Abstract
The human antimicrobial peptide cathelicidin LL-37 has, besides its antimicrobial properties, also been shown to regulate apoptosis in a cell type-specific manner. Mechanisms involved in LL-37-regulated apoptotic signaling are not identified. Here, we show that LL-37 reduces the human osteoblast-like MG63 cell number and cell viability in the micromolar concentration range with an IC50 value of about 5 µM. Treatment with 4 µM LL-37 increased the number of annexin V-positive cells and stimulated activation of caspase 3 showing that LL-37 promotes apoptosis. Treatment with 4 µM LL-37 caused an acute and sustained rise in intracellular Ca2+ concentration assessed by laser-scanning confocal microscopy of Fluo-4-AM-loaded MG63 cells. LL-37 increased Ca2+ also in the presence of the respective L- and T-type voltage-sensitive Ca2+ channel blockers nifedipine and NiCl2. LL-37 had no effect on Ca2+ in cells incubated with Ca2+-free solution. LL-37 (4 and 8 µM) reduced the MG63 cell number both in the presence and absence of Ca2+ in the medium. In conclusion, LL-37 reduces the osteoblast cell number by promoting apoptosis, and furthermore, LL-37 stimulates Ca2+ inflow via a mechanism independent of voltage-sensitive Ca2+ channels. Interestingly, LL-37-induced lowering of the cell number seems to be mediated via a mechanism independent of Ca2+.
Key Words: Annexin V, Apoptosis, Calcium, Caspase 3, LL-37, Osteoblast
Introduction
The human antimicrobial peptide cathelicidin LL-37 is stored as the proprotein form hCAP-18 in neutrophils and epithelial cells until activated by the serine protease proteinase 3 and subsequently released [1, 2]. LL-37 is an amphiphilic α-helical cationic peptide possessing hydrophobic as well as hydrophilic properties [3]. It exerts a direct antimicrobial activity through disrupting the cell wall of both Gram-negative and Gram-positive bacteria causing bacterial cell lysis and by neutralizing lipopolysaccharides [4, 5, 6]. The cationic LL-37 molecule binds to negatively charged microbial membrane lipids thereby showing membrane selectivity [3]. Furthermore, LL-37 potentiates chemokine production by microbial stimuli in keratinocytes and other epithelial cells, suggesting that it enhances the immune defense of the skin [7].
LL-37 has, besides its antimicrobial and immune modulator activities, also been shown to affect various cellular functions, such as phagocytosis [8], cell differentiation [9] and apoptosis [10, 11, 12, 13, 14, 15, 16]. Importantly, the effects of LL-37 on apoptosis seem to be cell type specific; LL-37 promotes apoptosis in vascular smooth muscle cells [10], periodontal ligament cells [11], neutrophils [12], T cells [13] and airway epithelium [14] but suppresses apoptosis in keratinocytes [15] and dermal fibroblasts [16]. For neutrophils, LL-37 has been reported to exert both pro- and antiapoptotic effects [12, 17].
In periodontitis, which is a progressive inflammatory disease, the end stage is characterized by destruction of the alveolar bone leading to loss of teeth. Interestingly, the LL-37 concentrations are high (µM) in the gingival crevicular fluid from patients suffering from chronic periodontitis [18], suggesting that this peptide may influence the disease process. The bone-forming osteoblasts represent a very important cell type in both periodontal health and disease responsible for maintaining the alveolar bone mass [19].
The signaling pathways and mechanisms involved in LL-37-regulated apoptosis are not completely understood [3]. The Ca2+ ion is thought to play a role in cell killing and apoptosis, and dysfunctional regulation of Ca2+ homeostasis can result in apoptosis [20, 21], suggesting that Ca2+ may be involved in LL-37-induced proapoptotic signaling. LL-37 has been reported to interact with different cellular proteins such as the purinergic P2X7 receptor [17, 22] and the epidermal growth factor receptor [23, 24, 25], but LL-37 interacts also with DNA [26]. In the present study, we investigate effects of LL-37 on human osteoblast-like MG63 cell viability and Ca2+ signaling demonstrating that LL-37 induces apoptosis also in this cell type. Additionally, we demonstrate that LL-37 causes a rapid and sustained rise in the intracellular Ca2+ concentration through inflow of Ca2+ from the extracellular space, suggesting that LL-37 alters Ca2+ handling. Interestingly, the LL-37-induced reduction of the osteoblast cell number and cell viability seems to be mediated through a mechanism independent of Ca2+.
Materials and Methods
Cell Culture and Experimental Procedure
The human osteoblast-like MG63 cell line and the primary human osteoblast cell line hFOB 1.19 from the American Type Tissue Culture Collection (ATCC, Manassas, Va., USA) were cultured in DMEM/Ham's F12 (1:1) cell culture medium (Life Technologies, Invitrogen, Carlsbad, Calif., USA) supplemented with antibiotics (penicillin 50 U/ml, streptomycin 50 µg/ml) and 10% fetal calf serum in accordance with instructions from ATCC. The cells were kept in a water-jacketed cell incubator at 37°C under 5% CO2 in air. The MG63 cells express markers for osteogenic differentiation such as alkaline phosphatase enzyme activity and form mineralized nodules showing that they are representative for native osteoblasts [27, 28]. The cells were trypsinized (0.25% trypsin/EDTA) and reseeded upon reaching confluence. Medium was exchanged every second day. Experiments were performed on subconfluent cells (80% confluence). The cells were used for experiments in passages 3-10. Incubation with LL-37 caused similar effects irrespective of passage number. Before experiments fetal calf serum was omitted in order to standardize the experimental conditions. For experiments assessing the importance of Ca2+ for LL-37-induced effects on cell number, MG63 cells were incubated in either DMEM cell culture medium containing 1.8 mM Ca2+ or Ca2+-free DMEM medium, both from Life Technologies. The Ca2+-free DMEM medium was further supplemented with 1.8 mM MgCl2 to achieve isoosmolar conditions and to supplement with divalent cations to compensate for those lost by the omission of Ca2+. In order to achieve culture conditions with low Ca2+ concentration, the Ca2+-containing DMEM medium was supplemented with 1.8 mM of the Ca2+ chelator ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA, Sigma Chemicals, St. Louis, Mo., USA).
Assessment of Cell Number and Cell Viability
The number of cells was calculated in a Bürker chamber as appropriate. Cell viability was assessed using trypan blue exclusion test. The cells were incubated for 2 min with 0.4% trypan blue (Sigma) and then washed 3 times in 0.9% NaCl. Cells containing trypan blue were counted as a measure of dead/dying cells. Cell morphology was assessed in digital photographs from a Nikon TMS microscope equipped with a digital camera (Pixelink, Nikon, Nikon Nordic AB, Solna, Sweden).
Determination of Active Caspase 3
After washing in phosphate-buffered saline (PBS), cellular proteins were extracted using a protein extraction buffer provided in the active caspase 3 ELISA kit from R&D (R&D Systems, Minneapolis, Minn., USA). The amount of active caspase 3 was determined by ELISA according to the manufacturer's instructions and normalized to total protein in each sample measured by a Bio-Rad protein assay kit (BioRad, Hercules, Calif., USA). Each sample was analyzed in duplicate.
Annexin V Flow Cytometry
Apoptosis was assessed by flow cytometry using Annexin V staining. Cells were washed with PBS and then incubated with FITC-labeled Annexin V and the fluorescent viability dye 7-aminoactinomycin D (7-AAD) using the FITC Annexin V apoptosis detection kit 1 (BD, Franklin Lakes, N.J., USA) according to the manufacturer's instructions. The proportion of Annexin V-positive/negative and 7-AAD-positive/negative cells was determined by flow cytometry using an Accuri C6 flow cytometer (BD). Cells in early stages of apoptosis are Annexin V positive and 7-AAD negative, whereas cells in later stages of apoptosis and necrosis stain positive for both Annexin V and 7-AAD. Gates for Annexin V- and 7-AAD-positive cells were set using fluorescence minus one control. 20,000 events were recorded in each sample.
Measurement of Intracellular Ca2+ Concentration
For determination of intracellular Ca2+ concentration, the cells were cultured on glass-bottom cell culture Petri dishes (MatTek, Ashland, Mass., USA). The cells were washed with PBS, incubated with the Ca2+-sensitive fluorescent dye Fluo-4-AM (3 µM, Invitrogen) for 40 min at room temperature, and then washed carefully. During the Ca2+ measurements the cells were incubated in an N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)-buffered salt solution containing 2.5 mM Ca2+. This Ca2+ concentration is somewhat higher than the plasma concentration of Ca2+ in healthy adults. We used HEPES-buffered salt solution with 2.5 mM Ca2+ only in these acute experiments. The composition of the HEPES-buffered salt solution was (mM): NaCl 135.5, KCl 5.9, CaCl2 2.5, MgCl2 1.2, HEPES 11.6 and glucose 11.5. In some experiments measurement of Ca2+ was performed under Ca2+-free conditions, i.e. in HEPES-buffered salt solution without CaCl2 and with the addition of 2 mM EGTA (Sigma). The experiments were performed at room temperature. Fluorescence was recorded using a laser-scanning confocal microscope (LSM 510 PASCAL, Carl Zeiss AG, Göttingen, Germany). The excitation and emission wavelengths were 488 and 505 nm, respectively. The Ca2+ measurements were performed on subconfluent (80% confluence) cells as an integrated signal from all cells (about 300 cells) within the visual field at ×100 magnification. The confocal pinhole setting was kept identical for all experiments.
Drugs
LL-37 was purchased from Bachem AG (Bubendorf, Switzerland) and dissolved in dimethyl sulfoxide (DMSO) according to the manufacturer's instructions. The L-type Ca2+ channel blocker nifedipine (Sigma), the thromboxane A2 analogue U46619 (Tocris Bioscience, Bristol, UK) and the P2X7 receptor antagonist AZ11645373 (Tocris) were dissolved in DMSO. The controls received vehicle, DMSO, as appropriate. The final concentration of DMSO was 0.1%.
Statistics
Values are presented as means ± SEM. Statistical significance was calculated using ANOVA and Student's two-tailed t test for unpaired comparisons with Bonferroni correction for post hoc analysis as appropriate. p values <0.05 were regarded to denote statistical significance.
Results
LL-37 Reduces MG63 Cell Number and Cell Viability
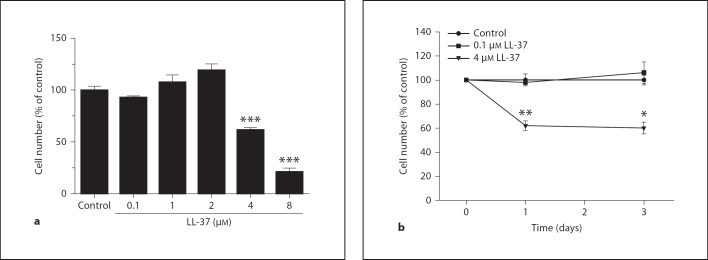
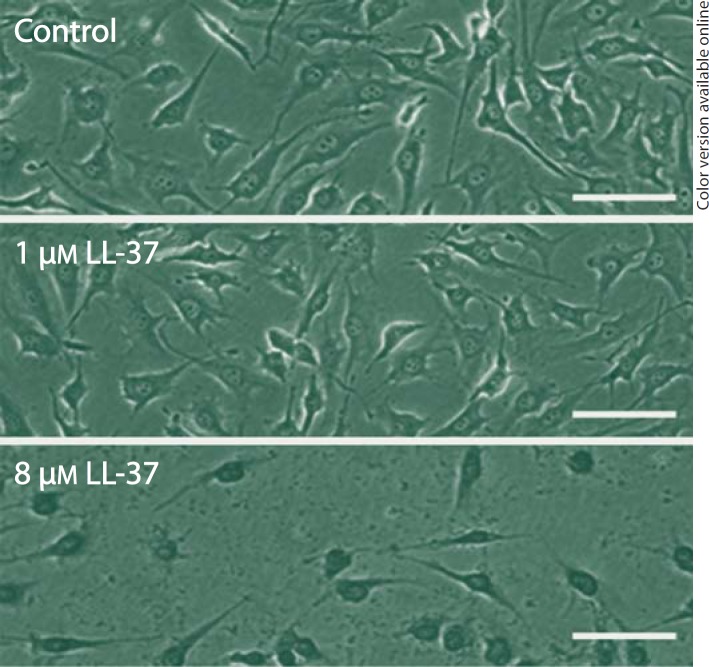
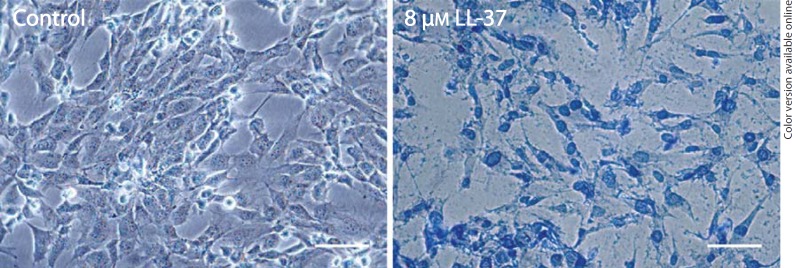
Treatment with LL-37 for 24 h reduced the number of human MG63 osteoblasts by 40 and 75% at 4 and 8 µM, respectively (fig. 1a). Lower concentrations of LL-37 (0.1-2 µM) had no effect on the number of cells (fig. 1a). LL-37 caused a concentration-dependent decrease in the cell number with an IC50 value of about 5 µM. Treatment with 4 µM LL-37 for 1 and 3 days reduced the MG63 cell number by about 40% (fig. 1b). A lower concentration (0.1 µM) of LL-37 had no effect on the cell number neither at 1 nor 3 days of treatment (fig. 1b). Staining with trypan blue to assess cell viability showed that treatment with LL-37 (8 µM) for 24 h reduced the cell number and caused alteration of cell morphology characterized by shrinkage of the cells representing classical signs of apoptosis (fig. 2). Furthermore, almost all cells remaining after treatment with 8 µM LL-37 contained trypan blue, which indicates that they represent dead/dying cells (fig. 2). No trypan blue-positive cells were observed in response to a lower concentration (1 µM) of LL-37 (fig. 2). The positive trypan blue staining observed in response to 8 µM LL-37 in MG63 cells was also confirmed in another human osteoblast cell line, hFOB 1.19 cells (fig. 3), demonstrating that micromolar concentrations of LL-37 reduce cell viability in two different human osteoblast cell lines.
Fig. 1.
a Treatment with LL-37 for 24 h reduces the MG63 osteoblast cell number in a concentration-dependent manner. LL-37 at 4 and 8 µM reduces the osteoblast cell number by 40 and 75%, respectively, while lower concentrations of LL-37 lack an effect. b Treatment with 4 µM LL-37 for 1 and 3 days reduces the MG63 cell number by about 40%. A lower concentration (0.1 µM) of LL-37 has no effect on the cell number neither at 1 nor 3 days of treatment. Values are means ± SEM of 4-15 observations in each group. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. controls.
Fig. 2.
Staining with trypan blue, assessing cell viability, shows that treatment with 8 µM LL-37 for 24 h reduces the MG63 cell number and causes alteration of cell morphology characterized by shrinkage of the cells. Furthermore, nearly all LL-37-treated cells contain trypan blue indicating that these cells represent dead/dying cells. No trypan blue-positive cells were observed in response to a lower concentration (1 µM) of LL-37. Control cells show normal morphology with no trypan blue-positive cells. Bars represent 25 µm.
Fig. 3.
Treatment with LL-37 (8 µM) for 24 h causes accumulation of trypan blue in primary human osteoblast hFOB 1.19 cells. Control cells show no trypan blue-positive cells. Trypan blue-positive cells represent dead/dying cells. Bars represent 20 µm.
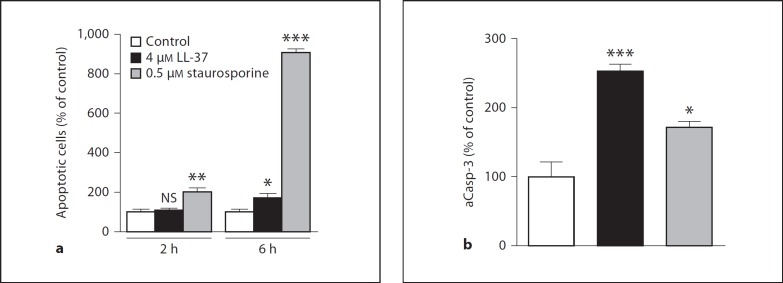
LL-37 Increases the Number of Annexin V-Positive Cells and Elevates Active Caspase 3 in MG63 Cells
Treatment with LL-37 (4 µM) for 6 h increased the proportion of Annexin V-positive MG63 cells by about 70% as demonstrated by flow cytometry (fig. 4a). The well-known proapoptotic agent staurosporine (0.5 µM) was included as a positive control [29]. Treatment with 0.5 µM staurosporine for 6 h increased the number of Annexin V-positive cells by about 9 times (fig. 4a). Staurosporine (0.5 µM) but not LL-37 (4 µM) increased the proportion of Annexin V-positive cells also at a shorter time point, i.e. 2 h (fig. 4a). Stimulation with LL-37 (4 µM) for 24 h increased the amount of active caspase 3 between 2 and 3 times in MG63 cells (fig. 4b). The positive control staurosporine (0.5 µM) increased active caspase 3 by about 2-fold (fig. 4b). Taken together these data show that LL-37 is proapoptotic for osteoblasts.
Fig. 4.
a Treatment with 4 µM LL-37 for 6 h increases the proportion of apoptotic MG63 cells. Apoptosis was determined by flow-cytometric analysis of Annexin V-positive cells. Staurosporine (0.5 µM) was included as positive control. b Treatment with 4 µM LL-37 for 24 h increases MG63 cellular active caspase 3 (aCasp-3) level 2-3 times. The amount of aCasp-3 was determined by ELISA and normalized to total protein in each sample. Values are means ± SEM of 3-4 observations in each group. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. controls. NS = Not significant.
LL-37 Increases MG63 Intracellular Ca2+ Concentration
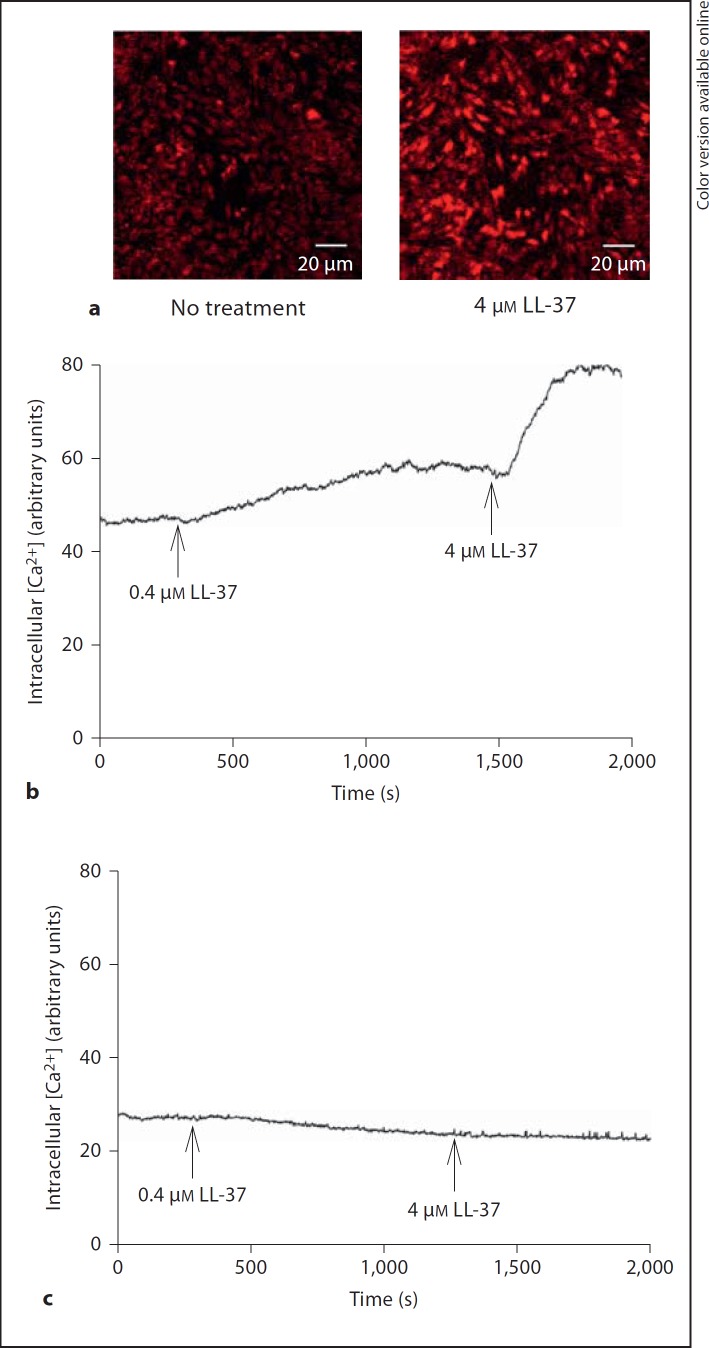
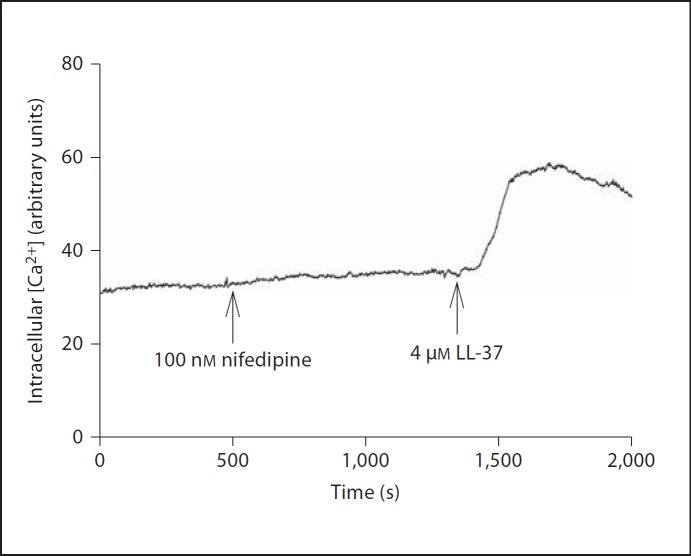
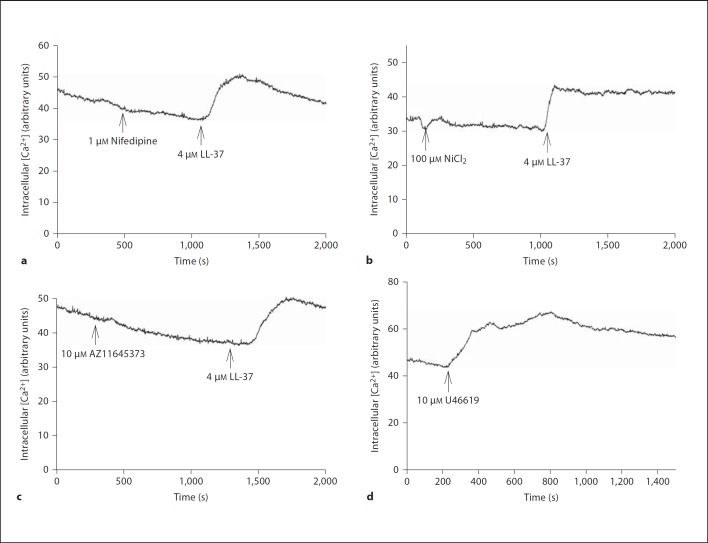
Defective cellular Ca2+ handling is thought to be involved in proapoptotic signaling [21], and therefore we assessed the effects of LL-37 on the intracellular Ca2+ concentration. Treatment with LL-37 (4 µM) caused an acute (within about 60 s) and sustained rise in intracellular Ca2+ concentration demonstrated by laser-scanning confocal microscopy of Fluo-4-AM-labeled MG63 cells incubated in Ca2+-containing (2.5 mM) HEPES-buffered salt solution (fig. 5a, b). A lower concentration of LL-37 (0.4 µM) also elevated Ca2+, but the Ca2+ response to 0.4 µM LL-37 was small compared to that of 4 µM (fig. 5b). The addition of vehicle control (0.1% DMSO) had no effect on Ca2+ (fig. 5a). LL-37 (0.4 and 4 µM) had no effect on intracellular Ca2+ concentration in Ca2+-free solution (fig. 5c), suggesting that the LL-37-induced increase in Ca2+ depends on inflow of extracellular Ca2+. Treatment with 100 nM and 1 µM of the L-type voltage-sensitive Ca2+ channel blocker nifedipine had no effect on the LL-37-induced Ca2+ response (4 µM LL-37), suggesting that LL-37 acts via another mechanism than by stimulation of Ca2+ inflow through L-type Ca2+ channels (fig. 6, 7a). The T-type Ca2+ channel blocker NiCl2 (100 µM) had no effect on the LL-37-induced (4 µM) Ca2+ response (fig. 7b), suggesting that inflow of Ca2+ by LL-37 is mediated through another pathway than via T-type Ca2+ channels. Furthermore, inclusion of the P2X7 receptor antagonist AZ11645373 (10 µM) had no impact on the rise in Ca2+ evoked by 4 µM LL-37 (fig. 7c). The thromboxane A2 analogue U46619 was included as positive control causing a rapid and powerful rise in intracellular Ca2+ concentration (fig. 7d).
Fig. 5.
a Treatment with LL-37 (4 µM) causes an acute and sustained rise in intracellular Ca2+ concentration assessed by laser-scanning confocal microscopy of Fluo-4-AM-loaded MG63 cells incubated in Ca2+ containing (2.5 mM) HEPES-buffered salt solution. No treatment (left panel) represents the Ca2+ signal in the presence of vehicle control (0.1% DMSO). Addition of 0.1% DMSO has no effect on Ca2+. The Ca2+ indicator Fluo-4-AM fluorescence is shown in red. b Line trace showing that both 0.4 and 4 µM LL-37 elevates Ca2+. c LL-37 (0.4 and 4 µM) has no effect on intracellular Ca2+ concentration in Ca2+-free solution. Ca2+-free conditions were achieved by removing CaCl2 from the HEPES-buffered salt solution and by inclusion of 2 mM EGTA. Each experiment was repeated at least twice.
Fig. 6.
LL-37 (4 µM) increases MG63 intracellular Ca2+ concentration monitored by laser-scanning confocal microscopy of Fluo-4-AM-loaded cells in the presence of the L-type Ca2+ channel blocker nifedipine (100 nM). Nifedipine was included at the arrow and present throughout the experiment. This trace shows one representative experiment out of two.
Fig. 7.
Treatment with 1 µM nifedipine (a), 100 µM NiCl2 (b) and 10 µM AZ11645373 (c) has no effect on the LL-37-induced (4 µM) Ca2+ response in MG63 cells. d The thromboxane A2 analogue U46619 (10 µM) was included as positive control causing a rapid and powerful rise in intracellular Ca2+ concentration. The intracellular Ca2+ concentration was monitored by laser-scanning confocal microscopy of Fluo-4-AM-loaded MG63 cells. Nifedipine, NiCl2 and AZ11645373 were included at the arrow and present throughout the experiment. Each experiment was repeated at least twice.
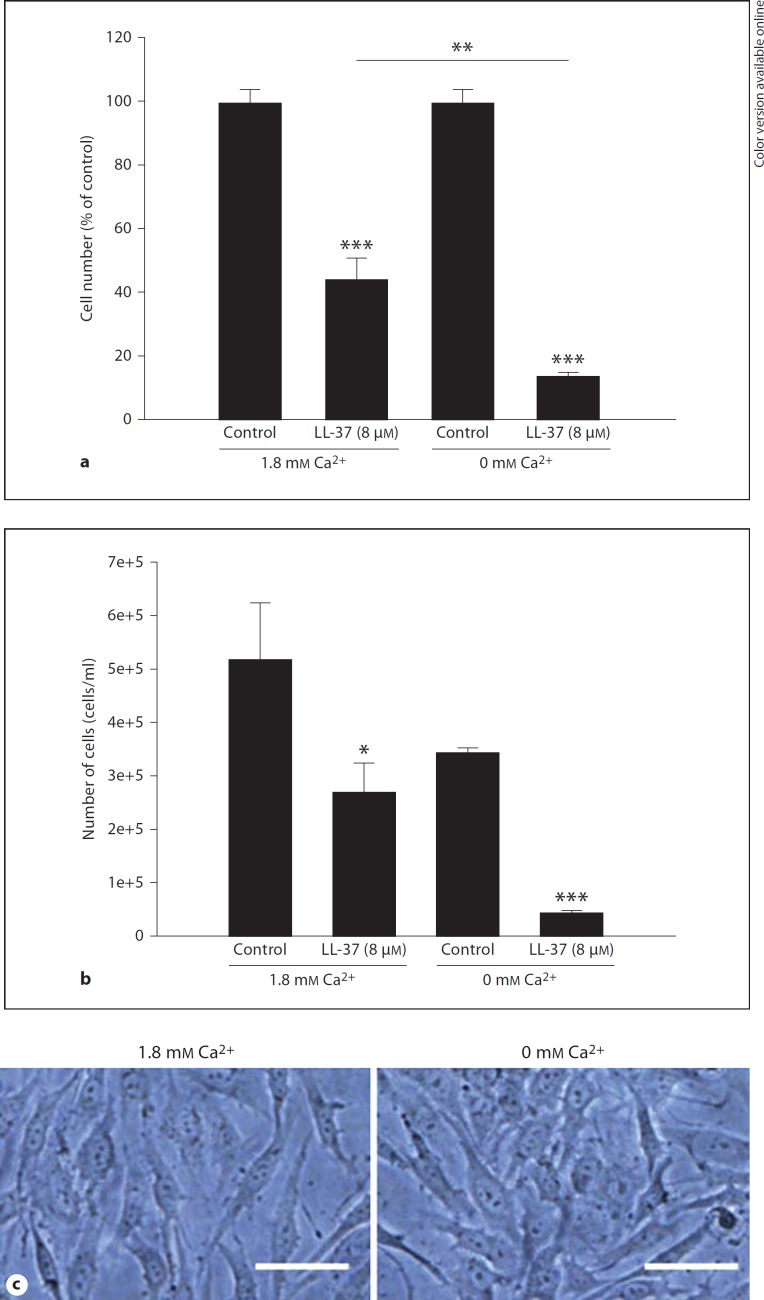
LL-37 Reduces MG63 Cell Number Both in the Presence and Absence of Extracellular Ca2+
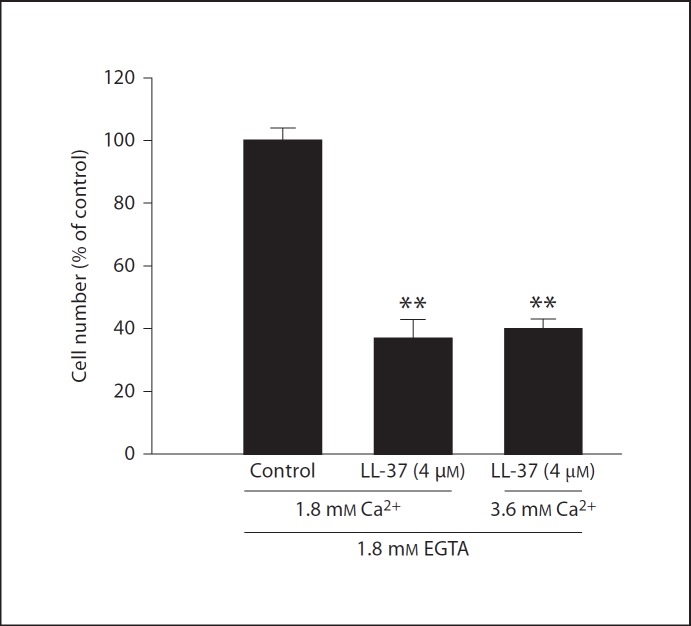
Treatment with LL-37 (8 µM) for 24 h reduced the number of MG63 cells by about 55% for cells cultured in DMEM culture medium containing 1.8 mM Ca2+ and by about 85% for cells cultured in Ca2+-free DMEM culture medium (fig. 8a). In fact, the LL-37-induced reduction of the cell number was more powerful (p < 0.01) in the absence than in the presence of extracellular Ca2+ (fig. 8a). A lower concentration of LL-37 (4 µM) reduced the cell number by about 15% in the presence (86 ± 2% for LL-37-treated cells vs. 100 ± 5% for control cells, p < 0.05, n = 3 in each group) and by about 35% in the absence (66 ± 7% for LL-37-treated cells vs. 100 ± 16% for control cells, p < 0.05, n = 3 in each group) of extracellular Ca2+. Omitting Ca2+ for 24 h reduced slightly, but not significantly, the number of cells as demonstrated when cell counts are plotted as absolute data (fig. 8b). The MG63 cells showed a similar morphology in the presence and absence of Ca2+ (fig. 8c). Treatment with LL-37 (4 µM) for 24 h reduced the cell number by about 60% when the Ca2+ chelator agent EGTA (1.8 mM) was included in the Ca2+-containing DMEM culture medium in order to lower Ca2+ (fig. 9). LL-37 (4 µM) also reduced the cell number by about 60% when extra Ca2+ (final Ca2+ concentration 3.6 mM) was included in the EGTA-containing medium (fig. 9). For these experiments EGTA was administered in an equimolar concentration to extracellular Ca2+ (1.8 mM) in order to bind most of the Ca2+ ions. Thus, LL-37-induced lowering of the osteoblast cell number is observed both when the cells are cultured under Ca2+-free conditions and when Ca2+ ions are absorbed with EGTA.
Fig. 8.
a Treatment with 8 µM LL-37 for 24 h reduces the MG63 cell number by about 55% for cells cultured in DMEM culture medium containing 1.8 mM Ca2+ and by 85% for cells cultured in Ca2+-free DMEM culture medium. b Omitting Ca2+ for 24 h reduces slightly, but not significantly, the number of cells as demonstrated when cell count data are plotted as absolute data. a, b Values are means ± SEM of 4-5 observations in each group. * p < 0.05, ** p < 0.001 vs. controls; ** p < 0.01 for LL-37-treated groups as indicated. c The MG63 cells show similar morphology in the presence and absence of Ca2+. Bars represent 20 µm.
Fig. 9.
Treatment with 4 µM LL-37 for 24 h reduces the cell number by about 60% for cells grown in culture medium (DMEM + 1.8 mM Ca2+) containing either 1.8 mM EGTA alone or 1.8 mM EGTA in combination with an excess of Ca2+ (3.6 mM Ca2+). Values are means ± SEM of 3 observations in each group. ** p < 0.01 vs. controls.
Discussion
In the present study, we demonstrate that the human antimicrobial peptide LL-37 induces apoptosis in human osteoblast-like MG63 cells and that this effect is associated with an elevated intracellular Ca2+ concentration. LL-37 causes, in the micromolar concentration range, an acute and sustained rise in intracellular Ca2+ in Ca2+-containing but not in Ca2+-free solution showing that the LL-37-induced increase in intracellular Ca2+ is due to an inflow of Ca2+ along its gradient from the extracellular space to the cytosol. The increase in Ca2+ by LL-37 was much stronger at 4 than at 0.4 µM, suggesting a concentration-dependent effect, although it is difficult to come to firm conclusions about the concentration dependence since we have investigated only two concentrations of LL-37. Membrane depolarization results in an inflow of Ca2+ from the extracellular space, causing a sustained and long-lasting increase in intracellular Ca2+[30] similar to that observed in response to LL-37 in the present study. The pattern of LL-37-induced rise in intracellular Ca2+ concentration is thus compatible with inflow of Ca2+ from the extracellular space. Osteoblasts (MC3T3-E1 cells and MG63 cells) have been reported to express voltage-sensitive Ca2+ channels [31, 32]. Our data show that neither the L-type Ca2+ channel blocker nifedipine nor the T-type Ca2+ channel blocker NiCl2 have an impact on the LL-37-evoked rise in Ca2+ in MG63 cells, suggesting that the inflow of Ca2+ triggered by LL-37 is through another mechanism than via voltage-sensitive L-type and T-type Ca2+ channels. We used relevant concentrations of nifedipine (0.1 and 1 µM), which fully inhibit the inflow of Ca2+ via L-type Ca2+channels in cultured vascular smooth muscle cells [30], and NiCl2 (100 µM) inhibiting T-type but not L-type Ca2+ channels [33]. Furthermore, inclusion of the selective and highly potent P2X7 receptor antagonist AZ11645373 [34] had no effect on the rise in Ca2+ evoked by LL-37, suggesting an alternative mechanism. Based on our findings presented here, we suggest that the LL-37-induced inflow of extracellular Ca2+ represents a novel signaling pathway for LL-37 that may involve LL-37-induced permeabilization of the cell membrane causing formation of transmembrane pores and/or that LL-37 acts as a detergent. These data implicate that LL-37 may work through a similar mechanism in human osteoblasts as in LL-37-induced permeabilization of the bacterial cell wall [3].
Here, we show for the first time that the antimicrobial peptide LL-37 reduces the human MG63 osteoblast cell number by promoting apoptosis. LL-37-induced apoptosis was demonstrated by both an enhanced proportion of Annexin V-positive cells and by an elevated active caspase 3 level in response to LL-37 treatment. Furthermore, LL-37-treated cells showed altered morphology such as cell shrinkage representing a classical sign of apoptosis. Previously, proapoptotic effects of LL-37 have been demonstrated in vascular smooth muscle cells, periodontal ligament cells, neutrophils, T cells and airway epithelial cells [10, 11, 12, 13, 14]. We demonstrate a reduction of the osteoblast cell number by LL-37 in the micromolar concentration range, while no effect on the cell number is observed at lower concentrations of LL-37. We have previously shown that LL-37 reduces lipopolysaccharide-induced MCP-1 and IL-6 expression at low concentrations (0.1 and 1 µM) and induces apoptosis only at high (>5 µM) concentrations in human periodontal ligament cells [11]. LL-37-induced proapoptosis is thus observed in different cell systems but only in the micromolar concentration range. Very high levels of LL-37 have been demonstrated in lesional tissue from patients suffering from autoimmune diseases such as psoriasis, rosacea and ulcerative colitis [35, 36, 37]. For example, the median concentration of LL-37 is 304 µM in psoriatic skin lesions [35]. The LL-37 levels are elevated locally in chronic periodontitis; in fact, they are well within the micromolar concentration range [18], suggesting that LL-37-induced apoptosis of osteoblasts may have an impact on alveolar bone homeostasis in patients suffering from this disease. Thus, we may conclude that the LL-37-induced apoptosis of osteoblasts, observed in the micromolar concentration range in the present study, is relevant for the in vivo situation considering the very high levels of LL-37 observed in various autoimmune and inflammatory diseases.
Ca2+ governs many important cellular processes and is thought to be involved in apoptosis [21, 38]. A rise in intracellular Ca2+ may originate from intracellular stores such as the endoplasmic reticulum but also from the extracellular space [21]. Here, we show that LL-37 elevates intracellular Ca2+ concentration in human MG63 osteoblasts through stimulation of Ca2+ inflow. The LL-37-evoked inflow of Ca2+ seems not to be critically important for the LL-37-induced reduction of the MG63 cell number, since LL-37 lowers the cell number both in the presence and absence of Ca2+ in the cell culture medium and, furthermore, LL-37 causes a rise in intracellular Ca2+ but no apoptosis at a low concentration (0.4 µM). Ca2+-independent apoptosis has been described in different experimental systems [39, 40]. Interestingly, the LL-37-evoked inward flow of Ca2+, occurring independently of LL-37-induced apoptosis, may represent an important mechanism regulating Ca2+-dependent cellular processes governed by LL-37. Thus, we demonstrate here that LL-37 alters human osteoblast Ca2+ handling and induces Ca2+-independent apoptosis.
In summary, we show that LL-37 alters cellular Ca2+ homeostasis by causing stimulation of Ca2+ inflow through a voltage-sensitive L- and T-type Ca2+ channel-independent mechanism in human osteoblasts. Furthermore, we demonstrate that LL-37 induces apoptosis of osteoblasts via a mechanism that is independent of Ca2+.
Acknowledgements
This study was supported by grants from the Swedish Research Council, the Faculty of Odontology at Malmö University, the Medical Faculty at Lund University, the Swedish Dental Society, the Patent Revenue Fund and the foundations of Crafoord, Magnus Bergvall, Lars Hierta, Sven and Lilly Thuréus and Greta and Johan Kock.
References
- 1.Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 2.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 3.Burton MF, Steel PG. The chemistry and biology of LL-37. Nat Prod Rep. 2009;26:1572–1584. doi: 10.1039/b912533g. [DOI] [PubMed] [Google Scholar]
- 4.Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 6.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nijnik A, Pistolic J, Filewod NCJ, Hancock REW. Signaling pathways mediating chemokine induction in keratinocytes by cathelicidin LL-37 and flagellin. J Innate Immun. 2012;4:377–386. doi: 10.1159/000335901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Shively JE. Generation of novel bone forming cells (monoosteophils) from the cathelicidin-derived peptide LL-37 treated monocytes. PLoS One. 2010;5:e13985. doi: 10.1371/journal.pone.0013985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciornei CD, Tapper H, Bjartell A, Sternby NH, Bodelsson M. Human antimicrobial peptide LL-37 is present in atherosclerotic plaques and induces death of vascular smooth muscle cells: a laboratory study. BMC Cardiovasc Disord. 2006;6:49. doi: 10.1186/1471-2261-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jönsson D, Nilsson BO. The antimicrobial peptide LL-37 is anti-inflammatory and proapoptotic in human periodontal ligament cells. J Periodont Res. 2012;47:330–335. doi: 10.1111/j.1600-0765.2011.01436.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Cherryholmes G, Shively JE. Neutrophil secondary necrosis is induced by LL-37 derived from cathelicidin. J Leukoc Biol. 2008;84:780–788. doi: 10.1189/jlb.0208086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mader JS, Ewen C, Hancock RE, Bleackley RC. The human cathelicidin, LL-37, induces granzyme-mediated apoptosis in regulatory T cells. J Immunother. 2011;34:229–235. doi: 10.1097/CJI.0b013e318207ecdf. [DOI] [PubMed] [Google Scholar]
- 14.Barlow PG, Beaumont PE, Cosseau C, Mackellar A, Wilkinson TS, Hancock RE, Haslett C, Govan JR, Simpson AJ, Davidson DJ. The human cathelicidin LL-37 preferentially promotes apoptosis of infected airway epithelium. Am J Respir Cell Mol Biol. 2010;43:692–702. doi: 10.1165/rcmb.2009-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamorro CI, Weber G, Gronberg A, Pivarcsi A, Stahle M. The human antimicrobial peptide LL-37 suppresses apoptosis in keratinocytes. J Invest Dermatol. 2009;129:937–944. doi: 10.1038/jid.2008.321. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Cho DH, Lee KJ, Cho CS, Bang SI, Cho BK, Park HJ. LL-37 suppresses sodium nitroprusside-induced apoptosis of systemic sclerosis dermal fibroblasts. Exp Dermatol. 2011;20:843–845. doi: 10.1111/j.1600-0625.2011.01327.x. [DOI] [PubMed] [Google Scholar]
- 17.Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol. 2006;176:3044–3052. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- 18.Turkoglu O, Emingil G, Kutukculer N, Atilla G. Gingival crevicular fluid levels of cathelicidin LL-37 and interleukin-18 in patients with chronic periodontitis. J Periodontol. 2009;80:969–976. doi: 10.1902/jop.2009.080532. [DOI] [PubMed] [Google Scholar]
- 19.Lerner UH. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J Dent Res. 2006;85:596–607. doi: 10.1177/154405910608500704. [DOI] [PubMed] [Google Scholar]
- 20.Orrenius S, Nicotera P. The calcium ion and cell death. J Neural Transm Suppl. 1994;43:1–11. [PubMed] [Google Scholar]
- 21.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 22.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 23.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 24.von Haussen J, Koczulla R, Shaykhiev R, Herr C, Pinkenburg O, Reimer D, Wiewrodt R, Biesterfeld S, Aigner A, Czubayko F, Bals R. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59:12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Coffelt SB, Waterman RS, Florez L, Bentrup KH, Zwezdaryk KJ, Tomchuck SL, LaMarca HL, Danka ES, Morris CA, Scandurro AB. Ovarian cancers overexpress the antimicrobial protein hCAP-18 and its derivative LL-37 increases ovarian cancer cell proliferation and invasion. Int J Cancer. 2008;122:1030–1039. doi: 10.1002/ijc.23186. [DOI] [PubMed] [Google Scholar]
- 26.Nygren PA. Alternative binding proteins: affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J. 2008;275:2668–2676. doi: 10.1111/j.1742-4658.2008.06438.x. [DOI] [PubMed] [Google Scholar]
- 27.Kraus D, Deschner J, Jäger A, Wenghoefer M, Bayer S, Jepsen S, Allam JP, Novak N, Meyer R, Winter J. Human β-defensins differently affect proliferation, differentiation, and mineralization of osteoblast-like MG63 cells. J Cell Physiol. 2012;227:994–1003. doi: 10.1002/jcp.22808. [DOI] [PubMed] [Google Scholar]
- 28.Kawano M, Ariyoshi W, Iwanaga K, Okinaga T, Habu M, Yoshioka I, Tominaga K, Nishihara T. Mechanism involved in enhancement of osteoblast differentiation by hyaluronic acid. Biochem Biophys Res Commun. 2011;405:575–580. doi: 10.1016/j.bbrc.2011.01.071. [DOI] [PubMed] [Google Scholar]
- 29.Dezaki K, Maeno E, Sato K, Akita T, Okada Y. Early-phase occurrence of K+ and Cl− efflux in addition to Ca2+ mobilization is a prerequisite to apoptosis in HeLa cells. Apoptosis. 2012;17:821–831. doi: 10.1007/s10495-012-0716-3. [DOI] [PubMed] [Google Scholar]
- 30.Byron KL, Taylor CW. Spontaneous Ca2+ spiking in a vascular smooth muscle cell line is independent of the release of intracellular Ca2+ stores. J Biol Chem. 1993;268:6945–6952. [PubMed] [Google Scholar]
- 31.Bergh JJ, Shao Y, Puente E, Duncan RL, Farach-Carson MC. Osteoblast Ca2+ permeability and voltage-sensitive Ca2+ channel expression is temporally regulated by 1,25-dihydroxyvitamin D3. Am J Physiol Cell Physiol. 2006;290:C822–C831. doi: 10.1152/ajpcell.00403.2005. [DOI] [PubMed] [Google Scholar]
- 32.Burns DM, Stehno-Bittel L, Kawase T. Calcitonin gene related peptide elevates calcium and polarizes membrane potential in MG-63 cells by both cAMP-independent and -dependent mechanisms. Am J Physiol Cell Physiol. 2004;287:C457–C467. doi: 10.1152/ajpcell.00274.2003. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharjee A, Whitehurst RM, Zhang M, Wang L, Li M. T-type calcium channels facilitate insulin secretion by enhancing general excitability in the insulin-secreting β-cell line, INS-1. Endocrinology. 1997;138:3735–3740. doi: 10.1210/endo.138.9.5390. [DOI] [PubMed] [Google Scholar]
- 34.Michel AD, Ng SW, Roman S, Clay WC, Dean DK, Walter DS. Mechanism of action of species-selective P2X7 receptor antagonists. Br J Pharmacol. 2009;156:1312–1325. doi: 10.1111/j.1476-5381.2009.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, Morhenn VB, Gallo RL. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 37.Schauber J, Rieger D, Weiler F, Wehkamp J, Eck M, Fellermann K, Scheppach W, Gallo RL, Stange EF. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2006;18:615–621. doi: 10.1097/00042737-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, Wieckowski MR, Pinton P. Mitochondrial Ca2+ and apoptosis. Cell Calcium. 2012;52:36–43. doi: 10.1016/j.ceca.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shabbir M, Ryten M, Thompson C, Mikhailidis D, Burnstock G. Characterization of calcium-independent purinergic receptor-mediated apoptosis in hormone-refractory prostate cancer. BJU Int. 2008;101:352–359. doi: 10.1111/j.1464-410X.2007.07293.x. [DOI] [PubMed] [Google Scholar]
- 40.Liu SI, Huang CC, Huang CJ, Wang BW, Chang PM, Fang YC, Chen WC, Wang JL, Lu YC, Chu ST, Chou CT, Jan CR. Thimerosal-induced apoptosis in human SCM1 gastric cancer cells: activation of p38 MAP kinase and caspase-3 pathways without involvement of [Ca2+]i elevation. Toxicol Sci. 2007;100:109–117. doi: 10.1093/toxsci/kfm205. [DOI] [PubMed] [Google Scholar]