ABSTRACT
Oral squamous cell carcinoma (OSCC), the subtype of head and neck cancers, is notorious for its high incidence and death rate. The role of long non-coding RNAs (lncRNAs) is discovered to be significant for the canceration and cancer progression. Long intergenic non-protein coding RNA 958 (LINC00958) is discovered as a carcinogene in multiple cancers, such as gastric cancer, pancreatic cancer, and glioma, but there has been no report about how LINC00958 functions in OSCC. The objective of our study is to unfold function and mechanism investigation on LINC00958 in OSCC. First, TCGA database showed the upregulation and prognostic significance of LINC00958 in head and neck squamous carcinoma. Then, we discovered in OSCC clinical samples that LINC00958 presented high expression and predicted poor prognosis. Also, LINC00958 was elevated in OSCC cells. In vitro gain- and loss-function experiments proved that LINC00958 facilitated cell growth, retarded apoptosis, accelerated migration, and epithelial-to-mesenchymal transition (EMT) in OSCC. Mechanistically, we confirmed the cytoplasmic expression of LINC00958 in OSCC cells, and revealed that LINC00958 sequestered miR-627-5p to upregulate YBX2 expression. Rescue assays indicated that LINC00958 regulated OSCC cell proliferation, motility and EMT through YBX2. Together, we showed that LINC00958 promoted OSCC progression through miR-627-5p/YBX2 axis, indicating LINC00958 as a new prognostic marker, and provided new perspectives for molecular targeted treatment for OSCC.
Keywords: LINC00958, miR-627-5p, YBX2, OSCC
Introduction
Head and neck cancer ranks sixth among the most prevalent malignancies worldwide.1 As a subtype of head and neck cancer, oral squamous cell carcinoma (OSCC) takes up approximately 90% of all the malignant neoplasms in oral cavity.2,3 In 2015, oral and pharynx cancer was reported to have 48,100 cases of incidence and 22,100 cases of death in China.4 Despite the progressing surgical regimes, the past over three decades hasn’t seen any significant improvement in the 50% overall survival in OSCC patients,5,6 which necessities the in-depth exploration on the molecular mechanism of OSCC.
Long non-coding RNAs (lncRNAs), longer than 200 nucleotides, are molecules lack of encoding proteins.7 The dysregulation of lncRNA is largely reported to make a difference in the tumor initiation and development.7 Long intergenic non-protein coding RNA 958 (LINC00958) was initially identified in bladder cancer as a potential oncogene,8 and was subsequently demonstrated by multiple reports to have an oncogenic performance in pancreatic cancer and glioma.9,10 Nevertheless, there hasn’t been any report referring to the role of LINC00958 in OSCC.
LncRNAs could serve as transcriptional or post-transcriptional regulators in carcinogenesis.11 At post-transcriptional level, mounting reports have showed that lncRNAs could function as competitive endogenous RNAs in cytoplasm to regulate cancers,12 including OSCC.13 Through targeting miRNAs, lncRNAs prevented the microRNAs (miRNAs) from the base-pairing binding with mRNAs, so as to induce the expression of mRNAs.14 MiR-627-5p has been documented in a previous study that it suppressed proliferation, migration, and invasion in glioma,15 but never has it been investigated in OSCC.
Y box binding protein 2 (YBX2), also known as dbpC, belongs to Y box binding proteins, a family of RNA binding proteins responsible for DNA repair, transcription, and translation.16 The involvement of Y box binding proteins in cancers has been revealed by several studies. For instance, LINC00312 interacted with YBX1 to promote migration and vasculogenic mimicry in lung adenocarcinoma.17 Recently, YBX2 has been shown to present high expression in human testicular seminoma and ovarian dysgerminomas,18 and also plays a role in breast cancer.19 However, its role in OSCC remains unexplored.
Present study sought to explore the function and modulatory mechanism of LINC00958 in OSCC.
Materials and methods
Human tissue samples
Seventy fresh OSCC tissues and the paired adjacent normal tissues were dissected from patients in the Oral and Maxillofacial Surgery Department of the Second Affiliated Hospital of Harbin Medical University. After surgery, all fresh tissues were immediately stored in liquid nitrogen for subsequent use. Patients involved in this study were diagnosed via pathology, and none of the patients received any other treatments, such as chemotherapy or radiotherapy. This study received the approval of the Second Affiliated Hospital of Harbin Medical University and have obtained the informed consents from all patients.
Cell lines and culture
The cell lines used in this study included the OSCC cell lines (SCC15, TSCCA, HSU3, and Fadu) and primary normal human oral keratinocytes (NHOK) cell line. These cell lines were provided by the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China), and were maintained in the RPMI‐1640 medium (Gibco, Waltham, MA, USA) with the supplementation of 10% FBS (Gibco, Waltham, MA, USA), 100 U/mL penicillin, as well as 100 μg/mL streptomycin. The culture condition was in humidified air under 37°C and contained 5% CO2.
Cell transfection
LINC00958 or YBX2 was knocked down using shRNAs (GenePharma, Shanghai, China) targeting LINC00958 or YBX2 (shLINC00958#1 and shLINC00958#2, or shYBX2#1 and shYBX2#2) with scramble shRNAs (shNC) as negative control, or overexpressed using pcDNA3.1 vector (Addgene, Inc., Cambridge, MA, USA) containing LINC00958 or YBX2 sequences (pcDNA3.1/LINC00958 or pcDNA3.1/YBX2) with pcDNA3.1 as control. MiR-627-5p was silenced using miR-627-5p inhibitor with NC inhibitor as control, or was overexpressed using miR-627-5p mimic with NC mimic as control. The constructed plasmids or vectors were introduced into SCC15 or Fadu cells as demanded with the use of Lipofectamine 2000 (Thermo Fisher Scientific, Inc., Rockford, IL, USA)
Quantitative real-time polymerase chain reaction
The extracts of total RNA were obtained from the OSCC tissues or cells applying the TRIzol reagent (Invitrogen). Quality and density of RNAs were determined by the NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Inc.). The complementary DNA (cDNA) was converted from RNA using Transcriptor First Strand cDNA Synthesis Kit (GeneCopoeia, Guangzhou, China). The real‐time PCR reaction was accomplished using the Power SYBR Green PCR Master Mix (Applied Biosystems, Foster, CA, USA).
Cell proliferation assays
For colony formation assay, OSCC cells planted in the six-well plates (5 × 102 cells/well) was incubated for about 14 days. After incubation, the cells were fixed and stained using 0.1% crystal violet (Sigma-Aldrich Co., St Louis, MO, USA). Colonies containing more than 50 cells were counted utilizing a light microscope (Olympus Corporation).
For cell counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) assay, OSCC cells plated in the 96-well plates were added with 10 µL CCK-8 solution after being incubated for 0, 24, 48, 72, and 96 h. Subsequently, cells were cultured for 2 h. Number of viable cells was evaluated by monitoring the variations in absorbance at 450 nm.
For EdU assay, the Cell Light™ EdU kit (Ribobio, Guangzhou, China) was used. OSCC cells were inoculated in 96-well plate (8 × 103/well). 2 days following transfection, each well was added with 50 µM EdU and subjected to incubation for 2 h. Then, cells fixed in 4% formaldehyde were incubated by 2 mg/mL Glycine. Following the permeabilization utilizing 0.5% Triton X-100, the cells were subjected to the staining with DAPI. Radio of EdU-positive cells was evaluated utilizing the fluorescence microscope (Nikon Eclipse Ti Microscope, Japan).
Caspase-3 activity assay
The caspase activities of OSCC cells were evaluated by the caspase activity assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Cells were collected after 48 h of transfection, washed utilizing PBS, and resuspended utilizing the cold lysis buffer. Five microliters of caspase-3 substrates were added into the supernatant following the centrifugation, and then the mixture was cultured for 4 h in the dark at 37°C. The absorbance value was evaluated utilizing the microplate reader (Infinite M200, Tecan, Männedorf, Switzerland).
Migration assays
Cell migration assay was conducted as former description.20 1 × 105 OSCC cells were plated into the upper insert containing serum-free medium (250 μL), and the medium in lower insert contained 10% FBS. Following the incubation for 24 h, the migrated cells were stained with 0.1% crystal violet. Then, cells were observed and evaluated from five deliberately selected fields utilizing the inverted microscope.
Subcellular fractionation analysis
Subcellular isolation of RNAs of OSCC cells was performed with the utilization of Cytoplasmic and Nuclear RNA Purification Kit (Norgenbiotek Corporation, Thorold, ON, Canada). The nuclear and cytoplasmic fractions were examined utilizing qRT-PCR.
Fluorescence In Situ Hybridization (FISH) and immunofluorescence (IF) staining
RNA FISH was performed using RNA FISH Kit (Exonbio Lab, Guangzhou, China) in OSCC cells as former description.21 The probes for LINC00958 were produced by Exonbio Lab (Guangzhou, China). FISH and IF staining were used to detect the localization of LINC00958 and expressions of E-cadherin and N-cadherin. Hybridization with LINC00958 probes were conducted at 37°C. The cells were incubated overnight with the probes or antibodies against E-cadherin (ab1416, Abcam, Cambridge, MA) and N-cadherin (ab76057, Abcam, Cambridge, MA) at 4°C. Following the incubation with secondary antibodies, the amplification utilizing Tyramide signal amplification (Exonbio Lab, Guangzhou, China) and staining with DAPI (Abcam, Cambridge, MA), the confocal microscope (Olympus FV1000, Tokyo, Japan) was used to capture the images.
Dual luciferase reporter assay
The 3ʹ untranslated regions (3ʹUTR) of LINC00958 or YBX2 with the binding sites for miR-627-5p or with the mutant sequences were separately subcloned into a pmirGLO Dual-Luciferase vectors containing firefly luciferase gene (Promega, Madison WI, USA) to establish the WT-LINC00958, Mut-LINC00958 or WT-YBX2, Mut-YBX2 reporters. The miR-627-5p mimics or NC mimics, or miR-627-5p inhibitors or NC inhibitors were co-transfected with WT-LINC00958, Mut-LINC00958, WT-YBX2, or Mut-YBX2 into OSCC cells respectively with the use of Lipofectamine 2000 (Thermo Fisher Scientific, Inc., Rockford, IL, USA). Dual-Luciferase Reporter Assay System (Promega, Madison, WI) was used to determine the luciferase activities 24 h after transfection, with Renilla luciferase activities as normalized control.
RNA immunoprecipitation (RIP)
RIP assays were conducted utilizing the RNA-Binding Protein Immunoprecipitation kit (Millipore, Burlington, MA). The immunoprecipitation reaction was conducted with antibodies against Ago2 (ab32381, Abcam, Cambridge, MA) with IgG (ab150077, Abcam, Cambridge, MA) as control. The enrichment of LINC00958 and YBX2 mRNA was determined by RT-PCR.
Western blot analysis
The extracts of total proteins were obtained from OSCC cells utilizing the radioimmunoprecipitation assay lysis buffer (Pierce, Rockford, IL, USA). Then the proteins at equivalent amount were separated by the SDS-PAGE and loaded onto the polyvinylidene fluoride membranes. Following the overnight incubation with primary antibodies and 1 h incubation with secondary antibodies, the membranes were washed and visualized utilizing the ECL Plus Detection Kit (Pierce, Rockford, IL, USA). All primary antibodies were provided by Abcam (Cambridge, MA), including anti-E-cadherin (ab1416), anti-N-cadherin (ab76057), anti-YBX2 (ab154829), and anti-GAPDH (ab181602) as internal control.
Statistical analysis
Statistic expression was conducted based on mean ± SD. Analysis of results was conducted utilizing the GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Student’s t tests or one-way ANOVA was used to evaluate the differences between two groups or among more than two groups. Differences were viewed to have statistical significance when P < .05. All experiments were repeated for three times.
Results
The upregulation and clinical significance of LINC00958 in OSCC
To figure out the implication of LINC00958 in OSCC, we first browsed the cancer genome atlas (TCGA) database, finding that LINC00958 presented a higher level in head and neck squamous carcinoma (HNSC) samples compared with the normal samples (Figure 1(a)), and its high expression indicated unfavorable outcome in HNSC patients (Figure 1(b)). To gain more evidence, we collected 70 samples of OSCC tissues and the matched adjacent non-cancerous tissues, and detected the expression of LINC00958. As a result, RT-qPCR analysis showed that LINC00958 was highly expressed in OSCC tissues (Figure 1(c)). Kaplan-Meier analysis revealed that high LINC00958 level resulted in dismal prognosis in OSCC patients (Figure 1(d)). Additionally, we detected the expression of LINC00958 in cells, finding that LINC00958 expression was elevated in OSCC cell lines (Figure 1(e)), among which SCC15 presented highest LINC00958 level whereas Fadu the lowest. Therefore, we concluded from these data that LINC00958 was upregulated in OSCC and had prognostic significance in OSCC patients.
Figure 1.
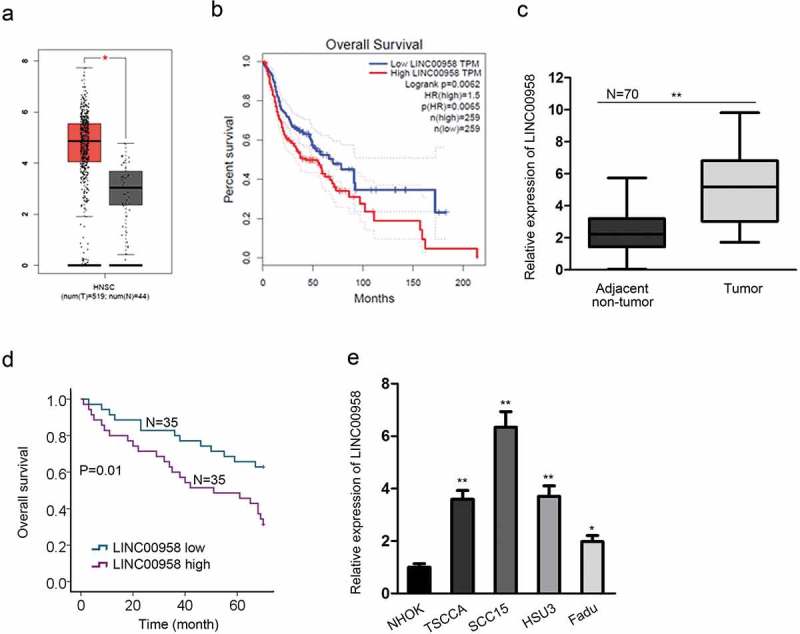
Upregulation and Clinical Significance of LINC00958 in OSCC. (a) TCGA data showed the upregulation of LINC00958 in head and neck squamous carcinoma (HNSC). (b) TCGA database showed that LINC00958 upregulation indicated poor prognosis in HNSC. (c) RT-qPCR analyses showed the upregulation of LINC00958 in OSCC tissues. (d) Kaplan-Meier analysis and log-rank test showed that high LINC00958 expression predicted poor prognosis in OSCC. (e) RT-qPCR analyses confirmed the upregulation of LINC00958 in OSCC cell lines.
*P < .05; **P < .01.
LINC00958 aggravated cell proliferation and attenuated apoptosis in OSCC
Then, we investigated the performance of LINC00958 in affecting OSCC cell proliferation through gain- and loss-of-function experiments. LINC00958 was silenced in SCC15 cells and overexpressed in Fadu cells (Figure 2(a)). We observed the attenuated cell proliferation in OCC15 cells transfected with shLINC00958#1 or shLINC00958#2, and shLINC00958#1 presented a better efficiency in attenuating cell proliferation (Figure 2(b), left). In contrast, overexpressing LINC00958 in Fadu cells improved cell proliferation (Figure 2(b), right). Therefore, we used shLINC0098#1 for subsequent assays. In consistency, colony formation and EdU assays showed that silencing LINC00958 reduced, whereas overexpressing LINC00958 induced the colony generation and EdU-positive ratio (Figure 2(c,d)). Caspase-3 activity was detected to reflect apoptosis level in SCC15 and Fadu cells. Consequently, we found that LINC00958 knockdown increased the caspase-3 activity, while LINC00958 overexpression presented opposite effects (Figure 2(e)). Altogether, LINC00958 could aggravate cell proliferation and attenuated apoptosis in OSCC.
Figure 2.
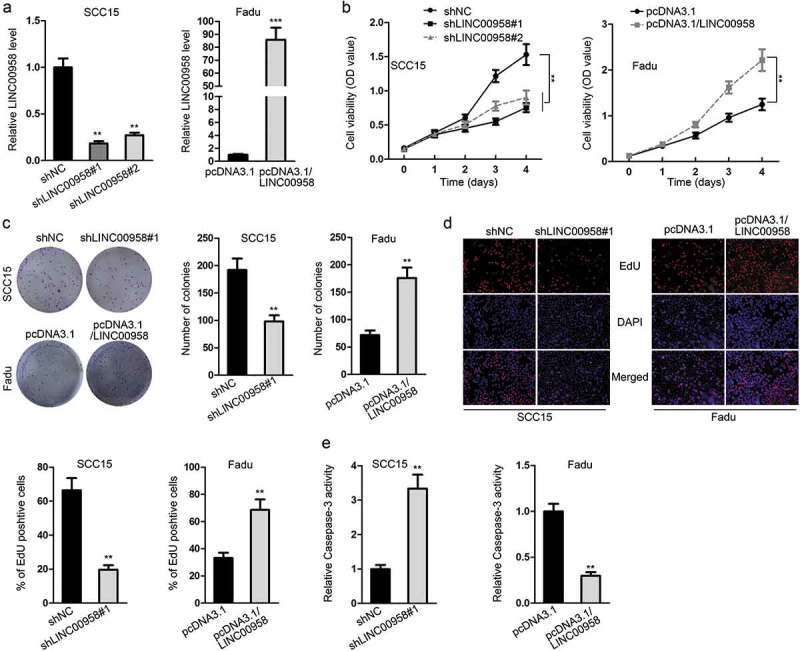
LINC00958 Aggravated Cell Proliferation and Attenuated Apoptosis in OSCC. (a) RT-qPCR results of LINC00958 silencing by shLINC00958#1 or shLINC00958#2 in SCC15 cells and its overexpression by pcDNA3.1/LINC00958 in Fadu cells. (b) CCK-8 results of cell proliferation in response to LINC00958 knockdown or overexpression. (c) Colony formation results of cell proliferation in response to LINC00958 knockdown or overexpression. (d) The ratio of EdU positive cells upon LINC00958 knockdown or overexpression. (e) Caspase-3 activity in OSCC cells upon LINC00958 knockdown or overexpression.
**P < .01; ***P < .001.
LINC00958 facilitated cell migration and EMT in OSCC
Also, we detected the effect of LINC00958 on cell motility in OSCC. Transwell results exhibited that LINC00958 silencing led to a weakened ability of migration cells in SCC16 cells, and LINC00958 overexpression in Fadu cells presented opposite results (Figure 3(a)). Results of RT-qPCR and western blot analyses showed that the epithelial marker E-cadherin level was increased in LINC00958-silenced SCC15 cells and decreased in LINC00958-overexpressed Fadu cells, whereas the mesenchymal marker N-cadherin level was decreased in LINC00958-silenced SCC15 cells and increased in LINC00958-overexpressed Fadu cells (Figure 3(b,c)). Same results were observed through IF staining (Figure 3(d)). Collectively, LINC00958 facilitated cell migration and epithelial-to-mesenchymal transition (EMT) in OSCC.
Figure 3.
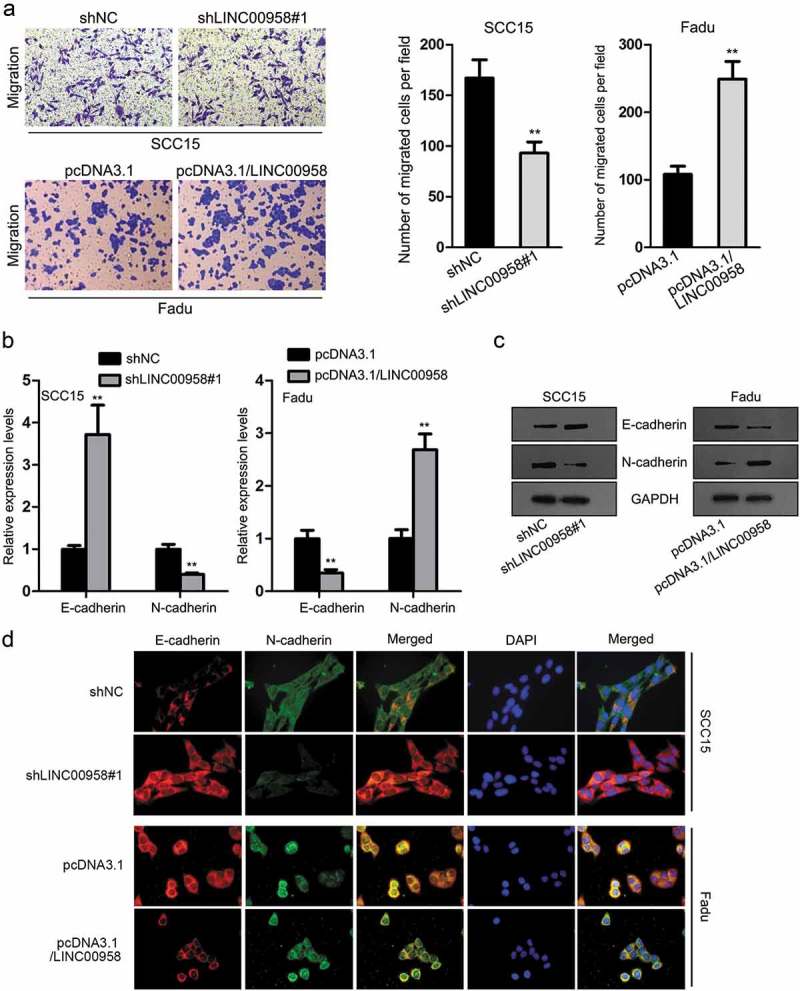
LINC00958 Facilitated Cell Migration and EMT in OSCC. (a) Cell migration upon LINC00958 knockdown or overexpression was assessed by transwell migration assay. (b–c) The mRNA and protein levels of E-cadherin and N-cadherin upon LINC00958 knockdown or overexpression were detected by RT-qPCR and western blot analyses. (d) IF results of the expression of E-cadherin and N-cadherin upon LINC00958 knockdown or overexpression.
**P < .01.
LINC00958 targeted miR-627-5p in OSCC
Since lncRNAs could function through regulating gene expression in cancers through different manners depending on its cellular localization,22 we first probed the location of LINC00958 in OSCC cells. We observed through subcellular fractionation and FISH staining results that LINC00958 was mainly located in cytoplasm (Figure 4(a,b)). It is axiomatically known that lncRNAs could regulate downstream gene expression through acting as ceRNAs.23 Hence, we speculated that LINC00958 might potentially act through ceRNA mechanism in OSCC. We searched StarBase and found out its target miRNAs. After silencing or overexpressing LINC00958 and detecting the expression of all the candidate miRNAs in OSCC cells, we found that only miR-627-5p and miR-3174 was upregulated in response to LINC00958 silencing and downregulated in response to LINC00958 overexpression (Supplementary Table 1). Therefore, we detected the expression of miR-627-5p and miR-3174 in OSCC tissues. Consequently, only miR-627-5p presented significant downregulation in OSCC tissues (Figure 4(c)). We also confirmed in OSCC samples that miR-627-5p expression was negatively associated with LINC00958 expression (Figure 4(d)). In addition, the downregulation of miR-627-5p was validated in OSCC cell lines (Figure 4(e)). These results indicated that miR-627-5p might related to LINC00958 and involved in OSCC. Therefore, we focused on the investigation of LINC00958-miR-627-5p interaction. The binding sites between LINC00958 and miR-627-5p and the mutant sequences on LINC00958 were presented in Figure 4(f). The overexpression of miR-627-5p in SCC15 cells and silencing miR-627-5p in Fadu cells were validated by RT-qPCR results (Figure 4(g)). Luciferase reporter assays showed that SCC15 cells transfected with miR-627-5p presented an increased luciferase activity on only WT-LINC00958 reporter, whereas Fadu cells transfected with opposite results on only WT-LINC00958 reporter (Figure 4(h)). RIP assays demonstrated that LINC00958 and miR-627-5p could be co-immunoprecipitated by anti-Ago2 (Figure 4(i)). These results indicated that LINC00958 sponged miR-627-5p in OSCC cells.
Figure 4.
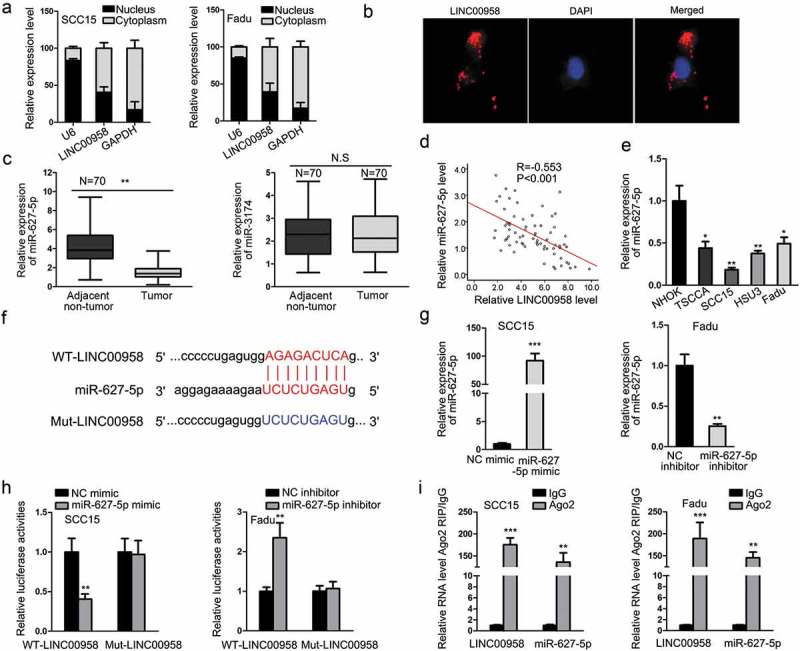
LINC00958 Targeted miR-627-5p in OSCC. (a–b) Subcellular fractionation and FISH assay showed the cytoplasmic localization of LINC00958 in OSCC cells. (c) RT-qPCR results showed that only miR-627-5p, instead of miR-3174, was downregulated in OSCC tissues. (d) Spearman’s correlation curve showed the negative correlation between LINC00958 and miR-627-5p in OSCC samples. (e) RT-qPCR results of the downregulation of miR-627-5p in OSCC cell lines. (f) The binding sites between LINC00958 and miR-627-5p and the mutant sites. (g) RT-qPCR results confirmed the overexpression of miR-627-5p by miR-627-5p mimic in SCC15 cells and the knockdown of miR-627-5p by miR-627-5p inhibitor in Fadu cells. (h) Luciferase reporter assay confirmed that miR-627-5p interacted with LINC00958. (i) RIP assay confirmed the interplay between miR-627-5p and LINC00958.
*P < .05; **P < .01; ***P < .001.
LINC00958/miR-627-5p targeted YBX2 in OSCC
In subsequence, we set out to find the downstream mRNA for LINC00958/miR-627-5p. Combining the prediction results of 4 databases (microT, miRmap, PITA, and PicTar), we identified seven mRNAs targeted by miR-627-5p, which were PHLPP2, FCHSD2, RANBP9, EBAG9, SKIDA1, UBN2, and YBX2 (Figure 5(a)). To find out the miRNA indeed correlated to miR-627-5p, we detected the expression of these genes in face of miR-627-5p overexpression and inhibition. It turned out that only YBX2 expression could be induced by miR-627-5p inhibition and be reduced by miR-627-5p overexpression (Figure 5(b)), which was further confirmed by western blot results (Figure 5(c)). We also examined the effect of LINC00958 on YBX2 expression, discovering that ectopic expression of LINC00958 induced, whereas silencing LINC00958 reduced the expression of YBX2 at mRNA and protein levels (Figure 5(d)). The binding sites between YBX2 and miR-627-5p and the mutant sites were presented in Figure 5(e). Luciferase reporter assay confirmed that miR-627-5p targeted YBX2 (Figure 5(f)). Then we further detected whether YBX2 was targeted by LINC00958/miR-627-5p. Results of luciferase reporter assays showed that co-transfection of pcDNA3.1/LINC00958 counteracted the inhibitive effect of miR-627-5p mimic on luciferase activity of WT-YBX2 rather than Mut-YBX2, and co-transfection of shLINC00958#1 counteracted the inductive effect of miR-627-5p inhibitor on the luciferase activity of WT-YBX2 rather than Mut-YBX2 (Figure 5(g)). Moreover, we examined the involvement of YBX2 in OSCC. It was discovered that YBX2 expression was elevated in OSCC tissues and cells (Figure 5(h)). Besides, YBX2 was negatively correlated with miR-627-5p and positively correlated with LINC00958 in OSCC samples (Figure 5(i)). Therefore, these results implicated that LINC00958/miR-627-5p targeted YBX2 in OSCC.
Figure 5.
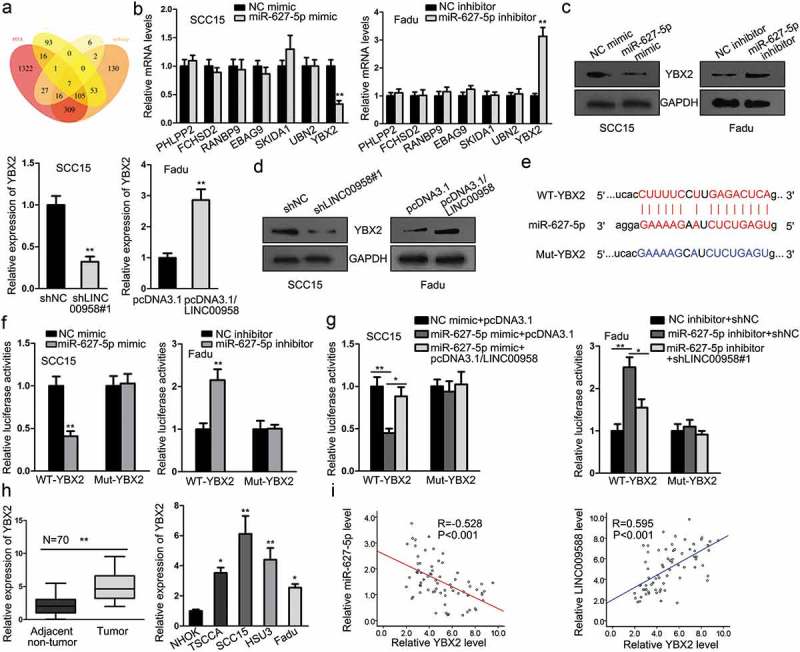
LINC00958/miR-627-5p Targeted YBX2 in OSCC. (a) The Venn pattern showed that 7 genes (PHLPP2, FCHSD2, RANBP9, EBAG9, SKIDA1, UBN2, and YBX2) were commonly predicted as the targets for miR-627-5p in four bioinformatics databases (microT, miRmap, PITA, and PicTar). (b) RT-qPCR results showed that among the seven candidate mRNAs, only YBX2 could be reduced or induced by overexpression or knockdown of miR-627-5p in OSCC cells. (c) Western blot analyses confirmed that protein levels of YBX2 could be reduced or induced by overexpression or knockdown of miR-627-5p in OSCC cells. (d) RT-qPCR and western blot analyses showed that mRNA and protein levels of YBX2 could be induced or reduced by overexpression or knockdown of LINC00958 in OSCC cells. (e) The binding sequences between YBX2 and miR-627-5p and the mutant sequences. (f) Luciferase reporter assays confirmed that miR-627-5p targeted YBX2 mRNA. (g) Luciferase reporter assay proved that LINC00958/miR-627-5p targeted YBX2 mRNA. (h) RT-qPCR results of the upregulation of YBX2 in OSCC tissues and cell lines. (i) Spearman’s correlation curve showed that YBX2 was positively correlated with LINC00958 and negatively correlated with miR-627-5p.
*P < .05; ***P < .01.
LINC00958 regulated cell growth, motility, and EMT through YBX2 in OSCC
In the end, to probe whether LINC00958 facilitated cell growth, motility and EMT through YBX2, we designed rescue assays. YBX2 was silenced in Fadu cells and was overexpressed in SCC15 cells (Figure 6(a)). Since shYBX2#1 showed better knockdown efficiency, we used it for subsequent experiments. Results of CCK-8 and colony formation assays showed that overexpression of YBX2 reversed the inhibitive influence of shLINC00958#1 on cell proliferation, and silencing YBX2 reversed the facilitative influence of pcDNA3.1/LINC00958 on cell proliferation (Figure 6(b,c)). Besides, the caspase-3 activity was hampered by shLINC00958#1, which was restored by the co-transfection of pcDNA3.1/YBX2 in SCC15 cells (Figure 6(d), left). In Fadu cells, the caspase-3 activity was improved by pcDNA3.1/LINC00958, which was restored by the co-transfection of shYBX2#1 (Figure 6(d), right). Transwell assays showed that cell migration retarded by shLINC00958#2 could be restored by YBX2 overexpression, and the cell migration induced by pcDNA3.1/LINC00958 could be reversed by YBX2 knockdown (Figure 6(e)). Furthermore, results of RT-qPCR and western blot analyses showed that the increased level of E-cadherin in LINC00958 silenced SCC15 cells was reversed by YBX2 overexpression, and the decreased level of E-cadherin in LINC00958 overexpressed Fadu cells was restored by YBX2 knockdown, whereas the level of N-cadherin presented opposite results (Figure 6(f,g)). In consequence, it was suggested that LINC00958 regulated cell growth, motility, and EMT through YBX2 in OSCC.
Figure 6.
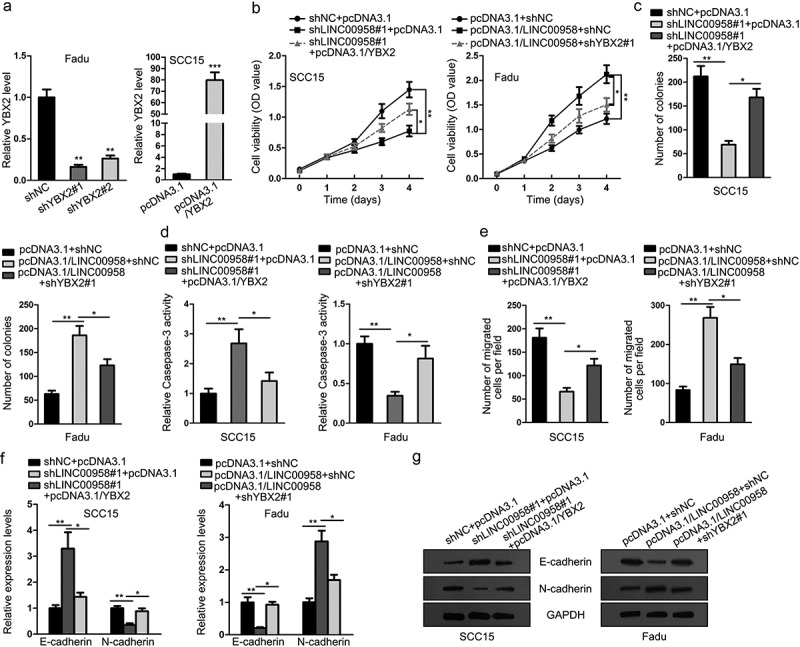
LINC00958 Regulated Cell Growth, Motility, and EMT through YBX2 in OSCC. (a) The silencing of YBX2 by shYBX2#1 or shYBX2#2 in Fadu cells and the overexpression of YBX2 by pcDNA3.1/YBX2 in SCC15 cells were confirmed by RT-qPCR analyses. (b–c) SCC15 cells were transfected with shNC+pcDNA3.1, shLINC00958#1+ pcDNA3.1, or shLINC00958#1+ pcDNA3.1/YBX2. Fadu cells were transfected with pcDNA3.1+ shNC, pcDNA3.1/LINC00958+ shNC, or pcDNA3.1/LINC00958+ shYBX2#1 for subsequent experiments. CCK-8 and colony formation results of SCC15 and Fadu cells in each group. (d) Caspase-activity of SCC15 and Fadu cells in each group was evaluated to assess cell apoptosis level. (e) Transwell migration results of SCC15 and Fadu cells in each group. (f–g) RT-qPCR and western blot analysis of E-cadherin and N-cadherin levels of SCC15 and Fadu cells in each group.
*P < .05; **P < .01; ***P < .01.
Discussion
OSCC, as the most prevalent class of head and neck cancer,2,3 has a dismal 5 year overall survival around 50% hardly improved over the last three decades.5,6 Therefore, the molecular mechanism of OSCC progression is in need of a better understanding so as to provide new potential prognostic and therapeutic targets for OSCC.
Emerging reports have illustrated that lncRNAs are responsible for the regulation of progression of OSCC. For instance, SNHG20 was an aggravator for OSCC tumorigenesis through regulating miR-197/LIN28 axis.24 MALAT1 promoted the development of OSCC through regulating miR-125b/STAT3.25 In present study, we focused on the role of long intergenic non-protein coding RNA 958 (LINC00958) in OSCC, because it has been demonstrated to function as an oncogene in bladder cancer, pancreatic cancer, and glioma.8–10 Also, we found through TCGA database that LINC00958 was highly expressed in HNSC and predicted poor outcome in HNSC samples, indicating that LINC00958 might also be associated with OSCC. Expectedly, we firstly uncovered the elevation of LINC00958 in OSCC tissues and cell lines, and revealed its clinical significance in OSCC patients, suggesting it as a prognostic marker in OSCC. Functionally, we found through in vitro experiments that LINC00958 could improve the proliferation, migration and EMT in OSCC cells.
As for mechanism, it is known that lncRNAs regulate gene expression in cancers at transcriptional and post-transcriptional level,11 and the manners by which lncRNAs regulate gene expression vary depending on the cellular location of lncRNAs.22 Herein, we confirmed in OSCC cells that LINC00958 was majorly located in cytoplasm. In cytoplasm, lncRNAs could post-transcriptionally administrate gene expression by sponging miRNAs in cancers,12 including in OSCC.13 We detected all the predicted targeted miRNAs for LINC00958 in StarBase database upon the dysregulation of LINC00958 and found only two responding miRNAs, which were miR-627-5p and miR-3174. Although previous study illustrated that miR-3174 promoted the apoptosis in gastric cancer,26 indicating its tumor-suppressive role, we showed that miR-3174 presented no significant downregulation in OSCC tissues. However, In consistent with another former report that miR-627-5p was a suppressor for cell proliferation, migration and invasion in glioma,15 we firstly showed in OSCC that miR-627-5p was significantly downregulated. Moreover, we confirmed that LINC00958 sponged and negatively correlated with miR-627-5p in OSCC cells.
In ceRNA network, lncRNAs protected downstream genes from the mRNA degradation or repression in translation caused by miRNAs,14 so as to upregulate the gene expression. We searched for four bioinformatics databases and identified seven commonly predicted target mRNAs for miR-627-5p. From these targets, we found that only YBX2 was submitted to the regulation of miR-627-5p. YBX2, also known as dbpC, is a member of Y box binding proteins regulating DNA repair, transcription, and translation.16 Previous studies showed that YBX2 presented high expression in human testicular seminoma and ovarian dysgerminomas,18 and also played a role in breast cancer.19 Our study was the first to correlate YBX2 to OSCC. We uncovered the upregulation of YBX2 in OSCC samples and cells, and firstly confirmed that LINC00958 sponged miR-627-5p to induce YBX2 expression in OSCC. Correlation analyses indicated that YBX2 was positively correlated with LINC00958 and was negatively correlated with miR-627-5p. Finally, rescue experiments suggested that LINC00958 promoted proliferation, migration, and EMT through YBX2.
In conclusion, our study provided LINC00958 as a new prognostic marker in OSCC and mechanistically uncovered that LINC00958 regulated miR-627-5p/YBX2 axis in OSCC, which is enlightening for the development of molecular targeted therapy for OSCC.
Abbreviations
- OSCC
Oral squamous cell carcinoma
- lncRNAs
Long non-coding RNAs
- EMT
epithelial-to-mesenchymal transition
- miRNAs
microRNAs
- NHOK
normal human oral keratinocytes
- cDNA
complementary DNA
- CCK-8
cell counting kit-8
- FISH
Fluorescence In Situ Hybridization
- RIP
RNA immunoprecipitation
- HNSC
head and neck squamous carcinoma
Acknowledgments
Thank you for all involved in this study.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- 1.Lala M, Chirovsky D, Cheng JD, Mayawala K.. Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC): a systematic literature review. Oral Oncol. 2018;84:108–120. doi: 10.1016/j.oraloncology.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J Clin. 2015. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 3.Johnson NW, Jayasekara P, Amarasinghe AA. Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontol 2000. 2011;57:19–37. doi: 10.1111/j.1600-0757.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Shpitzer T, Bahar G, Feinmesser R, Nagler RM. A comprehensive salivary analysis for oral cancer diagnosis. J Cancer Res Clin Oncol. 2007;133:613–617. doi: 10.1007/s00432-007-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G, An X, Zhao H, Zhang Q, Zhao H. Long non-coding RNA HNF1A-AS1 promotes cell proliferation and invasion via regulating miR-17-5p in non-small cell lung cancer. Biomed Pharmacother. 2017;98:594–599. doi: 10.1016/j.biopha.2017.12.080. [DOI] [PubMed] [Google Scholar]
- 8.Seitz AK, Christensen LL, Christensen E, Faarkrog K, Ostenfeld MS, Hedegaard J, Nordentoft I., Nielsen M.M., Palmfeldt J., Thomson M., et al. Profiling of long non-coding RNAs identifies LINC00958 and LINC01296 as candidate oncogenes in bladder cancer. Sci Rep. 2017;7:395. doi: 10.1038/s41598-017-00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Chen JZ, Zhang JQ, Chen HX, Qiu FN, Yan ML, Tian Y.F., Peng C.H., Shen B.Y., Chen Y.L., et al. Silencing of long noncoding RNA LINC00958 prevents tumor initiation of pancreatic cancer by acting as a sponge of microRNA-330-5p to down-regulate PAX8. Cancer Lett. 2019;446:49–61. doi: 10.1016/j.canlet.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Guo E, Liang C, He X, Song G, Liu H, Lv Z, Guan J, Yang D, Zheng J. Long noncoding RNA LINC00958 accelerates gliomagenesis through regulating miR-203/CDK2. DNA Cell Biol. 2018. doi: 10.1089/dna.2018.4163. [DOI] [PubMed] [Google Scholar]
- 11.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Yang L, Chen T, Liu X, Guo Y, Zhu Q, Tong X., Yang W., Xu Q., Huang D., et al. A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Mol Cancer. 2019;18:28. doi: 10.1186/s12943-019-1010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng B, Li Y, Jiang F, Wei C, Chen G, Zhang W, Zhao W., Yu D.. LncRNA GAS5 suppresses proliferation, migration, invasion, and epithelial-mesenchymal transition in oral squamous cell carcinoma by regulating the miR-21/PTEN axis. Exp Cell Res. 2019;374(2):365–373. doi: 10.1016/j.yexcr.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 14.Noh JH, Kim KM, McClusky WG, Abdelmohsen K, Gorospe M. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip Rev RNA; 2018. doi: 10.1002/wrna.1471. [DOI] [PMC free article] [PubMed]
- 15.Fan Z, Zheng J, Xue Y, Liu X, Wang D, Yang C, Ma J, Liu L, Ruan X, Wang Z, et al. NR2C2-uORF targeting UCA1-miR-627-5p-NR2C2 feedback loop to regulate the malignant behaviors of glioma cells. Cell Death Dis. 2018;9:1165. doi: 10.1038/s41419-018-1149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. The pleiotropic functions of the Y-box-binding protein, YB-1. BioEssays. 2003;25:691–698. doi: 10.1002/bies.10300. [DOI] [PubMed] [Google Scholar]
- 17.Peng Z, Wang J, Shan B, Li B, Peng W, Dong Y, Shi W, Zhao W, He D, Duan M, et al. The long noncoding RNA LINC00312 induces lung adenocarcinoma migration and vasculogenic mimicry through directly binding YBX1. Mol Cancer. 2018;17:167. doi: 10.1186/s12943-018-0920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohno Y, Matsuki Y, Tanimoto A, Izumi H, Uchiumi T, Kohno K, Shimajiri S., Sasaguri Y.. Expression of Y-box-binding protein dbpC/contrin, a potentially new cancer/testis antigen. Br J Cancer. 2006;94:710–716. doi: 10.1038/sj.bjc.6602987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stricker TP, Brown CD, Bandlamudi C, McNerney M, Kittler R, Montoya V, Peterson A, Grossman R, White KP.. Robust stratification of breast cancer subtypes using differential patterns of transcript isoform expression. PLoS Genet. 2017;13:e1006589. doi: 10.1371/journal.pgen.1006589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang P-Y, Hsieh P-L, Wang TH, Yu C-C, Lu M-Y, Liao Y-W, Lee TH, Peng CY.. Andrographolide impedes cancer stemness and enhances radio-sensitivity in oral carcinomas via miR-218 activation. Oncotarget. 2016;8:4196–4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Li J, Zhu Y, Dai Y, Zeng T, Liu L, Li J, Wang H, Qin Y, Zeng M, et al. MicroRNA-9 promotes tumor metastasis via repressing E-cadherin in esophageal squamous cell carcinoma. Oncotarget. 2014;5:11669–11680. doi: 10.18632/oncotarget.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K, Shi Z-M, Chang Y-N, Hu Z-M, Qi H-X, Hong W. The ways of action of long non-coding RNAs in cytoplasm and nucleus. Gene. 2014;547:1–9. doi: 10.1016/j.gene.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Han L, Liu Z, Gao N. Long noncoding RNA MNX1-AS1 contributes to lung cancer progression through the miR-527/BRF2 pathway. J Cell Physiol. 2019;234(8):13843–13850. doi: 10.1002/jcp.28064. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Zhao W, Wang Z, Xiang X, Zhang S, Liu L. Long non-coding RNA SNHG20 promotes the tumorigenesis of oral squamous cell carcinoma via targeting miR-197/LIN28 axis. J Cell Mol Med. 2019;23(1):680–688. doi: 10.1111/jcmm.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang SM, Hu WW. Long non-coding RNA MALAT1 promotes oral squamous cell carcinoma development via microRNA-125b/STAT3 axis. J Cell Physiol. 2018;233(4):3384–3396. doi: 10.1002/jcp.26185. [DOI] [PubMed] [Google Scholar]
- 26.Li B, Wang L, Li Z, Wang W, Zhi X, Huang X, Zhang Q, Chen Z, Zhang X, He Z, et al. miR-3174 contributes to apoptosis and autophagic cell death defects in gastric cancer cells by targeting ARHGAP10. Mol Ther Nucleic Acids. 2017;9:294–311. doi: 10.1016/j.omtn.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


