Abstract
Triggering receptor expressed on myeloid cells 1 (TREM-1) is an important mediator of innate inflammatory responses in microbial infections and sepsis. TREM-1 ligation on neutrophils (PMN) or monocytes results in the production of proinflammatory cytokines. Engagement of TREM-1 induces the activation of MAP kinases as well as rapid Ca2+ mobilization. However, a detailed understanding of TREM-1 signaling pathways is currently lacking. We evaluated the TREM-1 signaling hierarchy in monocytic cells and found that the acute myeloid leukemia cell line MUTZ-3 expresses TREM-1 in a natural and functional manner. We compared essential signaling molecules of the TREM-1, TLR and NLR cascade in MUTZ-3 cells as well as primary monocytes or PMN by Western blot analysis. These studies confirmed the essential role of phosphatidyl inositide 3-kinase (PI3K) and p38MAPK in the TREM-1 as well as the TLR or NLR cascade of monocytic cells. Importantly, PI3K and p38MAPK signals in monocytic cells both control Ca2+ mobilization and are directly connected in the TREM-1 signaling hierarchy, which contrasts previous results obtained in PMN. Taken together, our results indicate cell type-specific differences in the TREM-1 signaling cascade and contribute to an enhanced understanding of the regulation of innate inflammatory responses.
Key Words: Innate inflammatory response, TREM-1, Signal transduction, Monocytes, Polymorphonuclear neutrophils
Introduction
Neutrophils and monocytes are important innate immune effector cells combating microbial and fungal infections. Despite sharing common functions such as their rapid recruitment to a site of infection, microbicidal activity or the release of inflammatory mediators, both cell types harbor vast differences, i.e. in their clearly distinct morphologies and also functionalities. Likewise, the triggering receptor expressed on myeloid cells 1 (TREM-1), a 30-kDa transmembrane glycoprotein of the V-type immunoglobulin-like receptor family [1, 2], is constitutively expressed on polymorphonuclear neutrophils (PMN) and CD14+ monocytes [3]. TREM-1 plays an important role in the innate host defense against microbial infections and sepsis [4, 5, 6, 7]. However, currently, it is not clear which contribution is made by PMN and which by monocytes. As an important hint at the differential effects of TREM-1 in distinct cell types, the recent study by Klesney-Tait et al. [8] demonstrates an important role for TREM-1/3 in transepithelial migration of PMN in a mouse pneumonia model. In general, expression of TREM-1 is upregulated through stimulation with TLR agonists and cytokines [9]. Endogenous ligands for TREM-1 have been observed on PMN and platelets as well as in the sera of septic patients [10, 11, 12, 13]. However, the exact nature of the TREM-1 ligand and cell-specific function of the TREM-1 receptor have so far remained elusive.
Engagement of TREM-1 using agonistic monoclonal antibodies triggers the full spectrum of PMN effector mechanisms, including the oxidative burst, phagocytosis, degranulation and the release of inflammatory mediators like IL-8, MCP-1 or myeloperoxidase [3, 14]. In monocytes, TREM-1 ligation initiates upregulation of costimulatory molecules, e.g. CD86, and the production of proinflammatory chemokines including MCP-1, MCP-3, MIP-1α and IL-8 as well as cytokines like TNF-α [3, 15, 16]. Activation of TREM-1 in PMN or monocytes occurs in synergy with receptors for pathogen-associated molecular patterns (PAMP), including Toll-like receptors (TLR) or NACHT-LRR receptors (NLR) [5, 17]. In rodent models of pneumonia or sepsis, administration of a soluble TREM-1-Ig fusion protein or an antagonistic peptide rescues the animals from an otherwise lethal course, indicating an important role for TREM-1 in the amplification of inflammatory processes [5, 18].
For signal transduction, TREM-1 requires the adaptor molecule DAP12 that associates with the receptor in the transmembrane region [3]. In PMN and monocytes, the cross-linking of TREM-1 results in the phosphorylation of the phospholipase C (PLC)-γ, extracellular-signal-regulated kinases 1/2 (EKR1/2) and non-T-cell-activation linker (NTAL) as well as rapid Ca2+ mobilization [3, 19, 20, 21]. In PMN, TREM-1 ligation also activates the phosphatidylinositol 3-kinases (PI3K)/Akt pathway [19, 22, 23, 24]. Other downstream events in PMN include the phosphorylation of the Src kinase Lck/Yes-related novel (Lyn) and Janus kinase 2 (Jak2) as well as activation of the transcription factors signal transducers and activators of transcription 3 (Stat3) and Stat5 [20]. In addition, the Tec family kinase Bruton tyrosine kinase (Btk) has recently been identified as another positive regulator in the TREM-1/DAP12 pathway using a monocytic cell line that ectopically expresses TREM-1 and DAP12 [25]. However, the hierarchy of intracellular TREM-1 signaling remains incompletely understood and mechanisms of synergy with PRR are ill defined. Detailed knowledge will be important to reveal the true role of TREM-1 in the physiology and pathophysiology of inflammation.
In this work, we show that TREM-1 is variably expressed on acute myeloid leukemias (AML) and characterize the AML cell line MUTZ-3 as monocytoid cells that naturally express TREM-1 in a functional manner. By Western blot analyses and specific inhibitors, we show that PI3K and p38MAPK are also key regulators of the TREM-1 signaling in this monocytic cell line, primary monocytes and PMN. In contrast to TREM-1 signaling in PMN, where PI3K and p38MAPK contribute to PMN activation independently [19], PI3K and p38MAPK phosphorylation as well as Ca2+ flux are directly connected to each other in monocytic cells. Beyond this, PI3K and p38MAPK are central components of TLR- or NLR-mediated amplification of TREM-1-induced cytokine production. Taken together, our results establish MUTZ-3 as a monocytic cell line suitable for further characterization of monocytic TREM-1 signaling and map PI3K and p38MAPK as central components in the interaction of the TREM-1, TLR and NLR with distinct signaling cascades of TREM-1 in different myeloid cell types.
Materials and Methods
Materials
CHAPS, DTT, EDTA, NaCl, SDS, Tris, thiourea and urea were from Carl Roth (Karlsruhe, Germany), complete protease inhibitor cocktail from Roche (Basel, Switzerland) and NaF, Na-orthovanadate and PMSF from Sigma-Aldrich (Taufkirchen, Germany). Antibodies against Akt (clone C67E7), p-Akt (clone 193H12), p38MAPK, p-p38MAPK (clone 3D7) and HRP-linked secondary antibodies for Western blot analyses were from Cell Signaling (Danvers, Mass., USA). Anti-TREM-1 (clones 1C5 or 6B1) and mouse IgG1 (clone 4C9) used as isotype control were purified from hybridoma supernatants as described previously [26]. F(ab')2 fragments of clones 6B1 and 4C9 were prepared using a commercial kit according to the manufacturer's instructions (Pierce, Thermo Scientific, Rockford, Ill., USA). All antibodies were tested endotoxin-free (<0.1 EU/µg protein) using a limulus amoebocyte lysate assay (Pierce, Thermo Scientific). Human IgG from a healthy volunteer donor was purified by affinity chromatography using protein A columns (Pierce, Thermo Scientific) according to standard protocols. FITC-labelled anti-TREM-1 (clone 9H7, IgG1) was generated according to standard procedures [26] and is not competitive for antigen-binding with clone 6B1 (data not shown). The following TLR agonists were used: Palmitoyl-3-Cys-Ser-(Lys)4 (Pam3Cys) from EMC Microcollections (Tübingen, Germany), R-848 from InvivoGen (Toulouse, France), Salmonella typhimurium (LPS) from Sigma-Aldrich and GM-CSF from LABGEN/Natutec (Frankfurt, Germany). Signal transduction inhibitors were the PI3K inhibitor LY294002 (Cell Signaling) and the p38MAPK inhibitor SB203580 (Tocris, Bristol, UK). For flow cytometry, the following antibodies were used: FITC-labelled anti-CD66b, APC-labelled anti-CD14, PE-labelled anti-CD34 (all from Beckman-Coulter, Krefeld, Germany), PE-labelled anti-CD86, FITC-labelled IgG1 (both from Beckton Dickinson, Heidelberg, Germany) and PE-labelled anti-HLA-DR (eBioscience, San Diego, Calif., USA). All human studies were performed in accordance with the Declaration of Helsinki after obtaining informed consent from healthy volunteer donors or patients, and they were approved by the local ethics committee according to the institutional guidelines and as required by the national authorities.
Cell Culture
The human AML-derived cell line MUTZ-3 was cultured as described elsewhere [27] in MEM-α with ribonucleosides and deoxyribonucleosides supplemented with 20% (v/v) fetal calf serum (FCS), 2 mML-glutamine, 50 µM β-mercaptoethanol (all from Gibco, Life Technologies GmbH, Frankfurt, Germany) and 10% conditioned medium from the human bladder carcinoma cell line 5637, containing various cytokines including GM-CSF, G-CSF, SCF and M-CSF [28]. Medium was changed on days 1 and 3. On day 6, MUTZ-3 cells were stimulated as indicated in MEM-α without conditioned medium.
Purification of PMN and CD14+ Monocytes
Human peripheral blood PMN or mononuclear cells were purified from human blood via Polymorphprep (Axis-Shield, Oslo, Norway) as described previously [29]. Briefly, citrated whole blood was layered over Polymorphprep (ratio 1:1) and centrifuged at 500 g for 35 min at room temperature. PMN were harvested from the lower band, purity was routinely 92–98%, as assessed by CD66b+ cells via flow cytometry. Monocytes were further enriched from peripheral blood mononuclear cells (PBMC) by magnetic cell sorting (MACS) using CD14 MicroBeads (Miltenyi, Bergisch Gladbach, Germany). Monocytes were routinely >90% CD14+ as examined by flow cytometry. Monocytes and PMN were stimulated as indicated in Iscove's medium (Gibco) supplemented with 5% (v/v) FCS, 2 mML-glutamine, 50 µM β-mercaptoethanol and 1 mM Na-pyruvate (SERVA Electrophoresis, Heidelberg, Germany).
Cell Stimulation
Anti-TREM-1 (clone 6B1, 10 µg/ml) was coated on 96-well flat-bottom plates and for Western blot analyses on 12-well plates (both from Greiner Bio One, Frickenhausen, Germany). Ca2+ mobilization was analyzed after cross-linking of anti-TREM-1 (clone 6B1, 10 µg/ml) with secondary goat anti-mouse F(ab')2 (20 µg/ml, Dianova, Hamburg, Germany). Where indicated, LPS Pam3Cys; R-848 were added. For inhibitor studies, the indicated pharmacologic inhibitors were added 20 min before TREM-1 stimulation (LY294002 at 15 µM; SB203580 at 60 µM for MUTZ-3 or monocytes or 20 µM for PMN, respectively). All inhibitors were carefully titrated and only nontoxic concentrations were used (data not shown) as revealed by MTS assay (Promega, Madison, Wisc., USA) for MUTZ-3 cells or Nicoletti assay for monocytes. For PMN, the oxidative burst induced by PMA (0.5 µg/ml) in the presence or absence of the respective inhibitors was used as viability control. Only concentrations having no impact on the PMA-induced oxidative burst were used [19].
Flow Cytometry
Expression of TREM-1 on AML blasts was assessed from either leukapheresis or bone marrow samples with >90% blasts by morphology that had been frozen in FCS with 10% (v/v) DMSO (Sigma-Aldrich) in liquid nitrogen on the day of diagnosis. For flow cytometry, samples were thawed and washed 3 times in FACS buffer (PBS with 1% bovine serum albumin and 0.05% sodium azide), and subsequently labelled with anti-TREM-1 (clone 1C5) or an isotype-matched control (clone 4C9) and detected by a PE-conjugated goat anti-mouse antibody (Jackson ImmunoResearch, Suffolk, UK). The samples were washed twice and analyzed with a FACSCanto flow cytometer (Becton Dickinson) and FlowJo software V8.8.7 (Tree Star Inc., Ashland, Oreg., USA) gating on the live blast population after exclusion of debris. The fraction of TREM-1-positive cells was set according to the isotype-matched control.
MUTZ-3 cells or monocytes were incubated with the indicated monoclonal antibody (mAb) for 30 min at 4°C after Fc block (Miltenyi) for 10 min at 4°C, washed twice and analyzed with an LSR II flow cytometer (Becton Dickinson) and FlowJo software V8.8.7. To determine the surface expression of TREM-1, FITC-labelled anti-TREM-1 (clone 6B1) or IgG1 as isotype control were used after exclusion of dead cells with propidium iodide (Sigma-Aldrich). For intracellular detection of DAP12, cells were fixed with 2% paraformaldehyde and permeabilized with 95% methanol and subsequently stained with fluorescein-labelled anti-DAP12 (R&D Systems, Wiesbaden, Germany) or an isotype-matched control.
Detection of IL-8 or TNF-α Release
Supernatants from stimulated cells were collected after 16 h or 8 h, frozen at −20°C until required and analyzed by standard enzyme-linked immunosorbent assay (ELISA) for IL-8 or TNF-α (both from R&D Systems) according to the manufacturer's instruction.
Western Blot Analyses
Cells were lysed with modified urea buffer (7 M urea, 2 M thiourea, 5 mM DTT, 2% CHAPS, 10 mM PMSF, 0.5 mM Na-orthovanadat, 5 mM NaF and complete protease inhibitor cocktail) and protein concentration was quantified according to the method of Bradford. Lysates were subjected to 12% SDS PAGE. After electrophoresis, the proteins were transferred onto a PVDF membrane (Merck Millipore, Billerica, Mass., USA) and probed with appropriate primary and secondary antibodies conjugated with horseradish peroxidase and visualized by the ECL detection system (Pierce, Bonn, Germany) [19]. Quantitative signal density analyses were performed by Quantity One software V4.4.0 and are indicated as average density in arbitrary units (BioRad, Munich, Germany).
Ca2+ Mobilization Assay
The CD14+ subpopulation of MUTZ-3 cells was enriched by MACS using CD14 MicroBeads as described above. CD14+ MUTZ-3 cells, monocytes or PMN were loaded with the Ca2+-sensitive fluorogenic dye FLUO-3/AM (2 µM, Molecular Probes, Eugene, Oreg., USA) as previously described [19]. Briefly, 1 × 106 cells/ml were incubated with FLUO-3/AM in medium for 60 min at 37°C and washed twice. Stimuli were added as indicated and fluorescence signals acquired by flow cytometry.
Statistical Analysis
Different groups were analyzed by a two-tailed Student's t test for comparison between two groups or by one-way ANOVA with Bonferroni's post test as indicated. For all analyses, p < 0.05 was considered as statistically significant. All statistical analyses were performed using GraphPad Prism (version 5.0a for Mac OS X, GraphPad Software, San Diego, Calif., USA, www.graphpad.com).
Results
TREM-1 Is Variably Expressed on AML Blasts of Myelomonocytic Differentiation
The expression of TREM-1 has been associated with a mature stage of myeloid development [30]. High levels of TREM-1 mRNA expression levels on primary CD14+ monocytes, AML M5 blasts or differentiated U937 cells have been observed in studies that were, however, based on the detection of mRNA by PCR or Northern blot. Hence, no conclusions on TREM-1 expression at protein level can be made.
Therefore, we analyzed a series of patients diagnosed with AML at our center between 1999 and 2003 for TREM-1 expression by flow cytometry. Expression of TREM-1 was quantified as percentage of TREM-1-positive blasts. As summarized in table 1, these AML patient samples included AML at various stages of myeloid differentiation, indicated by FAB subtypes. We observed significant TREM-1 expression (defined as >20% positive blasts) in eight of 17 AMLs with a preference for myelomonocytic (M4) or monocytic differentiation (M5), which is in line with Gingras et al. [30]. Nevertheless, the TREM-1 expression levels were variable and were not consistent in all patients, possibly due to lack of gene expression or receptor shedding as described for mature monocytes [22, 31]. In conclusion, TREM-1 is expressed on protein level in primary AML samples.
Table 1.
Expression of TREM-1 on AML cells by flow cytometry
| Patient No. | Diagnosis | Age, years | TREM-1-positive cells, % |
|---|---|---|---|
| 1 | AML n.o.s. | 53 | 41.4 |
| 2 | AML n.o.s. | 42 | 12.8 |
| 3 | AML M2 | 31 | 2.4 |
| 4 | AML M2 | 67 | 25.7 |
| 5 | AML M2 | 57 | 6.3 |
| 6 | AML M3 | 41 | 2.1 |
| 7 | AML M4 | 73 | 87.5 |
| 8 | AML M4 | 71 | 8.8 |
| 9 | AML M4 | 56 | 53.6 |
| 10 | AML M4 | 46 | 36.8 |
| 11 | AML M4 | 55 | 10.0 |
| 12 | AML M4 | 70 | 14.9 |
| 13 | AML M4eo | 25 | 59.2 |
| 14 | AML M4eo | 30 | 3.8 |
| 15 | AML M5a | 51 | 23.6 |
| 16 | AML M5b | 62 | 39.2 |
| 17 | AML M6 | 53 | 4.3 |
n.o.s. = Not otherwise specified.
TREM-1 Is Functionally Expressed on the Monocytic AML Cell Line MUTZ-3
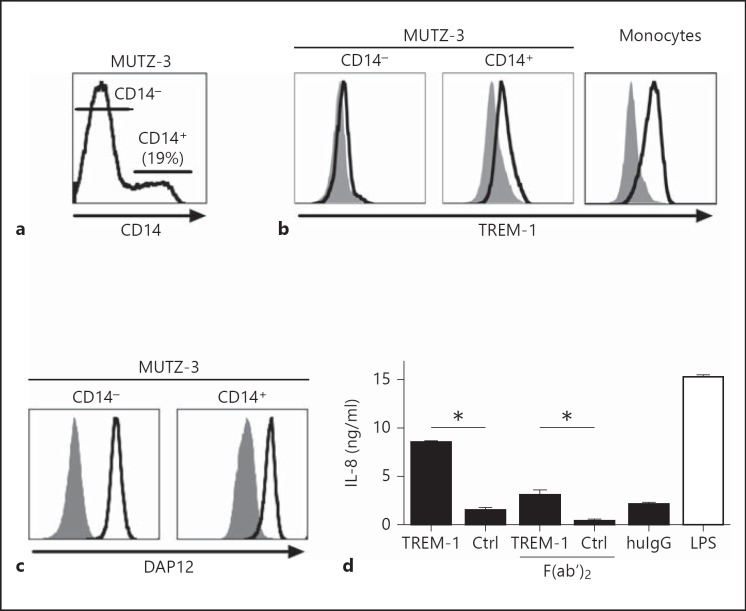
To understand the physiology of TREM-1 signal transduction in detail and to avoid in vitro artifacts related to ectopic receptor expression, we were interested in a cell line that naturally expresses TREM-1. Furthermore, the use of a cell line will reduce the risk of inconsistent results due to donor variability when using primary cells. Since we found TREM-1 expression in AML samples of monocytoid differentiation, we analyzed the AML cell line MUTZ-3 for expression of TREM-1 by flow cytometry. Consistent with previous reports [27, 32], MUTZ-3 cells harbor three distinct subpopulations: proliferating CD14-CD34+ cells as well as nonproliferating CD14-CD34- and CD14+CD34- cells (data not shown). Gating on CD14+ or CD14- subpopulations (fig. 1a, b), we detected TREM-1 expression only on the CD14+ subpopulation, but not on CD14- MUTZ-3 cells using a specific mAb compared to an isotype-matched control. The expression of TREM-1 on MUTZ-3 cells was stable, but was somewhat weaker than monocytes (fig. 1b). Since DAP12 is the essential adaptor protein required for TREM-1 signal transduction [3], we analyzed DAP12 expression in MUTZ-3 cells. As an important prerequisite for functional studies and in contrast to TREM-1 and CD14 expression, we found that DAP12 was uniformly expressed on MUTZ-3 cells (fig. 1c).
Fig. 1.
TREM-1 is expressed and functional on CD14+ MUTZ-3 cells. a Surface expression of CD14 on MUTZ-3 cells identifies a CD14+ and a CD14- subpopulation. b Labeling with anti-TREM-1 (clone 6B1, open histogram) versus an isotype-matched control mAb (Ctrl; clone 4C9, filled histogram) was determined gating on the CD14- or CD14+ subpopulations of MUTZ-3 cells or on purified CD14+ monocytes. c The expression of DAP12 (open histogram) versus an isotype-matched control Ab (filled histogram) was analyzed gating on the CD14- and CD14+ subpopulations of MUTZ-3 cells. d MUTZ-3 cells (5 × 105 cells/ml) were stimulated with coated anti-TREM-1 (clone 6B1), control mAb (clone 4C9), F(ab')2 fragments of anti-TREM-1 (clone 6B1) or control mAb (clone 4C9), human IgG (huIgG) (each at 10 µg/ml) or LPS (100 ng/ml). The release of IL-8 was quantified with ELISA after 16 h of stimulation. For all data shown, one representative result (mean + SD) of 3 independent experiments is depicted. * p < 0.05, a significant difference by two-tailed Student's t test.
In monocytes, TREM-1 ligation activates various effector mechanisms including the release of inflammatory mediators like IL-8 or TNF-α in synergy with various TLR agonists [14, 15]. To address whether TREM-1 on MUTZ-3 cells is functionally active, we stimulated the cells with anti-TREM-1 alone or in combination with LPS for comparison and analyzed the release of IL-8 or TNF-α. As shown in figure 1d, we found a TREM-1-specific release of IL-8 compared to the control Ab, while we were unable to detect TNF-α production after TREM-1 ligation of MUTZ-3 (not shown). Since MUTZ-3 cells express the high-affinity IgG receptor CD64, we wanted to confirm that the release of IL-8 was not caused by Fc receptor-mediated effects. Therefore, we used F(ab')2 fragments of the TREM-1-specific mAb or the control mAb for stimulation and found a comparable release of IL-8, allowing us to conclude that the observed effects are TREM-1-specific and not FcR-specific.
Ligation of TREM-1 Induces Phosphorylation of Akt and the MAP Kinase p38MAPK in Monocytic Cells
Next, we asked whether TREM-1-specific signal transduction pathways in MUTZ-3 cells are activated in a way comparable to that in primary monocytes. Engagement of TREM-1 induces the phosphorylation of ERK1/2 and PLC-γ in monocytes or PMN [3, 21]. In addition, we and others have previously shown a major role for PI3K and p38MAPK after TREM-1 ligation in PMN [19, 20].
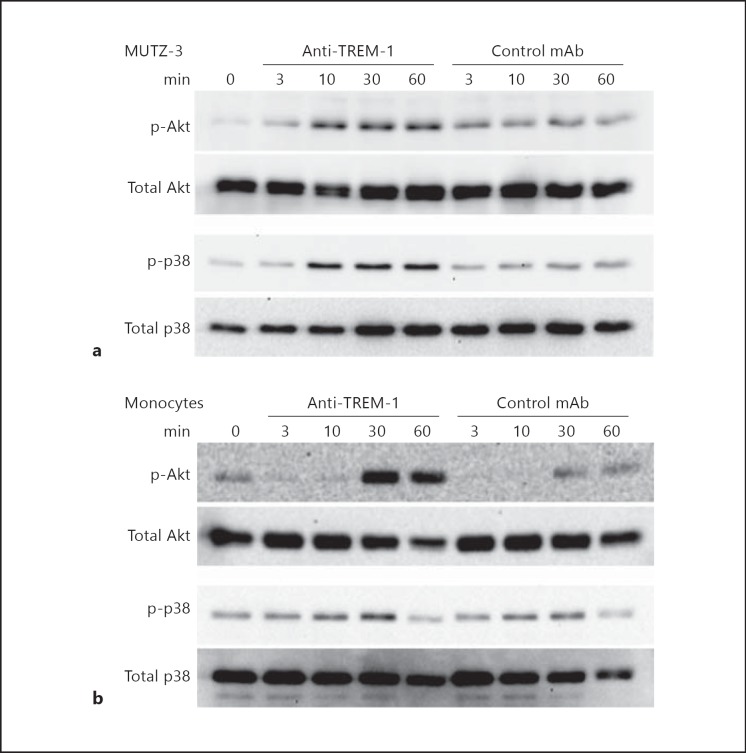
We were therefore interested in TREM-1-specific phosphorylation patterns of Akt as a downstream target of PI3K and the MAP kinase p38MAPK. MUTZ-3 cells were stimulated with anti-TREM-1 and phosphorylation of the respective signaling molecules was analyzed by Western blot over time. As shown in figure 2a and in the densitometric analysis in online supplementary figure 1A (for all online suppl. material, see www.karger.com/doi/10.1159/000355892), we observed an increased phosphorylation of Akt as well as of p38MAPK starting 10 min after receptor ligation while only minor background activity was detectable with the isotype-matched control Ab. This indicates that both molecules are involved in TREM-1 signaling in MUTZ-3 cells. For comparison, we analyzed the activation of Akt and p38MAPK in primary monocytes (fig. 2b and the densitometric analysis in online suppl. fig. 1B). Here we observed a similar TREM-1 specific phosphorylation pattern for p38MAPK starting after 10 min as described for MUTZ-3 cells. In contrast, the maximal Akt phosphorylation in primary monocytes occurred later, at 30 min after TREM-1 ligation. Nevertheless, these results clearly indicate that TREM-1 signaling patterns in monocytic MUTZ-3 cells and primary monocytes are very similar and both involve Akt and the MAP kinase p38MAPK.
Fig. 2.
TREM-1 ligation results in phosphorylation of Akt and p38MAPK in monocytic cells. MUTZ-3 cells (a) or CD14+ monocytes (b) (both 1 × 106 cells/ml) were stimulated with anti-TREM-1 (clone 6B1) or control mAb (clone 4C9) (both 10 µg/ml) for the indicated time points. Whole cell lysates were resolved by 12% SDS-PAGE and subsequently analyzed by Western blot against phosphorylated (p-) and total Akt or p38MAPK as indicated. These results are representative of 3 independent experiments.
PI3K and p38MAPK Are Central Regulators of TREM-1 Signal Transduction
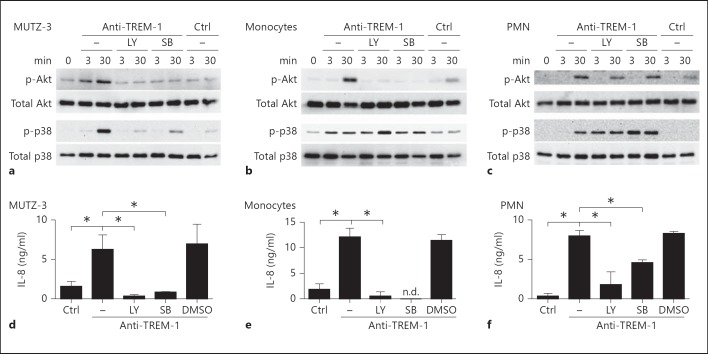
In PMN, engagement of TREM-1 leads to the activation of two distinct signaling pathways that are either dependent on PI3K or p38MAPK [19]. In our previous work in PMN, we were able to differentiate early (at 3 min) from late (at 30 min) phosphorylation patterns of Akt or MAP kinases after TREM-1 ligation. Therefore, we chose to compare the activation of Akt and p38MAPK at these time points in MUTZ-3 cells, primary monocytes or PMN, respectively. The cells were stimulated with anti-TREM-1 in the absence or presence of specific inhibitors for PI3K or p38MAPK. As expected, phosphorylation of Akt in MUTZ-3 cells was abrogated in the presence of the PI3K inhibitor at early or later time points (fig. 3a, upper panel and the densitometric analysis in online suppl. fig. 2A). Surprisingly, activation of p38MAPK was also strongly reduced, indicating that PI3K is involved in the activation of this MAP kinase in MUTZ-3 cells (lower panel). Conversely, activation of Akt was also diminished in the presence of the p38MAPK inhibitor, suggesting a bidirectional connection between these two kinases.
Fig. 3.
PI3K and p38MAPK have an essential role in TREM-1-mediated activation of Akt in monocytic cells. MUTZ-3 cells (a, d), monocytes (b, e) or PMN (c, f) were stimulated with anti-TREM-1 (clone 6B1) or control mAb (clone 4C9; Ctrl) (both 10 µg/ml) in the absence or presence of PI3K inhibitor (LY294002; LY) and p38MAPK inhibitor (SB203580; SB). a-c The phosphorylation of Akt and p38MAPK was analyzed after 3 and 30 min. These results are representative of at least 2 independent experiments. d-f The release of IL-8 was determined with ELISA after 16 h for MUTZ-3 cells (106 cells/ml) and 8 h for PBMC or PMN (both 1 × 106 cells/ml). The summarized results of 3 independent experiments plus SD are depicted. n.d. = Not detectable. * p < 0.05, a significant difference by one-way ANOVA with Bonferroni's post test between the indicated groups.
To validate our results in primary cells, we treated monocytes or PMN as before and analyzed the phosphorylation of Akt and p38MAPK after TREM-1 ligation. As shown before in MUTZ-3 cells, the phosphorylation of Akt in monocytes (fig. 3b and the densitometric analysis in online suppl. fig. 2B) or PMN (fig. 3c and the densitometric analysis in online suppl. fig. 2C) was strongly reduced in the presence of the PI3K inhibitor. However, in contrast to MUTZ-3 cells, the PI3K inhibitor did not affect the activation of p38MAPK in monocytes. On the contrary, the early phosphorylation of p38MAPK was enhanced in the presence of the respective inhibitor, indicating a compensatory activation of the p38 pathway. Nevertheless, in the presence of the p38MAPK inhibitor, the activation of Akt was completely abrogated in primary monocytes identically to how it was observed for MUTZ-3 cells, clearly placing Akt downstream of p38MAPK in monocytic cells.
In contrast, in PMN, we were still able to detect phosphorylation of Akt in the presence of the p38MAPK inhibitor (fig. 3c), indicating that the activation of Akt can occur at least partially independently of p38MAPK in PMN. Importantly, PMN viability in the presence of the respective inhibitors or vehicle control was not affected (online suppl. fig. 3). Moreover, PI3K and p38MAPK inhibitor completely abrogated the TREM-1-specific release of IL-8 in MUTZ-3 cells, monocytes or PMN in the presence of either inhibitor, respectively (fig. 3d), indicating that TREM-1 signaling pathways in monocytic MUTZ-3 cells, primary monocytes and PMN are related but apparently do not follow the same signaling patterns.
TREM-1 Signaling Induces Ca2+ Mobilization in Monocytic Cells and PMN
TREM-1 ligation induces a rapid Ca2+ mobilization in monocytes and PMN [3, 19]. Therefore we asked whether the engagement of TREM-1 also involves Ca2+ mobilization in MUTZ-3 cells. For this purpose, CD14+ MUTZ-3 cells, monocytes or PMN were loaded with the Ca2+-sensitive fluorogenic dye FLUO3-AM. TREM-1 ligation was induced by anti-TREM-1 crosslinked via anti-mouse F(ab')2 fragments as indicated. As shown in figure 4 (left panel), a robust Ca2+ flux was detectable over time after TREM-1 ligation on MUTZ-3 cells, monocytes or PMN, respectively, while there was no detectable background activity using an isotype-matched control Ab. Thus TREM-1 specific Ca2+ mobilization also occurs in MUTZ-3 cells. Since we mapped PI3K and p38MAPK as key regulators of the TREM-1 signaling cascade in PMN, we also asked whether activation of PI3K or p38MAPK is required for Ca2+ mobilization in monocytic cells. So we preincubated MUTZ-3 cells or primary monocytes with the previously mentioned pharmacological inhibitors for PI3K or p38MAPK, respectively, and monitored Ca2+ flux as before. We were unable to detect any significant Ca2+ signal in MUTZ-3 cells, monocytes or PMN after TREM-1 ligation in the presence of the PI3K inhibitor (LY) (fig. 4). In contrast, in the presence of the p38MAPK inhibitor, the Ca2+ signal in PMN was unaffected, while we detected no or only minor residual Ca2+ flux in MUTZ-3 cells or monocytes, respectively. This indicates that PI3K as well as p38MAPK signals are essential for Ca2+ mobilization in monocytic cells as opposed to PMN, where Ca2+ flux is only dependent on PI3K signals but not on p38MAPK signals [19].
Fig. 4.
TREM-1 induced Ca2+ mobilization is dependent on PI3K and p38MAPK in monocytic cells. The CD14+ subpopulation of MUTZ-3 cells enriched by MACS (upper panel), CD14+ monocytes (middle panel) or PMN (lower panel) (each 106 cells/ml) were loaded with the Ca2+-sensitive fluorogenic dye FLUO3-AM. Anti-TREM-1 (clone 6B1) or control mAb (clone 4C9) (both 10 µg/ml) and goat anti-mouse F(ab')2 (20 µg/ml) were added as indicated and Ca2+ flux was monitored over time in the absence or presence of PI3K (LY294002) or p38MAPK inhibitor (SB203580). The results from 1 representative of 3 independent experiments are depicted.
TREM-1 Cooperates with TLR, NLR and GM-CSF for IL-8 and TNF-α Production
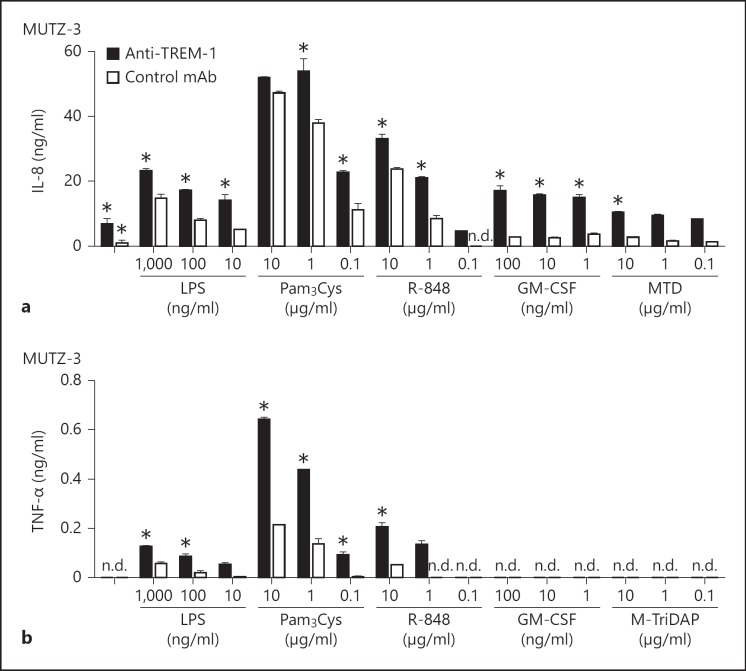
In monocytes, TREM-1 ligation induces the release of IL-8 or TNF-α in synergy with various TLR agonists [14, 15]. In MUTZ-3 cells, we found IL-8 but not TNF-α production after TREM-1 ligation (fig. 1d). To address whether the mechanisms of synergy between TREM-1 and other inflammatory stimuli are intact, we stimulated MUTZ-3 cells with anti-TREM-1 alone or in combination with various TLR agonists or GM-CSF and analyzed the release of IL-8 and TNF-α. Beyond the TREM-1-specific IL-8 release compared to control Ab, we detected cooperative IL-8 production upon ligation of TREM-1 and the TLRs 2 (Pam3Cys), 4 (LPS) and 7/8 (R-848) in a concentration-dependent manner. Interestingly, IL-8 production was also increased upon TREM-1 ligation in the presence of GM-CSF (fig. 5a). In contrast, we were unable to detect a release of TNF-α upon TREM-1 ligation alone (fig. 5b). Nevertheless, we found a significant increase in TNF-α production by MUTZ-3 cells after additional stimulation with agonists for TLR2, TLR4 or TLR7/8, indicating a truly synergistic activation pathway for TREM-1 and TLR. Interestingly, we did not observe any TNF-α production upon costimulation with anti-TREM-1 and GM-CSF, suggesting that TNF-α is no target gene of TREM-1- or GM-CSF-induced activation in MUTZ-3 cells, in contrast to IL-8. Nevertheless, stimulation of TREM-1 along with TLR agonists or GM-CSF occurs in a cooperative manner in MUTZ-3 cells, resembling the well-known IL-8 release pattern of primary monocytes or PMN (not shown) [3].
Fig. 5.
TREM-1 cooperates with TLR and GM-CSF, but not with NLR for IL-8 and TNF-α production in MUTZ-3 cells. MUTZ-3 cells (0.5 × 105 cells/ml) were stimulated with anti-TREM-1 (clone 6B1) or control Ab (clone 4C9) (both 10 µg/ml) in combination with TLR agonists, GM-CSF or M-Tri-DAP (MTD) as indicated. The release of IL-8 (a) or TNF-α (b) was quantified with ELISA after 16 h of stimulation. One representative of 3 independent experiments is depicted (mean + SD). n.d. = Not detectable. * p < 0.05, a significant difference between the indicated groups or compared to cells stimulated with anti-TREM-1 alone by one-way ANOVA with Bonferroni's post test.
Monocytes show an increased expression of costimulatory molecules upon ligation of TREM-1 [15]. To further investigate TREM-1-specific activation of monocytic MUTZ-3 cells, we analyzed the surface activation markers CD11b, CD11c, HLA-DR and CD86. The cells were incubated with anti-TREM-1 alone or in combination with TLR agonists or GM-CSF, respectively. In contrast to primary monocytes, we were unable to observe an altered expression of any of these markers compared to control Ab (data not shown). These data indicate that TREM-1 engagement on MUTZ-3 cells triggers the release of IL-8 or TNF-α in synergy with TLR agonists, but not the upregulation of surface activation markers.
PI3K and p38MAPK Are Essential for TREM-1, TLR and NLR Mediated Activation
Since we found an essential role for PI3K and p38MAPK in TREM-1-mediated IL-8 production in monocytes and MUTZ-3 cells, we were interested to know whether these two kinases were also involved in TLR- or NLR-mediated synergism with TREM-1. For this purpose, we stimulated MUTZ-3 cells with anti-TREM-1 or LPS in the additional absence or presence of the specific inhibitors for PI3K or p38MAPK. As shown in figure 6a (and in the densitometric analysis in online suppl. fig. 4A), we detected Akt as well as p38MAPK phosphorylation upon TREM-1 ligation that was strongly enhanced in the additional presence of LPS. Importantly, Akt phosphorylation was completely abrogated in the presence of the PI3K inhibitor and the p38MAPK inhibitor. In contrast, we observed no diminished p38MAPK phosphorylation in the presence of either inhibitor, suggesting altered signaling pathways upon TREM-1/TLR coligation compared to TREM-1 ligation alone (fig. 3a). Importantly, the synergistic release of IL-8 was significantly reduced by the PI3K inhibitor as well as the p38MAPK inhibitor, clearly indicating that both kinases are cooperatively involved in TREM-1/TLR-induced activation of MUTZ-3 cells (fig. 6c). Interestingly, while the IL-8 production after TREM-1 ligation alone was completely abrogated in the presence of the PI3K or p38MAPK inhibitors (fig. 3), we only observed partial effects upon TREM-1/TLR4 coligation, suggesting that alternative pathways are activated.
Fig. 6.
PI3K and p38MAPK are essential for LPS enhanced TREM-1 activation of monocytic cells and PMN. MUTZ-3 cells (a, c), CD14+ monocytes (b, d) or PMN (e) were stimulated with anti-TREM-1 (clone 6B1) or control Ab (Ctrl; 4C9) (both 10 µg/ml) in the absence or presence of LPS (100 ng/ml), PI3K inhibitor (LY294002; LY) and p38MAPK inhibitor (SB203580; SB). a, b The phosphorylation of Akt and p38MAPK were analyzed after 3 and 30 min. These results are representative of 2 independent experiments. c-e The release of IL-8 was determined with ELISA after 16 h for MUTZ-3 cells (5 × 105 cells/ml) and after 8 h for PBMC and PMN (both 1 × 106 cells/ml). The summarized results of 3 independent experiments plus SD are shown. n.d. = Not detectable. * p < 0.05, a significant difference by one-way ANOVA with Bonferroni's post test.
For comparison, we performed the analogous Western blot analyses (fig. 6b and the densitometric analysis in online suppl. fig. 4B) and IL-8 assays for primary monocytes or PMN after TREM-1 and TLR4 ligation with, in principle, identical results, confirming that the observed signaling pathways are comparable in the monocytic MUTZ-3 line and primary monocytes or PMN.
Since we observed the cooperative IL-8 production in MUTZ-3 cells or monocytes after coligation of TREM-1/NLR (fig. 5), we were interested in whether the mechanisms are also true for the cooperative effects of TREM-1 and NLRs. As shown in figure 7a (and in the densitometric analysis in online suppl. fig. 5A), the analysis by Western blot revealed the phosphorylation of Akt or p38MAPK after stimulation with anti-TREM-1 or the NOD1/2 agonist M-TriDAP, while we were unable to detect an enhanced pAkt signal after coligation. In contrast, we found an enhanced p38MAPK phosphorylation after coligation, which was even more evident in monocytes (fig. 7b and the densitometric analysis in online suppl. fig. 5B), suggesting that PI3K and p38MAPK are also important parts in the amplification of TREM-1/NLR signals. Similarly to the results obtained before with the TLR4 agonist, we observed a complete abrogation of Akt phosphorylation in the presence of the PI3K or p38MAPK inhibitor, while p38MAPK phosphorylation was only partially affected. However, IL-8 production by MUTZ-3 cells after TREM-1/NLR coligation was completely abrogated in the presence of either inhibitor, indicating nonredundant pathways for the cooperation of TREM-1 and NLRs.
Fig. 7.
PI3K and p38MAPK are essential for NLR-enhanced TREM-1 activation in monocytic cells. MUTZ-3 cells (a, c), CD14+ monocytes (b, d) or PMN (e) were stimulated with anti-TREM-1 (clone 6B1) or control mAb (Ctrl; clone 4C9) (both 10 µg/ml) in the absence or presence of the NLR agonist M-TriDAP (MTD, 10 µg/ml), PI3K inhibitor (LY294002; LY) and p38MAPK inhibitor (SB203580; SB). a, b The phosphorylation of Akt and p38MAPK was analyzed after 3 and 30 min. These results are representative of 2 independent experiments. c-e The release of IL-8 was determined with ELISA after 16 h for MUTZ-3 cells (0.5 × 105 cells/ml) and after 8 h for PBMC or PMN (both 1 × 106 cells/ml). The summarized results of 3 independent experiments plus SD are depicted. * p < 0.05, a significant difference by one-way ANOVA with Bonferroni's post test.
Collectively, these data suggest that PI3K and p38MAPK are central and common components of the TREM-1, TLR and NLR signaling pathways that are linked in a nonredundant fashion.
Discussion
Even though TREM-1 is expressed by PMN as well as by monocytes in a similar fashion and acts as an amplifier of inflammatory responses in both cell types [3, 5, 18, 33], there may still be significant differences in the function of TREM-1 in PMN or monocytes, respectively. To appropriately acknowledge the role of TREM-1 in innate host defense, it is important to understand the key signaling pathways involved in TREM-1-mediated activation.
Since previous studies that associated TREM-1 expression with a mature stage of myeloid development have been based on the detection of mRNA [30], we analyzed various AML of distinct FAB subtypes by flow cytometry for TREM-1 expression on protein level. Our data confirm that TREM-1 is expressed on AML blasts, although the level of expression seems variable, making it difficult to use TREM-1 as a diagnostic or prognostic marker. Further studies with more patients are necessary to evaluate this question.
While TREM-1 expression levels may vary in primary patient samples, we presently identify the AML cell line MUTZ-3 as a monocytic cell line expressing TREM-1 in a natural way. Consistent with a mature stage of myeloid development [30], we find that only the CD14+ subpopulation of MUTZ-3 is positive for TREM-1. To determine whether or not TREM-1 is also functional in MUTZ-3 cells, we show a TREM-1-specific release of IL-8 after receptor ligation which is comparable to primary monocytes or PMN as a valid functional read out for TREM-1 engagement [3, 14]. In line with previous data [14, 19, 24], we observe cooperative effects for IL-8 secretion after TREM-1 and TLR coligation as already mentioned for PMN and monocytes. In monocytes, TREM-1 ligation also mediates the release of TNF-α and the upregulation of surface activation markers like CD86 or HLA-DR [5, 15]. In contrast, we do not observe this in MUTZ-3 cells (fig. 5 and data not shown). However, while stimulation with various TLR agonists is also not able to induce upregulation of the indicated activation markers, we observed TNF-α production upon TREM-1/TLR coligation, indicating that the signaling pathways important for TREM-1/TLR synergy are still active. Nevertheless, this also suggests that TNF-α production or the upregulation of CD86 and HLA-DR in MUTZ-3 cells are, in short-term cultures, not regulated in a comparable way to primary monocytes, despite the fact that MUTZ-3 can differentiate in a cytokine-dependent manner into diverse dendritic-cell or osteoclast populations [27, 32, 34, 35].
Nevertheless, MUTZ-3 expresses TREM-1 in a natural and functional manner and might serve as model system to study TREM-1-induced signaling and transcription in monocytic cells. A technical advantage of studying TREM-1 signaling in a cell line like MUTZ-3 cells may include the independence on volunteer donors and donor variability that regularly impedes the interpretation of results when using primary cells. On the other hand, monocytic MUTZ-3 cells may also be of advantage over transfectants [25], as ectopic overexpression of TREM-1 may affect signaling pathways in an uncontrollable way. However, at this point, this is merely speculative and needs to be clarified with a direct comparison.
In line with previous data from PMN and monocytes [3, 14, 20], we observe TREM-1-specific phosphorylation of Akt and p38MAPK in MUTZ-3 cells and monocytes. In contrast to our previous results obtained in PMN [19], we have been unable to detect differences in the kinetics of Akt versus p38MAPK phosphorylation in MUTZ-3 cells or monocytes, already pointing to subtle differences in monocytic versus neutrophilic TREM-1 signaling. Our inhibitor assays clearly show the crucial importance of PI3K and p38MAPK for TREM-1-induced IL-8 production in MUTZ-3 cells as well as primary monocytes or PMN. However, the phosphorylation pattern of MUTZ-3 cells in the presence of the PI3K or the p38MAPK inhibitor indicates that PI3K and p38MAPK are regulated depending on each other (fig. 3). This clearly differs from PMN where we observe p38MAPK-independent phosphorylation of Akt. We observe that the TREM-1-specific production of IL-8 in PMN is dependent on p38MAPK. This differs from the regulation of the oxidative burst [19]. Beyond this, we find an enhanced p38MAPK phosphorylation in PMN in the presence of the p38MAPK inhibitor, suggesting a compensatory activation pathway by this inhibitor. However, this remains merely speculative and requires further investigation. In addition, comparing the phosphorylation patterns of MUTZ-3 cells with primary monocytes and PMN, we find that p38MAPK activation occurs upstream of PI3K/Akt phosphorylation in primary monocytes, pointing to a distinct hierarchy of signaling molecules in monocytic versus granulocytic cells. Even more differences become apparent when analyzing the cooperative signaling events induced by TREM-1 and TLR or NLR ligands in monocytic cells versus PMN.
In general, the activation of Akt is described as a direct downstream targeting of PI3K [36, 37], but there are findings suggesting that p38MAPK can also regulate PI3K-mediated Akt phosphorylation and activation [38]. Moreover, analyses of signaling cascades of various chemoattractants suggest a crosstalk between the p38MAPK and PI3K/Akt pathways in PMN [39, 40]. Monocyte migration towards TGF-β1 also requires the combined signaling of PI3K and p38MAPK [41]. Nevertheless, further work is needed to clarify whether p38MAPK directly activates Akt or if there is a crosstalk between PI3K and p38MAPK in monocytic cells and PMN after TREM-1 ligation.
Finally, we detect TREM-1-specific Ca2+ mobilization in MUTZ-3 cells (fig. 4) which is in line with previous reports demonstrating Ca2+ flux in monocytes [3] or PMN [19]. In contrast to PMN, where Ca2+ mobilization is only dependent on PI3K but not on p38MAPK [19], inhibition of PI3K and p38MAPK abrogates intracellular Ca2+ flux almost completely in MUTZ-3 cells and in monocytes. This clearly supports the hypothesis of a difference in the hierarchy of TREM-1-signaling molecules in monocytic cells and PMN.
A general caveat of such studies using pharmacological inhibitors is that there are potential off-target activities for which it is difficult to control. Therefore further studies using knock down approaches by RNAi or shRNA will be needed to further characterize these signaling patterns. However, transfection of PMN or primary monocytes may be difficult as these cell types become easily activated upon manipulation, i.e. by electroporation, also impeding interpretable results. Hence, it is important to establish MUTZ-3 as a TREM-1-expressing cell line suitable for such knockdown approaches.
We have established the myeloid cell line MUTZ-3 as a suitable model system to further study the TREM-1 signaling cascade in monocytic cells. We confirm that PI3K and p38MAPK are essential signaling molecules in primary monocytes as well as in MUTZ-3 cells with subtle differences in the hierarchy of PI3K and p38MAPK, while there are clear differences between TREM-1-induced Ca+ signaling in monocytic cells and PMN. These results enhance our current understanding of how the innate inflammatory responses are regulated and might contribute to the development of future concepts to treat severe inflammatory conditions such as sepsis.
Supplementary Material
Supplementary data
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Ra 988/4-2) to H.S. and M.P.R. and STA984/4-1 (to M.S. and M.P.R.), University Medical Center Mainz MAIFOR Program (to M.P.R.) and by the Federal Ministry of Education and Research (BMBF 01EO1003). The authors thank Andrea Drescher and Annekatrin Klaric for excellent technical assistance.
References
- 1.Radaev S, Kattah M, Rostro B, Colonna M, Sun PD. Crystal structure of the human myeloid cell activating receptor TREM-1. Structure. 2003;11:1527–1535. doi: 10.1016/j.str.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Kelker MS, Debler EW, Wilson IA. Crystal structure of mouse triggering receptor expressed on myeloid cells 1 (TREM-1) at 1.76 Å. J Mol Biol. 2004;344:1175–1181. doi: 10.1016/j.jmb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Bouchon A, Dietrich J, Colonna M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 4.Ford JW, McVicar DW. TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol. 2009;21:38–46. doi: 10.1016/j.coi.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 6.Gibot S, Massin F, Marcou M, Taylor V, Stidwill R, Wilson P, et al. TREM-1 promotes survival during septic shock in mice. Eur J Immunol. 2007;37:456–466. doi: 10.1002/eji.200636387. [DOI] [PubMed] [Google Scholar]
- 7.Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol. 2003;3:445–453. doi: 10.1038/nri1106. [DOI] [PubMed] [Google Scholar]
- 8.Klesney-Tait J, Keck K, Li X, Gilfillan S, Otero K, Baruah S, et al. Transepithelial migration of neutrophils into the lung requires TREM-1. J Clin Invest. 2013;123:138–149. doi: 10.1172/JCI64181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213:701–713. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Haselmayer P, Grosse-Hovest L, Landenberg von P, Schild H, Radsak MP. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood. 2007;110:1029–1035. doi: 10.1182/blood-2007-01-069195. [DOI] [PubMed] [Google Scholar]
- 11.Mezayen El R, Gazzar El M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007;111:36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibot S, Buonsanti C, Massin F, Romano M, Kolopp-Sarda M-N, Benigni F, et al. Modulation of the triggering receptor expressed on the myeloid cell type 1 pathway in murine septic shock. Infect Immun. 2006;74:2823–2830. doi: 10.1128/IAI.74.5.2823-2830.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derive M, Bouazza Y, Sennoun N, Marchionni S, Quigley L, Washington V, et al. Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis. J Immunol. 2012;188:5585–5592. doi: 10.4049/jimmunol.1102674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radsak MP, Salih HR, Rammensee H-G, Schild H. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J Immunol. 2004;172:4956–4963. doi: 10.4049/jimmunol.172.8.4956. [DOI] [PubMed] [Google Scholar]
- 15.Bleharski JR, Kiessler V, Buonsanti C, Sieling PA, Stenger S, Colonna M, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170:3812–3818. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 16.Tessarz AS, Cerwenka A. The TREM-1/DAP12 pathway. Immunol Lett. 2008;116:111–116. doi: 10.1016/j.imlet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Netea MG, Azam T, Ferwerda G, Girardin SE, Kim S-H, Dinarello CA. Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. J Leukoc Biol. 2006;80:1454–1461. doi: 10.1189/jlb.1205758. [DOI] [PubMed] [Google Scholar]
- 18.Gibot S, Kolopp-Sarda M-N, Béné MC, Bollaert P-E, Lozniewski A, Mory F, et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200:1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haselmayer P, Daniel M, Tertilt C, Salih HR, Stassen M, Schild H, et al. Signaling pathways of the TREM-1- and TLR4-mediated neutrophil oxidative burst. J Innate Immun. 2009;1:582–591. doi: 10.1159/000231973. [DOI] [PubMed] [Google Scholar]
- 20.Fortin CF, Lesur O, Fulop T. Effects of TREM-1 activation in human neutrophils: activation of signaling pathways, recruitment into lipid rafts and association with TLR4. Int Immunol. 2007;19:41–50. doi: 10.1093/intimm/dxl119. [DOI] [PubMed] [Google Scholar]
- 21.Tessarz AS, Weiler S, Zanzinger K, Angelisová P, Horejsí V, Cerwenka A. Non-T cell activation linker (NTAL) negatively regulates TREM-1/DAP12-induced inflammatory cytokine production in myeloid cells. J Immunol. 2007;178:1991–1999. doi: 10.4049/jimmunol.178.4.1991. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Pina V, Martinez E, Fernandez-Ruiz I, del Fresno C, Soares-Schanoski A, Jurado T, et al. Role of MMPs in orchestrating inflammatory response in human monocytes via a TREM-1-PI3K-NF- B pathway. J Leukoc Biol. 2012;91:933–945. doi: 10.1189/jlb.0711340. [DOI] [PubMed] [Google Scholar]
- 23.Fortin CF, Lesur O, Fulop T. Effects of aging on triggering receptor expressed on myeloid cells (TREM)-1-induced PMN functions. FEBS Lett. 2007;581:1173–1178. doi: 10.1016/j.febslet.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 24.Dower K, Ellis DK, Saraf K, Jelinsky SA, Lin L-L. Innate immune responses to TREM-1 activation: overlap, divergence, and positive and negative cross-talk with bacterial lipopolysaccharide. J Immunol. 2008;180:3520–3534. doi: 10.4049/jimmunol.180.5.3520. [DOI] [PubMed] [Google Scholar]
- 25.Ormsby T, Schlecker E, Ferdin J, Tessarz AS, Angelisová P, Köprülü AD, et al. Btk is a positive regulator in the TREM-1/DAP12 signaling pathway. Blood. 2011;118:936–945. doi: 10.1182/blood-2010-11-317016. [DOI] [PubMed] [Google Scholar]
- 26.Radsak MP, Taube C, Haselmayer P, Tenzer S, Salih HR, Wiewrodt R, et al. Soluble triggering receptor expressed on myeloid cells 1 is released in patients with stable chronic obstructive pulmonary disease. Clin Dev Immunol. 2007;2007:52040. doi: 10.1155/2007/52040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quentmeier H, Duschl A, Hu ZB, Schnarr B, Zaborski M, Drexler HG. MUTZ-3, a monocytic model cell line for interleukin-4 and lipopolysaccharide studies. Immunology. 1996;89:606–612. doi: 10.1046/j.1365-2567.1996.d01-780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quentmeier H, Zaborski M, Drexler HG. The human bladder carcinoma cell line 5637 constitutively secretes functional cytokines. Leuk Res. 1997;21:343–350. doi: 10.1016/s0145-2126(96)00132-4. [DOI] [PubMed] [Google Scholar]
- 29.Radsak MP, Hilf N, Singh-Jasuja H, Braedel S, Brossart P, Rammensee H-G, et al. The heat shock protein Gp96 binds to human neutrophils and monocytes and stimulates effector functions. Blood. 2003;101:2810–2815. doi: 10.1182/blood-2002-07-2261. [DOI] [PubMed] [Google Scholar]
- 30.Gingras M-C, Lapillonne H, Margolin JF. TREM-1, MDL-1, and DAP12 expression is associated with a mature stage of myeloid development. Mol Immunol. 2002;38:817–824. doi: 10.1016/s0161-5890(02)00004-4. [DOI] [PubMed] [Google Scholar]
- 31.Gómez-Piña V, Soares-Schanoski A, Rodríguez-Rojas A, del Fresno C, García F, Vallejo-Cremades MT, et al. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol. 2007;179:4065–4073. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 32.Santegoets SJAM, Masterson AJ, van der Sluis PC, Lougheed SM, Fluitsma DM, van den Eertwegh AJM, et al. A CD34+ human cell line model of myeloid dendritic cell differentiation: evidence for a CD14+CD11b+ Langerhans cell precursor. J Leukoc Biol. 2006;80:1337–1344. doi: 10.1189/jlb.0206111. [DOI] [PubMed] [Google Scholar]
- 33.Knapp S, Gibot S, de Vos A, Versteeg HH, Colonna M, van der Poll T. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J Immunol. 2004;173:7131–7134. doi: 10.4049/jimmunol.173.12.7131. [DOI] [PubMed] [Google Scholar]
- 34.Masterson AJ, Sombroek CC, de Gruijl TD, Graus YMF, van der Vliet HJJ, Lougheed SM, et al. MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood. 2002;100:701–703. doi: 10.1182/blood.v100.2.701. [DOI] [PubMed] [Google Scholar]
- 35.Ciraci E, Barisani D, Parafioriti A, Formisano G, Arancia G, Bottazzo G, et al. CD34 human hematopoietic progenitor cell line, MUTZ-3, differentiates into functional osteoclasts. Exp Hematol. 2007;35:967–977. doi: 10.1016/j.exphem.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, et al. The protein-kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 37.Burgering BMT, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 38.Rane MJ. p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J Biol Chem. 2000;276:3517–3523. doi: 10.1074/jbc.M005953200. [DOI] [PubMed] [Google Scholar]
- 39.Heit B, Tavener S, Raharjo E, Kubes P. An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J Cell Biol. 2002;159:91–102. doi: 10.1083/jcb.200202114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller BJ, et al. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- 41.Olieslagers S, Pardali E, Tchaikovski V, Dijke ten P, Waltenberger J. TGF-β1/ALK5-induced monocyte migration involves PI3K and p38 pathways and is not negatively affected by diabetes mellitus. Cardiovasc Res. 2011;91:510–518. doi: 10.1093/cvr/cvr100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data