use adoptive immunotherapy by activating TIL, the clinical efficacy would not be satisfactory, which made the anti-tumor immunity of TIL run into difficulties.
Treg plays a key role in cellular immunity dominated by tumor immunity process. In 1999, Sakaguchi et al. removed CD25+ cells from CD4+ T cells, and transferred them to nude mice, resulting in multiple autoimmune diseases in nude mice. However, if CD4+CD25+ T cells and CD4+ single positive T cells were both transferred to nude mice, no autoimmune disease was observed, which confirmed that CD4+CD25+ T cells could maintain self-tolerance. Subsequently, in vitro studies also showed that CD4+CD25+ T cells had low reactivity and immunosuppressive function. According to their function, CD4+CD25+ T cells were used as Treg.8 The degree of its infiltration in tumor tissues reflects the extent of tumor immune suppression reaction in the body, playing an important role in immune regulation of the body, and determines whether the immune response would eventually activate the immune or induce tolerance. Jone et al. reported that the removal of Treg cells by anti-CD25 mAb could inhibit the growth of malignant melanoma, which provided a new immunotherapy for human tumor treatment.9 A group of scholars also tested a blocker of Treg cells, that might be used in the treatment of malignant melanoma.10
At present, the common TIL isolation methods included collagenase digestion, lymphocyte separation medium, and density gradient centrifugation. After two weeks of in vitro culture, isolated TIL was transfused into patients for immunotherapy. There was a great number of Treg cells in TIL extracted by conventional methods, which could induce autogenic apoptosis of TIL, reduce the anti-tumor effect of TIL, and limit the clinical transformation of adoptive immunotherapy of TIL. Therefore, exploration of an efficient mechanism to isolate TIL with higher purity has become a hot research topic. In this study, an oligoclonal HCC-TIL-based method was used to selectively culture and amplify TIL in presence of low Treg content and high anti-tumor activity. Through in vitro and in vivo experiments, the anti-tumor activity of TIL and the relationship with various cytokines were analyzed, and the possible mechanism of TIL in killing the tumor was discussed as well. Thus, the present study provides experimental and theoretical basis for the adoptive immunotherapy of TIL for HCC.
Materials and methods
HCC tissue sample
Fresh HCC tissues and normal liver tissues from hepatic hemangioma were obtained from surgical resection, respectively, and confirmed by pathology in the Eastern Hepatobiliary Surgery Hospital, the Second Military Medical University (Shanghai, China). All patients provided written informed consent as well.
Cell line and cell culture
Mice H22 hepatoma cells were purchased from the Shanghai Cell Institute of the Chinese Academy of Sciences (Shanghai, China). TILs were cultured from fresh HCC tissue samples, while autologous hepatoma cells suspensions were simultaneously prepared.11 Cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS) and antibiotics in an atmosphere of 5% CO2 at 37°C.
The preparation of TIL for tissue culture of hepatocellular carcinoma
(1) Conventional TIL culture: fresh HCC tissue samples were washed with phosphate buffered saline (PBS) and RPMI 1640 medium, moved into aseptic utensils, and cut into pieces with the volume of 1 mm3. Then, the samples were digested by various enzymes including 3600 U DNA enzyme, 50 U collagenase, and 125 U hyaluronidase, and filtered by using a steel mesh. The density gradient centrifugation was performed for lymphocyte separation liquid, the white cell layer enriched with TIL between 75% and 100% separation liquid layers was absorbed, and 0.2% trypan blue staining was performed to count the live cells, and then cultured and amplified. (2) The oligoclonal TIL–based method: The tumor fragments with the size about 2.0 mm3 were taken from different parts of the HCC tissue, which included the area from the tumor center to the border area of the tumor (2 mm from the edge of the tumor). The tissue fragments were placed in a 24-well plate with a specific culture medium [complete medium (pH 7.2) containing IL-2 (6000 IU/ml)]. The complete medium was composed of RPMI 1640, 25 mmol/L HEPES, 100 U/ml penicillin, 100 U/ml streptomycin, 2 mmol/L glutamine, 5.5 × 10−5 mol/L β-mercaptoethanol, and 10% FBS. The tissue was cultured under the conditions of 5% CO2 and constant temperature of 37°C. The oligoclonal TIL was sequentially cultured and observed, and the proportion of Treg in TIL was detected by flow cytometry.
Flow cytometry analysis
Flow cytometry was used to detect the proportion of Treg in oligoclonal TIL culture, and CD8+ T proportion in conventional and oligoclonal TIL culture. Treg cells were bound by a cocktail of CD4 and CD25 antibodies and were identified using anti-FoxP3 antibody. CD8+ T cells were labeled with fluorescent dye (PE)-conjugated monoclonal antibody against CD8. After staining with these surface markers, the cells were fixed, permeabilized, and samples were analyzed with a BD FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Detection of anti-tumor activity of hepatoma TIL in vitro by using the MTT method
The HCC tissue sample was divided into two identical parts. The conventional hepatoma TIL and oligoclonal hepatoma TIL were prepared and cultured according to the above-mentioned methods. The 2-week conventional hepatoma TIL cells and oligoclonal hepatoma TIL cells were selected as the effector cells, and the autologous hepatoma cells were used as target cells. The control well (RPIM1640 complete medium containing 10% FBS), the experimental and control wells were set in a 96-well plate. Then, 100 μl (2.5 × 104/ml) target cells and 100 μl (2.5 × 105/ml) effector cells were added into the experimental well. The control well-included target cells and effector cells. The cells were incubated in 5% CO2 at 37°C for 24 h, and 20 μl MTT solution was added into each well. After 4 h of culture, the anti-tumor activity of liver cancer TIL in vitro was detected by MTT method. The experiment was repeated for 3 times.
Colony formation assay
The autologous hepatoma cells were isolated from HCC patients (n = 6) and normal liver cells were isolated from hepatic hemangioma patients (n = 3). The conventional hepatoma TIL and oligoclonal hepatoma TIL were prepared, cultured and treated on HCC cell/normal liver cells according to the above-mentioned methods. After co-culture for two weeks. Cell colonies were stained with 0.005% crystal violet and analyzed using a microscope. The experiment was carried out in triplicate wells for three times.
Adoptive immunotherapy for H22 liver cancer-bearing mice
Male (4-week-old) BALB/C mice were purchased from The Animal Experimental Center of the Second Military Medical University (Shanghai, China), and were fed with food and water. The animal welfare guidelines for the care and use of laboratory animals were followed, and the experimental protocol was approved by the Animal Care Committee of Second Military Medical University (Shanghai, China). The mice were maintained in SPF environment of Experimental Animal Center and sacrificed by anesthetization. Mice H22 hepatoma cell line in the logarithmic growth phase was collected, and the cell concentration was adjusted. 0.2 mL (containing 5 × 106 HCC cells) was used to generate an animal model.12 The cells were subcutaneously inoculated on the forelimb axillary fossa of 6-week-old male BALB/C mice. When the tumor grew to an average diameter of about 1.0 cm, the models were used as tumor-bearing mice for immunotherapy. A total of 15 tumor-bearing mice with tumor diameter of about 1.0 cm were randomly selected and divided into three groups: control group, conventional treatment group, and oligoclonal treatment group. 0.3 mL of PBS solution, conventionally cultured TIL, and oligoclonal cultured TIL were administrated by tail vein injection, respectively. The number of TIL cells was 1 × 107, and the injection was once per week with a total of 3 times. After 30 days, the mice were sacrificed. Tumor volumes were measured, and tumor tissues and peripheral blood were collected as well.
Detection of cytokine using ELISA
The secretion of interferon gamma (IFN-γ) in oligoclonal TIL culture and cytokine levels (interleukin (IL)-10, transforming growth factor beta (TGF-β), and IFN-γ) in peripheral blood of mice in the different treatment groups were measured by an enzyme-linked immunosorbent assay (ELISA) method. The experimental data were then statistically analyzed.
Immunohistochemistry (IHC) staining
The IHC method was used to detect the distribution of Treg cells in liver tissue of the patient with liver cancer and the expression of FoxP3 and IL-10 in tumor tissue of tumor-bearing mice. HE staining was used to detect lymphocytic infiltration in hepatoma tissues of tumor-bearing mice.
Statistical analysis
The measurement data were represented as mean ± standard deviation (SD). SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) was used for performing t-test or analysis of variance (ANOVA); enumeration data were analyzed using chi-square test; linear regression and correlation analysis were performed for investigating correlation between TIL and Treg cells. P < 0.05 was statistically considered significant.
Results
Distribution of characteristics of FoxP3+Treg cells in HCC
IHC results showed that Treg cells were mainly distributed in tumor stroma, while those were rare in para-cancerous tissue (Figure 1, n = 30). The number of Treg cells in a single high magnification field was 34.32 ± 6.24 in tumor parenchyma, and that was 4.32 ± 2.66 in para-cancerous tissue. The results showed that the difference in the number of Treg cells between tumor parenchyma and para-cancerous tissue was statistically significant. The density of Treg cells in tumor parenchyma was higher than that in para-cancerous tissue.
Figure 1.
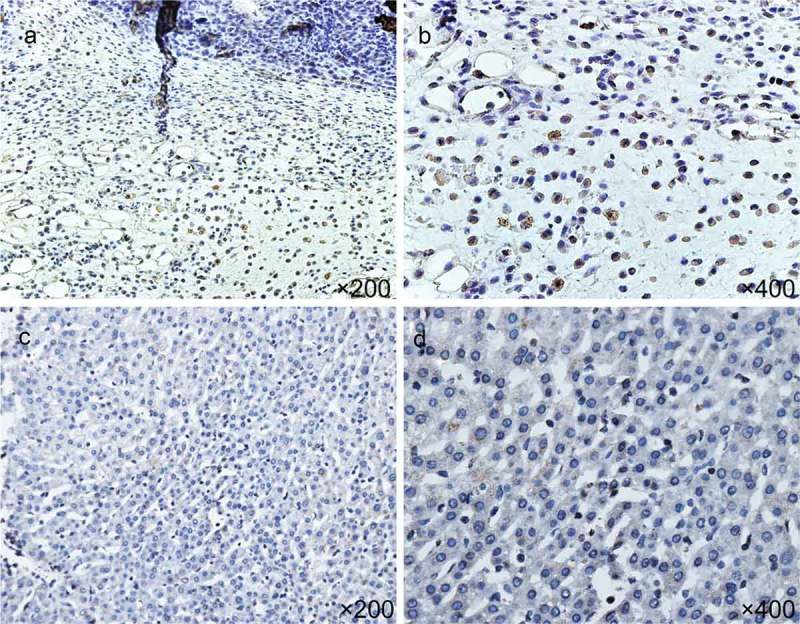
Expression of FoxP3+ Treg cells in HCC tissues: a and b, junction of tumor parenchyma and non-tumor tissues; c and d: adjacent tissues (n = 30).
Isolation, culture, and identification of liver cancer TIL
After the oligoclonal TIL was cultured for 3–4 h, a small amount of isolated TIL was found. After 24 h, dense lymphocyte groups were observed around the tissue, and a certain number of erythrocytes, fibroblasts, and tumor cells were found. With the prolongation of culture time, the number of lymphocytes significantly increased, and the heterozygous cells gradually died and dissolved. After 2-week of culture, the number of TIL could reach 1.2 × 106/mL. Lymphocyte confluence in the culture well could be visible at this time as well (Figure 2a).
Figure 2.
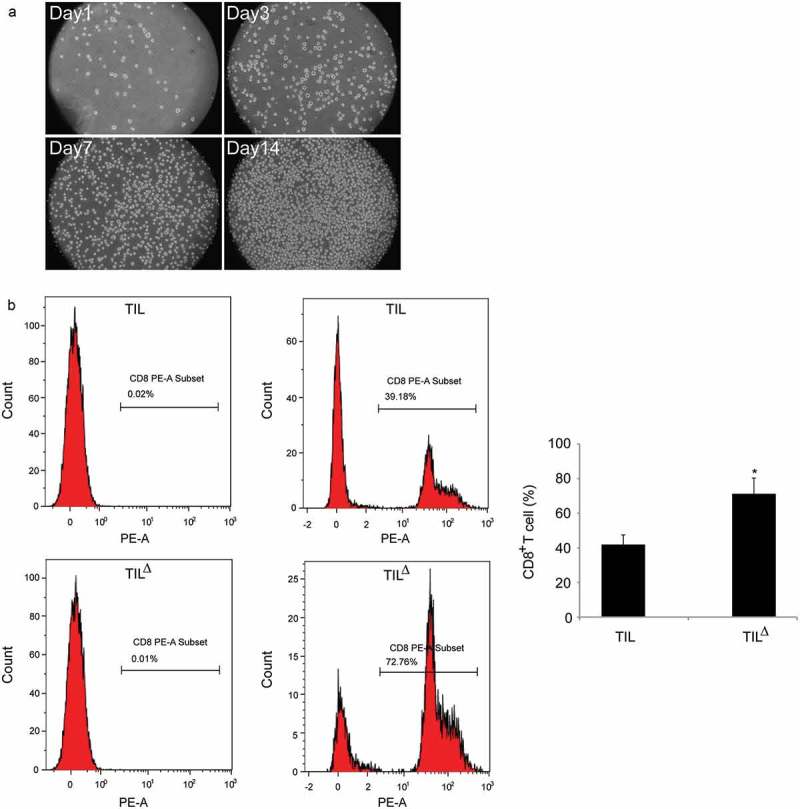
Isolation, culture, and identification of TIL from HCC tissues: a, morphological observation of TIL at different culture time-periods; b, proportion of CD8+ T cells obtained using different culture methods detected by flow cytometry (TIL: conventional cultured TIL; TIL△: oligoclonal cultured TIL; * P < 0.05).
The proportion of subgroup cells in TIL cells from different tumor sources was different, and CD8+ T cells were one of the main subgroups, and also the main effector cells, which mediated the anti-tumor effect of TIL. The percentage of CD8+ T cells after the 2-week culture of TIL was measured, and the results showed that the percentage of CD8+ T cells (71.25 ± 9.09)% obtained by the oligoclonal TIL culture method was remarkably higher than that of the conventional TIL culture (41.89 ± 5.57)% (P < 0.05) (Figure 2b).
Anti-tumor activity of oligoclonal HCC-TIL in vitro
The killing activity of different oligoclonal hepatoma TIL on autologous hepatoma cells was detected. The results showed that each oligoclonal TIL had certain killing activity on autologous hepatoma cells. The oligoclonal TIL with cytotoxic activities higher than 50% were further amalgamated, cultivated, and amplified for detection of the killing activity of autologous hepatoma cells. The results showed that the isolated TIL had a clear inhibitory effect on autologous liver cancer cells. Under the same target ratio, the killing rate of 2-week cultured oligoclonal TIL was higher than that of conventional TIL, and the corresponding killing rate for each method was (72.56 ± 6.69) % and (46.24 ± 4.03) % (P < 0.05), respectively (Figure 3a).
Figure 3.
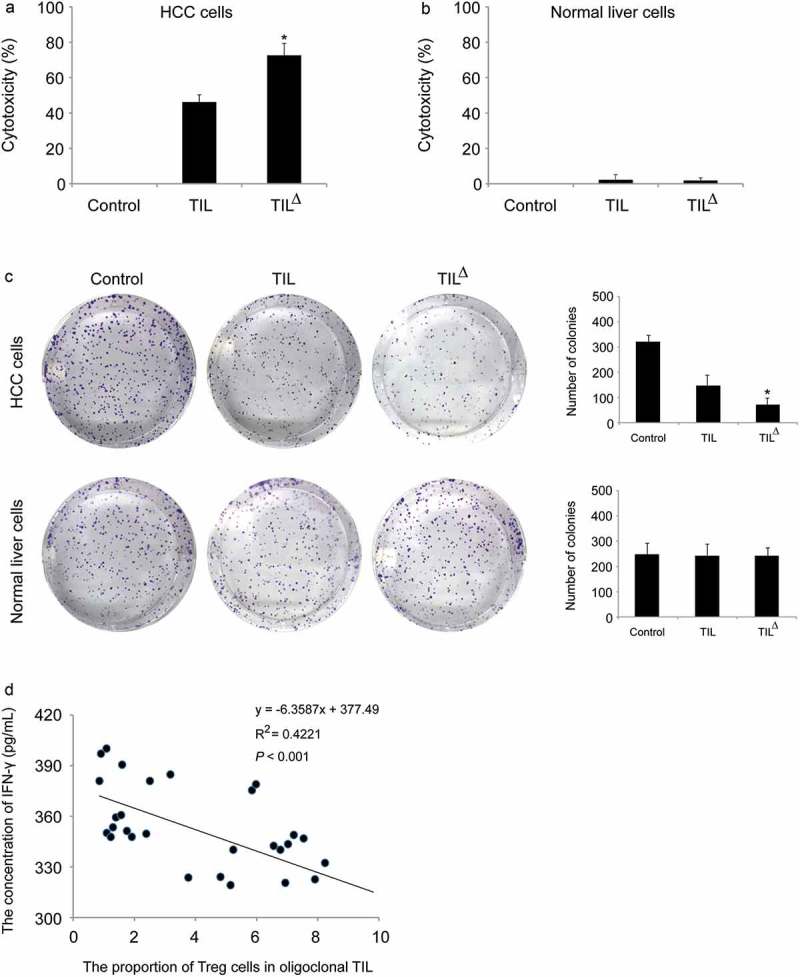
In vitro antitumor activity of TIL from oligoclonal HCC: a, in vitro killing rate of different TIL culture methods on autologous tumor cells (Control: control group; TIL: conventional TIL treatment group; TIL△: oligoclonal TIL treatment group; n = 30); b, in vitro killing rate of different TIL culture methods on normal liver cells (Control: control group; TIL: conventional TIL treatment group; TIL△: oligoclonal TIL treatment group; n = 3); c, the neoplasia ability was detected by colony formation assay. *P < 0.05 (HCC cells, n = 6; normal liver cells, n = 3);d, correlation analysis of Treg ratio in TIL and concentration of IFN-γ.
Treg content in oligoclonal TIL affects the anti-tumor activity of TIL
In cytotoxicity assay, 30 cases of 2-week cultured oligoclonal TIL were selected, then 5 × 105 TIL cells were placed in the 96-well plates for each case, and 5 × 104 autologous tumor cells were implanted in each well that co-cultured overnight. Normal liver cells were isolated from three hepatic hemangioma patients. The cytotoxicity of TIL on normal liver cells was also measured by MTT assay. Our results revealed that oligoclonal TIL had stronger cytotoxicity on autologous tumor cells but had little effect on normal liver cells (Figure 3a and b). The results of colony formation assay were consistent with those of cytotoxicity assay (Figure 3c). The concentration of IFN-γ was detected using ELISA method, and the percentage of Treg cells in the oligoclonal TIL was detected by using flow cytometry. IFN-γ is a major cytokine released by TIL, and its content is positively related to the anti-tumor activity of TIL. Therefore, the detection of IFN-γ in oligoclonal TIL could be used to analyze its anti-tumor activity. The results showed that the proportion of Treg cells in TIL was negatively correlated with the release of IFN-γ (P < 0.001) (Figure 3d), which indicated the higher proportion of Treg cells in TIL, the lower IFN-γ secretion ability, and poorer anti-tumor activity.
Effect of oligoclonal TIL on immunotherapy of tumor-bearing mice
The tumor growth of tumor-bearing mice was significantly inhibited after 30 days of TIL treatment (Figure 4a-b). The tumor volume in the oligoclonal TIL treatment group was less than that of the conventional TIL treatment group and the control group, and the tumor inhibition rate in the oligoclonal TIL group was significantly higher than that of the conventional TIL treatment group, which suggested that the adoptive immunotherapy of TIL was effective, and the oligoclonal HCC-TIL could significantly inhibit the growth of liver cancer xenografts.
Figure 4.
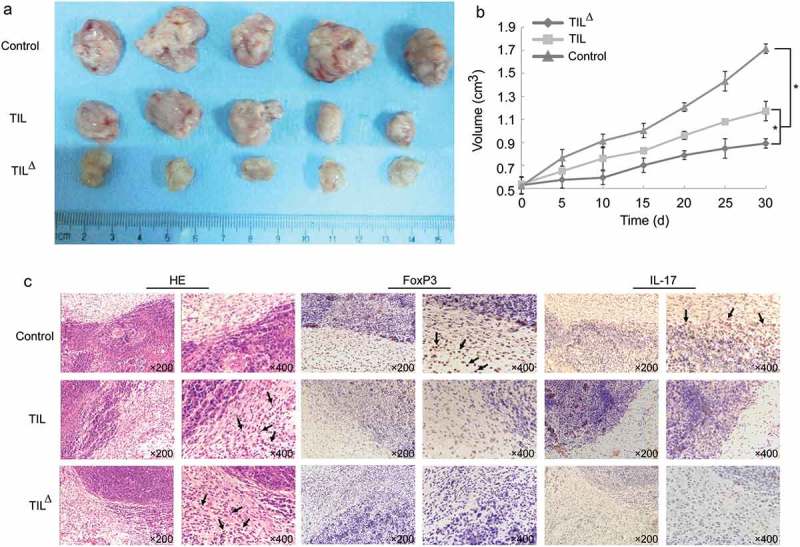
Effect of adoptive immunotherapy of TIL on transplanted mice: a, tumor tissue obtained from different treatment groups of tumor-bearing mice after 30 days of TIL treatment; b, tumor volume of mice in different treatment groups; c, expression of FoxP3 and IL-17, and TIL infiltration in different treatment groups (* P < 0.05) (Arrows represent tumor infiltrating lymphocytes, FoxP3, and IL-17 positive cells).
IHC results showed that the positive rate of TIL infiltration in the tumor tissues of the oligoclonal TIL treatment group was higher than that in the conventional TIL treatment group and the control group, while the positive rates of FoxP3 and IL-17 in the tumor tissues were lower (Figure 4c), indicating that the oligoclonal hepatoma TIL therapy was associated with the decrease of FoxP3 and IL-17 and the inhibition of proliferative Treg cells.
The level of cytokine in peripheral blood was detected by ELISA (Figure 5). The contents of TGF-β and IL-10 in peripheral blood of the oligoclonal TIL treatment group were lower than the conventional TIL treatment group and the control group (P < 0.05), while the content of the peripheral blood IFN-γ was higher than the conventional TIL treatment group and the control group (P < 0.05), indicating that the oligoclonal hepatoma TIL treatment was associated with the decrease of TGF-β and IL-10 as well as the increase of IFN-γ.
Figure 5.
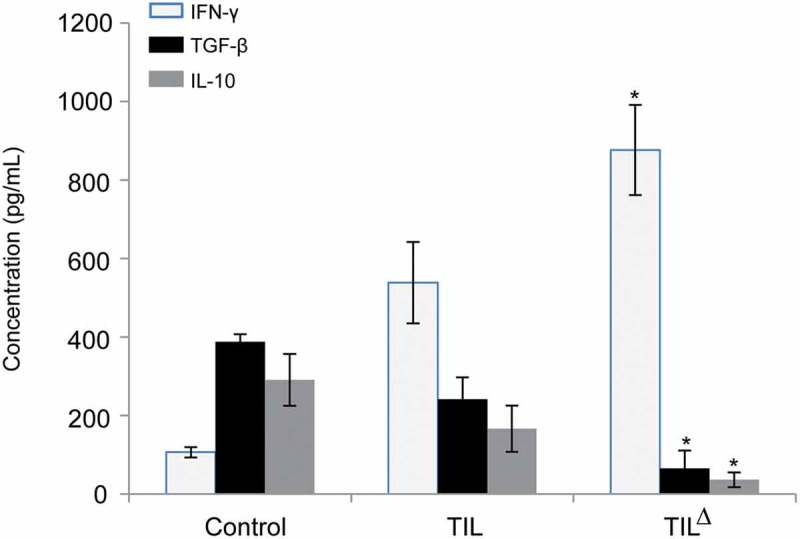
Changes of cytokines in peripheral blood in different treatment groups of tumor-bearing mice (* P < 0.05).
Discussion
The occurrence and development of HCC is a complex process involving several factors. Among them, the immune microenvironment plays an important role in promoting the recurrence and metastasis of HCC.13 The presence of TIL is associated with favorable long-term outcome in breast cancer, and is the main effector cells of the body’s own anti-tumor activity and adoptive immunotherapy. Since Rosenberg reported for the first time that TIL had specific cytotoxicity to tumors in 1986, TIL based on anti-tumor immunotherapy has gradually become a hot spot in the treatment of HCC.14 Studies indicated that patients with more lymphocytic infiltration in HCC tissues had lower recurrence and metastasis rate after surgery.15,16 The recurrence of HCC after liver transplantation was associated with immunosuppression, and TIL subgroup could predict postoperative recurrence.17 In fact, the number and quality of TIL in the body was not enough to maintain the immune response to HCC, and with the resection of HCC, TIL was consequently removed, so it could not effectively play the role of anti-recurrence and anti-metastasis on HCC. Therefore, it was particularly important to the culture and amplify TIL in vitro, and perform its autotransfusion for adoptive immunotherapy.
The conventional method for TIL preparation of HCC was to cut the whole liver cancer tissue into small pieces, digest them with a variety of enzymes, and obtain TIL by density gradient centrifugation. The purity and inhibitory effect of TIL obtained by this method were not remarkable. The possible reasons were as follows: first, numerous operating steps might cause cell damage; second, improper sampling could not specifically amplify TIL with high anti-tumor activity. TIL involves a group of heterogeneous lymphocytes mainly composed of T cells, which are unevenly distributed in liver cancer tissues, and contain Treg cells. Treg cells are mainly CD4+CD25+ regulatory T cells, which directly differentiate from the thymus or are induced from T cells by TGF-β in peripheral blood. As a specific marker for Treg, FoxP3 is associated with the function of Treg, and is considered as an important evidence for the existence of Treg.18 The content of Treg in HCC tissues was higher than that in paracancerous tissues, and changed with culture time in vitro.19 Treg cells could inhibit the antitumor activity of TIL by inducing apoptosis of TIL.20–22
By screening the liver cancer tissues with more TIL and less Treg, and utilizing a mild separation method to prepare TIL for culture and amplification, it was feasible to obtain TIL with stronger anticancer activity. We referred to the method of Rosenberg14 to establish a preparation method for TIL in oligoclonal HCC, which made TIL actively infiltrate from the small liver cancer tissues, avoiding the mechanical operation and the enzyme digestion process to damage the cells, thereby maintaining the integrity and activity of TIL. As TIL had the characteristics of suspension growth, the impurity cells could be effectively removed, and TIL with higher purity could be obtained by controlling the culture conditions and repeatedly subculturing. The anti-tumor activity of different oligoclonal TIL was identified by detecting the secretion level of IFN-γ, and then the oligoclonal TIL with high antitumor activity was selected and cultured.
The subgroup composition of TIL and its secreted cytokines determined its anti-tumor activity, while the tumor microenvironment determined the differences among subgroups.23 In vitro experiments showed that the number and antitumor activity of CD8+T cells obtained by oligoclonal TIL culture method were both higher than those of CD8+ T cells obtained by conventional TIL culture method. After being cultured in the medium containing IL-2, TIL was co-cultured with tumor cells, then high-level of IFN-γ was detected in the culture medium, and its proportion was negatively correlated with the proportion of Treg in TIL. The cytotoxicity of TIL obtained by conventional TIL culture was relatively low, which might be related to the low purity of TIL and the high proportion of Treg.
Animal experiments showed that the adoptive immunotherapy of oligoclonal TIL had obvious therapeutic effects on liver cancer-bearing mice and could effectively inhibit tumor growth. IHC method was used to detect the expression of lymphocyte infiltration and the expressions of FoxP3 and IL-17 in mice liver cancer tissues, and analyze the secretion of IL-10, TGF-β, and IFN-γ in peripheral blood cells. It was speculated that, on the one hand, the in vitro activated TIL could be transformed into cytotoxic T lymphocytes (CTL) after transfused into the tumor-bearing mice, resulting in DNA fragmentation or apoptosis of the tumor cells by acting on the “death locus” and the perforin pathway through the Fas pathway;24 on the other hand, the release of various cytokines in the body could help restore the immune function of the body. Among these cytokines, FoxP3 and TGF-β were associated with Treg cells, while IL-10, IL-17, and IFN-γ were associated with cellular immunity of the body.
The changes in the expression level of cytokine showed that the in vitro activated TIL could play an obvious antitumor role in vivo after being injected into the tail vein of tumor-bearing mice, and its antitumor activity was higher than that of TIL in the body, suggesting that TIL in the body might be inhibited by the tumor microenvironment. Cytokine that was associated with tumor immune escape, such as FoxP3, TGF-β, IL-10, and IL-17, showed a decreasing trend after treatment, demonstrating that TIL adoptive immunotherapy could effectively inhibit the differentiation of T cells into Treg, Th17, and Th2 cells. However, the increase of IFN-γ secretion expressed that more T cells differentiated into Th1 cells, which was conducive to the production of the body’s own immune response to kill tumor cells. Collectively, the findings from the present study provide an overview, as depicted in Supplementary Figure 1.
In conclusion, compared with conventional TIL culture method, oligoclonal TIL culture method could obtain TIL with higher purity, and its anti-tumor activity was associated with the number of Treg cells. Oligoclonal TIL not only had cytotoxicity to autologous liver cancer cells in vitro, but also inhibited the growth of transplanted liver cancer in mice in vivo. Its mechanism was associated with the inhibition of Treg amplification, increased secretion of cytokine IFN-γ, and decreased secretion of TGF-β, IL-10, and IL-17 as well.
Funding Statement
This work was supported by the National Nature Science Foundation of China [81101716]; State Key Project on Infectious Diseases of China [2012ZX10002-016]; National Natural Science Foundation of China (CN) [81301830].
Acknowledgments
This study was supported by the National Nature Science Foundation of China (Grant Nos. 81101716 and 81301830) and the State Key Project on Infectious Diseases of China (Grant No. 2012ZX10002-016).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Clark T, Maximin S, Meier J, Pokharel S, Bhargava P.. Hepatocellular carcinoma: review of epidemiology, screening, imaging diagnosis, response assessment, and treatment. Curr Probl Diagn Radiol. 2015;44(6):479–486. doi: 10.1067/j.cpradiol.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Rahbari NN, Mehrabi A, Mollberg NM. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253(3):453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 3.Pang YL, Zhang HG, Peng JR, Pang XW, Yu S, Xing Q, Yu X, Gong L, Yin YH, Zhang Y, et al. The immunosuppressive tumor microenvironment in hepatocellular carcinoma. Can-cer Immunol Immunother. 2009;58(6):877–886. doi: 10.1007/s00262-008-0603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21(1):3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirabe K, Motomura T, Muto J, Toshima T, Matono R, Mano Y, Takeishi K, Ijichi H, Harada N, Uchiyama H, et al. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: pathology and clinical management. Int J Clin Oncol. 2010;15(6):552–558. doi: 10.1007/s10147-010-0131-0. [DOI] [PubMed] [Google Scholar]
- 6.Weber J, Atkins M, Hwu P, Radvanyi L, Sznol M, Yee C. Immunotherapy task force of the NCI investigational drug steering committee. White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clin Cancer Res. 2011;17(7):1664–1673. doi: 10.1158/1078-0432.CCR-10-2272. [DOI] [PubMed] [Google Scholar]
- 7.Hiraoka N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: molecular biology. Int J Clin Oncol. 2010;15(6):544–551. doi: 10.1007/s10147-010-0130-1. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. [PubMed] [Google Scholar]
- 9.Oble DA, Loewe R, Yu P, Mihm MC Jr.. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun. 2009;9:3. [PMC free article] [PubMed] [Google Scholar]
- 10.Ripley RT, Davis JL, Klapper JA, Mathur A, Kammula U, Royal RE, Yang JC, Sherry RM, Hughes MS, Libutti SK, et al. Liver resection for metastatic melanoma with postoperative tumor-infiltrating lymphocyte therapy. Ann Surg Oncol. 2010;17(1):163–170. doi: 10.1245/s10434-009-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green CJ, Charlton CA, Wang LM, Silva M, Morten KJ, Hodson L. The isolation of primary hepatocytes from human tissue: optimising the use of small non-encapsulated liver resection surplus. Cell Tissue Bank. 2017;18(4):597–604. doi: 10.1007/s10561-017-9641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Sheng YY, Wei JW, Gao XM, Zhu Y, Jia HL, Dong QZ, Qin LX. MicroRNA-219-5p promotes tumor growth and metastasis of hepatocellular carcinoma by regulating cadherin 1. Biomed Res Int. 2018;15:4793971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, Spandidos DA, Libra M. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol. 2012;40(6):1733–1747. doi: 10.3892/ijo.2012.1408. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986;233(4770):1318–1321. [DOI] [PubMed] [Google Scholar]
- 15.Shirabe K, Matsumata T, Maeda T, Sadanaga N, Kuwano H, Sugimachi K. A long-term surviving patient with hepatocellular carcinoma including lymphocytes infiltration–a clinicopathological study. Hepatogastroenterology. 1995;42(6):996–1001. [PubMed] [Google Scholar]
- 16.Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27(2):407–414. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 17.Parmiani G, Anichini A. T cell infiltration and prognosis in HCC patients. J Hepatol. 2006;45(2):178–181. doi: 10.1016/j.jhep.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 19.Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, Coleman N, Alexander GJ. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41(4):722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 20.Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010;127(4):748–758. doi: 10.1002/ijc.25464. [DOI] [PubMed] [Google Scholar]
- 21.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 22.Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20(2):241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahir G, Moser M. Tumor microenvironment and lymphocyte infiltration. Cancer Immunol Immunother. 2012;61(6):751–759. doi: 10.1007/s00262-012-1253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Da T, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


