Abstract
Neutrophils activated by Mycobacterium tuberculosis (Mtb) form neutrophil extracellular traps (NETs), containing DNA and several biologically active cytosolic and granular proteins. These NETs may assist in the innate immune defense against different pathogens. We investigated whether the NET-forming neutrophils mediate an activating signal to macrophages during the early multicellular inflammatory reaction and granuloma formation. Mtb-induced NETs were found to be reactive oxygen species dependent and phagocytosis dependent. A neutrophil elastase inhibitor also delayed NET formation. However, NET formation occurred independently of Mtb-induced apoptosis. We observed close interactions between macrophages and Mtb-activated neutrophils, where macrophages bound and phagocytosed NETs. Significant secretion of the cytokines interleukin (IL)-6, tumor necrosis factor-α, IL-1β and IL-10 were detected from macrophages cocultured with NETs from Mtb-activated but not phorbol myristate acetate-activated neutrophils. NETs binding heat shock protein 72 (Hsp72) or recombinant Hsp72 were able to trigger cytokine release from macrophages. Only Mtb-induced NETs contained Hsp72, suggesting that these NETs can transfer this danger signal to adjacent macrophages. We propose that Hsp72 sequestered in NETs plays an important role in the interaction between neutrophils and macrophages during the early innate immune phase of an Mtb infection. The immunomodulatory role of NETs and proteins derived from them may influence not only chronic inflammation during tuberculosis but also immune regulation and autoimmunity.
Key Words: Mycobacterium tuberculosis, Neutrophil extracellular traps, Heat shock protein 72, Neutrophil, Macrophage, Innate immunity
Introduction
Neutrophils play an essential role in the innate immune response to infection. They are the first cells to be recruited and participate in the defense by engulfment of microorganisms and by the release of reactive oxygen species (ROS) and microbicidal granule molecules [1]. Neutrophils are abundant in numbers, and their potent cargo can cause tissue damage if not tightly controlled. This is balanced by the short life span of the neutrophils after which apoptosis ensues, and the cells are cleared by efferocytosis by macrophages [2]. The discovery of neutrophil extracellular traps (NETs) released by activated neutrophils has thrown light upon yet another defense mechanism of neutrophils [3, 4, 5]. NETs are formed during the unique cell death pathway (NETosis), which has been described as being dependent on ROS generated by NADPH oxidase [6, 7] but not on caspase activation [3].
During human tuberculosis infection, Mycobacterium tuberculosis (Mtb) encounters cells of the innate immune system in the lower respiratory tract. Although macrophages play a vital role in controlling and eliminating the intracellular pathogen, large numbers of neutrophils are recruited to the lungs in the initial stages of an Mtb infection [8, 9]. Neutrophils are not able to kill virulent Mtb [10]; however, neutrophil recruitment appears to be important for early granuloma formation and containment of the infection [11]. A crosstalk between neutrophils and other immune cells has been described [12], where the release of granule proteins attracts other immune cells and also modulates the function of tissue-resident cells. Neutrophil apoptosis and elimination by macrophages is usually considered an anti-inflammatory response where inflammation is resolved [13, 14, 15]. During this process, macrophages can acquire antimicrobial peptides from ingested apoptotic neutrophils, which contribute to a more efficient killing of intracellular Mtb [16]. In another context, neutrophil-derived elastase from apoptotic neutrophils was able to activate macrophages via modulation of Toll-like receptor 4 (TLR4) and an increase in tumor necrosis factor (TNF)-α that led to increased killing of Leishmania major[17]. We have earlier shown that Mtb infection increases the rate of neutrophil apoptosis and that pathogen-induced apoptotic neutrophils trigger a proinflammatory response in macrophages [18, 19, 20], which was mediated by the release of heat shock protein 72 (Hsp72) [20, 21].
NET formation has been demonstrated in neutrophils in response to different microbes, signaling through TLRs, Fc receptors, chemokine and cytokine receptors [4], as well as in response to stimulating agents such as phorbol myristate acetate (PMA) [5, 7, 22, 23]. NETs have been reported to have bactericidal effects. NET induction has been observed in response to Mtb; however, the NETs were unable to kill mycobacteria [24]. NETs have been shown to cause tissue damage, and the bactericidal and tissue-damaging effects can be assigned to the granular proteins and histones associated with the NETs [23, 25, 26]. It was also recently shown that NETs can activate the NLRP3 inflammasome in lupus macrophages [27].
Since NETs are formed in different inflammatory settings, their formation could play an important role in the initial stages of an Mtb infection in vivo by trapping the mycobacterium, preventing spreading to other organs, thus allowing for macrophages to engulf and kill the bacteria. NETs sequester the toxic contents from dying neutrophils which prevents damage to surrounding tissue [5], and macrophages may acquire these antimicrobials by phagocytosis of NETs. Therefore, NETs may play a vital role in the partnership between neutrophils and macrophages during granuloma formation in tuberculosis. In the present study, we show that Mtb-induced NETs bind Hsp72 and trigger a proinflammatory activation of macrophages mediated by Hsp72.
Materials and Methods
Antibodies and Reagents
TACS™ Annexin V-FITC was obtained from R&D Systems (McKinley Place, Minn., USA). Penicillin-streptomycin (PEST), DMEM cell culture media, RPMI-1640 without phenol red, L-glutamine and HEPES were obtained from Gibco (Grand Island, N.Y., USA). BD™-Cytometric Bead Array (CBA) Human Inflammation Kit was obtained from BD Biosciences Pharmingen (San Diego, Calif., USA). Cell isolation components Polymorphprep™ and Lymphoprep™ were purchased from Axis-Shield (Oslo, Norway). Heparin was obtained from LEO Pharma (Malmö, Sweden). Cytochalasin D (CytD), neutrophil elastase inhibitor (NEi; MeOSuc-AAPV-cmk) and Anti-Neutrophil Elastase rabbit primary antibody were all from Calbiochem (Nottingham, UK). Anti-Hsp70/Hsp72 primary antibody (Stressgen SPA-812-D) and human recombinant Hsp70/Hsp72 (recHsp72; Stressgen NSP-555-D) came from Enzo Life Sciences (Farmingdale, N.Y., USA). Primary antibodies were detected using Alexa Flour 594 secondary IgG antibody from Molecular Probes (Eugene, Oreg., USA). Diphenylene iodonium (DPI), PMA and dimethyl sulfoxide were purchased from Sigma Aldrich (Saint Louis, Mo., USA). 5-Chloromethyl-fluorescein diacetate (CFMDA, CellTracker Green) and Sytox®Green were obtained from Invitrogen (Eugene, Oreg., USA). Fluorescent mounting medium was purchased from Dako (Carpinteria, Calif., USA). Heparinized peripheral blood, buffy coats and normal human serum (NHS) were delivered from the blood bank at Linköping University Hospital (Linköping, Sweden). Krebs Ringer Glucose (KRG) with or without 1 mM CaCl2, and PBS, pH 7.3, were prepared in house.
Monocyte Isolation
Human peripheral monocytes were isolated from buffy coats from healthy blood donors. The buffy coats were diluted 1:1 with 0.9% NaCl and separated over a dextran gradient, Lymphoprep, followed by multiple washes in PBS containing heparin, followed by multiple washes in KRG without Ca2+. The monocytes were subsequently resuspended in DMEM containing 1% PEST and 10 mM HEPES and transferred to sterile 75-cm2 cell culture flasks at approximately 107 cells/ml. The cells were allowed to adhere for 1 h at 37°C, after which nonadherent cells were removed by multiple washes with KRG. The remaining monocytes were cultured in DMEM containing 10% NHS pooled from five donors, for 6-8 days, in order to differentiate to macrophages. More than 98% of macrophages were routinely positive for CD64 at day 7 of culture. The culture medium was changed every 3-4 days. Macrophages were harvested by cell scraping in cold PBS, before counting and resuspension in DMEM containing 2% NHS for use in experiments.
CFMDA staining of macrophages for microscopy was achieved by adding 1.5 µM CFMDA in DMEM for 30 min. The medium was then changed to fresh DMEM, and cells were further incubated for 30 min before cell scraping and counting.
Neutrophil Isolation
Human neutrophils were isolated from heparinized whole blood over a Lymphoprep and Polymorphprep gradient. Red blood cells were lysed by hypotonic shock and neutrophils were washed multiple times in KRG.
Bacteria
Mtb H37Rv (γ-irradiated whole cells, NR 14819, LOT 59585333) was provided by BEI Resources (Manassas, Va., USA). Mtb H37Rv lspA−/− strain was a gift from Dr. Joel Ernst, New York University School of Medicine. The mutant lspA−/− was cultured statically in Middlebrook 7H9 supplemented with ADC, 0.05% Tween-80 and 0.5% glycerol for 14 days and subsequently heat inactivated at 80°C for 60 min. Single-cell suspensions of bacteria were prepared using a Dounce homogenizer, sedimentation and multiple passages through a 27-gauge syringe and stored at −70°C. Before use, aliquots were passed multiple times through a 27-gauge syringe.
NET Formation and Quantitation
For microscopy studies of NET formation, 5 × 105 neutrophils were seeded in wells on 13-mm glass coverslips, stained with 2 µM cell-permeant DNA-binding dye Sytox Green, stimulated with 25 nM PMA, Mtb [multiplicity of infection (MOI 10)] or left untreated. After 4 h, fluorescence images were taken of the unfixed neutrophils. Images were analyzed using ImageJ software. The ratio of live versus dead cells (Sytox+) was attained by manual counting, comparing fluorescent and phase-contrast images. The DNA area stained by Sytox Green was measured for at least 100 cells per cover slip, and the experiment was repeated three times in triplicates with different donors. Using Microsoft Excel, the distribution of neutrophils across the range of the nuclear area was obtained using the frequency function. The mean frequency distribution for the different stimuli was plotted in a graph using GraphPad Prism. For quantitation experiments, 105 Sytox Green-stained neutrophils were seeded in black 96-well plates with RPMI-1640 (phenol red free) supplemented with 10 mM HEPES, 1% PEST and 2% human serum albumin. Neutrophils were allowed to adhere for 30 min before addition of NEi (50 µM) or DPI (10 µM). Neutrophils were incubated with inhibitors for 30 min before stimulation with 25 nM PMA, Mtb (MOI 10), left untreated or lysed with 0.2% Triton X-100 as a 100% lysis control. Fluorescence was measured at 0, 3, 6, 9 and 18 h using a plate reader, Hidex Chameleon Multilable Detection Platform (SisLab) with a filter setting of 485/535 nm (excitation/emission).
Immunostaining and Microscopy
For fluorescence microscopy, 5 × 105 neutrophils were seeded on 13-mm glass coverslips in DMEM containing 1% PEST and 10mM HEPES. In experiments where inhibitors were used, neutrophils were preincubated with 10 µM CytD, 10 µM DPI or 50 µM NEi for 30 min prior to stimulation. Cells were stimulated with 25 nM PMA, Mtb (MOI 10) or left untreated for 1 h, after which they were washed thrice with KRG. For detecting Hsp72, cells were incubated for 5 h and then fixed with 4% PFA for 30 min. For the study of neutrophil elastase, neutrophils were stimulated as described, followed by incubation for 9 h, addition of CFMDA-stained macrophages (at a ratio of 2 neutrophils per macrophage), coincubation for 1 h and fixation. Cells were then blocked with 2% BSA and 10% goat serum in PBS, incubated with primary antibodies, washed, incubated with secondary antibody and incubated with DAPI before mounting with fluorescent mounting medium. Specimens were analyzed using a Zeiss LSM 700 confocal system coupled to a Zeiss Axio Observer Z1 fluorescence microscope and Zeiss Zen software, or a Nikon Eclipse E800 microscope coupled to a Nikon DS Ri1 camera and Nikon NIS-Elements software.
Macrophage Activation and Cytokine Analysis
Neutrophils were treated with 50 μM NEi, 10 µM DPI or left untreated for 30 min before stimulation with 25 nM PMA or Mtb (MOI 10) for 1 h. Neutrophils were washed and resuspended in DMEM containing 2% NHS and incubated along with inhibitors for 3 h allowing for NET formation to start. Medium containing inhibitors was removed and macrophages were added to the neutrophils at a ratio of 1:2. Unstimulated macrophages and macrophages stimulated with Mtb (MOI 5) were used as negative and positive controls, respectively.
To evaluate the role of Hsp72 in our system, 1 µg recHsp72 was added to neutrophils after PMA-induced NET formation had occurred for 3 h. Macrophages were subsequently added to the wells at a ratio of 1:2.
Coincubation of macrophages and PMN was allowed for 18 h upon which culture media was collected, centrifuged (10,000 g, 5 min) and stored in aliquots at −70°C. Samples were analyzed for cytokine content using CBA. TNF-α, interleukin (IL)-1β, IL-10 and IL-6 were analyzed according to the manufacturer's instructions using the respective CBA Flex Set.
Statistical Analysis
Data presented are expressed as mean values ± SEM. Statistical significance and differences between groups were calculated using paired Student's t test (significant at * p < 0.05 and ** p < 0.01) with the Graphpad Prism 5 software.
Results
Mtb-Activated Neutrophils Acquired More Clustered NETs than PMA-Activated Cells
It has previously been reported that Mtb can induce NET formation in neutrophils [24]. We confirmed these results with our experiments and compared the morphology of these Mtb-induced NETs with the commonly studied PMA-induced NETs. Mtb-activated neutrophils acquired more clustered NETs than PMA-activated cells (fig. 1a). By staining with the cell-impermeable DNA-staining Sytox Green, we could identify dead neutrophils and quantify the morphology of the NETs produced. By plotting the DNA area against the number of NET-forming cells, a frequency graph was created depicting the distribution of cells (fig. 1b). We could observe that Mtb activation led to more neutrophils with a larger DNA area compared to PMA-activated and unstimulated cells. The cells with a DNA area >400 µm2 were classified as NETotic cells [28]. Forty-seven percent of the Mtb-activated neutrophils positively stained with Sytox Green had formed NETs compared to 34% of the PMA-activated and 20% of the unstimulated cells (fig. 1c).
Fig. 1.
Mtb induces NET formation in neutrophils. a Neutrophils were activated with Mtb (MOI 10), PMA (25 nM) or left inactivated and incubated for 3 h. Mtb-activated neutrophils acquire more clustered NETs than neutrophils chemically induced with PMA. b Quantitation of the DNA area in neutrophils stained with cell-impermeable Sytox Green with a frequency graph showing the percentage of Sytox-positive cells against the distribution of the DNA area. Mtb activation leads to more neutrophils with a larger DNA area compared to PMA activation and unstimulated neutrophils. c Bar graph showing the percentage of netting neutrophils with a DNA area >400 µm2 as quantified from b. Mean values or representative micrographs from duplicates from 3 independent experiments are shown. * p < 0.05 and ** p < 0.01, compared to unstimulated control.
Kinetics of NET Formation in Neutrophils
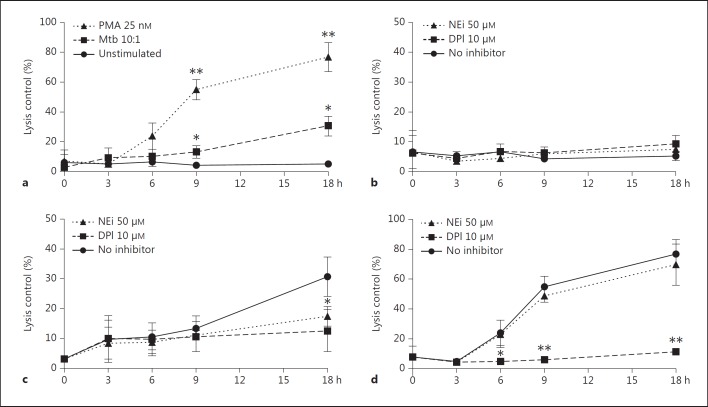
To follow the kinetics of NET formation, neutrophils were preincubated with the NADPH oxidase inhibitor DPI or NEi before activation with Mtb or PMA. NET formation occurred more rapidly in PMA- and Mtb-activated neutrophils compared to the unstimulated cells (fig. 2a). After 9 h, a significant difference was observed for both PMA- and Mtb-treated cells. After 18 h, 77% of PMA- and 31% of Mtb-activated neutrophils were stained positive for Sytox Green compared to 5% of the unstimulated control. When adding DPI, NET formation was abrogated in Mtb- and PMA-stimulated cells (fig. 2b, c, d), indicating that NET formation is NADPH oxidase dependent. The elastase inhibitor had a significant effect on lowering NET formation in Mtb-activated neutrophils (fig. 2c), but no such effect on PMA-activated neutrophils (fig. 2d).
Fig. 2.
Time kinetics of NET formation in neutrophils. a NETs were quantitated measuring fluorescence in neutrophils stained with cell-impermeable Sytox Green and activated with Mtb (MOI 10), PMA (25 nM) or left inactivated. The ratio of NETotic cells compared to a lysis control was followed for 18 h. b-d Effect of DPI or NEi on NET formation in untreated (b), Mtb-treated (c) and PMA-treated neutrophils (d). Mean values and error bars representing SEM from triplicates from 3 separate experiments are shown. * p < 0.05; ** p < 0.01.
Phagocytosis-Dependent NET Formation in Mtb-Activated Neutrophils
We then investigated if NET formation in Mtb-activated neutrophils was dependent on phagocytosis of bacteria. Neutrophils were incubated with 10 µM of CytD, an inhibitor of actin polymerization and phagocytosis, before Mtb was added. In the Mtb-stimulated wells without CytD, NETs were formed (fig. 3a), whereas CytD-treated cells closely resembled the unstimulated control (fig. 3b, c). This demonstrates that Mtb-induced NET formation is phagocytosis dependent.
Fig. 3.
Mtb-induced NETs are phagocytosis dependent but not dependent on the bacterial 19-kDa lipoprotein. a Neutrophils exposed to wild-type Mtb (MOI 10) for 6 h. b, c Neutrophils treated with 10 µM CytD and activated with wild-type Mtb (b), or left inactivated (c). d Neutrophils exposed to an Mtb lspA−/− mutant lacking the 19-kDa bacterial cell wall lipoproteins. Representative micrographs from duplicates from 3 independent experiments are shown.
Apoptosis Is Not a Prerequisite for NET Formation
Persson et al. [21] also showed that an Mtb lspA−/− mutant lacking the 19-kDa lipoprotein did not induce apoptosis in neutrophils. Using this Mtb mutant, we investigated whether apoptosis was a prerequisite for Mtb-induced NET formation. We activated the neutrophils with wild-type and lspA−/− Mtb. The lspA−/− Mtb induced NETs to the same extent as the wild-type Mtb (fig. 3d), suggesting that Mtb-induced apoptosis is not required for NET formation.
Macrophages Bind and Phagocytose NETs
It is well established that macrophages recognize and ingest apoptotic neutrophils. To evaluate if macrophages interacted with NET-forming cells, neutrophils were activated with Mtb and allowed to form NETs during a 9-hour incubation. Macrophages prestained with CFMDA were then added. After 1 h of coincubation, cells were fixed, and subsequently, stained with DAPI and immunostained for elastase to identify NETs. Confocal microscopy was used to capture images of the macrophage interaction with NETs (fig. 4). The elastase-containing NETs could be seen in close proximity of macrophages, which bound (fig. 4a, b) and phagocytosed NETs, as can be seen as red spots in the macrophage cytoplasm (fig. 4c).
Fig. 4.
Macrophages interact with NETs. Neutrophils were activated with Mtb (MOI 10) and incubated for 6 h. Macrophages (green) prestained with CFMDA were added to the wells, and coincubation was allowed for 1 h before cells were fixed. DNA was visualized with DAPI (blue), and neutrophil elastase was detected by antibody staining (red). a, b Arrows point to events of macrophage binding to elastase in NETs. c Arrow points to phagocytosed neutrophil elastase in macrophages.
Macrophages Release Increased Amounts of Proinflammatory Cytokines in Response to Mtb-Induced NETs
Pathogen-induced apoptosis in neutrophils induce a proinflammatory response in macrophages [19]. To evaluate if this response is mediated via NETs, we measured the release of IL-6, TNF-α, IL-1β and IL-10 from macrophages coincubated with NETotic neutrophils. We observed an increased cytokine release in macrophages incubated with Mtb-NETotic neutrophils but not in PMA-NETotic neutrophils (fig. 5a). DPI treatment of neutrophils during Mtb activation resulted in reduced NET formation (fig. 2), and their ability to stimulate macrophages was greatly reduced (fig. 5b). IL-6 and IL-10 were both significantly reduced (p < 0.01 and p < 0.05, respectively). IL-1β was also reduced, but to a lesser extent. However, the neutrophil ability to stimulate TNF-α in macrophages was not affected by DPI inhibition of NETs. The NEi did not show any effect on modulating the macrophage response to NETotic neutrophils, neither when using PMA-activated (data not shown) nor Mtb-activated (fig. 5b) neutrophils. There was no detectable cytokine release from neutrophils under the same stimulation and inhibition conditions (data not shown).
Fig. 5.
a Macrophages release increased amounts of cytokines in response to Mtb-activated neutrophils. b Effects of DPI and NEi on cytokine release. Neutrophils were preincubated for 30 min with DPI or NEi and activated with Mtb (MOI 10), PMA (25 nM) or left inactivated for 4 h at which macrophages were added to the wells and cells coincubated for 18 h. Mean values and error bars representing SEM from duplicates from 8 separate experiments are shown.* p < 0.05; ** p < 0.01.
Mtb-Activated Neutrophils Release Hsp72 That Bind to NETs
It was previously reported by Persson et al. [20] that Hsp72 released from neutrophils during phagocytosis of Mtb mediate a proinflammatory activation of macrophages. Therefore, we investigated whether Hsp72 was released during NET formation. We could confirm the observation that phagocytosis of Mtb induced Hsp72 release and that Hsp72 was associated with NETs (fig. 6a). The unstimulated control neutrophils did not release any Hsp72 (fig. 6d). Cells preincubated with DPI before Mtb activation did not produce NETs and did not release significant amounts of Hsp72 (fig. 6b). Although PMA induces NET formation, no Hsp72 was associated with the NETs (fig. 6c). The results suggest that Hsp72 is released concomitantly with NETs in response to Mtb stimulation. The NETs bind and concentrate Hsp72, thereby effectively presenting these danger molecules to macrophages and triggering local cytokine production.
Fig. 6.
Hsp72 release in NET-forming neutrophils. a Mtb-activated neutrophils. b Mtb-activated neutrophils with DPI. c PMA-induced neutrophils. d Unstimulated control neutrophils. Neutrophils were activated with Mtb (MOI 10), PMA (25 nM) or left inactivated for 6 h before fixation. DNA was visualized with DAPI (blue), and Hsp72 was detected by antibody staining (red).
Hsp72 Triggers Cytokine Release in Macrophages
We used human recHsp72 to confirm the effect of Hsp72 on macrophage cytokine release. recHsp72 alone had a significant effect on triggering the release of IL-10, IL-6 and TNF-α in macrophages (fig. 7). TNF-α release increased further when recHsp72 was added in the presence of PMA-NETs. Macrophages stimulated with only recHsp72 did not release IL-1β. However, the combination of PMA-NETs and recHsp72 triggered IL-1β release, supporting the hypothesis that NETs require Hsp72 for its stimulatory capacity.
Fig. 7.
Release of IL-6, TNF-α, IL-1β and IL-10 from macrophages stimulated with human recHsp72 (1 µg/ml). Neutrophils were activated with PMA (25 nM) for 3 h to form NETs, whereafter recHsp72 and macrophages were added to the wells, and cells were coincubated for 18 h. Mean values and error bars representing SEM from duplicates from 3 separate experiments are shown.* p < 0.05; ** p < 0.01.
Discussion
Mtb-induced NET formation has been previously described by Ramos-Kichik et al. [24]. Considering our previous observations that Mtb-induced apoptotic neutrophils can induce a proinflammatory activation of macrophages [18, 19, 20, 21], we asked whether NETs could mediate this activating signal to macrophages. Macrophages and neutrophils coexist at the inflammation site and work in concert to remove pathogens [29]. They are both important cells in the defense against tuberculosis; however, very little has been published on the interaction between NET-forming cells and macrophages. It has been shown that apoptotic neutrophils can supply macrophages with antimicrobial molecules [30] and that this transfer of molecules aids in the killing of intracellular pathogens such as Mtb [16]. Neutrophils also transfer ingested intracellular pathogens to macrophages during efferocytosis [30].
PMA-induced NETs are commonly used to model NETosis in neutrophils [3, 6, 28, 31, 32, 33, 34]. Analyzing NET formation, we could conclude that Mtb activation led to more clustered NETs than PMA activation. This can be due to the fact that a soluble stimulus will give a rapid and more homogenous activation, while bacteria are engulfed and come in contact with a limited number of cells [21]. The content of granular proteins in the NETs could also differ between the stimuli. The structure and concentration of Mtb-induced NETs would benefit the sequestration of bacteria and limit their spread to surrounding tissues [3, 6]. However, NETs show no mycobicidal activity [24].
When inhibiting neutrophil phagocytosis of Mtb, we observed an inhibition of NETosis, showing that Mtb-induced NETs are phagocytosis dependent. It has been shown that the actin cytoskeleton participates in chromatin release, rupture of the cell membrane and deployment of NETs and that lipopolysaccharide-induced NETs could be inhibited by CytD [35], suggesting that inhibition of actin polymerization could also have a more direct effect on NET formation. However, since PMA-induced NETs were not CytD sensitive, this effect of Mtb is likely to be phagocytosis dependent.
It has previously been shown that the TLR ligand 19-kDa mycobacterial lipoprotein enhances the oxidative burst and activation status of neutrophils stimulated with fMLP [36] and that the mutant strain lacking this cell wall component was not effective in promoting Mtb-induced apoptosis [21]. The fact that the lspA−/− Mtb mutant lacking surface lipoproteins induced NETs to the same extent as the wild-type Mtb suggests that Mtb-induced NET formation can occur independently of TLR signaling and apoptosis.
NETs contain elastase that can degrade bacterial virulence factors [3]. The role of elastase in neutrophil apoptosis has been considered, and a recent study demonstrated that the secretory leukocyte protease inhibitor SLP1 could inhibit neutrophil apoptosis [37]. Upon PMA activation and ROS production, neutrophil-derived elastase translocates to the nucleus and aids in chromatin decondensation leading to NETosis [28]. Using the cell-permeable NEi (MeOSuc-AAPV-cmk) we could observe an inhibition of Mtb-induced NETosis but no significant inhibition of PMA-induced NETosis, showing that NETs are also formed in the absence of apoptosis.
Elastase from apoptotic neutrophils can activate macrophages and augment their killing capacity of the intracellular pathogen L. major[17]. We could observe macrophages binding to NETs and also ingesting elastase from neutrophils. This suggests that elastase could be involved in signaling between NETotic cells and macrophages and that phagocytosis of elastase from NETs may be a mode in which macrophages acquire potent proteases.
Given that elastase has proinflammatory qualities [38] and NETs are richly decorated with elastase [3], we examined whether inhibiting elastase activity would have an effect on macrophage activation by NETotic cells. We observed no effect when inhibiting elastase either in samples of PMA- or Mtb-activated NETs, although elastase inhibition did affect the Mtb-induced NET formation per se.
The stress-induced Hsp72 is a protein with potent chaperone- and cytokine-like activity [39], and exosome-released Hsp72 from Mtb-induced apoptotic neutrophils act as a proinflammatory signal on macrophages [20]. Hsp70 stimulates monocytes via TLR2 and TLR4, which activate the nuclear factor-κB pathway resulting in the release of TNF-α, IL-1β, IL-6, IL-12 and nitric oxide [40, 41]. TLRs have also been found to induce phagosome maturation leading to the degradation of phagocytosed bacteria [42]. When inducing NET formation with Mtb, we observed an increased release of Hsp72 that did not occur in PMA-activated NETs. Hsp72 was concentrated to the NET strands, suggesting that Mtb-induced NETs can acquire specific proinflammatory properties from sequestered Hsp72. NETs could be inhibited with the NADPH oxidase inhibitor DPI, which blocks the release but not the intracellular expression of Hsp72 [20].
We could observe that the difference in NET content of Hsp72 between Mtb- and PMA-activated NETs also affects the activation of macrophages with the release of several proinflammatory cytokines and IL-10. IL-10 is an important regulatory element that could dampen inflammation through the inhibition of TNF-α and interferon-γ production, and thus, is suppressing the accompanying Th1 response [43]. That IL-10 is released together with proinflammatory cytokines in response to Mtb-induced NETs shows that during macrophage activation, there is a balanced immune response, where either pro- or anti-inflammatory cytokines may dominate. The PMA-activated neutrophils did not induce the same proinflammatory response in macrophages as Mtb-activated neutrophils, despite the fact that necrotic bodies are proinflammatory and that the PMA-activated cells were necrotic to the same extent as Mtb-activated cells. This suggests that the composition of NETs is important and that Mtb-induced NETs can transfer signals to macrophages, partly through Hsp72.
In the presence of NETs, recHsp72 greatly increased the release of IL-6, TNF-α, IL-1β and IL-10 in macrophages. Hsp72 alone also increased the release of IL-6, IL-10 and TNF-α, but the effect on TNF-α release was further increased when NETs were present. The direct effect of Hsp72 could be due to the fact that Hsp72 binds directly to the surface of the wells. However the release of IL-1β required NETs to be present. Kahlenberg et al. [27] recently showed that NETs are able to activate the NLRP3 inflammasome in lipopolysaccharide-primed macrophages resulting in the release of IL-1β. IL-1β release requires a priming signal to activate the nuclear factor-κB pathway resulting in synthesis of pro-IL-1β [44]. A second signal for the inflammasome would activate caspase 1 to cleave pro-IL-1β into its active form. The combination of Hsp72 and NETs may provide both these signals.
When NET formation was inhibited with the NADPH oxidase inhibitor DPI, the Mtb-activated neutrophils lost their stimulatory properties, leading to a significant decrease in IL-6, IL-1β and IL-10, but not TNF-α, released from macrophages. Mtb-activated neutrophils released only small amounts of Hsp72 when treated with DPI [20]. With no NETs present, the Hsp72 is not concentrated but diluted in the extracellular space reducing its signaling activity. It can also be argued that extracellular Mtb are captured in the NET preparations and that these bacteria would have a proinflammatory effect on macrophages. The combination of more extracellular mycobacteria in the NETs, and the accumulation of Hsp72 in the NETs, could work in concert in augmenting the proinflammatory activation of macrophages. The fact that TNF-α release did not decrease when Mtb-induced NETs were blocked with DPI is unclear. DPI does not inhibit spontaneous apoptosis or intracellular Hsp72 in neutrophils [20]. With a subpopulation of spontaneously apoptotic neutrophils present, these may affect the outcome of TNF-α release.
Corleis et al. [10] recently showed that Mtb-induced necrosis in PMN lead to the release of DNA, suggesting NET formation. The necrotic cell death was ROS dependent and triggered by virulence factors encoded by the RD1 genomic region. RD1 depletion rendered the bacteria sensitive to ROS-mediated killing, which implies that necrosis induction is a survival strategy for virulent Mtb. However, the escape of bacteria from necrotic and NET-forming neutrophils could in turn activate macrophages, resulting in a proinflammatory milieu leading to the eradication of the mycobacterium instead of latent intracellular survival inside macrophages.
NETs are important for the antimicrobial defense against certain pathogens but have also been implicated in autoimmune antigen presentation and the inflammation process in various diseases [45]. How NETs and their components are formed may vary greatly in different disease settings. The main task of the neutrophils is to eliminate microbes. Although mycobacteria are resistant to killing by neutrophils, NETs are able to trap them. In the process of neutrophil activation by Mtb, Hsp72 is released and NETs are formed. We show that these NETs can trigger a proinflammatory cytokine response in adjacent macrophages. An effective innate immune response is crucial for fighting tuberculosis and shaping the subsequent adaptive immune response. Understanding the scenario of when innate immune cells encounter the mycobacterium is important when looking for new effective prevention and treatment options for tuberculosis.
Acknowledgements
This work was supported by the Swedish Research Council, Ragnar Söderbergs Foundation, Sida and the Swedish Heart and Lung Foundation. The authors thank Robert Blomgran for critical revision of the manuscript.
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Wartha F, Henriques-Normark B. Etosis: a novel cell death pathway. Sci Signal. 2008;1:pe25. doi: 10.1126/stke.121pe25. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make nets. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 8.Appelberg R, Silva MT. T cell-dependent chronic neutrophilia during mycobacterial infections. Clin Exp Immunol. 1989;78:478–483. [PMC free article] [PubMed] [Google Scholar]
- 9.Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corleis B, Korbel D, Wilson R, Bylund J, Chee R, Schaible UE. Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell Microbiol. 2012;14:1109–1121. doi: 10.1111/j.1462-5822.2012.01783.x. [DOI] [PubMed] [Google Scholar]
- 11.Seiler P, Aichele P, Bandermann S, Hauser AE, Lu B, Gerard NP, Gerard C, Ehlers S, Mollenkopf HJ, Kaufmann SH. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur J Immunol. 2003;33:2676–2686. doi: 10.1002/eji.200323956. [DOI] [PubMed] [Google Scholar]
- 12.Soehnlein O, Weber C, Lindbom L. Neutrophil granule proteins tune monocytic cell function. Trends Immunol. 2009;30:538–546. doi: 10.1016/j.it.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 15.Gregory CD, Brown SB. Apoptosis: eating sensibly. Nat Cell Biol. 2005;7:1161–1163. doi: 10.1038/ncb1205-1061. [DOI] [PubMed] [Google Scholar]
- 16.Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT, Krutzik SR, Bloom BR, Ganz T, Modlin RL, Stenger S. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177:1864–1871. doi: 10.4049/jimmunol.177.3.1864. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro-Gomes FL, Moniz-de-Souza MC, Alexandre-Moreira MS, Dias WB, Lopes MF, Nunes MP, Lungarella G, DosReis GA. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J Immunol. 2007;179:3988–3994. doi: 10.4049/jimmunol.179.6.3988. [DOI] [PubMed] [Google Scholar]
- 18.Perskvist N, Long M, Stendahl O, Zheng L. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. J Immunol. 2002;168:6358–6365. doi: 10.4049/jimmunol.168.12.6358. [DOI] [PubMed] [Google Scholar]
- 19.Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. J Immunol. 2004;173:6319–6326. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
- 20.Persson YA, Blomgran-Julinder R, Rahman S, Zheng L, Stendahl O. Mycobacterium tuberculosis-induced apoptotic neutrophils trigger a pro-inflammatory response in macrophages through release of heat shock protein 72, acting in synergy with the bacteria. Microbes Infect. 2008;10:233–240. doi: 10.1016/j.micinf.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Persson A, Blomgran-Julinder R, Eklund D, Lundstrom C, Stendahl O. Induction of apoptosis in human neutrophils by Mycobacterium tuberculosis is dependent on mature bacterial lipoproteins. Microb Pathog. 2009;47:143–150. doi: 10.1016/j.micpath.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 22.von Kockritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med. 2009;87:775–783. doi: 10.1007/s00109-009-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Kichik V, Mondragon-Flores R, Mondragon-Castelan M, Gonzalez-Pozos S, Muniz-Hernandez S, Rojas-Espinosa O, Chacon-Salinas R, Estrada-Parra S, Estrada-Garcia I. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb) 2009;89:29–37. doi: 10.1016/j.tube.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe., T Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker H, Albrett AM, Kettle AJ, Winterbourn CC. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol. 2012;91:369–376. doi: 10.1189/jlb.0711387. [DOI] [PubMed] [Google Scholar]
- 27.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;90:1217–1226. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 30.Silva MT, Silva MN, Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989;6:369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 31.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 32.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, Wahn V, Papayannopoulos V, Zychlinsky A. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–959. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel S, Kumar S, Jyoti A, Srinag BS, Keshari RS, Saluja R, Verma A, Mitra K, Barthwal MK, Krishnamurthy H, Bajpai VK, Dikshit M. Nitric oxide donors release extracellular traps from human neutrophils by augmenting free radical generation. Nitric Oxide. 2010;22:226–234. doi: 10.1016/j.niox.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neeli I, Dwivedi N, Khan S, Radic M. Regulation of extracellular chromatin release from neutrophils. J Innate Immun. 2009;1:194–201. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neufert C, Pai RK, Noss EH, Berger M, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J Immunol. 2001;167:1542–1549. doi: 10.4049/jimmunol.167.3.1542. [DOI] [PubMed] [Google Scholar]
- 37.Subramaniyam D, Hollander C, Westin U, Erjefalt J, Stevens T, Janciauskiene S. Secretory leukocyte protease inhibitor inhibits neutrophil apoptosis. Respirology. 2011;16:300–307. doi: 10.1111/j.1440-1843.2010.01901.x. [DOI] [PubMed] [Google Scholar]
- 38.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 39.Asea A. Stress proteins and initiation of immune response: chaperokine activity of hsp72. Exerc Immunol Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- 40.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 41.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. Hsp70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 42.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from Toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 43.O'Leary S, O'Sullivan MP, Keane J. IL-10 blocks phagosome maturation in Mycobacterium tuberculosis-infected human macrophages. Am J Respir Cell Mol Biol. 2011;45:172–180. doi: 10.1165/rcmb.2010-0319OC. [DOI] [PubMed] [Google Scholar]
- 44.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]