Abstract
L-ficolin is a soluble pattern recognition molecule expressed by the liver that contributes to innate immune defense against microorganisms. It is well described that binding of L-ficolin to specific pathogen-associated molecular patterns activates the lectin complement pathway, resulting in opsonization and lysis of pathogens. In this study, we demonstrated that in addition to this indirect effect, L-ficolin has a direct neutralizing effect against hepatitis C virus (HCV) entry. Specific, dose-dependent binding of recombinant L-ficolin to HCV glycoproteins E1 and E2 was observed. This interaction was inhibited by soluble L-ficolin ligands. Interaction of L-ficolin with E1 and E2 potently inhibited entry of retroviral pseudoparticles bearing these glycoproteins. L-ficolin also inhibited entry of cell-cultured HCV in a calcium-dependent manner. Neutralizing concentrations of L-ficolin were found to be circulating in the serum of HCV-infected individuals. This is the first description of direct neutralization of HCV entry by a ficolin and highlights a novel role for L-ficolin as a virus entry inhibitor.
Key Words: Hepatitis C virus, Ficolin, Virus entry, Neutralization, Glycosylation
Introduction
Chronic hepatitis C virus (HCV) infection affects approximately 130 million people. Chronic infection is a risk factor for cirrhosis and hepatocellular carcinoma, resulting in the requirement for liver transplantation [1]. Entry inhibitors are an attractive treatment in the clinical setting of liver transplantation, where they might prevent infection of transplanted tissue. Current therapies target the HCV NS3 protease and the NS5B polymerase [2] but cannot prevent initial infection.
Binding of HCV envelope glycoproteins E1 and E2 to cellular CD81 and SR-B1 is essential for HCV entry, and antibodies that inhibit these interactions neutralize entry [reviewed in [3]]. These glycoproteins are targets for therapeutic intervention and host immunity [4, 5]. They possess up to 5 and 11 N-linked glycosylation sites, respectively [6]. These glycans are structurally heterogeneous [7] and contribute to glycoprotein biosynthesis, modulation of infectivity and evasion of neutralizing antibodies [8, 9]. Many glycosylation sites are highly conserved across genetically diverse HCV isolates [10]. This makes them attractive targets for antiviral drug development. The lectins cyanovirin-N [11], griffithsin [12] and mannose-binding lectin (MBL) [10] all inhibit entry by binding to these glycans.
Ficolins are a family of serum proteins functionally and structurally related to the collectins, sharing a quaternary structure with MBL and complement component C1q [13]. They consist of disulfide-linked 35-kDa polypeptides organized into trimers. Oligomers of these three polypeptides form functional dodecamers [13]. They bind glycan-containing pathogen-associated molecular patterns and activate the complement cascade. The carbohydrate-binding activity is attributed to the C-terminal fibrinogen-like binding domain, which has general specificity for N-acetyl groups on the outer walls of microorganisms [14]. In humans, three ficolins have been identified, namely L-ficolin, M-ficolin and H-ficolin [15]. H-ficolin and L-ficolin are expressed by hepatocytes. L-ficolin has a broad binding specificity for targets including galactose, β-glucan, acetylated compounds, N-acetylglucosamine (GlcNAc) and N-acetylcysteine (CysNAc). Binding is mediated by four binding sites in the C-terminal fibrinogen-like binding domain, some of which require calcium for interaction [16]. L-ficolin associates with MBL-associated serine proteases, resulting in complement activation, phagocytosis and clearance of pathogens bearing N-acetylated structures such as GlcNAc [17, 18], a major component of bacterial cell walls that is also incorporated into virus glycoproteins. This early innate recognition may play a critical step in priming adaptive immune responses to infection [19].
The contribution of ficolins to controlling virus infections is poorly understood. Porcine ficolin-α reduces the infectivity of porcine reproductive and respiratory syndrome virus (PRRSV) [20]. Human L-ficolin also inhibits the infectivity of influenza A in vivo [21]. A role for L-ficolin in immune recognition of HCV virions may exist, as binding of a monomeric recombinant L-ficolin to the HCV envelope glycoproteins has been demonstrated to activate the complement cascade resulting in cell lysis [22]. However, no direct antiviral effect on HCV has been described for L-ficolin. Here, the ability of a purified recombinant oligomeric L-ficolin to directly neutralize HCV was investigated. Interaction of L-ficolin with recombinant HCV envelope glycoproteins in the context of HCV pseudoparticles (HCVpp) and cell-cultured HCV particles (HCVcc) was found to result in direct inhibition of virus entry.
Materials and Methods
Purification of Plasma L-Ficolin
L-ficolin was isolated from citrated human plasma using GlcNac-Sepharose-4B beads according to the method of Cseh et al. [23].
Expression of Recombinant FLAG-Tagged L-Ficolin
Human L-ficolin cDNA was amplified from an IMAGE clone BCO 69572 (Open Biosystem) to create a recombinant amino-terminal FLAG-tagged L-ficolin (primers available upon request). The modified L-ficolin was cloned into pcDNA-DEST26 (Invitrogen) and expressed in HEK 293T cells cultured in DMEM. Culture supernatants were clarified and L-ficolin was purified using anti-FLAG M2 affinity resin (Sigma). L-ficolin was eluted with 175 μg/ml FLAG peptide. Fractions were collected and dialyzed with TBS (50 mM Tris-HCl, pH 8.0, 0.15 M NaCl). Protein separation was performed on a 10% SDS-PAGE gel, followed by staining with SimplyBlue (Life Technologies). Western blotting was performed on separated proteins with anti-L-ficolin mAb GN5 (Hycult) or anti-FLAG mAb (Sigma). Total protein concentration was determined using a bicinchoninic acid (BCA) assay (Pierce), while active L-ficolin was measured using an acetylated BSA binding assay (online suppl. methods; for all online suppl. material, see www.karger.com/doi/10.1159/000362209).
L-Ficolin HCV Glycoprotein Binding Assay and Inhibition
HCV glycoproteins E1/E2 and 6xHis-tagged soluble E2 constructs were described previously [24, 25]. Maxisorp plates were coated with anti-L-ficolin antibody GN5 (Hycult) in PBS and incubated at 4°C overnight. Plates were blocked with PBS-Tween and 5% milk and incubated with 5 μg/ml L-ficolin. Lysates containing HCV glycoproteins E1/E2 derived from genotype 1 (H77c, accession number AF011751), diluted 1/10 in PBS-Tween, or purified sE2 (4 μg/ml) in PBS-Tween were added. A cell lysate from untransfected 293T cells was included as a negative control. After washing, 1 µg/ml biotinylated mAb AP33 [26] was added for 1 h. After washing 3 times, wells were incubated for 30 min with 0.5 µg/ml horseradish peroxidase-conjugated streptavidin. Binding was detected at 620 nm after incubation with tetramethylbenzidine (Sigma). Inhibition experiments were performed with an additional step after L-ficolin incubation, adding 100 µl/well of serial dilutions of either GlcNAc, CysNAc or D-mannose. After 1 h, the plates were washed 3 times before addition of HCV glycoproteins.
Neutralization of Pseudovirus Entry
HCVpp were prepared as previously described [27], incorporating E1/E2 glycoproteins from HCV genotypes 1a (H77c, AF011751), 2a (JFH1, AB047639), 3a (UKN3A13.6, AY894683) and 4a (UKN4.11.1, AY734986). Pseudoparticles possessing the vesicular stomatitis virus (VSV) G protein were also produced. The infectivity of purified pseudoparticles was assessed using Huh7 cells, incubating particles with purified L-ficolin preparations (quantified by either BCA assay or functional binding assay) for 1 h in DMEM before addition to target cells. A fraction from the L-ficolin purification process containing no detectable protein (determined by BCA assay) was used as a control. Luciferase activity was assessed after 72 h. Neutralization assays with human immunodeficiency virus (HIV)-1 pseudoviruses were performed essentially in the same manner, using glycoproteins from strain HXB2. Infection of TZM-bl cells was performed as previously described [28]. To assess any effect of L-ficolin on Huh7 cells, fractions containing L-ficolin oligomers (or controls) were incubated with cells at 4°C for 1 h prior to washing with PBS and addition of pseudoviruses.
Neutralization of HCVcc Infection
Neutralization assays with JFH1 HCVcc were performed as previously described [27], using similar conditions to those of HCVpp assays. Neutralization was performed with 100 focus-forming units of virus and different concentrations of purified L-ficolin protein. The effect of calcium was determined using an approximate median inhibitory concentration of oligomeric L-ficolin in the presence of 2 or 7 mM CaCl2. Infection was determined by staining for the presence of HCV NS5A using antibody 9E10 [29]. Neutralization was calculated as the percentage of an uninhibited control.
Statistical Analysis
Unpaired t tests or one-way analysis of variance tests were used as appropriate to determine differences between mean binding/neutralization values. Serum concentrations of L-ficolin in different groups of individuals were compared using a Mann-Whitney U test.
Results
In vitro Expressed Recombinant L-Ficolin Forms Oligomers Similar to Serum-Purified L-Ficolin
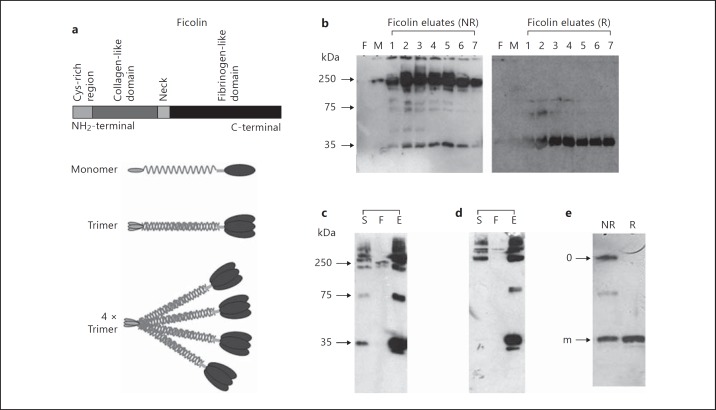
Natural L-ficolin exists as oligomers of a 35-kDa subunit, up to and including a dodecamer form (fig. 1a). These oligomers were observed for fractions of L-ficolin purified from human serum after passage through a GlcNAc-Sepharose matrix and elution with soluble GlcNAc (fig. 1b, left panel). Proteins observed at 35 and 70 kDa and above 250 kDa represented monomers, dimers and higher-order oligomers, respectively. Consistent with previous reports [30], proteins were also observed at molecular masses greater than the expected dodecamer, suggesting that L-ficolin is able to form covalently linked higher-molecular weight oligomers. Under reducing conditions, the oligomers of serum L-ficolin were reduced to a molecular mass of 35 kDa (fig. 1b, right panel), with traces of dimer or trimer. Recombinant FLAG-tagged, affinity-purified L-ficolin was observed as a similar mixture of monomers and oligomers, both when probed with anti-L-ficolin mAb (fig. 1c) and with anti-FLAG mAb (fig. 1d), indicating that the recombinant protein possesses a similar structure to the in vivo form. Under these nonreducing conditions, higher oligomers were observed, including a possible dodecamer complex. Treatment with dithiothreitol resulted in only the monomeric recombinant protein being resolved by Western blot (fig. 1e).
Fig. 1.
Expression of recombinant, oligomeric human L-ficolin. a Schematic illustrating the structure of L-ficolin. Each polypeptide monomer possesses a cysteine-rich N-terminal domain, a collagen-like domain, a neck region and a C-terminal fibrinogen-like domain. Monomers oligomerize into dodecamers formed of trimeric subunits. b L-ficolin was purified from human serum using GlcNAc-Sepharose. The flow-through (F), mannose wash (M) and L-ficolin eluate fractions (lanes 1-7) were detected by Western blot with mAb GN5 following nonreducing (NR) or reducing (R) SDS-PAGE. Serum L-ficolin oligomers appeared as a 35-kDa monomer, 70-kDa dimers and approximately 250-kDa oligomers, as well as higher-order multimers. c, d Expression of recombinant L-ficolin. In vitro expressed L-ficolin was purified using an anti-FLAG M2 affinity resin. L-ficolin samples were resolved using nonreducing PAGE and Western blotting with anti-L-ficolin antibody GN5 (c) or anti-FLAG mAb M2 (d). Each blot presents reactivity with start material (S), flow through (F) and eluted L-ficolin (E). Monomeric (35 kDa), dimeric (approx. 70 kDa) and multimeric forms (≥250 kDa) of recombinant L-ficolin were observed. e Eluted material was analyzed under nonreducing conditions (NR) or reduced in the presence of dithiothreitol (R), demonstrating the reduction of oligomers (o) to monomers (m).
Recombinant L-Ficolin Interacts with HCV Glycoproteins
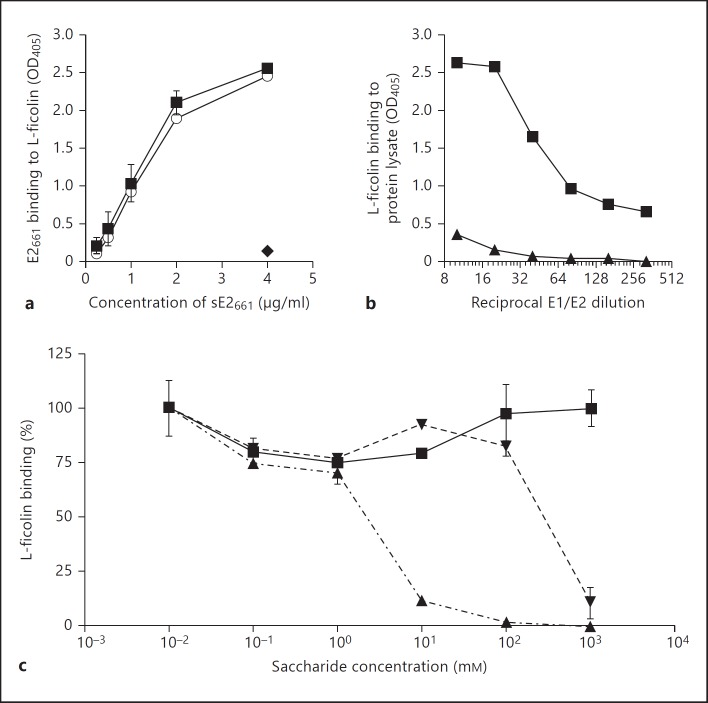
Two recombinant glycoprotein constructs derived from the HCV infectious clone H77c [31] were used to model the interaction between L-ficolin and HCV virions. Binding of both recombinant L-ficolin and L-ficolin purified from serum to the E2 glycoprotein ectodomain (aa 363-661) was comparable (fig. 2a). This was confirmed by dose-dependent binding of recombinant L-ficolin to E1/E2 heterodimers (aa 170-746; fig. 2b).
Fig. 2.
Recombinant L-ficolin binds to HCV glycoproteins E1 and E2 in an acetyl-specific manner. a L-ficolin binding to recombinant HCV glycoproteins was evaluated by ELISA. E2 was captured using mAb GN5-immobilized L-ficolin and detected by anti-E2 antibody. An equivalent, dose-dependent interaction was observed between the soluble ectodomain of HCV E2 (sE2661) and both recombinant L-ficolin (◼) and purified serum L-ficolin (⚪). The highest concentration of sE2661 was incubated with mAb GN5 in the absence of L-ficolin as a control (◆). b Dose-dependent binding of L-ficolin to 293T cell lysates from cells transfected with E1/E2 (◼) or mock-transfected cells (▲). c Immobilized L-ficolin was preincubated with the indicated concentrations of L-ficolin ligands GlcNAc (▼), CysNAc (▲) or D-mannose (◼) prior to binding sE2. Binding is presented as a proportion of binding in the absence of inhibitor. Median inhibitory concentration values were 260 mM (GlcNAc) and 1.8 mM (CysNAc). No inhibition by D-mannose was observed.
Preincubating L-ficolin with ligands resulted in competition for E2 binding (fig. 2c). Both CysNAc and GlcNAc inhibited the binding interaction between recombinant L-ficolin and the E2 ectodomain in a dose-dependent manner. Fifty percent inhibition was achieved with 1.8 mM CysNAc and 260 mM GlcNAc. At the greatest concentration tested (1 M), the MBL ligand D-mannose had no effect on binding.
L-Ficolin Neutralizes Genetically Diverse HCV Strains
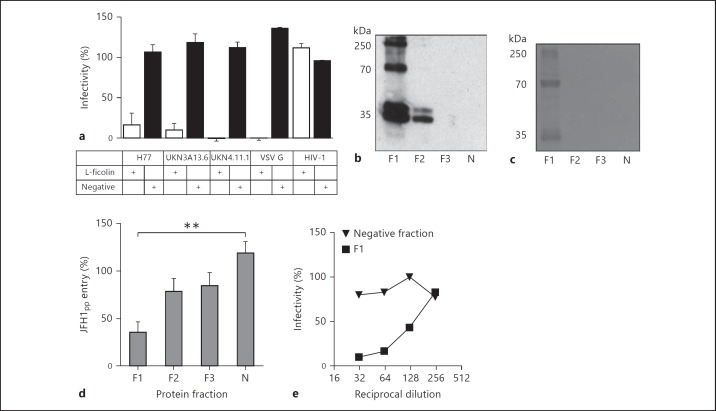
Having demonstrated an interaction between recombinant L-ficolin and the HCV glycoproteins, the effect of L-ficolin on entry of HCVpp possessing the HCV glycoproteins isolated from patient viruses was investigated. Before performing these experiments, the expression of L-ficolin by target Huh7 cells was assessed. No cellular expression of L-ficolin in the target cells was observed, either by immunofluorescence or quantitative RT-PCR of L-ficolin mRNA (data not shown). Glycoproteins derived from genetically diverse HCV viruses were tested in a pseudoparticle entry assay (fig. 3a). Active L-ficolin in the preparation was quantified using a functional assay binding to acetylated BSA. At a concentration of 1 μg/ml active protein, almost complete inhibition of entry of pseudoparticles reconstituted with strains H77 (genotype 1a), UKN3A13.6 (genotype 3a) and UKN4.11.1 (genotype 4) was observed (fig. 3a). A control fraction from the purification procedure containing no L-ficolin (as determined by Western blot and BCA assay) had no inhibitory effect when diluted equivalently. Entry of pseudoparticles bearing VSV G was also neutralized by L-ficolin, but this L-ficolin preparation had no effect on entry of retroviral pseudoparticles bearing HIV glycoproteins into CD4+/CCR5+ TZM-bl cells, eliminating the possibility of nonspecific toxicity (fig. 3a). Recombinant L-ficolin was further separated into 3 fractions (F1, F2 and F3) containing a mixture of oligomers/monomers, or only monomers, as analyzed by Western blot (fig. 3b) and stained SDS-PAGE (fig. 3c). Consistent with the greater sensitivity of Western blotting, protein was only observed in sample F1 in the stained gel. No contaminating protein was observed. Total protein was quantified in these samples by BCA assay. Samples F1, F2 and F3 possessed 157, 46 and 10 µg/ml protein, respectively. Each sample was diluted to 1 µg/ml total protein and assessed for neutralizing potency against HCVpp possessing the glycoproteins from strain JFH1 (fig. 3d). F1 neutralized HCVpp entry by >60%. F2 and F3 demonstrated no significant inhibition. A negative control fraction possessed no neutralizing activity. To exclude the possibility that the small neutralizing effect of F2 and F3 might be due to the presence of residual FLAG peptide in the eluted material, neutralization experiments with this peptide demonstrated no effect on the entry of HCVpp (not shown). Given that the negative fraction was treated identically and had no effect on entry, it is most likely that the neutralizing effect was due to small quantities of oligomeric L-ficolin in these fractions. The sample possessing detectable oligomeric L-ficolin consistently had significantly greater neutralizing effect than any other sample tested. This sample was further found to inhibit the entry of HCVcc of strain JFH-1. Sample F1, at a stock of 157 µg/ml, was serially diluted in parallel with the negative fraction before incubation with HCVcc. Neutralization by F1 was dose dependent, with a median effective concentration of 1.2 µg/ml (fig. 3f), while no inhibition was observed for the negative fraction.
Fig. 3.
L-Ficolin-mediated neutralization of HCV entry. a Retroviral pseudoparticles possessing envelope glycoproteins E1/E2 from strains of HCV representing genotypes 1a (H77c), 3a (UKN3A13.6) and 4a (UKN4.11.1), the VSV G protein or HIV-1 gp160 were treated for 1 h at room temperature with purified recombinant L-ficolin (gray bars) or a control fraction from purification containing no L-ficolin (black bars), before infecting Huh7 cells. All of the HCVpp and the VSV G pseudoparticles were neutralized by L-ficolin. No inhibition of entry of pseudoparticles bearing HIV-1 glycoproteins into TZM-bl cells was observed. Data are presented as the proportion of infectivity in the absence of inhibitor. b FLAG-tagged L-ficolin expressed in the supernatants of HEK 293T cells was fractionated by affinity purification on FLAG resin and pooled into 3 independent fractions (F1, F2 and F3), as well as a negative fraction containing no L-ficolin (N). These fractions contained 157, 46, 10 and 0 µg/ml total protein, respectively. c When analyzed by staining an SDS-PAGE gel, protein was only detected in sample F1. d The fractions were each diluted to 1 µg/ml total protein and assessed for neutralization of entry of HCVpp bearing JFH1 E1/E2 glycoproteins. Data are presented as the proportion of an uninhibited control. Significant neutralization was only exhibited for the fraction containing detectable oligomeric L-ficolin (** p < 0.01). e The F1 fraction was found to neutralize entry of the JFH1 strain of HCVcc in a dose-dependent manner (median inhibitory concentration = 1.2 µg/ml), while no inhibition was observed with the negative control sample.
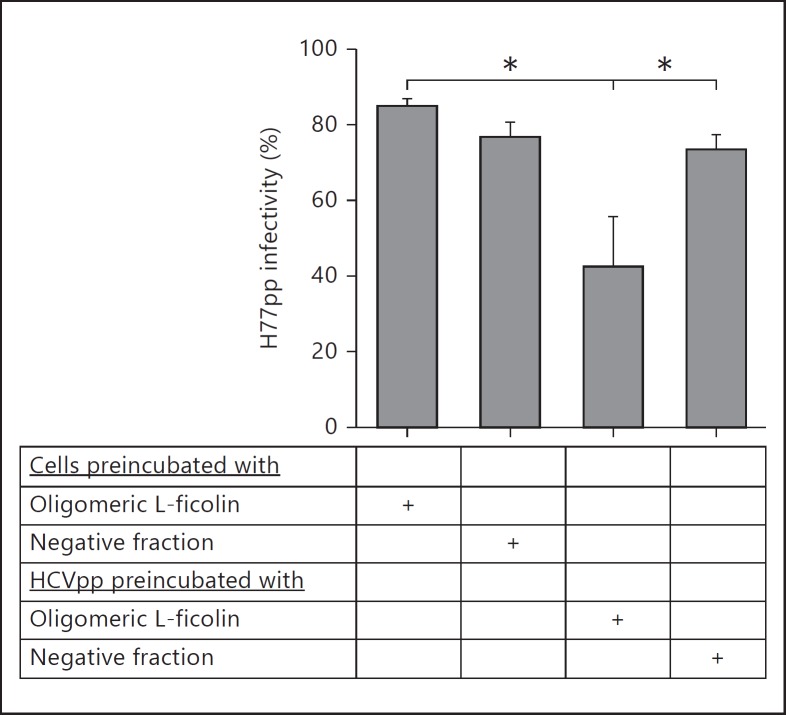
To eliminate the possibility that the observed neutralizing effect was a result of L-ficolin interacting with the target cells, preincubation experiments were performed with a preparation of L-ficolin. Neutralization was only observed when pseudoparticles were preincubated with the L-ficolin-containing sample (fig. 4).
Fig. 4.
Neutralization of HCV entry by L-ficolin requires interaction with virus particles. Protein fractions containing oligomeric L-ficolin, or a control with no detectable L-ficolin diluted equivalently, were incubated with either HCVpp (strain H77) or target Huh7 cells. Following washing, cells were infected with HCVpp. Neutralization >50% was only observed when L-ficolin was preincubated with virus particles. A significant difference was observed between virus and cells treated with L-ficolin compared to cells treated alone (* p < 0.05, one-way analysis of variance). No significant differences in HCV entry were observed when cells were treated with L-ficolin or a negative control.
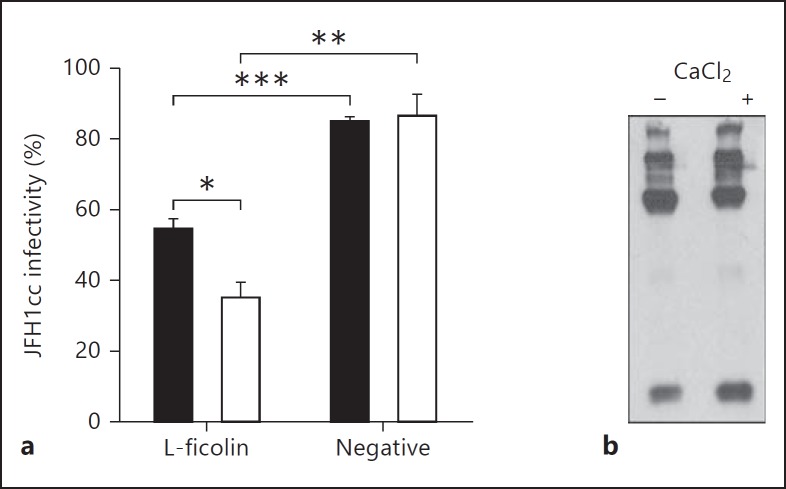
L-Ficolin Neutralizes HCVcc in a Calcium-Dependent Manner
L-Ficolin binding has been described to be partially calcium dependent [32]. To determine if this is the case for recognition of HCV, neutralization experiments were performed using HCVcc in the presence of different concentrations of CaCl2. HCVcc particles were prepared in media containing a basal level of 2 mM CaCl2 or media supplemented to a final concentration of 7 mM CaCl2. Using 1.6 µg/ml L-ficolin in this assay, enhanced neutralization was observed with increasing calcium chloride concentration, indicating that this activity is, at least in part, calcium dependent. This is likely to be caused by interactions between the S1 binding site and the GlcNAc present on the surface of HCV particles. When L-ficolin treated with 7 mM CaCl2 was analyzed by Western blot, no difference in patterns of oligomerization was observed (fig. 5b). Consistent with previous reports [30], the action of calcium is likely to be on binding activity rather than disulfide-mediated oligomerization.
Fig. 5.
Inhibition of HCV entry is calcium dependent. a Infection assays were prepared with cell culture-grown HCV. Neutralization was performed with approximately 50% neutralizing concentrations of oligomeric L-ficolin in the presence of 2 mM CaCl2 (black bars) or 7 mM CaCl2 (gray bars). Neutralization was compared to a positive uninhibited control and a sample treated with an equivalent L-ficolin-negative fraction from purification. Greater neutralization of HCVcc was observed with L-ficolin fractions in the presence of 7 mM CaCl2 (** p < 0.01, *** p < 0.001, one-way analysis of variance). * p < 0.05. b Ficolin was incubated in PBS ± 7 mM CaCl2 before analysis by SDS-PAGE/Western blotting with mAb GN5. No difference in oligomerization was observed in the presence of CaCl2.
Inibition of HCV Entry by L-Ficolin Ligands
To further assess the binding specificity to the observed neutralization, inhibition experiments with GlcNAc and CysNAc were performed. At the concentrations demonstrated to inhibit interaction of E2 with L-ficolin, both GlcNAc (data not shown) and CysNAc (online suppl. fig. 1) were also found to inhibit entry in the absence of L-ficolin. As such, any blocking effect of L-ficolin neutralization was not resolvable. This unexpected result could be evidence that cellular receptors involved in HCV entry recognize acetyl-containing molecular entities similar to the ficolin, so that acetyl-containing inhibitors (GlcNAc and CysNAc) also competitively inhibit these interactions. To investigate this further, CysNAc was incubated with either HCV, VSV or HIV-1 pseudoviruses, or target cells, prior to infection (online suppl. fig. 1B). Treatment of both HCVpp and VSVpp with CysNAc resulted in a reduction in infectivity, but no effect on HIV-1 entry was observed. It is possible that VSV and HCV share interactions with receptors that interact with this ligand. Unexpectedly, treatment of target cells with CysNAc resulted in a significant increase in entry of HIV-1pp but not HCVpp or VSVpp. This finding is worthy of further investigation and may be linked to the plasma-membrane fusion event of HIV-1 compared to the endosomal fusion of VSV and HCV. The lack of inhibition of pseudovirus entry with CysNAc-pretreated cells suggests that this blocking effect is mediated on the virus, rather than the cells, and is not attributable to cell cytotoxicity. Visual inspection of Huh7 cells treated with CysNAc (online suppl. fig. 1C) confirmed that no cell death occurred in treated cells but revealed a visible change in cell morphology and size, which may be linked to altered receptor expression and resistance to HCV entry.
L-Ficolin Neutralizes HCV Entry at Physiologically Relevant Levels
As many proteins can inhibit viruses at high concentrations, we next determined if the concentration of L-ficolin that resulted in in vitro neutralization was physiologically relevant in both healthy donors and a cohort of HCV-infected patients. Serum L-ficolin was quantified by ELISA in sera taken from healthy donors and patients with chronic HCV infection (online suppl. fig. 2). There was no significant difference between the median L-ficolin concentrations in healthy donors (4.6 μg/ml, SD ±1.5) and patients with chronic HCV infection (4.2 μg/ml, SD ±1.9; p = 0.29, Mann-Whitney U test). This confirmed that the concentration of L-ficolin that neutralizes HCV entry in vitro is biologically relevant and that individuals with chronic HCV infection do not have impaired capacity to produce L-ficolin.
Discussion
The soluble innate immune effector L-ficolin has been implicated in the control of a range of infectious diseases, acting as an opsonin and activating complement upon binding to glycosylated targets [33]. Here, we demonstrated that a recombinant, oligomeric L-ficolin mediates direct neutralization of HCV entry.
A key advance was the expression of correctly folded oligomeric recombinant L-ficolin in human cells, in contrast to previous studies using bacterially expressed protein that yielded only monomers [22]. The monomeric form of L-ficolin was described to activate the complement cascade and facilitate complement-mediated lysis of HCV-infected cells but not to inhibit HCV entry. The N-terminal FLAG tag used for purification had no significant effect on the oligomerization of the recombinant L-ficolin polypeptides, and this oligomer possessed binding equivalent to that of serum-purified protein. This construct provides a useful tool for further investigations of the direct antiviral properties of L-ficolin.
Acetylated sugars are defined ligands for L-ficolin [14]. It is likely that the high-mannose oligosaccharides present on the surface of E1/E2 possessing a GlcNAc2 stem are binding targets for L-ficolin [34, 35]. There is evidence that two of the N-linked glycosylation sites might possess complex glycans containing terminal GlcNAc residues at residues 423 and 430 [7]. These asparagines have been implicated in the entry of HCVpp [8] and HCVcc [9], respectively. The neutralization of entry of HCVcc and HCVpp representing HCV genotypes 1, 2, 3 and 4 is consistent with a role for conserved E1/E2 glycans in HCV entry. The genotype 3a clone used in this study has previously been described to be resistant to neutralization by broadly neutralizing antibodies [27, 36]. L-ficolin effectively neutralized this isolate, indicating a possible therapeutic application for inhibiting entry of antibody neutralization-resistant HCV strains. Consistent with previous reports demonstrating interaction of L-ficolin with GlcNAc [17] and CysNAc [32], both ligands inhibited interaction of recombinant L-ficolin with glycoprotein E2, suggesting interaction of the fibrinogen-like domain and N-linked glycans. Four discrete binding sites in the L-ficolin fibrinogen-like domain have been identified (S1-4), which possess different binding specificities [37]. This may account for the difference observed in competition assays with the ligands GlcNAc and CysNAc; although they both bind to the S2 site, GlcNAc also binds around the S1 site, while CysNAc is able to bind to site S3 and, at high concentrations, to other sites with little structural interaction [37]. The unexpected discovery that both GlcNAc and CysNac inhibited HCV entry in vitro also raises new questions about the nature of virus-cell interactions. It is possible that these ligands alter the expression of host receptors, and this is worthy of further investigation.
The calcium-dependent nature of the neutralizing activity suggested that the accessibility of the binding site on L-ficolin is modulated by the presence of a Ca2+ ion, as previously described [16]. As the fibrinogen-like domain possesses multiple binding sites with differential specificities, the exact interactions between the HCV glycoproteins and the binding surface of L-ficolin remain to be determined. We also eliminated the possibility that L-ficolin interactions with target cells make them refractory to virus entry, demonstrating a virion-specific effect. The exact molecular interaction between L-ficolin and E1/E2 is currently under investigation.
Recombinant oligomeric L-ficolin inhibited entry of both HCV and VSV pseudoviruses. It is still not understood what common factor determines this sensitivity to L-ficolin neutralization, but this molecule has potential for application as an entry inhibitor for other enveloped viruses. It is plausible that neutralization specificity is associated with specific modifications to carbohydrates on the glycoprotein surface. This neutralizing activity is consistent with the ability of porcine ficolin-α to bind and neutralize PRRSV. Ficolin-α reduced the cytopathic effect of PRRSV and inhibited replication of infectious viral particles [20]. However, soluble lectins have also been demonstrated to inhibit antibody neutralization of HIV-1 [38], suggesting that complex interactions might take place at the surface of the virion.
It remains to be demonstrated if binding of this oligomeric L-ficolin construct to HCV might result in secondary antiviral properties that are therapeutically useful. L-ficolin might contribute to viral clearance through activation of serine proteases activating the complement cascade. Complement is an essential component of the antiviral immunity to other flaviviruses [39] and is likely to contribute to protection against HCV infection [22]. Indirect evidence supports an antiviral role for the complement cascade, as both HCV NS5A and core proteins inhibit complement component C4 transcription. Protectin (CD59) is also incorporated into HCV virions [40], suggesting that HCV has evolved multiple strategies to evade complement-dependent lysis. It is possible that L-ficolin-mediated complement activation might contribute to in vivo elimination of HCV particles as well as infected cells.
We assayed the concentration of L-ficolin expressed in the serum of healthy individuals and people chronically infected with HCV, finding that the range is consistent with that previously reported in healthy cohorts [41]. While we appreciate that the localized concentration of L-ficolin might be higher at the site of synthesis in the liver, this provides evidence that neutralizing concentrations of L-ficolin are circulating in human sera. The proportion of HCV virions that circulate as complexes with L-ficolin remains to be determined. It was also noted that any liver damage caused by chronic HCV infection did not result in reduced L-ficolin expression in these individuals.
This study highlights the potential for an oligomeric recombinant L-ficolin as a novel therapeutic entry inhibitor for HCV infection. The broad, potent antiviral activity might also be applied to inhibition of entry of other enveloped viruses possessing acetylated glycoproteins. We have also identified a previously unreported antiviral activity of the L-ficolin ligand CysNAc.
Supplementary Material
Supplementary data
Acknowledgements
This work was funded by a studentship to M.R.H. from the Egyptian government, EU FP7 (Health-F4-2012-305600) and the MRC DPFS (G0801169). Clinical samples were provided with ethical approval (MREC/98/3/55) by the Trent Clinical HCV Cohort. We thank Arvind Patel, Charles Rice, Jens Bukh and Francois-Loic Cosset for kind provision of valuable reagents. Current addresses: R.J.P.B.: TWINCORE Zentrum für Experimentelle und Klinische Infektionsforschung GmbH, Feodor-Lynen-Strasse 7, DE-30625 Hannover (Germany); A.K.: Crucell, Archimedesweg 4-6, NL-2333 CN Leiden (The Netherlands); T.P.H.: Pfizer, Pharmacokinetics, Dynamics and Metabolism, 1 Burtt Road, Andover, MA 01810 (USA).
References
- 1.Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, Kaldor JM. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 2.Londono MC, Crespo G, Forns X. Pretransplant and posttransplant treatment of hepatitis C virus infection with protease inhibitors. Curr Opin Organ Transplant. 2013;18:271–278. doi: 10.1097/MOT.0b013e3283614aca. [DOI] [PubMed] [Google Scholar]
- 3.Edwards VC, Tarr AW, Urbanowicz RA, Ball JK. The role of neutralizing antibodies in hepatitis C virus infection. J Gen Virol. 2012;93:1–19. doi: 10.1099/vir.0.035956-0. [DOI] [PubMed] [Google Scholar]
- 4.Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 5.Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology. 2004;39:5–19. doi: 10.1002/hep.20032. [DOI] [PubMed] [Google Scholar]
- 7.Iacob RE, Perdivara I, Przybylski M, Tomer KB. Mass spectrometric characterization of glycosylation of hepatitis C virus E2 envelope glycoprotein reveals extended microheterogeneity of N-glycans. J Am Soc Mass Spectrom. 2008;19:428–444. doi: 10.1016/j.jasms.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goffard A, Callens N, Bartosch B, Wychowski C, Cosset FL, Montpellier C, Dubuisson J. Role of N-linked glycans in the functions of hepatitis C virus envelope glycoproteins. J Virol. 2005;79:8400–8409. doi: 10.1128/JVI.79.13.8400-8409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helle F, Vieyres G, Elkrief L, Popescu CI, Wychowski C, Descamps V, Castelain S, Roingeard P, Duverlie G, Dubuisson J. Role of N-linked glycans in the functions of hepatitis C virus envelope proteins incorporated into infectious virions. J Virol. 2010;84:11905–11915. doi: 10.1128/JVI.01548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown KS, Keogh MJ, Owsianka AM, Adair R, Patel AH, Arnold JN, Ball JK, Sim RB, Tarr AW, Hickling TP. Specific interaction of hepatitis C virus glycoproteins with mannan binding lectin inhibits virus entry. Protein Cell. 2010;1:664–674. doi: 10.1007/s13238-010-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helle F, Wychowski C, Vu-Dac N, Gustafson KR, Voisset C, Dubuisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J Biol Chem. 2006;281:25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 12.Meuleman P, Albecka A, Belouzard S, Vercauteren K, Verhoye L, Wychowski C, Leroux-Roels G, Palmer KE, Dubuisson J. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob Agents Chemother. 2011;55:5159–5167. doi: 10.1128/AAC.00633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 14.Krarup A, Mitchell DA, Sim RB. Recognition of acetylated oligosaccharides by human L-ficolin. Immunol Lett. 2008;118:152–156. doi: 10.1016/j.imlet.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita M, Fujita T. Ficolins and the lectin complement pathway. Immunol Rev. 2001;180:78–85. doi: 10.1034/j.1600-065x.2001.1800107.x. [DOI] [PubMed] [Google Scholar]
- 16.Garlatti V, Belloy N, Martin L, Lacroix M, Matsushita M, Endo Y, Fujita T, Fontecilla-Camps JC, Arlaud GJ, Thielens NM, Gaboriaud C. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 2007;26:623–633. doi: 10.1038/sj.emboj.7601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita M, Endo Y, Taira S, Sato Y, Fujita T, Ichikawa N, Nakata M, Mizuochi T. A novel human serum lectin with collagen- and fibrinogen-like domains that functions as an opsonin. J Biol Chem. 1996;271:2448–2454. doi: 10.1074/jbc.271.5.2448. [DOI] [PubMed] [Google Scholar]
- 18.Le Y, Lee SH, Kon OL, Lu J. Human L-ficolin: plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett. 1998;425:367–370. doi: 10.1016/s0014-5793(98)00267-1. [DOI] [PubMed] [Google Scholar]
- 19.Roozendaal R, Carroll MC. Complement receptors CD21 and CD35 in humoral immunity. Immunol Rev. 2007;219:157–166. doi: 10.1111/j.1600-065X.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 20.Keirstead ND, Lee C, Yoo D, Brooks AS, Hayes MA. Porcine plasma ficolin binds and reduces infectivity of porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. Antiviral Res. 2008;77:28–38. doi: 10.1016/j.antiviral.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan Q, Chen H, Wang F, Jeza VT, Hou W, Zhao Y, Xiang T, Zhu Y, Endo Y, Fujita T, Zhang XL. L-ficolin binds to the glycoproteins hemagglutinin and neuraminidase and inhibits influenza A virus infection both in vitro and in vivo. J Innate Immun. 2012;4:312–324. doi: 10.1159/000335670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Ali MA, Shi Y, Zhao Y, Luo F, Yu J, Xiang T, Tang J, Li D, Hu Q, Ho W, Zhang X. Specifically binding of L-ficolin to N-glycans of HCV envelope glycoproteins E1 and E2 leads to complement activation. Cell Mol Immunol. 2009;6:235–244. doi: 10.1038/cmi.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cseh S, Vera L, Matsushita M, Fujita T, Arlaud GJ, Thielens NM. Characterization of the interaction between L-ficolin/p35 and mannan-binding lectin-associated serine proteases-1 and −2. J Immunol. 2002;169:5735–5743. doi: 10.4049/jimmunol.169.10.5735. [DOI] [PubMed] [Google Scholar]
- 24.Michalak JP, Wychowski C, Choukhi A, Meunier JC, Ung S, Rice CM, Dubuisson J. Characterization of truncated forms of hepatitis C virus glycoproteins. J Gen Virol. 1997;78:2299–2306. doi: 10.1099/0022-1317-78-9-2299. [DOI] [PubMed] [Google Scholar]
- 25.Yamada E, Montoya M, Schuettler CG, Hickling TP, Tarr AW, Vitelli A, Dubuisson J, Patel AH, Ball JK, Borrow P. Analysis of the binding of hepatitis C virus genotype 1a and 1b E2 glycoproteins to peripheral blood mononuclear cell subsets. J Gen Virol. 2005;86:2507–2512. doi: 10.1099/vir.0.81169-0. [DOI] [PubMed] [Google Scholar]
- 26.Owsianka A, Tarr AW, Juttla VS, Lavillette D, Bartosch B, Cosset FL, Ball JK, Patel AH. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol. 2005;79:11095–11104. doi: 10.1128/JVI.79.17.11095-11104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarr AW, Urbanowicz RA, Hamed MR, Albecka A, McClure CP, Brown RJ, Irving WL, Dubuisson J, Ball JK. Hepatitis C patient-derived glycoproteins exhibit marked differences in susceptibility to serum neutralizing antibodies: genetic subtype defines antigenic but not neutralization serotype. J Virol. 2011;85:4246–4257. doi: 10.1128/JVI.01332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindenbach BD, Evans MJ, Syder AJ, Wolk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA, Rice CM. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623–626. doi: 10.1126/science.1114016. [DOI] [PubMed] [Google Scholar]
- 30.Hummelshoj T, Thielens NM, Madsen HO, Arlaud GJ, Sim RB, Garred P. Molecular organization of human Ficolin-2. Mol Immunol. 2007;44:401–411. doi: 10.1016/j.molimm.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 31.Yanagi M, Purcell RH, Emerson SU, Bukh J. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into the liver of a chimpanzee. Proc Natl Acad Sci USA. 1997;94:8738–8743. doi: 10.1073/pnas.94.16.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krarup A, Thiel S, Hansen A, Fujita T, Jensenius JC. L-ficolin is a pattern recognition molecule specific for acetyl groups. J Biol Chem. 2004;279:47513–47519. doi: 10.1074/jbc.M407161200. [DOI] [PubMed] [Google Scholar]
- 33.Matsushita M, Endo Y, Fujita T. Cutting edge: complement-activating complex of ficolin and mannose-binding lectin-associated serine protease. J Immunol. 2000;164:2281–2284. doi: 10.4049/jimmunol.164.5.2281. [DOI] [PubMed] [Google Scholar]
- 34.Duvet S, Cocquerel L, Pillez A, Cacan R, Verbert A, Moradpour D, Wychowski C, Dubuisson J. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J Biol Chem. 1998;273:32088–32095. doi: 10.1074/jbc.273.48.32088. [DOI] [PubMed] [Google Scholar]
- 35.Goffard A, Dubuisson J. Glycosylation of hepatitis C virus envelope proteins. Biochimie. 2003;85:295–301. doi: 10.1016/s0300-9084(03)00004-x. [DOI] [PubMed] [Google Scholar]
- 36.Tarr AW, Lafaye P, Meredith L, Damier-Piolle L, Urbanowicz RA, Meola A, Jestin JL, Brown RJ, McKeating JA, Rey FA, Ball JK, Krey T. An alpaca nanobody inhibits hepatitis C virus entry and cell-to-cell transmission. Hepatology. 2013;58:932–939. doi: 10.1002/hep.26430. [DOI] [PubMed] [Google Scholar]
- 37.Garlatti V, Martin L, Gout E, Reiser JB, Fujita T, Arlaud GJ, Thielens NM, Gaboriaud C. Structural basis for innate immune sensing by M-ficolin and its control by a pH-dependent conformational switch. J Biol Chem. 2007;282:35814–35820. doi: 10.1074/jbc.M705741200. [DOI] [PubMed] [Google Scholar]
- 38.Marzi A, Mitchell DA, Chaipan C, Fisch T, Doms RW, Carrington M, Desrosiers RC, Pohlmann S. Modulation of HIV and SIV neutralization sensitivity by DC-SIGN and mannose-binding lectin. Virology. 2007;368:322–330. doi: 10.1016/j.virol.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Mehlhop E, Diamond MS. Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J Exp Med. 2006;203:1371–1381. doi: 10.1084/jem.20052388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amet T, Ghabril M, Chalasani N, Byrd D, Hu N, Grantham A, Liu Z, Qin X, He JJ, Yu Q. CD59 incorporation protects hepatitis C virus against complement-mediated destruction. Hepatology. 2012;55:354–363. doi: 10.1002/hep.24686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kilpatrick DC, Fujita T, Matsushita M. P35, an opsonic lectin of the ficolin family, in human blood from neonates, normal adults, and recurrent miscarriage patients. Immunol Lett. 1999;67:109–112. doi: 10.1016/s0165-2478(98)00147-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data