Abstract
Periodontal disease is caused by microorganisms and host-derived inflammation involving increased cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production. We previously demonstrated that human β-defensin-3 induces COX-2 and PGE2 in human gingival fibroblasts (HGFs). We, therefore, aimed to examine the inducible effects of LL-37, the only cathelicidin expressed in humans, on COX-2 expression and PGE2 synthesis in HGFs and to elucidate the relevant signaling pathways. The COX-2 expression was upregulated by LL-37 in dose- and time-dependent manners. Accordingly, the synthesis of PGE2 in cell-free culture supernatants was raised by LL-37 (p < 0.01) and blocked by NS-398, a specific COX-2 inhibitor (p < 0.01). P2X inhibitors and a neutralizing antibody against P2X7 purinergic receptor significantly abrogated COX-2 induction and PGE2 production by LL-37 (p < 0.01). LL-37 upregulated COX-2 expression and PGE2 synthesis via activation of extracellular signal-regulated kinase (ERK) and p46 c-Jun N-terminal kinase (JNK), while interleukin-1β did so via nuclear factor-ĸB and all three mitogen-activated protein kinases. In summary, LL-37 can control arachidonic acid metabolism by induction of COX-2 expression and PGE2 synthesis via the P2X7 receptor, ERK, and p46 JNK. The pro-inflammatory effects of LL-37 may be essential for initiating oral mucosal inflammation in periodontal disease.
Key Words: Cathelicidin, Cyclooxygenase 2, LL-37, Mitogen-activated protein kinase, P2X7, Prostaglandin E2, Purinergic receptors
Introduction
The oral cavity is a unique environment, in which antimicrobial peptides may play a key role in maintaining the balance between health and disease [1]. Major antimicrobial peptides, which are important defense molecules in innate immunity, consist of members of the cathelicidin and defensin families [2]. The only member of the cathelicidin family found in humans is human cationic antimicrobial peptide-18 (hCAP-18), which is stored in the secondary granules of neutrophils as an inactive precursor [3]. Moreover, hCAP-18 is expressed in the epithelium of the tongue, esophagus, cervix, and vagina [4]. The levels of LL-37, derived from hCAP-18 by proteolytic cleavage, are also detectable in saliva [5] and gingival crevicular fluid (GCF) [6]. Furthermore, findings from some in vivo studies have demonstrated that LL-37 is usually associated with periodontal disease, a common oral inflammatory disorder [7, 8, 9]. For example, the levels of mRNA expression coding for hCAP-18 in tissue samples with chronic periodontitis appear to be upregulated compared with healthy control tissue [8]. Likewise, the levels of LL-37 are elevated in GCF of patients with chronic periodontitis [6, 9]. Inflammation is known to play a crucial role in the pathogenesis of periodontal disease, particularly the release of potent mediators of inflammation, such as pro-inflammatory cytokines and prostaglandins (PGs), which are derivatives of arachidonic acid metabolism [10].
PGs are bioactive prostanoids and play important roles in regulating diverse cellular functions under physiological and pathological conditions [11, 12]. Two major steps for PG biosynthesis include the release of arachidonic acid from membrane phospholipids by phospholipases and the conversion of arachidonic acid to PGH2 by cyclooxygenase (COX). There are two major isozymes of COX: COX-1 and COX-2. COX-1 is constitutively expressed in many tissues, whereas COX-2 is induced by a variety of pro-inflammatory agents [13]. PGE2, a main product derived from COX-2 induction, is a potent mediator of inflammation and associated with periodontal disease [14]. In human gingival fibroblasts (HGFs), PGE2 production is induced by interleukin (IL)-1β [15] and lipopolysaccharides [13] via COX-2 upregulation.
Moreover, we previously reported that, among three human β-defensins (hBDs) that are expressed in the oral cavity [16], only hBD-3 can induce COX-2 mRNA and protein expression, resulting in elevated PGE2 production in HGFs [17]. Although LL-37 was reported to upregulate the expression of COX-2 and inhibitor of apoptosis-2, implicated in the COX-2/PGE2 anti-apoptotic pathway in skin keratinocytes [18], it is still unknown whether LL-37 can activate COX-2 expression in HGFs. Furthermore, it would be interesting to further investigate the relevant receptor and the signaling pathway(s) mediating COX-2 and PGE2 induction by LL-37 treatment in comparison to IL-1β treatment, as a positive control. In this study, we, therefore, aimed to study the pro-inflammatory effects as well as the relevant receptor and the signaling pathways of LL-37 on COX-2 expression and PGE2 production in HGFs.
Materials and Methods
Reagents and Antibodies
LL-37 peptide (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) was synthesized by Fmoc (fluoren-9-ylmethoxycarbonyl) chemistry using a MilliGen 9050 peptide synthesizer (MilliGen/Biosearch, Bedford, Mass., USA), as described previously [19]. Peptides were purified by preparative RP-HPLC. The purity of the peptides was at least 95% and the authenticity of the peptides was confirmed by a Microflex LRF matrix-assisted laser desorption/ionization time-of-flight mass spectrometer equipped with an additional gridless reflectron (Bruker Daltonik, Bremen, Germany). IL-1β was obtained from R&D Systems, Inc., Minneapolis, Minn., USA. A specific p38 mitogen-activated protein kinase (MAPK) inhibitor (SB203580), a specific c-Jun N-terminal kinase (JNK) inhibitor (SP600125), a highly selective MAPK kinase (MEK1/2) inhibitor (U0126), a nuclear factor (NF)-ĸB inhibitor (MG132), a broad-spectrum antagonist of P2 purinergic receptors (suramin), and the suramin analog (NF279) were purchased from Calbiochem, Darmstadt, Germany. A specific inhibitor of COX-2 activity (NS-398) was obtained from Cayman Chemical, Ann Arbor, Mich., USA. All reagents were dissolved in dimethyl sulfoxide (DMSO), whose final concentration was <0.1% (vol/vol).
The polyclonal antibodies against human COX-1, COX-2, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and the monoclonal antibodies against the p50 and p65 subunits of NF-ĸB were purchased from Santa Cruz Biotechnology, Santa Cruz, Calif., USA. The polyclonal antibodies against each MAPK and its phosphorylated form were purchased from Cell Signaling Technology, Danvers, Mass., USA. The neutralizing rabbit polyclonal antibody (IgG) that binds to the extracellular portion of the P2X7 receptor to inhibit activation was obtained from Alomone Laboratories (Jerusalem, Israel). The rabbit polyclonal antibody (IgG) to the P2X4 receptor (Santa Cruz Biotechnology) was used as a negative control.
Cell Culture
HGFs were isolated from gingival biopsies overlying impacted third molars, which were collected from patients (n = 4) after obtaining written informed consent, as described previously [20]. The research design was approved by the Human Experimentation Committee (No. 33/2010), Faculty of Dentistry, Chiang Mai University. Briefly, the gingival biopsies, vigorously shaken in transport medium containing antibiotic and antifungal agents, were cut into small pieces 2 mm wide and 2 mm long. The biopsies were placed on a 60-mm culture dish (Nunc, Roskilde, Denmark) and cultured in Dulbecco's modified Eagle's medium (Invitrogen, Grand Island, N.Y., USA), containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen), until a sufficient number of HGFs had been obtained from the biopsies. HGFs were then trypsinized, and their number was expanded for subsequent experiments. For cell treatment, HGFs were starved from FBS for 6 h prior to treatment with non-toxic doses of LL-37, as previously reported [21], or 1 ng/ml of IL-1β in the presence or absence of pharmacological inhibitors for specific times, since it has previously been shown that FBS can induce COX-2 expression and PGE2 production [22].
RT-PCR
Total RNA was harvested according to the manufacturer's protocols, using an Illustra RNAspin Mini kit (GE Healthcare, Little Chalfont, UK). The RT-PCR protocol has been described previously [17]. Briefly, 3 µg of total RNA were used for synthesis of complementary DNA (cDNA) by SuperScript™ first-strand cDNA system reagents (Fermentas, Hanover, Md., USA) and a primer pair specific for COX-1, COX-2, or GAPDH, as described in table 1. The PCR amplification for COX-1 and COX-2 comprised 30 and 35 cycles, respectively, of an initial denaturation for 3 min at 94°C, followed by the cycles of amplification (94°C for 1 min, 55°C for 1 min, and 72°C for 2 min). The PCR amplification for GAPDH consisted of 20 cycles of denaturation at 95°C for 45 s, annealing at 60°C for 1 min, and extension at 72°C for 2 min. The PCR products were resolved on 1.5% agarose gel, and photographs were taken by a CCD (charge-coupled device) camera, attached to the ChemiDoc XRS gel documentation system (Bio-Rad Laboratories, Hercules, Calif., USA). The sizes of PCR products were as predicted when compared to a molecular weight DNA standard (Fermentas).
Table 1.
Primers used for PCR, their sequences, annealing temperatures and base pair fragments
| Gene | Primer sequence | Annealing temperature | Fragment length |
|---|---|---|---|
| COX-1 | TGCCCAGCTCCTGGCCCGCCGCTT GTGCATCAACACAGGCGCCTCTTC |
55°C | 304 bp |
| COX-2 | TTCAAATGAGATTGTGGGAAAAT AGATGNATCTCTGCCTGAGTATCTT |
55°C | 305 bp |
| GAPDH | ACCACAGTCCATGCCATCACTGC TCCACCACCCTGTTGCTGTAGC |
60°C | 452 bp |
Real-Time PCR
Five percent (vol/vol) of cDNA was used for a real-time PCR assay using SensiFast™ SYBR No-ROX reagents (Bioline Reagent, Ltd., London, UK) and the Roche LightCycler® 480 II instrument (Roche Diagnostics, Ltd., Rotkreuz, Switzerland) to calculate the ratio of COX-2 relative to GAPDH expression in each sample from four separate experiments. The relative COX-2 mRNA induction was determined by comparing the mean ratio of LL-37-treated cells compared with that of untreated control cells, which was set to 1. Moreover, the percentage of COX-2 inhibition in the inhibitor- or the neutralizing antibody-treated samples was determined from comparison with that in the LL-37-treated or the IL-1β-treated sample, which was set to 100%.
Nuclear Extraction
HGFs (about 5 × 106 cells) were harvested with trypsin-EDTA and then centrifuged at 500 g for 10 min. Cells were washed with phosphate-buffered saline and transferred to a 1.5-ml microcentrifuge tube for centrifugation. The nuclear and cytoplasmic proteins were isolated by NE-PER® nuclear and cytoplasmic extraction reagents (Thermo Scientific, Rockford, Ill., USA). Briefly, the cell pellet was supplemented with ice-cold cytoplasmic extraction reagents I and II, incubated on ice for 1 min, and then centrifuged at 16,000 g for 5 min. The cytoplasmic supernatant was transferred to a clean pre-chilled tube. The insoluble fraction or the nuclear pellet was solubilized in ice-cold nuclear extraction reagent by vigorously shaking for 15 s every 10 min for a total of 40 min, and centrifuged at 16,000 g for 10 min. The nuclear supernatant was immediately transferred to a clean pre-chilled tube on ice. Finally, 10 µl of the nuclear and cytoplasmic extracts were used for immunoblotting.
Immunoblotting
Whole-cell lysates of control and treated HGFs were extracted in RIPA buffer [23]. Forty micrograms of cell lysates were resolved on 10% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked, probed with primary antibody against COX-1, COX-2, GAPDH, and the phosphorylated or non-phosphorylated form of MAPK at 1:500, and then incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) at 1:2,000. Regarding the nuclear and cytoplasmic extracts, the membranes were probed with primary antibody against the p50 or the p65 subunit of NF-ĸB at 1:500. The LumiGLO Reserve chemiluminescence reagent (KPL, Gaithersburg, Md., USA) was used as a substrate, and the signal was captured with a CCD camera, attached to the ChemiDoc XRS system.
ELISA for PGE2
Cell-free culture supernatants were collected and analyzed for PGE2 levels with a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Inc.). In brief, 150 µl of standard or samples and 50 µl of primary antibody solution were added to each well. Subsequently, 50 µl of PGE2 conjugate was added and incubated for 2 h at room temperature. The well was then washed, and 200 µl of substrate solution were added and incubated for 30 min under light protection. The reaction was stopped, and the developing color was determined by optical density using the Titertek Multiskan M340 multiplate reader (ICN Flow, Costa Mesa, Calif., USA), set to 450 nm within 30 min.
Statistical Analyses
Any differences in the fold of COX-2 mRNA induction, in the increase in PGE2 levels, and in the increase in the p65 or the p50 subunit of NF-ĸB in the nuclear extract between untreated and treated samples were expressed as means ± SD and tested by Student's t test at p < 0.05 or p < 0.01. In addition, any differences in the percentage of COX-2 mRNA inhibition and in the PGE2 concentrations between the control or the inhibitor-treated sample and the LL-37-treated or the IL-1β-treated sample were expressed as means ± SD and tested by one-way ANOVA at p < 0.05 or p < 0.01.
Results
Upregulation of COX-2 Expression and PGE2 Production by LL-37 in Dose- and Time-Dependent Manners
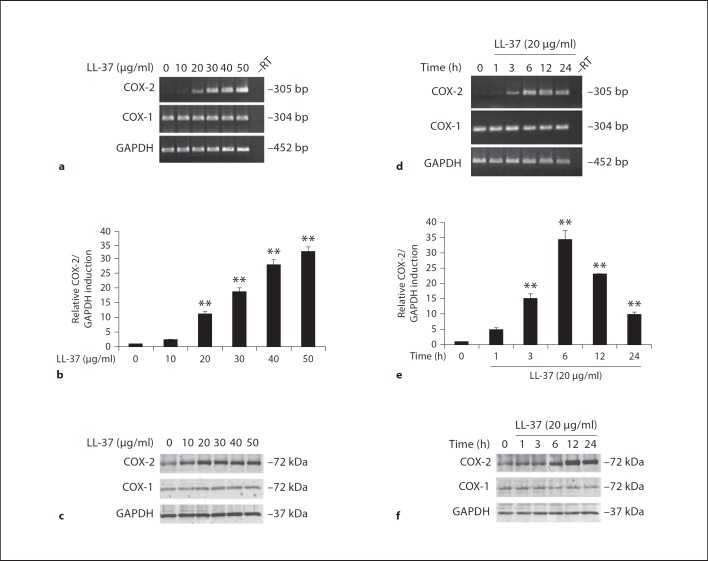
We have previously shown that LL-37 at concentrations <50 µg/ml (approximately equivalent to 10 µM) did not significantly alter the percentage of cell survival when compared to control untreated HGFs [21]. Therefore, these concentrations were chosen for treating HGFs in this study. HGFs were treated with various doses (0–50 µg/ml) of LL-37 for 24 h, or with 20 µg/ml of LL-37 for various times (0–24 h). LL-37 significantly induced COX-2 mRNA expression in a dose-dependent manner at p < 0.01 (fig. 1a, b, respectively), consistent with upregulation of COX-2 protein by LL-37 in a dose-dependent manner (fig. 1c). The time-course experiment showed that 20 µg/ml of LL-37 transiently induced COX-2 mRNA expression with a maximal induction observed at 6 h of treatment at p < 0.01 (fig. 1d, e). Likewise, expression of COX-2 protein was transiently induced, with a noticeably maximal increase at 12 h (fig. 1f). Whereas COX-2 expression was up-regulated by LL-37, COX-1 mRNA and protein expression remained unchanged upon LL-37 treatment (fig. 1a, c, d, f). Expression of GAPDH, a housekeeping gene control, was equal among the different samples (fig. 1a, c, d, f).
Fig. 1.
COX-2 mRNA and protein upregulation by LL-37 treatment. HGFs were treated with the indicated concentrations (0–50 µg/ml) of LL-37 for 12 h (a–c) or with 20 µg/ml of LL-37 for various times (0–24 h; d–f), and then total RNA and protein were extracted. Expression of mRNA for COX-1, COX-2, and GAPDH was analyzed by RT-PCR (a, d) and by real-time PCR (b, e) using a specific primer pair, and protein expression was assayed by immunoblotting (c, f) using antibodies specific to COX-1, COX-2, and GAPDH. The sizes of PCR products and proteins were as predicted, and the –RT sample was a negative control where the reverse transcriptase was omitted from the reverse transcription. The relative COX-2 mRNA induction (b, e) in LL-37-treated samples was expressed as mean ± SD from 4 separate experiments (** p < 0.01). The COX-2 mRNA expression in each sample was first normalized by GAPDH expression, and then the ratio of each experimental sample was compared to that of the control untreated sample, i.e., LL-37 at 0 µg/ml (b) or at 0 h (e), which was set to 1. a, c, d, f Representative images are from 4 independent experiments.
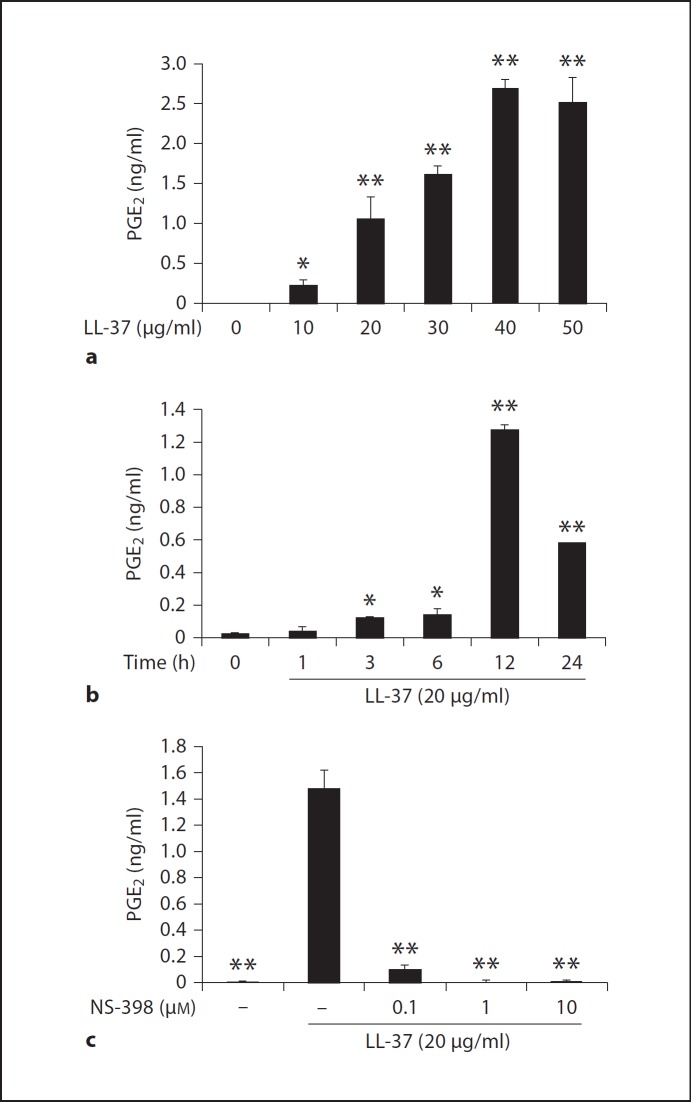
Consistent with COX-2 mRNA and protein induction, LL-37 treatment significantly raised PGE2 levels in cell-free culture supernatants collected from HGF samples in figure 1 (p < 0.01; fig. 2a, b). Interestingly, the maximally elevated PGE2 levels were observed in the HGF sample that was treated with 40 µg/ml of LL-37 (fig. 2a) and with LL-37 treatment for 12 h (fig. 2b), consistent with maximal COX-2 protein expression seen at 12 h in figure 1f. In addition, PGE2 levels raised by LL-37 resulted from COX-2 induction, since pretreatment of HGFs with NS-398 significantly blocked the elevation of PGE2 levels by LL-37 (p < 0.01) with the maximal inhibition seen at 1 µM of NS-398 (fig. 2c). Taken together, similar to the inducible effects of hBD-3 on COX-2 expression, resulting in elevated PGE2 production in HGFs [17], LL-37 can also upregulate both COX-2 expression and PGE2 synthesis in HGFs. Furthermore, both hBD-3- and LL-37-challenged HGFs produce PGE2 via de novo synthesis of COX-2. However, from the time-course experiment, some differences were noted between the inducible effects of hBD-3 and those of LL-37. Whereas hBD-3 cumulatively induces COX-2 expression and PGE2 synthesis [17], LL-37 does so transiently, suggesting different signaling pathways used to mediate COX-2 expression between hBD-3 and LL-37.
Fig. 2.
Elevated PGE2 levels by LL-37 treatment via enhanced COX-2 expression. a, b The cell-free culture supernatants collected from the dose and kinetic experiments in figure 1 were analyzed for the PGE2 levels by ELISA. c HGFs were pretreated with the indicated doses of NS-398 for 30 min before treatment with 20 µg/ml of LL-37 for 12 h. The cell-free conditioned media were collected and the PGE2 levels were measured by ELISA from 4 independent experiments and expressed as mean ± SD of PGE2 concentrations (in ng/ml; * p < 0.05, ** p < 0.01).
LL-37 Induces COX-2 Expression and PGE2 Synthesis via the P2X7 Purinergic Receptor
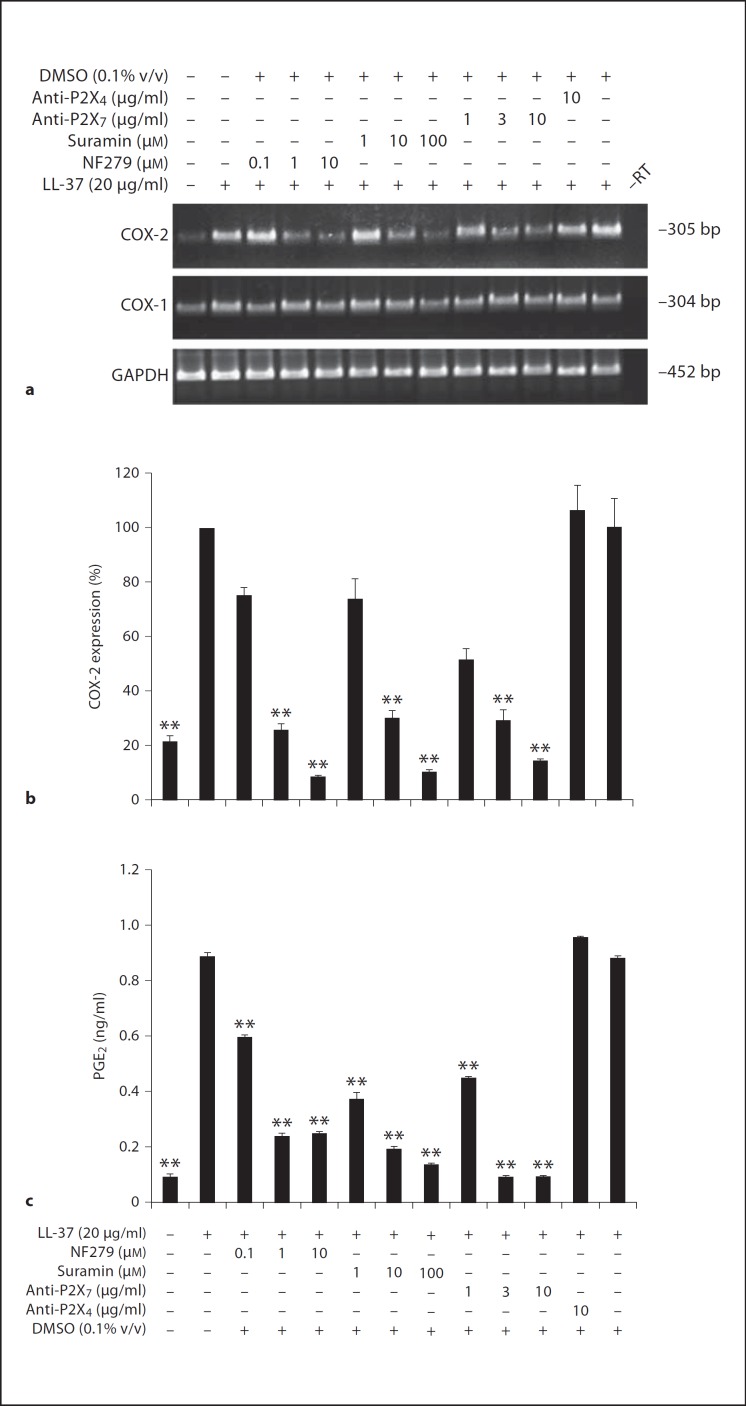
We have previously shown that IL-8 upregulation by LL-37 in HGFs is via the P2X7 purinergic receptor, and among seven P2X purinergic receptors, the P2X7 protein is the only P2X receptor expressed in HGFs [21]. Consequently, it was probable that the P2X7 receptor might function as a receptor for LL-37 in mediating the pro-inflammatory effect. HGFs were pretreated with various doses of suramin, a broad-spectrum antagonist of P2 purinergic receptor [24], of NF279, a selective P2X inhibitor [25], of the neutralizing antibody against P2X7 receptor, of DMSO, a solvent control, or of the antibody to P2X4 receptor for 30 min before LL-37 treatment for 12 h. Pretreatment with suramin, NF279, and the neutralizing antibody significantly blocked COX-2 mRNA induction in a dose-dependent manner (p < 0.01; fig. 3a, b), while COX-1 mRNA expression was unaffected (fig. 3a). Accordingly, the increased PGE2 production in cell-free culture supernatants by LL-37 was significantly inhibited by suramin, NF279, and the neutralizing antibody against the P2X7 receptor (anti-P2X7) at p < 0.01 (fig. 3c). In contrast, pretreatment with DMSO or the antibody to P2X4 receptor did not inhibit COX-2 and PGE2 induction by LL-37 (fig. 3).
Fig. 3.
Upregulation of COX-2 expression and PGE2 production by LL-37 via the P2X7 purinergic receptor. HGFs were pretreated with various doses of NF279, suramin, the neutralizing antibody against the P2X7 receptor (anti-P2X7), the antibody to P2X4 receptor (anti-P2X4), or DMSO for 30 min prior to LL-37 treatment for 12 h. Total RNA was isolated and mRNA expression was analyzed by RT-PCR (a) and real-time PCR (b), and the cell-free conditioned media were collected and assayed for the PGE2 levels by ELISA (c). a A representative image of RT-PCR is from four separate experiments. b The percentages of COX-2 mRNA expression normalized by GAPDH mRNA expression in inhibitor-treated or untreated samples were compared with the LL-37-treated sample, whose percentage was set to 100%, and shown as mean ± SD (** p < 0.01, n = 4). c The PGE2 levels were measured from four independent experiments and expressed as mean ± SD of PGE2 concentrations (in ng/ml; ** p < 0.01).
LL-37 Treatment Does Not Significantly Cause Nuclear Translocation of the p50 and p65 Subunits of NF-ĸB, but Phosphorylates ERK and p46 JNK MAPK Pathways
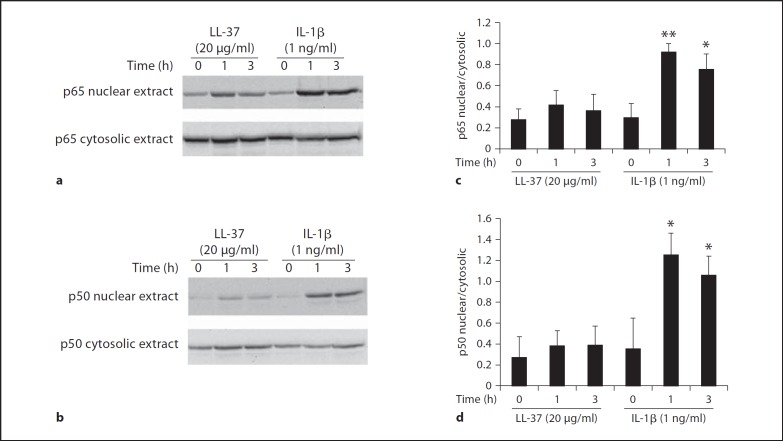
To address the possible signaling pathways for LL-37, we first investigated the activation of NF-ĸB and MAPK pathways, two major signaling pathways that are important in immune responses, by LL-37 treatment in comparison to IL-1β treatment. The nuclear and cytosolic extracts of HGFs were subjected to immunoblotting to determine the presence of the p50 and p65 subunits of NF-ĸB in the extracts. It was demonstrated that the levels of both p65 and p50 subunits of NF-ĸB were not significantly increased in the nuclear extract of HGFs treated with 20 µg/ml of LL-37 compared with the control untreated HGFs (fig. 4), suggesting that COX-2 and PGE2 induction by LL-37 does not mediate through NF-ĸB. As a positive control, treatment with 1 ng/ml of IL-1β resulted in a significant increase in both p50 and p65 subunits of NF-ĸB at 1 and 3 h (p < 0.05 or p < 0.01; fig. 4). Note that the levels of the p50 and p65 subunits of NF-ĸB in the cytosolic extracts were approximately equal among the different samples (fig. 4).
Fig. 4.
LL-37 treatment does not cause significant increases in the p50 and p65 subunits of NF-ĸB in the nuclear extract. HGFs were treated with 20 µg/ml of LL-37 or 1 ng/ml of IL-1β, as a positive control, for 1 and 3 h or left untreated. The nuclear and cytosolic extraction was performed and their extracts were analyzed for the presence of the p65 (a) and p50 (b) subunits of NF-ĸB by immunoblotting using the specific antibodies to the p65 and p50 subunits. a, b Representative images are from three independent experiments. The bar graphs (c, d) show means ± SD (n = 3) from the densitometry of the expression of the p65 subunit (c) and of the expression of the p50 subunit (d) in the nuclear extract relative to its respective expression in the cytosolic extract. Note the significant increases in the relative ratios of the p65 and p50 subunits in IL-1β-treated samples (* p < 0.05, ** p < 0.01, vs. untreated sample).
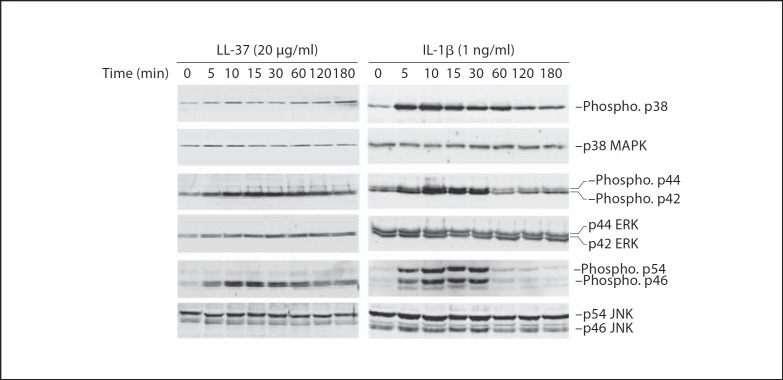
To further explore the involvement of MAPK activation by phosphorylation, HGFs were treated with 20 µg/ml of LL-37 or 1 ng/ml of IL-1β for various times (0–180 min), and the whole-cell lysates were subjected to immunoblotting. It was demonstrated that LL-37 treatment transiently phosphorylated p44 and p42 ERK and p46 JNK MAPK (fig. 5), whereas IL-1β treatment resulted in transient phosphorylation of all three MAPK pathways (fig. 5). Note that the levels of the non-phosphorylated form of all three MAPK pathways were equal among the samples (fig. 5).
Fig. 5.
Phosphorylation of ERK and p46 JNK MAPK pathways by LL-37 treatment. HGFs were incubated with either 20 µg/ml of LL-37 or 1 ng/ml of IL-1β for 0–180 min. The extracted whole-cell lysates were analyzed for the levels of the phosphorylated and the non-phosphorylated form of MAPK pathways, including p38, p44 and p42 ERK, and p54 and p46 JNK, by immunoblotting. A representative image is from 3 independent experiments. Note the phosphorylated p46 JNK is seen as doublets in LL-37- and IL-1β-treated samples, while the phosphorylated p54 JNK is seen as one band only in IL-1β-treated samples.
Upregulation of COX-2 Expression and PGE2 Levels by LL-37 via ERK and JNK MAPK
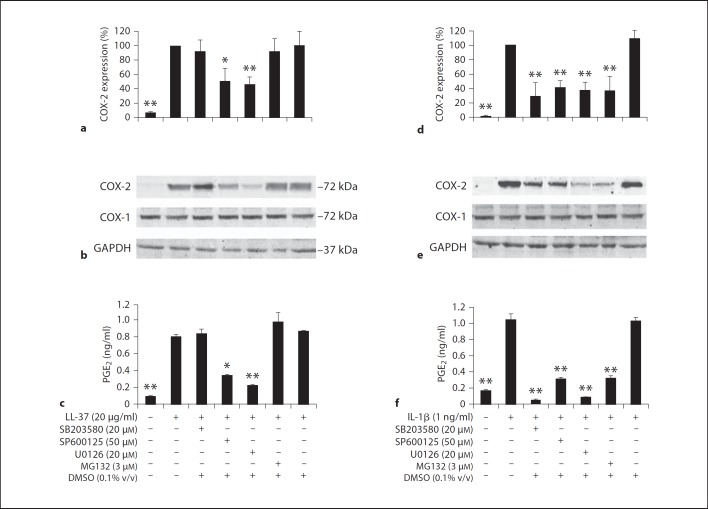
To next explore the involvement of each signaling pathway in the induction of COX-2 expression and PGE2 levels, HGFs were pretreated with specific inhibitors against each pathway or DMSO for 30 min prior to treatment with 20 µg/ml of LL-37 or 1 ng/ml of IL-1β for 12 h. Upregulation of COX-2 mRNA and protein expression and PGE2 production by LL-37 treatment were significantly abolished (p < 0.05 and p < 0.01, respectively) by SP600125, a specific JNK inhibitor, and U0126, a specific inhibitor of MEK1/2 that acts upstream of the ERK pathway (fig. 6a, b, c). However, pretreatment with SB203580 or MG132 did not inhibit COX-2 mRNA and protein induction or PGE2 production by LL-37 (fig. 6a, b, c), suggesting that neither p38 MAPK nor NF-ĸB is involved in COX-2 induction and PGE2 production by LL-37. In contrast, IL-1β induced COX-2 expression, leading to elevated PGE2 synthesis, via the phosphorylation of p38, ERK, and p54/46 JNK MAPK, and the activation of both p50 and p65 subunits of NF-ĸB, because COX-2 mRNA and protein induction and PGE2 synthesis were significantly inhibited by pretreatment with SB203580, U0126, SP600125, and MG132 at p < 0.01 (fig. 6d, e, f). In contrast, pretreatment with DMSO did not inhibit COX-2 and PGE2 induction by either LL-37 or IL-1β treatment (fig. 6). Taken together, LL-37 upregulates COX-2 expression, resulting in increased PGE2 production, via the ERK and p46 JNK MAPK pathways, whereas IL-1β does so via all three MAPKs and the NF-ĸB pathway.
Fig. 6.
Induction of COX-2 expression and PGE2 levels by LL-37 via ERK and JNK MAPK. HGFs were pretreated with an indicated dose of SB203580, SP600125, U0126, MG132, or DMSO for 30 min prior to treatment with 20 µg/ml of LL-37 (a–c) or with 1 ng/ml of IL-1β (d–f) for 12 h. Total RNA was isolated and the degrees of COX-2 expression relative to those of GAPDH expression were analyzed by real-time PCR (a, d), and total protein was extracted and analyzed for COX-1, COX-2, and GAPDH expression by immunoblotting (b, e). Note: COX-1 protein expression was unaffected by treatment with either stimulants or inhibitors. The cell-free conditioned media were collected and analyzed for PGE2 concentrations by ELISA (c, f). The percentages of COX-2 mRNA expression (a, d) in inhibitor-treated and control samples were determined by comparison with that of the LL-37- or the IL-1β-treated sample that was set to 100%. The percentages of COX-2 expression (a, d) and the PGE2 concentrations (in ng/ml; c, f) were determined from 4 separate experiments and expressed as mean ± SD (* p < 0.05; ** p < 0.01). b, e Representative images are from four independent experiments.
Discussion
CAPs are observed both at epithelial surfaces and within the granules of phagocytic cells. They are an important component of the innate immune system, since in addition to their ability to kill microorganisms, they are able to modulate inflammatory responses. In the present study, we showed that LL-37 upregulated the inflammatory mediators in arachidonic acid metabolism, particularly COX-2 induction and PGE2 synthesis in HGFs. Our findings identify an additional function and a role of LL-37 in the modulation of mucosal immunity in the oral cavity, since both COX-2 and PGE2 play an important role in the pathogenesis of periodontal disease [14]. Similarly to IL-1β-induced PGE2 formation, which is mediated by enhanced COX-2 gene expression in gingival fibroblasts [15], PGE2 levels were also raised by LL-37 via the specificity of COX-2 activation, consistent with our previous result showing that elevated PGE2 levels by hBD-3 treatment result from induced COX-2 expression [17]. The inducible effect of LL-37 on COX-2 expression and PGE2 production in HGFs is relevant to the increased activity of phospholipase A2, an upstream molecule involved in arachidonic acid metabolism, induced by LL-37 in mouse submandibular acinar cells [26].
The fact that COX-1 mRNA levels were not affected by treatment with LL-37, and the fact that the selective COX-2 inhibitor, NS-398, completely blocked the synthesis of PGE2 by LL-37 in figure 2c further suggest that COX-2, rather than COX-1, is involved in the elevated PGE2 production by LL-37. Moreover, because of the clinical observations that the concentrations of LL-37 are raised to the low µg/ml levels in the GCF of patients with chronic periodontitis [9], which are in the same range of LL-37 concentrations used to treat HGFs in this study, and that the levels of PGE2 are elevated in the GCF of patients with periodontitis [27], it is likely that elevated levels of LL-37 in the GCF of patients with chronic periodontitis may further enhance the production of PGE2 from gingival fibroblasts, causing greater inflammation in periodontitis. This warrants further investigation.
In HGFs, LL-37 activated COX-2 expression and PGE2 production via the ERK and p46 JNK signaling pathways, a finding which is in agreement with the role of ERK activation in COX-2 and PGE2 induction by LL-37 treatment in human dermal fibroblasts [28]. Moreover, it has been shown in several other cell types, including human corneal epithelial cells, human dermal fibroblasts, human mast cells, and glial cells, that treatment with LL-37 or its ortholog in rats, i.e. rCRAMP, results in the activation of the ERK pathway [29, 30, 31]. However, some slight differences in terms of involvement of other MAPK pathways than the ERK pathway are still noted in each individual cell type, suggesting cell type specificity. It has recently been reported that LL-37 induces IL-6 expression in human bronchial epithelial cells via activation of the NF-ĸB pathway [32]. However, we showed that this pathway was not involved in COX-2 induction and PGE2 production in HGFs. Our work has instead demonstrated that LL-37 induces COX-2 expression and PGE2 synthesis in HGFs via ERK and JNK activation. By contrast, IL-1β, a potent pro-inflammatory cytokine, induces COX-2 and PGE2 not only via all three MAPK pathways, including p38, ERK, and JNK, but also via activation of the p50 and p65 subunits of NF-ĸB, consistent with the result from a recent study [33], which shows that induction of COX-2 and PGE2 by another pro-inflammatory cytokine, i.e. tumor necrosis factor-α, is found to be involved in both MAPK and NF-ĸB signaling pathways in gingival fibroblasts. Taken together, the underlying signaling mechanisms involved in the induction of gene expression in each individual study can vary, but are specific, depending upon the distinct types of cells, stimulants, and genes of interest.
LL-37 can enter cells using pathways typical of cell-penetrating peptides involving the cell cytoskeletal machinery [34] and then interacts with GAPDH through p38 MAPK signaling [35]. In addition, LL-37 has been reported to be directly chemotactic for human neutrophils, monocytes, and T cells through formyl peptide receptor-like 1, a Gi protein-coupled receptor [36], and to activate keratinocyte migration via transactivation of the epidermal growth factor receptor by phosphorylation of ERK MAPK [37]. LL-37 has also been found to induce IL-8 production through phosphorylation of ERK1/2 via the P2X7 receptor in HGFs [21], submandibular gland cells [26], and human embryonic kidney 293 cells [38]. Accordingly, it is possible that LL-37 may either interact with its cognate receptors or internalize into the cells, or both, to upregulate COX-2 expression and PGE2 production in HGFs. In this study, we, however, showed that the P2X7 purinergic receptor was involved in COX-2 induction and PGE2 production mediated by LL-37 in HGFs. Therefore, it is probable that the inducible effect of LL-37 on pro-inflammatory genes is commonly mediated via the P2X7 receptor.
The dose of LL-37 used in this study (20 µg/ml or ∼4 µM) is shown to be non-toxic to several human cell types tested to date [21, 24, 39]. In fact, general toxicity of LL-37 to eukaryotic cells is reported at concentrations >13 µM[40]. However, we cannot exclude that LL-37 may possibly increase ATP levels in cell-free culture supernatants that will result in P2X7 receptor activation. Therefore, the effect of LL-37 may be indirect rather than direct on the P2X7 receptor, and this needs to be further investigated. In this study, the levels of PGE2 production by induced COX-2 expression were not only enhanced by IL-1β but also by a neutrophil- and epithelial-derived antimicrobial peptide, LL-37, in HGFs in a paracrine fashion. HGFs are the major cell type found in periodontal connective tissues that can provide a tissue framework for tooth anchorage. They also induce the production of various types of inflammatory mediators to promote periodontal disease development and progression. LL-37 can thus exert an inductive effect on arachidonic acid metabolism and IL-8 expression [21] in HGFs that may enhance the innate immune responses of periodontal tissues. In summary, the cationic antimicrobial peptide LL-37 is involved in the induction of COX-2 and PGE2 via the P2X7 purinergic receptor, ERK and p46 JNK MAPK, and LL-37 can be considered one of the pro-inflammatory mediators in the regulation of prostanoid formation in addition to an antimicrobial peptide.
Disclosure Statement
All authors report no conflicts of interest related to this study.
Acknowledgments
This study was supported by the Intramural Fund, Faculty of Dentistry, Chiang Mai University, the Royal Golden Jubilee PhD program to P.C. (grant No. PHD/0250/2549), the Discovery-Based Development Grant (P-10-11290), National Science and Technology Development Agency, the Thailand Research Fund (RMU5380014), the Center of Excellence for Innovation in Chemistry (PERCH-CIC), and a grant from the University of Amsterdam for research into the focal point ‘Oral Infections and Inflammation’ to J.G.M.B. and K.N. The authors thank Dr. M. Kevin O. Carroll, Professor Emeritus of the University of Mississippi School of Dentistry, USA, and Faculty Consultant at Chiang Mai University Faculty of Dentistry, Thailand, for his critical reading of this paper.
References
- 1.Dale BA, Fredericks LP. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr Issues Mol Biol. 2005;7:119–133. doi: 10.1093/jac/dki103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg A, Krisanaprakornkit S, Dale BA. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998;9:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- 3.Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr Issues Mol Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- 4.Frohm Nilsson M, Sandstedt B, Sørensen O, Weber G, Borregaard N, Ståhle-Bäckdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami M, Ohtake T, Dorschner RA, Gallo RL. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res. 2002;81:845–850. doi: 10.1177/154405910208101210. [DOI] [PubMed] [Google Scholar]
- 6.Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol Immunol. 2008;23:328–335. doi: 10.1111/j.1399-302X.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosokawa I, Hosokawa Y, Komatsuzawa H, Goncalves RB, Karimbux N, Napimoga MH, Seki M, Ouhara K, Sugai M, Taubman MA, Kawai T. Innate immune peptide LL-37 displays distinct expression pattern from beta-defensins in inflamed gingival tissue. Clin Exp Immunol. 2006;146:218–225. doi: 10.1111/j.1365-2249.2006.03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Türkoğlu O, Kandiloğlu G, Berdeli A, Emingil G, Atilla G. Antimicrobial peptide hCAP-18/LL-37 protein and mRNA expressions in different periodontal diseases. Oral Dis. 2011;17:60–67. doi: 10.1111/j.1601-0825.2010.01704.x. [DOI] [PubMed] [Google Scholar]
- 9.Türkoğlu O, Emingil G, Kütükçüler N, Atilla G. Gingival crevicular fluid levels of cathelicidin LL-37 and interleukin-18 in patients with chronic periodontitis. J Periodontol. 2009;80:969–976. doi: 10.1902/jop.2009.080532. [DOI] [PubMed] [Google Scholar]
- 10.Wixted JJ, Fanning P, Rothkopf I, Stein G, Lian J. Arachidonic acid, eicosanoids, and fracture repair. J Orthop Trauma. 2010;24:539–542. doi: 10.1097/BOT.0b013e3181f17b33. [DOI] [PubMed] [Google Scholar]
- 11.Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. 2006;36:37–49. doi: 10.1016/j.semarthrit.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguchi K, Shitashige M, Yanai M, Morita I, Nishihara T, Murota S, Ishikawa I. Prostaglandin production via induction of cyclooxygenase-2 by human gingival fibroblasts stimulated with lipopolysaccharides. Inflammation. 1996;20:555–568. doi: 10.1007/BF01487046. [DOI] [PubMed] [Google Scholar]
- 14.Morton RS, Dongari-Bagtzoglou AI. Cyclooxygenase-2 is upregulated in inflamed gingival tissues. J Periodontol. 2001;72:461–469. doi: 10.1902/jop.2001.72.4.461. [DOI] [PubMed] [Google Scholar]
- 15.Yucel-Lindberg T, Ahola H, Nilsson S, Carlstedt-Duke J, Modéer T. Interleukin-1 beta induces expression of cyclooxygenase-2 mRNA in human gingival fibroblasts. Inflammation. 1995;19:549–560. doi: 10.1007/BF01539135. [DOI] [PubMed] [Google Scholar]
- 16.Abiko Y, Saitoh M, Nishimura M, Yamazaki M, Sawamura D, Kaku T. Role of beta-defensins in oral epithelial health and disease. Med Mol Morphol. 2007;40:179–184. doi: 10.1007/s00795-007-0381-8. [DOI] [PubMed] [Google Scholar]
- 17.Chotjumlong P, Khongkhunthian S, Ongchai S, Reutrakul V, Krisanaprakornkit S. Human beta-defensin-3 up-regulates cyclooxygenase-2 expression and prostaglandin E2 synthesis in human gingival fibroblasts. J Periodontal Res. 2010;45:464–470. doi: 10.1111/j.1600-0765.2009.01259.x. [DOI] [PubMed] [Google Scholar]
- 18.Chamorro CI, Weber G, Grönberg A, Pivarcsi A, Ståhle M. The human antimicrobial peptide LL-37 suppresses apoptosis in keratinocytes. J Invest Dermatol. 2009;129:937–944. doi: 10.1038/jid.2008.321. [DOI] [PubMed] [Google Scholar]
- 19.den Hertog AL, van Marle J, van Veen HA, Van't Hof W, Bolscher JG, Veerman EC, Nieuw Amerongen., AV Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem J. 2005;388:689–695. doi: 10.1042/BJ20042099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krisanaprakornkit S, Kimball JR, Weinberg A, Darveau RP, Bainbridge BW, Dale BA. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907–2915. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montreekachon P, Chotjumlong P, Bolscher JG, Nazmi K, Reutrakul V, Krisanaprakornkit S. Involvement of P2X(7) purinergic receptor and MEK1/2 in interleukin-8 up-regulation by LL-37 in human gingival fibroblasts. J Periodontal Res. 2011;46:327–337. doi: 10.1111/j.1600-0765.2011.01346.x. [DOI] [PubMed] [Google Scholar]
- 22.Noguchi K, Shitashige M, Endo H, Kondo H, Yotsumoto Y, Izumi Y, Nitta H, Ishikawa I. Involvement of cyclooxygenase-2 in serum-induced prostaglandin production by human oral gingival epithelial cells. J Periodontal Res. 2001;36:124–130. doi: 10.1034/j.1600-0765.2001.360209.x. [DOI] [PubMed] [Google Scholar]
- 23.Krisanaprakornkit S, Kimball JR, Dale BA. Regulation of human beta-defensin-2 in gingival epithelial cells: the involvement of mitogen-activated protein kinase pathways, but not the NF-ĸB transcription factor family. J Immunol. 2002;168:316–324. doi: 10.4049/jimmunol.168.1.316. [DOI] [PubMed] [Google Scholar]
- 24.Zuyderduyn S, Ninaber DK, Hiemstra PS, Rabe KF. The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J Allergy Clin Immunol. 2006;117:1328–1335. doi: 10.1016/j.jaci.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Damer S, Niebel B, Czeche S, Nickel P, Ardanuy U, Schmalzing G, Rettinger J, Mutschler E, Lambrecht G. NF279: a novel potent and selective antagonist of P2X receptor-mediated responses. Eur J Pharmacol. 1998;350:R5–R6. doi: 10.1016/s0014-2999(98)00316-1. [DOI] [PubMed] [Google Scholar]
- 26.Pochet S, Tandel S, Querriére S, Tre-Hardy M, Garcia-Marcos M, De Lorenzi M, Vandenbranden M, Marino A, Devleeschouwer M, Dehaye JP. Modulation by LL-37 of the responses of salivary glands to purinergic agonists. Mol Pharmacol. 2006;69:2037–2046. doi: 10.1124/mol.105.021444. [DOI] [PubMed] [Google Scholar]
- 27.Preshaw PM, Heasman PA. Prostaglandin E2 concentrations in gingival crevicular fluid: observations in untreated chronic periodontitis. J Clin Periodontol. 2002;29:15–20. doi: 10.1034/j.1600-051x.2002.290103.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Cho DH, Lee KJ, Cho CS, Bang SI, Cho BK, Park HJ. LL-37 suppresses sodium nitroprusside-induced apoptosis of systemic sclerosis dermal fibroblasts. Exp Dermatol. 2011;20:843–845. doi: 10.1111/j.1600-0625.2011.01327.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Human cathelicidin LL-37 increases vascular permeability in the skin via mast cell activation, and phosphorylates MAP kinases p38 and ERK in mast cells. J Dermatol Sci. 2006;43:63–66. doi: 10.1016/j.jdermsci.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Brandenburg LO, Jansen S, Wruck CJ, Lucius R, Pufe T. Antimicrobial peptide rCRAMP induced glial cell activation through P2Y receptor signalling pathways. Mol Immunol. 2010;47:1905–1913. doi: 10.1016/j.molimm.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Yin J, Yu FS. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Invest Ophthalmol Vis Sci. 2010;51:1891–1897. doi: 10.1167/iovs.09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pistolic J, Cosseau C, Li Y, Yu JJ, Filewod NC, Gellatly S, Rehaume LM, Bowdish DM, Hancock RE. Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-ĸB signaling pathway. J Innate Immun. 2009;1:254–267. doi: 10.1159/000171533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Båge T, Lindberg J, Lundeberg J, Modéer T, Yucel-Lindberg T. Signal pathways JNK and NF-ĸB, identified by global gene expression profiling, are involved in regulation of TNFα-induced mPGES-1 and COX-2 expression in gingival fibroblasts. BMC Genomics. 2010;11:241. doi: 10.1186/1471-2164-11-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau YE, Rozek A, Scott MG, Goosney DL, Davidson DJ, Hancock RE. Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect Immun. 2005;73:583–591. doi: 10.1128/IAI.73.1.583-591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mookherjee N, Lippert DN, Hamill P, Falsafi R, Nijnik A, Kindrachuk J, Pistolic J, Gardy J, Miri P, Naseer M, Foster LJ, Hancock RE. Intracellular receptor for human host defense peptide LL-37 in monocytes. J Immunol. 2009;183:2688–2696. doi: 10.4049/jimmunol.0802586. [DOI] [PubMed] [Google Scholar]
- 36.De Yang, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H, Yang L, Higashiyama S, Yoshimura A, Sugai M, Hashimoto K. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 38.Tomasinsig L, Pizzirani C, Skerlavaj B, Pellegatti P, Gulinelli S, Tossi A, Di Virgilio F, Zanetti M. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J Biol Chem. 2008;283:30471–30481. doi: 10.1074/jbc.M802185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Supanchart C, Thawanaphong S, Makeudom A, Bolscher JGM, Nazmi K, Kornak U, Krisanaprakornkit S. The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis. J Dent Res. doi: 10.1177/0022034512460402. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]