Abstract
Circulating exosome-containing small RNAs have been demonstrated in vitro to be taken up by recipient cells and to alter gene expression through RNA interference. Here, we show that exosomes purified from various cancer cell lines as well as gel-purified exosomal and cellular miRNAs can induce pro-inflammatory cytokine expression in human peripheral blood mononuclear cells. Thus, circulating miRNAs may trigger innate immunity via pathogen recognition receptors, a new miRNA-activated pathway that merits some consideration.
Key Words: Toll-like receptors, Circulating miRNAs, Innate immunity, Cytokines
Introduction
Immune cells such as monocytes, macrophages, and dendritic cells sense microbe-derived nucleic acids through a subset of Toll-like receptors (TLRs) expressed in endosomal compartments [1]. These receptors include TLR-3, which recognizes double-stranded RNA, TLR-7 and TLR-8, which recognize single-stranded RNA, and TLR-9, which recognizes the unmethylated CpG motifs [2]. While short synthetic RNA such as small interfering RNAs (siRNAs) were originally thought to escape innate immune surveillance [3], we and others have demonstrated that they can be potent activators of endosomal TLR-7 and TLR-8 [4, 5, 6, 7]. In humans, TLR-7 and TLR-8 sense both double- and single-stranded siRNAs in a sequence-dependent manner, which leads to production of pro-inflammatory cytokines such as TNF-α and type 1 interferons [5, 8]. Usually endosomal TLRs do not respond to self-RNAs. This discrimination is mainly achieved by the endosomal localization of TLR-7 and TLR-8 [2]. Moreover, self-RNAs contain several naturally occurring 2′ modifications such as 2′O-methyl and pseudouridine modifications, which have been shown to abrogate TLR-7 and TLR-8 signaling [9, 10]. Similarly, 2′O-methyl, 2′-deoxy, and 2′-fluoro substitutions to the ribose backbone of synthetic RNAs suppressed immune stimulation, thus underlying the role of RNA modifications in immune escape [11, 12].
miRNAs are small noncoding RNA molecules that can regulate gene expression posttranscriptionally via RNA interference (RNAi). Though estimated to represent only 2% of the genome, miRNAs have been proposed to regulate as many as 60% of human genes [13]. Indeed, their sequence-specific requirements for mRNA recognition allow them to target multiple different transcripts and to function as pleiotropic regulators of gene expression [14, 15]. Recent studies have shown that, in addition to being expressed endogenously, miRNAs can be secreted from cells. These extracellular miRNAs are associated with lipid carriers known as exosomes, which are membrane-derived vesicles that originate from endosomal multivesicular bodies [15, 16]. The capacity of exosomes to transport miRNAs between cells and subsequently to modulate gene expression in the recipient cells further highlights their role as regulators of gene expression via the RNAi pathway [17, 18]. However, upon release from cells, exosomes may activate pathogen recognition receptors on target cells and/or endosomal TLRs subsequent to internalization by endocytosis. Having noted that DOTAP-formulated synthetic miRNA-21 can induce the expression of innate immune genes [[19], Cekaite and Sioud, unpubl. data], in the present study we investigated the immune stimulatory potency of exosome-derived miRNAs and cellular miRNAs from normal and cancer cells on TLR activation in human peripheral blood mononuclear cells (PBMCs). We show that miRNA-derived from cancer cells can stimulate innate immunity, while those obtained from blood leukocytes such as monocytes are immunologically inert.
Materials and Methods
Cells
PBMCs were obtained from buffy coats of healthy individuals and isolated by density gradient centrifugation (Lymphoprep; Nycomed Pharm, Oslo, Norway). Blood monocytes were prepared using plastic adherence as previously described [5]. Cancer cell lines were obtained from the American Type Culture Collection. Cells were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum and penicillin-streptomycin.
Exosomes and miRNA Isolation
Freshly isolated human monocytes and cancer cell lines were cultured in RPMI medium for 48 h. Subsequently, exosomes were isolated from the culture media using an ExoQuick-TC isolation kit (System Biosciences, Mountain View, Calif., USA) according to the manufacturer's instructions. Exosome pellets were gently washed and then resuspended in sterile PBS buffer. RNA was prepared from precipitated exosomes using Trizol extraction reagent (Gibco BRL). Cellular miRNAs from cancer cell lines and monocytes were prepared using a mirVana miRNA isolation kit (Ambion, Carlsbad, Calif., USA). Exosomal RNAs and cellular enriched miRNAs were analyzed using 15% polyacrylamide gels and the bands corresponding to miRNAs (18-40 nucleotides in length) were cut out, crushed into a fine slurry, and then incubated in RNase-free water at 37°C for 5 h with agitation as described elsewhere [20]. Subsequent to centrifugation at 12,000 g for 10 min, supernatants were collected and concentrated to 50 μl using a centricon-3 device and RNA concentrations were determined by a NanoDrop ND-1000 spectrophotometer.
Transfection and Exosome Treatment
PBMCs were seeded on 96-well plates at 2 × 105 cells/200 μl and transfected with exosome-derived miRNAs, cellular miRNAs, or synthetic miRNAs using N-[1-(2, 3-dioleoyloxy)propyl]-N,N,N,N-trimetylammonium methylsulfate (DOTAP; Roche Applied Biosciences) as described previously [5]. Purified exosomes from culture supernatants derived from 106 cells were added directly to PBMCs without DOTAP. After incubation for 18 h, the production of TNF-α and IFN-α in culture supernatants was analyzed with BD OptEIA ELISA sets according to the manufacturer's instructions (BD Biosciences).
Synthetic RNA Sequences
The following synthetic miRNA sequences were obtained from Eurofins MWG (Ebersberg, Germany). The expression of these miRNAs was altered during monocyte differentiation into dendritic cells [19].
miR21: 5′-UAGCUUAUCAGACUGAUGUUGA-3′
miR24: 5′-GUGCCUACUGAGCUGAUAUCAGU-3′
miR155: 5′-UUAAUGCUAAUCGUGAUAGGGGUU-3′
miR223: 5′-UCAGUUUGUCAAAUACCCCA-3′
miR320: 5′-CGCCUUCUCUUCCCGGUUCUUCC-3′
Western Blots
Around 106 cells were cultured at 37°C for 48 h and then exosomes were prepared from culture supernatants. Exosome pellets were solubilized in sample buffer (50 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5 mM EDTA, 0.02% bromophenol blue) and then separated on 10% SDS-PAGE. After electrophoresis, proteins were transferred onto nitrocellulose membranes and then incubated with mouse anti-human CD63 (TS63) monoclonal antibody. After washing, immunodetection was performed using HRP-conjugated anti-mouse IgG antibodies combined with the ECL detection system (GE Healthcare, Buckinghamshire, UK).
Statistical Analysis
Statistical analyses were conducted with Student's t test. p < 0.05 was considered statistically significant.
Results and Discussion
Exosomes Prepared from Breast Cancer Cell Lines Are Immunostimulatory
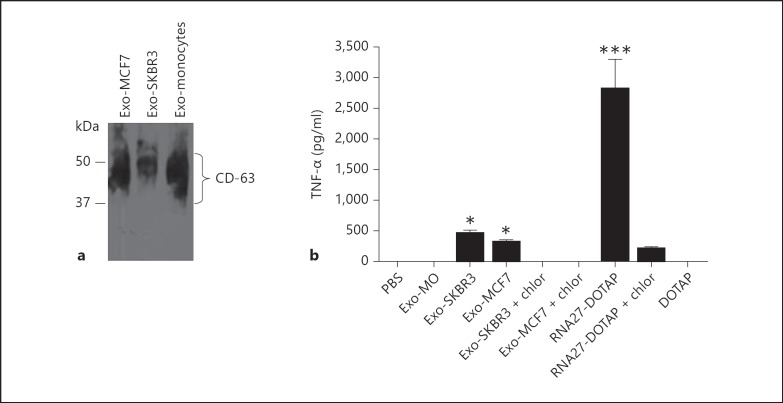
Recent studies support the involvement of circulating miRNAs in gene regulation in recipient cells through RNAi. To address the question whether they directly activate innate immune receptors, we first isolated exosomes from cell culture supernatants and then confirmed their identity by analyzing the presence of CD63, a commonly used marker of exosomes [17]. Strong signals for CD63 were present in all exosome preparations (fig. 1a as a representative example). Notably, the antibody recognizes the lysosomal integral membrane protein antigen, a 30- to 60-kDa (smear) protein [17].
Fig. 1.
Immunostimulatory potency of exosomes released from blood monocytes and breast cancer cell lines. a Western blot identification of the CD63 marker in exosome preparations. Purified exosomes from breast cancer cells lines (MCF7, SKBR3) and blood monocyte culture supernatants were solubilized in loading buffer and then analyzed by 10% SDS-PAGE. The membrane was probed with a monoclonal antibody against the CD63 marker. b SKBR3 and MCF7 exosomes induced TNF-α production in PBMCs. Human PBMCs were incubated with exosomes prepared from blood monocytes (Exo-MO), SKBR3 (Exo-SKBR3), and MCF7 (Exo-MCF7) culture supernatants as described in Materials and Methods. RNA27 formulated with DOTAP was used as a positive control [5]. Chloroquine (chlor, 2 μM) was added to the cells in order to inhibit endosome acidification. After overnight incubation, TNF-α was measured in culture supernatants by ELISA. Data are presented as means ± SD from triplicate determinations and are representative of at least 3 independent experiments. * p < 0.05, *** p < 0.001, in comparison to PBS-treated cells.
To determine whether exosomes activate innate immunity, we stimulated PBMCs with exosomes prepared from human monocytes and breast cancer cell lines. Exosomes derived from 106 cells were added directly to PBMCs. As a positive control, we have included RNA-27, which is known to activate endosomal TLR-7 and TLR-8 when formulated with DOTAP [5]. Exosomes prepared from monocytes did not induce any detectable level of TNF-α, whereas their counterparts prepared from breast SKBR3 and MCF7 cancer cells did (fig. 1b as a representative example). Likewise, exosomes prepared from ovarian SKOV-3 and colon SW480 cancer cell lines activated TNF-α production, while those prepared from PBMCs and monocyte-derived dendritic cells did not (data not shown). Collectively, the data suggest that exosome-dependent variation in RNA contents or lipid composition may play a role in triggering TLR signaling in PBMCs. As expected, RNA27 formulated with DOTAP exhibited strong immunostimulatory activity (p < 0.001). TNF-α induction with SKBR3 and MCF7 exosome preparations is more likely mediated by endosomal TLRs. Indeed, addition of chloroquine, an inhibitor of endosomal acidification, abolished immune stimulation (fig. 1b). Previous studies have shown that TLR-7 and TLR-8 activation by their ligands such as siRNAs occurs by acidification of the endosomal compartment following endocytosis of the ligands [2, 5]. This is thought to lead to receptor homodimerization, presumably through the ionization of histidine side chains [21]. The activation of TLRs with circulating exosomes is consistent with a recent study showing that miRNA21 and miRNA29a secreted by lung cancer cells in exosomes can activate innate immunity via TLR7/8 [22]. In addition, a study by Lehmann et al. [23] showed the activation of TLR7 in macrophages and microglia by extracellular miRNA let-7 leads to cytokine expression and neurodegenation [23]. It should be noted that we and others have shown that any immunostimulatory RNA sequence, regardless of its origin and structure (e.g. siRNA, miRNA, viral RNA, mRNA), can activate TLR7/8 once it is delivered to the endosomes [5, 6, 7, 8].
Exosome-Derived miRNAs and Cellular miRNAs Activated Endosomal TLRs
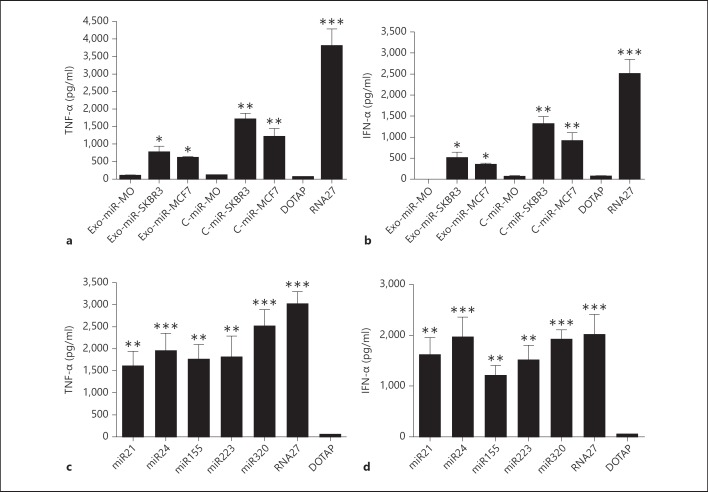
The potential activation of TLRs by exosome-derived miRNAs from cancer cell lines raises the question of whether cellular miRNA formulated with lipids would activate innate immunity. Therefore, we have examined the ability of gel-purified miRNAs to activate endosomal TLRs in PBMCs. In these experiments, cellular miRNAs from fleshly isolated blood monocytes, SKBR3 and MCF7 cancer cells were enriched using an miRNA isolation kit and then polyacrylamide gel-purified. We also gel-purified exosomal miRNAs. Both DOTAP-delivered exosomal miRNAs and cellular miRNAs from SKBR3 and MCF7 cells induced TNF-α and IFN-α production (fig. 2a, b as a representative example). Cytokine induction was also dependent on endosomal TLRs, because activity was abolished when PBMCs were co-treated with chloroquine (data not shown). Monocyte miRNAs did not stimulate immune response when formulated with DOTAP, suggesting that the immunostimulatory activity is cell-specific and may depend on the nature of the miRNAs present in the preparations [22]. Immunostimulation was also obtained with miRNAs prepared from cancer SKOV-3 and SW480 cells, but not with miRNAs prepared from PBMCs and monocyte-derived DCs (data not shown).
Fig. 2.
Induction of TNF-α and IFN-α by gel-purified miRNAs. Human PBMCs were transfected with purified exosomal miRNAs (Exo-miR), cellular miRNAs (C-miR) (a, b), or synthetic miRNAs (miR21, miR24, miR155, miR223, miR320) formulated with DOTAP (c, d). RNA27 was used as a positive control. All molecules were tested at 150 nM. After overnight incubation, TNF-α and IFN-α were measured in culture supernatants by ELISA. Data are presented as means ± SD from triplicate determinations and are representative of least 5 independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001, in comparison to DOTAP-treated cells. MO = Monocytes.
The activation of innate immunity by exosomal and cellular miRNA preparations prompted us to investigate the immunostimulatory potency of synthetic miRNAs. Using microarray we have noted that several innate immune genes can be activated by synthetic miRNA-21 [[19], Cekaite and Sioud, unpubl. data]. As shown in figure 2c and d, all tested sequences induced cytokine production in PBMCs when delivered to the endosomes via DOTAP. This response is not surprising given the high number of uridines in the tested miRNA sequences [11, 24]. The induction of TNF-α expression by exosomal and cellular miRNAs from SKBR3, MCF7, SKOV-3, and SW480 cancer cell lines implies that cellular and exosomal miRNAs do not contain naturally occurring modifications to avoid immune activation [9]. Certain nucleosides in native RNA become modified posttranscriptionally as part of their maturation process [10]. With respect to RNA modifications, we and others have shown that 2′-O-methyl nucleoside-modified synthetic RNAs can function as TLR-7 and TLR-8 antagonists [12, 25]. As such, these new agents could present therapeutic strategies for neutralizing TLR aberrant activation in inflammatory diseases and cancers. Comparable to siRNAs, chemically synthesized miRNAs formulated with cationic lipid DOTAP also triggered cytokine production. Avoiding the activation of innate immunity by synthetic miRNAs is important in the development of miRNA-based therapies.
The malignant potential of tumor cells is not only given by their genetic and epigenetic changes but it can also be promoted by chronic inflammation [26]. Tumor-infiltrating innate immune cells are composed of diverse cellular populations including macrophages, granulocytic and monocytic myeloid-derived suppressor cells, and myeloid-derived dendritic cells [27]. Inflammatory mediators released from TLR-expressing immune cells are key promoters of cancer-related inflammation. Indeed, several mouse and human studies have shown that high tumor-associated macrophages are mostly associated with poor patient prognosis and resistance to therapies [28, 29]. Based on these observations, the potential induction of inflammatory cytokines in innate immune cells by cancer-secreted miRNAs could play a major role in tumor growth and metastasis. Further understanding of the interactions between tumor cells and innate immune cells through secreted miRNAs may help to identify new therapeutic targets.
In summary, the observation that exosomes prepared from various cancer cell lines, but not normal blood leukocytes, can activate TLRs is interesting and adds more complexity to the cellular pathways that can be regulated by miRNAs. The induction of cytokines by exosome-derived miRNAs and cellular miRNAs, when formulated with DOTAP, would support the notion that endogenous miRNAs from cancer cells are not naturally modified in order to avoid TLR activation. Some gene silencing data obtained with exogenously delivered miRNAs should be re-evaluated with regard to the potential ability of miRNAs to activate innate immunity through TLR7/8. Defining which exosomal and cellular miRNAs may directly modulate immune activation through pathogen recognition receptors will help us to further understand miRNA-activated pathways.
Acknowledgements
We thank Dr. Morten Oksvold for the generous gift of the anti-CD63 monoclonal antibody. This study was supported in part by the Norwegian Cancer Society, and by the Gene Therapy program at the Norwegian Radium Hospital (M. Sioud).
References
- 1.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 3.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 4.Sioud M, Sørensen DR. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem Biophys Res Commun. 2003;312:1220–1225. doi: 10.1016/j.bbrc.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, Hartmann G. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–269. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 7.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNAs. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 8.Sioud M. Innate sensing of self and non-self. Trends in Mol Med. 2006;12:167–176. doi: 10.1016/j.molmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Hem M. Post-transcriptional nucleotide modification and alternative folding of RNA. Nucleic Acids Res. 2006;34:721–728. doi: 10.1093/nar/gkj471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sioud M. Single-stranded small interfering RNAs are more immunostimulatory than their double-stranded counterparts: a central role for 2′-hydroxyl uridines in immune responses. Eur J Immunol. 2006;36:1222–1230. doi: 10.1002/eji.200535708. [DOI] [PubMed] [Google Scholar]
- 12.Sioud M, Furseth G, Cekaite L. Suppression of immunostimulatory siRNA-driven innate immune activation by 2′-modified RNAs. Biochem Biophys Res Comm. 2007;361:122–126. doi: 10.1016/j.bbrc.2007.06.177. [DOI] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Turchinovick A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialzed function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 17.Vikers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23:91–97. doi: 10.1097/MOL.0b013e328350a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY. Secreted monocytic miR150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Cekaite L, Clancy T, Sioud M. Increased miR-21 expression during human monocyte differentiation into DCs. Front Biosci. 2010;2:818–828. doi: 10.2741/e143. [DOI] [PubMed] [Google Scholar]
- 20.Sioud M, Rosok O. Profiling microRNA expression using sensitive cDNA probes and filter arrays. Biotechniques. 2004;37:574–576. doi: 10.2144/04374ST01. [DOI] [PubMed] [Google Scholar]
- 21.Gibbart RJ, Morley PJ, Gay NJ. Conserved features in the extracellular domain of human toll-like receptor 8 are essential for Ph-dependent signalling. J Biol Chem. 2006;281:27503–27511. doi: 10.1074/jbc.M605003200. [DOI] [PubMed] [Google Scholar]
- 22.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann SM, Krüger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D, Habbel P, Kälin R, Franzoni E, Rybak A, Nguyen D, Veh R, Ninnemann O, Peters O, Nitsch R, Heppner FL, Golenbock D, Schott E, Ploegh HL, Wulczyn FG, Lehnardt S. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;5:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 24.Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Sousa CR. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur J Immunol. 2006;36:3256–3262. doi: 10.1002/eji.200636617. [DOI] [PubMed] [Google Scholar]
- 25.Robbins M, Judge A, Liang L, McClintock K, Yaworski E, MacLachlan I. 2′-O-methyl-modified RNAs act as TLR7 antagonists. Mol Ther. 2007;15:1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- 26.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophages subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aharinejad S, Sioud M, Lucas T, Abraham D. Targeting stromal-cancer cell interactions with siRNAs. Methods Mol Biol. 2009;487:243–266. doi: 10.1007/978-1-60327-547-7_12. [DOI] [PubMed] [Google Scholar]
- 28.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirström K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, Politi LS, Gentner B, Brown JL, Naldini L, de Palma M. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]