Abstract
Activation of complement may cause severe tissue damage in antibody-mediated allograft rejection and other antibody-mediated clinical conditions; therefore, novel potent complement inhibitors are needed. Previously, we described binding of the inhibitory receptor LAIR-1 and its soluble family member LAIR-2 to collagen. Here, we investigated binding of LAIR-1 and LAIR-2 to the complement proteins C1q and MBL, which both have collagen-like domains, and evaluated the effect of this binding on complement function. We demonstrate specific binding of recombinant LAIR proteins to both C1q and MBL. Surface plasmon resonance experiments showed that LAIR-2-Fc protein bound C1q and MBL with the highest affinity compared to LAIR-2-HIS. We, therefore, hypothesized that LAIR-2-Fc is a potent complement inhibitor. Indeed, LAIR-2-Fc inhibited C4 fixation to IgG or mannan, reduced activation of C4 by aggregated IgG in plasma and inhibited iC3b deposition on cells. Finally, LAIR-2-Fc inhibited complement-mediated lysis of cells sensitized with anti-HLA antibodies in an ex vivo model for antibody-mediated transplant rejection. Thus, LAIR-2-Fc is an effective novel complement inhibitor for the treatment and prevention of antibody-mediated allograft rejection and antibody-mediated clinical conditions.
Key Words: Complement inhibition, LAIR, MBL, C1q
Introduction
The complement system is a double-edged sword: on the one hand it is a vital part of the immune system contributing to the defense against microbes, but on the other hand it can contribute to severe pathology. For example, in antibody-mediated rejection (AMR) of transplanted organs by anti-HLA or anti-blood group antibodies complement is involved [1, 2]. Deposition of C4d in kidney allografts is often used as a diagnostic hallmark for AMR [3]. Also, reperfusion of transplanted organs triggers complement activation, which contributes to the ischemia-reperfusion injury [1]. This can lead to major complications such as damage to the graft and even stimulate cell-mediated rejection [4]. Most vital organs such as the heart, lung and kidneys are susceptible to complement-mediated injury [4]. Yet, application of complement inhibitors to protect grafts from these processes has not been approved, possibly because potent complements that effectively prevent deleterious complement activation in the grafts and improve transplant survival are currently not available in the clinic.
The complement system is activated via three pathways: the classical, the lectin and the alternative pathway. Activation of complement by IgG or IgM antibodies and in ischemia-reperfusion conditions mainly involves the classical and lectin pathways. The classical pathway is triggered by the binding of the C1 complex to IgG or IgM antibodies complexed with antigens. Binding of C1 is mediated by its subcomponent C1q, which is a multimeric protein containing 6 subunits, and each consists of a trimer of 3 different polypeptide chains. Each chain contains a C-terminal globular head region bound to an N-terminal collagen-like region [5]. The lectin pathway is initiated by binding of MBL (mannose-binding lectin) to carbohydrate structures on pathogens, which is followed by activation of the MBL-associated serine proteases. The MBL-associated serine proteases in turn activate C4 and C2. Similar to C1q, MBL is an oligomeric protein made up of up to 6 subunits, and each consists of a homotrimer of the same polypeptide chain. This chain also contains a C-terminal globular domain, coupled to an N-terminal collagen-like region. The alternative pathway can be activated by various triggers and amplifies complement activation at the level of C3 initiated by any pathway [4].
LAIR-1 (leukocyte-associated immunoglobulin-like receptor-1) is an inhibitory immune receptor expressed on most immune cells and LAIR-2 is a homologous protein released by cells. Ligation of LAIR-1 generates a threshold for activation of various cellular functions [6]. Collagens serve as ligands for LAIR-1 [7, 8], and we recently found that in particular dimeric recombinant LAIR proteins have a high affinity for collagen [9]. Recently, Son et al. [10] reported C1q as ligand for LAIR-1 on dendritic cells. In this report, we assessed binding of LAIR proteins to C1q as well as to MBL and explored the potential of LAIR proteins as complement inhibitors. We demonstrate that LAIR-1 and LAIR-2 bind to the collagen-like regions of C1q and of MBL. Moreover, we show that upon increasing the affinity of LAIR to C1q and MBL by dimerization of the protein, LAIR-2 can efficiently inhibit complement activation via classical and lectin pathways. Thus, dimerized LAIR-2, produced as LAIR-2-Fc protein, is a promising new complement inhibitor, which may be applied in organ transplantation to prevent AMR and ischemia-reperfusion injury.
Materials and Methods
Biological Samples
Approval for the study was obtained from the Institutional Review Board at the University Medical Center Utrecht. PBMCs were isolated by Ficoll-Paque centrifugation using standard procedures. Normal aged serum was prepared by incubating fresh serum for 1 week at 37°C in the presence of 0.02% (w/v) sodium azide.
Cell Lines, Recombinant Proteins and Antibodies
2B4 NFAT-GFP T cell reporters, K562, stably transduced with hLAIR-1 and EL-4 CD20 have been described previously [8, 11]. Recombinant LAIR and signal-inhibitory receptor on leukocyte-1 (SIRL) proteins were expressed in HEK293 cells as described [9]. Fc- and HIS-tagged proteins of LAIR-1, LAIR-2 and SIRL-1 were made. Recombinant human MBL was purchased from R&D Systems, human C1q and collagen I from Sigma-Aldrich, bovine serum albumin (BSA; fraction V) from Roche Diagnostics, horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Fc fragment specific) from Jackson Immunoresearch, anti-MBL from Bioporto, HRP-conjugated secondary anti-mouse antibodies from DAKO, goat anti-mouse IgG Alexa fluor 488-conjugated antibody from Life Technologies, monoclonal antibody anti-C4-1, C1q-85 (globular head specific) and C1q-52 (collagenous tail specific) from Sanquin, and herceptin and rituximab from Roche Diagnostics.
Goat anti-human C4 polyclonal antibody was biotinylated using EZ-link sulfo-NHS-LC-biotin according the manufacturer's protocol. Aggregated IgG was prepared by heating a therapeutic human IgG preparation (GammaQuin, Sanquin) at a concentration of 80 mg/ml in phosphate-buffered saline (PBS) for 20 min at 63°C [12].
Binding ELISA
Maxisorp plates (Thermo Fisher Scientific) were coated overnight at 4°C with 50 µl/well of collagen I (in 2 mM acetic acid in PBS), BSA (in PBS), C1q, globular head domains of C1q (C1q heads) or MBL (in 10 mM carbonate buffer, pH 9.6). When indicated, 10 μg/ml of specific globular head or collagenous tail antibodies were added 60 min prior to the addition of LAIR proteins. Wells were incubated with 10 μg LAIR-1-Fc, 625 ng LAIR-2-Fc or 10 μg SIRL-1-Fc in 50 µl PBS supplemented with 10 mg/ml BSA and 10 mM ethylenediaminetetraacetic acid (EDTA) for 30 min at room temperature (RT; quantities of recombinant proteins had been established in pilot experiments). HRP-conjugated goat anti-human IgG was added and incubated for 60 min at RT. Plates were washed and ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) reagent (100 µl/well; Roche Diagnostics)] was added. Substrate conversion was measured at 405 nm.
Immune Precipitation
Protein A/G plus-Sepharose beads (Santa Cruz Biotechnology) were incubated with 13 µg LAIR-1-Fc or LAIR-2-Fc (10 µl of packed Sepharose/sample) for 4 h at 4°C. Beads were washed twice with lysis buffer and subsequently 10 µg MBL were added in 500 µl wash buffer [13], incubated for 3 h at 4°C, washed and 30 µl SDS-sample buffer were added. Beads were centrifuged and samples were subjected to SDS-PAGE. Proteins were transferred to Immobilon-P membranes (Millipore) and detected using anti-MBL antibodies and HRP-conjugated secondary antibodies. Enhanced chemiluminescent substrate (Thermo Fisher Scientific) was used to visualize bound antibodies.
Flow Cytometry
K562 wild-type- or hLAIR-1-expressing cells were incubated with 100 ng MBL in the presence of 10%, v/v, human pooled serum in PBS with 10 mM EDTA for 30 min at RT. Cells were washed with PBS containing 10 mg/ml BSA and 0.1%, w/v, sodium azide and incubated with anti-MBL antibody for 30 min at RT. Binding was detected using goat anti-mouse IgG Alexa fluor 488-conjugated antibody.
2B4 T cell hybridoma cells stably transduced with an NFAT-GFP reporter and hLAIR-1-CD3ζ were used to study functional activity of LAIR-1 ligands as described previously [8].
Complement deposition from mouse serum onto a target cell was measured as described before [11]. Cells were analyzed on an LSR II FACS using FACSDiva software (BD Biosciences).
BIAcore
Surface plasmon resonance (BIAcore; GE Healthcare) binding studies were performed with the use of a BIAcore T100 system. Approximately 1,100 response units (RU) of C1q in 10 mM MES buffer (pH 6.1) and 900 of MBL in 10 mM sodium acetate (pH 4.5) buffer were coated for the LAIR-2-HIS proteins and 1,100 RU of C1q and 1,500 of MBL for the LAIR-2-IgG proteins were immobilized on a series S CM5 sensor chip using the amine coupling kit according to the manufacturer's instructions. After coupling, the chip was pulsed for 30 s with EDC/NHS followed by a 30-second pulse with ethanolamine. Analysis was performed in buffer [125 mM NaCl, 2.5 mM CaCl2, 0.005% (w/v) Tween 20 and 25 mM HEPES, pH 7.4] at 25°C and at a flow rate of 20 µl/min. Injections with increasing concentrations of recombinant proteins (0, 500, 1,000, 2,000, 3,000, 4,000, 5,000, 6,000, 8,000, 10,000, 12,000, 16,000, 20,000 and 24,000 nM for LAIR-2-HIS and 0, 31.25, 62.5, 125, 250, 500, 1,000, 2,000, 3,000, 4,000, 6,000 and 8,000 nM for LAIR-2-IgG) were allowed to bind for 5 min for LAIR-2-HIS and 10 min for LAIR-2-IgG, after which regeneration by either flowing buffer for 10 min for the LAIR-2-HIS or 10 mM formic acid for LAIR-2-IgG occurred. Baseline stability was checked after every experiment. Proteins were injected until binding equilibrium was reached. Binding data were analyzed with BIAcore T100 evaluation software (version 2.01). Affinity constants were determined by steady state analysis.
Complement Activity Assays
The Wieslab complement system screen (Euro Diagnostica) was used to determine the effects of LAIR proteins on the activity of the complement system. The assays were performed following the manufacturer's protocol with minor modifications. Fresh human serum (20 µl) was mixed with 13 µg of fusion protein in 20 µl PBS and incubated for 30 min at 37°C. Absorbance was read at 405 nm. Background values were subtracted.
Serum samples mixed with LAIR proteins (final concentration 0.6, 0.3 or 0.15 μg/ml) were also tested for CH50 titers in a routine diagnostic laboratory (Sanquin).
Fluid-Phase Activation of C4
Activated C4 in plasma or serum samples was measured with an enzyme-linked immunosorbent assay (ELISA) in which monoclonal antibody anti-C4-1 against a neo-epitope exposed on C4b, C4bi and C4c was used as a catching antibody [14]. Results were related to those obtained with dilutions of aged serum and expressed as arbitrary units per milliliter. Samples tested for the generation of fluid-phase C4b/c were prepared by mixing 30 µl normal fresh serum with 30 µl protein to be tested [LAIR-1-Fc at 1.72 mg/ml, SIRL-Fc at 2.76 mg/ml, LAIR-2-Fc at 1.3 mg/ml, and non-aggregated human IgG (GammaQuin, Sanquin) at 1.3 mg/ml as control]. As a positive control, 30 µl normal fresh serum were mixed with 30 µl veronal-buffered saline (VB; Lonza).
Complement-Dependent Cytotoxicity Crossmatch Test
A crossmatch assay for anti-HLA antibodies was modified to assess the effects of the LAIR proteins on the complement-dependent cytotoxicity. Heat-inactivated (30 min at 56°C) patient serum (1 µl) containing anti-HLA antibodies was incubated in Terasaki plates (Greiner BIO-One) with PBMCs (1 µl of 2-5 × 106 cells/ml) of a selected HLA-typed donor for 1 h at RT. Meanwhile samples to be tested were prepared by mixing 5 µl fresh serum, 15 µl VB and 5 µl VB containing LAIR proteins. A positive control was made by adding 5 µl normal fresh serum to 20 µl VB without protein; a negative control was made by mixing 5 µl normal fresh serum, 15 µl VB and 5 µl 100 mM EDTA. Subsequently, all samples were incubated for 20 min at RT. Next, 10 µl of each sample were added to the wells in duplicate and incubated for 2 h at RT. Then, 5 µl of eosin 5% (VWR) were added and after 3 min, 5 µl of 25% formaldehyde (Sigma-Aldrich) supplemented with 5 mg CaCO3 (Sigma-Aldrich). Finally, 8 μl paraffin oil (Fagron) were added. Lysis was scored by light microscopy.
Results
LAIR-1 and LAIR-2 Bind MBL and C1q
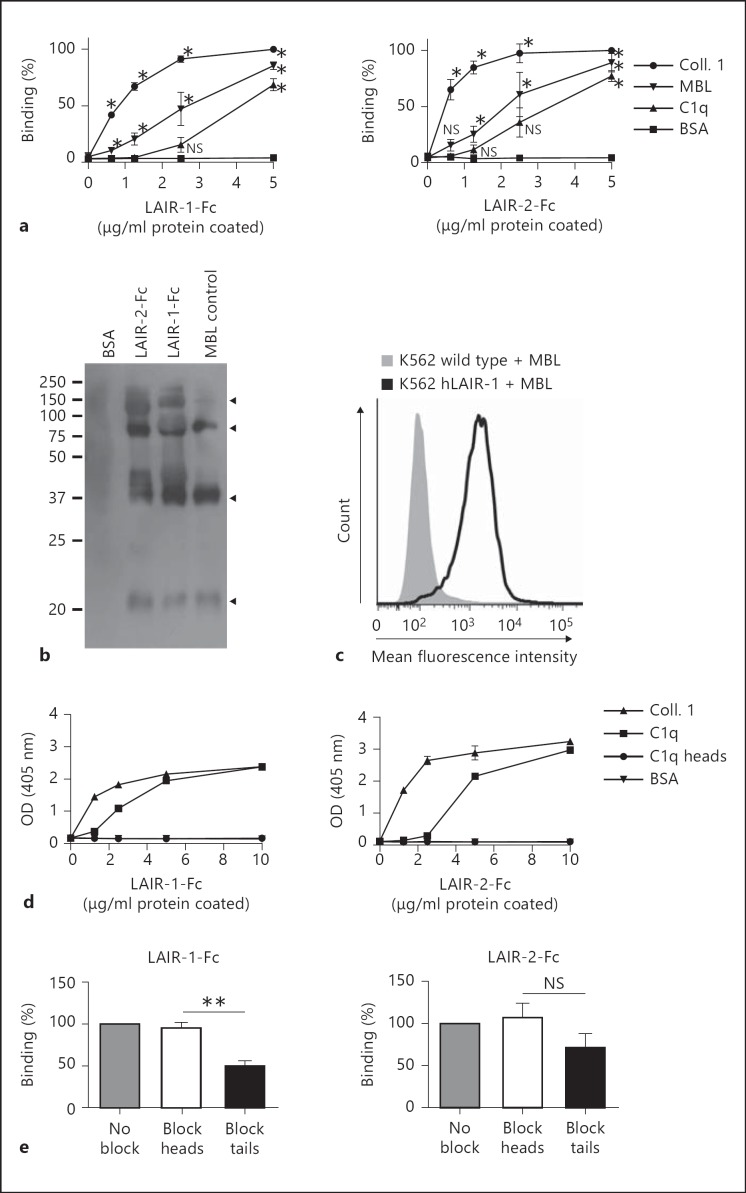
LAIR-1 binds collagens [8, 7]. C1q and MBL both contain collagen-like regions. Son et al. [10] recently showed binding of C1q to LAIR-1 and LAIR-2. We investigated binding of LAIR-1 and LAIR-2 to both complement proteins. LAIR-Fc fusion proteins, which are dimeric, bound to both solid-phase C1q and MBL (fig. 1a), whereas SIRL-1, an Ig-like inhibitory receptor that does not bind to collagen [13], did not bind (data not shown). The globular head domains of MBL bind to activators in a divalent cation-dependent manner. Addition of EDTA had neither an effect on LAIR binding to MBL nor C1q, suggesting that the binding site of MBL for LAIR proteins is different from that for activators. This is consistent with the notion that the binding sites for the LAIR proteins are located in the collagen-like domains of MBL and C1q. Our data confirm the recently reported C1q binding to LAIR-1, though in our hands LAIR-2 binds to human C1q with a higher affinity than LAIR-1. Furthermore, we extend this concept to MBL [10]. Immune precipitation experiments (fig. 1b) as well as flow cytometry on LAIR-1 transfectants (fig. 1c) further confirmed binding of MBL to LAIR.
Fig. 1.
Recombinant LAIR proteins bind complement MBL. Significance was tested using ANOVA with Bonferroni correction (* p ≤ 0.05, ** p ≤ 0.005; NS = Nonsignificant). a Binding of LAIR-1-Fc (10,000 ng) and LAIR-2-Fc (625 ng) to plate-bound MBL and C1q. Detection with HRP-conjugated anti-human IgG antibody. Mean percent binding ± SD relative to 5 μg/ml of coated collagen are shown. n = 3. b LAIR-1-Fc, LAIR-2-Fc and BSA (as a control) were coupled to Sepharose beads incubated with MBL. Precipitates were subjected to electrophoresis. MBL was detected with Western blotting (n = 2). Arrowheads indicate specific bands (c); K562 cells with or without stable expression of LAIR-1 were incubated with MBL and analyzed by flow cytometry. A representative experiment is shown (n = 3). d Binding of LAIR-1-Fc (10,000 ng) and LAIR-2-Fc (625 ng) to plate-bound collagen 1, C1q, C1q globular heads or BSA. One of 3 representative experiments is shown. OD = Optical density. e Blocking of LAIR-1-Fc (n = 3) and LAIR-2-Fc (n = 2) binding to plate-bound C1q (2.5 μg/ml) by specific globular head or collagenous tail antibodies (10 μg/ml). Means ± SD.
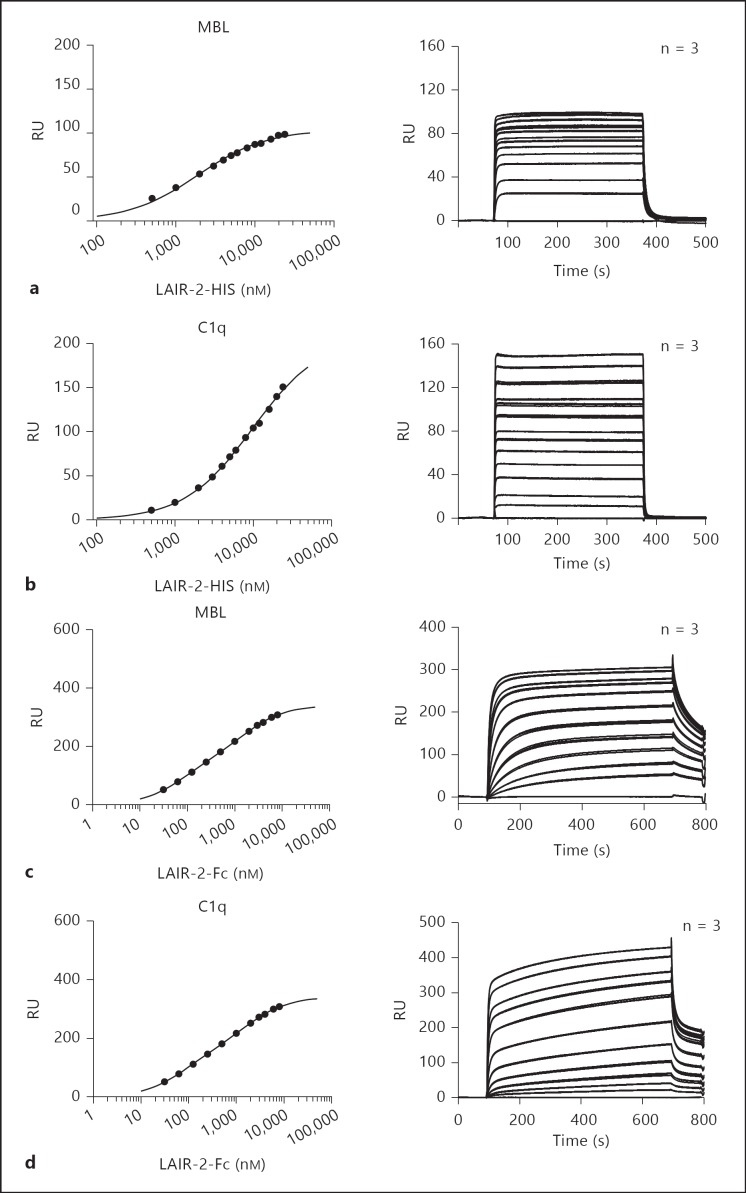
LAIR proteins did not bind to isolated C1q globular heads, a finding which supports the requirement of the collagenous tails for LAIR binding (fig. 1d). To further confirm that the collagenous tail is required for LAIR binding to C1q, we performed blocking experiments with specific globular head or collagenous tail antibodies. The binding of LAIR-1 to C1q was significantly reduced by the addition of collagenous tail-specific antibodies, while no effect of the addition of globular head-specific antibodies could be found (fig. 1e). LAIR-2 binding to C1q was slightly but not significantly reduced by collagenous tail-specific antibodies, probably because of the high-affinity interaction between LAIR-2 and C1q. Sixteen times more LAIR-1-Fc was needed than LAIR-2-Fc to obtain comparable optical densities in the binding experiments (fig. 1a). This indicates that LAIR-2 has a higher affinity for C1q and MBL than LAIR-1. Equilibrium binding data obtained with surface plasmon resonance analysis revealed that monomeric LAIR-2-HIS specifically bound to MBL (fig. 2a) and C1q (fig. 2b), albeit with low affinity (table 1). To increase the affinity, we generated dimers of either LAIR protein by producing either protein as an Fc fusion protein [9]. Indeed, LAIR-2-Fc fusion proteins bound MBL and C1q with a higher affinity (fig. 2c; table 1), though the affinity for C1q is lower than for MBL (fig. 2d; table 1).
Fig. 2.
LAIR-2-Fc binds MBL and C1q with high affinity. Binding of LAIR-2-HIS to immobilized MBL (a) or C1q (b) and binding of LAIR-2-Fc to immobilized MBL (c) or C1q (d) was measured by surface plasmon resonance analysis. Recombinant proteins were injected at a flow rate of 20 µl/min at 25°C through a BIAcore flow cell containing 1,100 RU C1q for the HIS and Fc fusion proteins, 900 RU MBL for the HIS proteins or 1,500 RU MBL for the Fc proteins. In the left panels, individual symbols representing resonance at equilibrium along with the corresponding concentration of the protein are shown. Triplicate binding curves of LAIR-2-HIS at a concentration of 0, 500, 1,000, 2,000, 3,000, 4,000, 5,000, 6,000, 8,000, 10,000, 12,000, 16,000, 20,000 and 24,000 nM and LAIR-2-Fc at a concentration of 0, 31.25, 62.5, 125, 250, 500, 1,000, 2,000, 3,000, 4,000, 6,000 and 8,000 nM are shown in the right panels. Values are means ± SEM.
Table 1.
CalculatedKD values
| LAIR-2 |
||||
|---|---|---|---|---|
| monomer | dimer | |||
| MBL |
KD KD 2 |
1,889±52 | 76±4 1,719±141 |
|
| C1q |
KD KD 2 |
9,812±251 | 186±22 2,608±168 |
|
Calculated affinities (KD in nM) from the binding at equilibrium (Req) of the surface plasmon resonance experiments. Values are means ± SEM.
These data are in line with our previous findings that LAIR-2 has a higher affinity for collagen than LAIR-1, and dimeric recombinant LAIR proteins have a higher affinity than monomeric forms [9].
Together, these data show that that LAIR-1 and LAIR-2 are capable of binding the collagen domain of C1q and MBL. LAIR-2-Fc binds MBL with a similar affinity as collagen, and C1q with a somewhat lower affinity.
LAIR-2-Fc Inhibits Complement Activation
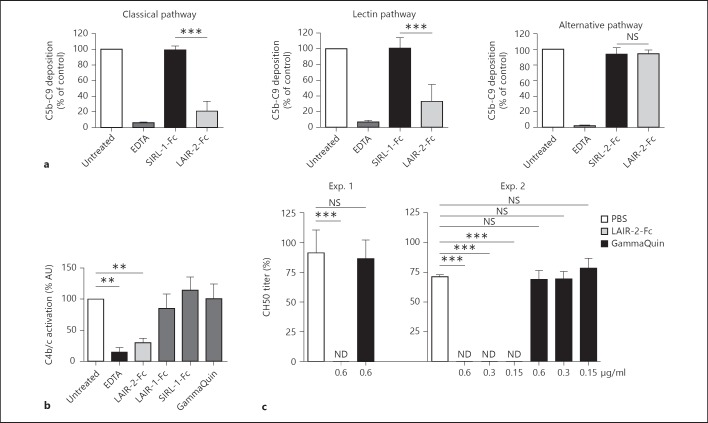
We next questioned whether binding of LAIR proteins to C1q and MBL may interfere with complement function of the classical and lectin pathways, respectively. We tested the effect of LAIR proteins added to fresh serum on complement activity assessed with the Wieslab complement system screen [15]. This screen uses deposition of C5b-9 as readout and measures the activity of the three complement pathways separately. To assess the effect on this deposition, serum was supplemented with recombinant LAIR proteins and tested. Untreated serum was set at 100%. The addition of a control recombinant protein had no effect on the activity of complement while the addition of LAIR-2-Fc significantly reduced C5b-9 deposition generated via the classical and lectin pathway but not that via the alternative pathway (fig. 3a), consistent with the absence of a protein with a collagen-like region in the alternative pathway. Thus, binding of LAIR-2-Fc to MBL and C1q interferes with activation of the lectin and classical pathways of the complement system, respectively. LAIR-2-Fc inhibited C4b/c production in fluid phase, while none of the control proteins did, confirming the specificity of the complement inhibition by LAIR-2 (fig. 3b). Finally, the data obtained with the Wieslab complement screening assays were confirmed by evaluating the effects of LAIR protein on the CH50 titer of normal human serum. A final concentration of 0.6 μg/ml LAIR-2 rendered the CH50 titers of normal serum undetectable, while the addition of PBS or human IgG at the same concentration (GammaQuin) had no effect (fig. 3c). Titration experiments rendering final LAIR-2 concentrations as low as 0.15 μg/ml showed undetectable CH50 titers in serum samples while using a more sensitive CH50 assay (fig. 3c). Together these data show that LAIR-2-Fc specifically inhibits the classical and lectin pathways of the complement system.
Fig. 3.
LAIR-2-Fc inhibits complement deposition. Significance was tested using ANOVA with Bonferroni correction (** p ≤ 0.005, *** p ≤ 0.001; NS = Nonsignificant). a Complement C5b-C9 deposition was measured in a pathway-specific Wieslab complement assay. Sera of 4 individual donors were either left untreated, incubated with EDTA to completely abrogate complement deposition or incubated with a control-Fc (SIRL-1) or LAIR-2-Fc. Complement deposition of the untreated condition was set at 100%; means ± SD are shown. Results for the classical, lectin and alternative pathways are shown. b C4b/c detection in arbitrary units (AU) after fluid-phase complement activation. Sera were either left untreated, incubated with EDTA or incubated with LAIR-1-Fc, a control-Fc (SIRL-1), GammaQuin or LAIR-2-Fc. AU of the untreated condition were set at a 100%. Means ± SD are shown, n = 3. c CH50 titer. Final concentration of LAIR-2-Fc and GammaQuin are depicted on the x-axis. ND = Not detectable. n = 3 donors for experiment (Exp.) 1 and Exp. 2; means ± SD are shown. Limit of detection for Exp. 1: 40% and Exp. 2: 12%.
LAIR-2-Fc Inhibits Complement-Mediated Cytotoxicity in an ex vivo Model for AMR
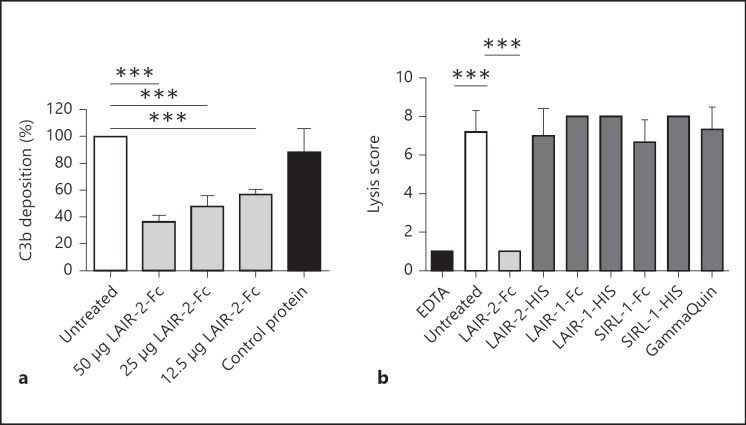
To test whether LAIR-2-Fc also inhibits cellular complement deposition, we sensitized a CD20-expressing cell line with a CD20 antibody. After incubation with fresh mouse serum, we detected mouse iC3b/C3b and C3c deposition on the cells by flow cytometry. Incubation of the mouse serum with LAIR-2-Fc significantly diminished iC3b/C3b and C3c deposition on the sensitized cells whereas a control protein did not (fig. 4a). Thus, LAIR-2-Fc inhibits human as well as mouse complement.
Fig. 4.
LAIR-2-Fc inhibits complement-mediated cell lysis in vitro. Significance was tested using ANOVA with Bonferroni correction (*** p ≤ 0.001). a Mouse iC3b/C3b/C3c deposition on EL-4 CD20 cells was measured by FACS. Untreated control was set at 100%, at least n = 3 for all conditions, except for the control protein (n = 2). Means ± SD are shown. b Cell lysis in an ex vivo transplant model. Cells were either left untreated, incubated with EDTA or incubated with LAIR-2-Fc, LAIR-2-HIS, LAIR-1-Fc, LAIR-1-HIS, a control Fc protein, a control HIS protein or GammaQuin. Lysis was scored by light microscopy. Minimal lysis was scored as 1, maximal lysis as 8. All conditions n = 3, except for the HIS proteins (n = 2). Means ± SD are shown.
We then tested the effect of LAIR-2-Fc in a human ex vivo model for antibody-mediated transplant rejection. Human PBMCs were sensitized with anti-HLA antibodies and lysed by subsequent addition of fresh human serum as a source of complement. LAIR-2-Fc as well as control proteins were added to this serum and the effect on cell lysis was measured. The addition of LAIR-2-Fc significantly reduced the lysis of the cells sensitized with anti-HLA antibodies, while the addition of control proteins or control IgG had no effect (fig. 4b).
Discussion
In this study, we show binding of recombinant LAIR proteins to the complement activators C1q and MBL. Consistent with our previous data on the interaction with collagen [9], the affinity of the dimeric forms of LAIR for C1q and MBL is higher than that of the monomeric proteins, and the affinity of LAIR-2 for these complement proteins is higher than that of LAIR-1. Our observation that LAIR-2-Fc but not LAIR-1-Fc inhibits complement is also in line with our finding that LAIR-2-Fc but not LAIR-2-Fc is an efficient platelet inhibitor [16]. This may reflect differences in affinity, but it also could be that LAIR-1 binds to different sites on C1q and MBL than LAIR-2. Our finding that LAIR-1 binds to MBL and C1q fixed to ELISA plates (fig. 1a), but showed no binding during surface plasmon resonance, may reflect that binding of MBL and C1q to the CM5 chip specifically blocked the LAIR-1 binding sites. Alternatively, immobilization to the CM5 chip may have led to conformational changes, thereby influencing the accessibility of the binding sites.
Son et al. [10] recently showed that LAIR-1 has a higher affinity for C1q than LAIR-2, which is not consistent with our data. Possibly, this contrasting result reflects that they evaluated monomeric LAIR proteins whereas we included dimeric proteins, which have a higher affinity.
An interesting implication of MBL binding to LAIR-1 is the possibility that it acts as a functional ligand for LAIR-1. Indeed, we were able to demonstrate functional ligation of LAIR-1 by MBL in a reporter assay, revealing MBL as a potential circulating ligand for LAIR-1 (online suppl. fig. 1; see www.karger.com/doi/10.1159/000354976). Together with the findings of Son et al. [10] that C1q can act as a functional ligand for LAIR-1, this extends LAIR-mediated control of immune cell function by collagens in the tissues to ligands in the circulation [17].
Our in vitro data indicate that monomeric sLAIR-1 and LAIR-2 are unlikely to have a physiological role on the complement system considering their relatively low affinities for MBL and C1q. However, dimerization of LAIR-2 led to a substantial increase in the affinity for these two complement proteins, and indeed LAIR-2-Fc reduces complement deposition on complement activators and inhibits lysis of cells sensitized with anti-HLA antibodies in a human ex vivo AMR model. Complement can induce significant tissue injury when activated during hyperacute rejection of transplanted organs, when complement split products such as C4d and C3d are deposited on the vascular endothelium [18]. Our data suggest that administration of LAIR-2-Fc fusion protein may constitute a therapeutic option for patients suffering from such complications. The C5 inhibitor eculizumab is currently being tested for its ability to inhibit complement-mediated graft rejection in the clinic and shows promising results [19]. However, this inhibitor, in contrast to a LAIR-2-based inhibitor, does not inhibit C4 and C3 activation. Observations in patients with paroxysmal nocturnal hemoglobinuria indicate that cells still can be killed in a complement-dependent fashion when the system is blocked at the level of C5, probably through interaction with phagocytes via receptors for fixed C3 [17].
We previously reported that LAIR-2-Fc is able to inhibit collagen-induced platelet aggregation [16]. This unique combination of inhibition of complement and thrombosis make LAIR-2 fusion proteins an interesting therapeutic option to prevent damage following reperfusion of transplanted organs as well as to prevent AMR.
In conclusion, AMR and ischemia-reperfusion injury are two major problems in the field of organ transplantation. Both processes are dependent on activation of the classical and lectin pathways. We here present LAIR-2-Fc as a potent inhibitor of these two pathways of the complement system. Administration of LAIR-2-Fc may therefore be an interesting novel therapeutic strategy to prevent or treat AMR as well as ischemia-reperfusion of kidney allografts.
Supplementary Material
Supplementary data
Acknowledgments
We thank Martin de Smet and Amelia Lacna for technical assistance, and Louis Bont for the help with statistical analysis. This study was funded by the Dutch Arthritis Foundation (grant 06-1-403).
References
- 1.Diepenhorst GM, van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann Surg. 2009;249:889–899. doi: 10.1097/SLA.0b013e3181a38f45. [DOI] [PubMed] [Google Scholar]
- 2.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohmig GA, Exner M, Habicht A, Schillinger M, Lang U, Kletzmayr J, Saemann MD, Horl WH, Watschinger B, Regele H. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091–1099. doi: 10.1681/ASN.V1341091. [DOI] [PubMed] [Google Scholar]
- 4.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat Rev Immunol. 2012;12:431–442. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 5.Sellar GC, Blake DJ, Reid KB. Characterization and organization of the genes encoding the A-, B- and C-chains of human complement subcomponent C1q. The complete derived amino acid sequence of human C1q. Biochem J. 1991;274((Pt 2)):481–490. doi: 10.1042/bj2740481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyaard L. The inhibitory collagen receptor LAIR-1 (CD305) J Leukoc Biol. 2008;83:799–803. doi: 10.1189/jlb.0907609. [DOI] [PubMed] [Google Scholar]
- 7.Tang X, Narayanan S, Peruzzi G, Apara A, Natarajan K, Margulies DH, Coligan JE, Borrego F. A single residue, arginine 65, is critical for the functional interaction of leukocyte-associated inhibitory receptor-1 with collagens. J Immunol. 2009;182:5446–5452. doi: 10.4049/jimmunol.0804052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebbink RJ, De Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJ, Meyaard L. Collagens are functional, high-affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203:1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olde Nordkamp MJM, van Roon JA, Douwes M, De Ruiter T, Urbanus RT, Meyaard L. Enhanced secretion of leukocyte-associated immunoglobulin-like receptor (LAIR)-2 and soluble LAIR-1 in rheumatoid arthritis: LAIR-2 is a more efficient antagonist of the LAIR-1-collagen inhibitory interaction than is soluble LAIR-1. Arthritis Rheum. 2011;63:3749–3757. doi: 10.1002/art.30612. [DOI] [PubMed] [Google Scholar]
- 10.Son M, Santiago-Schwarz F, Al-Abed Y, Diamond B. C1q limits dendritic cell differentiation and activation by engaging LAIR-1. Proc Natl Acad Sci USA. 2012;109:E3160–E3167. doi: 10.1073/pnas.1212753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boross P, Jansen JH, de Haij S, Beurskens FJ, van der Poel CE, Bevaart L, Nederend M, Golay J, van de Winkel JG, Parren PW, Leusen JH. The in vivo mechanism of action of CD20 monoclonal antibodies depends on local tumor burden. Haematologica. 2011;96:1822–1830. doi: 10.3324/haematol.2011.047159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hack CE, Belmer AJ. The IgG detected in the C1q solid-phase immune-complex assay is not always of immune-complex nature. Clin Immunol Immunopathol. 1986;38:120–128. doi: 10.1016/0090-1229(86)90129-7. [DOI] [PubMed] [Google Scholar]
- 13.Steevels TAM, Lebbink RJ, Westerlaken GHA, Coffer PJ, Meyaard L. Signal inhibitory receptor on leukocytes-1 (SIRL-1) is a novel functional inhibitory immune receptor expressed on human phagocytes. J Immunol. 2010;184:4741–4748. doi: 10.4049/jimmunol.0902039. [DOI] [PubMed] [Google Scholar]
- 14.Wolbink GJ, Bollen J, Baars JW, ten Berge RJ, Swaak AJ, Paardekooper J, Hack CE. Application of a monoclonal antibody against a neoepitope on activated C4 in an ELISA for the quantification of complement activation via the classical pathway. J Immunol Methods. 1993;163:67–76. doi: 10.1016/0022-1759(93)90240-8. [DOI] [PubMed] [Google Scholar]
- 15.Fredrikson GN, Truedsson L, Sjoholm AG. New procedure for the detection of complement deficiency by ELISA. Analysis of activation pathways and circumvention of rheumatoid factor influence. J Immunol Methods. 1993;166:263–270. doi: 10.1016/0022-1759(93)90367-g. [DOI] [PubMed] [Google Scholar]
- 16.Lenting PJ, Westerlaken GHA, Denis CV, Akkerman JW, Meyaard L. Efficient inhibition of collagen-induced platelet activation and adhesion by LAIR-2, a soluble Ig-like receptor family member. Plos One. 2010;5:e12174. doi: 10.1371/journal.pone.0012174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill A, Rother RP, Arnold L, Kelly R, Cullen MJ, Richards SJ, Hillmen P. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 opsonization. Haematologica. 2010;95:567–573. doi: 10.3324/haematol.2009.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wasowska BA. Mechanisms involved in antibody- and complement-mediated allograft rejection. Immunol Res. 2010;47:25–44. doi: 10.1007/s12026-009-8136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stegall MD, Chedid MF, Cornell LD. The role of complement in antibody-mediated rejection in kidney transplantation. Nat Rev Nephrol. 2012;8:670–678. doi: 10.1038/nrneph.2012.212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data