Abstract
TREM-1 (triggering receptor expressed on myeloid cells) is a surface molecule expressed on neutrophils and macrophages which has been implicated in the amplification of inflammatory responses triggered during infection. In the present study, we have investigated the clinical significance of TREM-1 in Streptococcus pyogenes-induced severe sepsis in both experimentally infected mice as well as in patients with streptococcal toxic shock. We found that S. pyogenes induced a dose-dependent upregulation of TREM-1 in in vitro cultured phagocytic cells and in the organs of S. pyogenes-infected mice. Furthermore, we reported a positive correlation between serum levels of soluble TREM-1 (sTREM-1) and disease severity in infected patients as well as in experimentally infected mice. Hence, sTREM-1 may represent a useful surrogate marker for streptococcal sepsis. We found that modulation of TREM-1 by administration of the TREM-1 decoy receptor rTREM-1/Fc substantially attenuated the synthesis of inflammatory cytokines. More importantly, treatment of S. pyogenes-infected septic mice with rTREM-1/Fc or the synthetically produced conserved extracellular domain LP17 significantly improved disease outcome. In summary, our data suggest that TREM-1 may not only represent a valuable marker for S. pyogenes infection severity but it may also be an attractive target for the treatment of streptococcal sepsis.
Key Words: Prognostic markers, Sepsis, Streptococcal septic shock, Streptococcus pyogenes, Therapeutic intervention, TREM-1
Introduction
Sepsis is a serious medical condition caused by the response of the body to an infection [1, 2]. Sepsis can present with a broad spectrum of severity, with septic shock being the most severe form of this syndrome. Early intervention and diagnosis significantly decreased sepsis-associated morbidity and mortality [3, 4]. In this regard, several potential biomarkers have been evaluated for the diagnosis and identification of patients at risk for a poor outcome. Among the candidates evaluated, TREM-1 (triggering receptor expressed on myeloid cells-1) has recently attracted attention as a diagnostic/prognostic biomarker for sepsis [5, 6]. TREM-1 belongs to the immunoglobulin superfamily and is expressed on monocytes and neutrophils [7, 8, 9]. Expression of TREM-1 can be induced by bacterial products such as lipopolysaccharides and lipoteichoic acid [7, 8, 9] and has been reported to be highly upregulated on neutrophils and peritoneal macrophages of mice subjected to cecal ligation and puncture [7, 8, 9]. TREM-1 is believed to be a potent amplifier of the inflammatory response to invading pathogens since activation of this receptor during infection results in enhanced production of proinflammatory cytokines [7, 8, 9]. Interestingly, modulation of TREM-1 has been shown to downregulate the inflammatory response and improve disease outcome in murine models of sepsis [8, 10] as well as in a rat model of overwhelming pseudomonas pneumonia [11]. These observations suggest that anti-TREM-1 interventions may be an efficient therapeutic strategy for the treatment of sepsis. On the other hand, Lagler et al. [12] reported that TREM-1 confers protection and is required for the control of pneumococcal pneumonia infection. Therefore, the beneficial effect of TREM-1 modulation seems to be highly dependent of the type of infection.
Soluble TREM-1 (sTREM-1) is shed from activated phagocytic cells and can be quantified in human plasma and body fluids [13]. The plasma sTREM-1 level appears to be a reliable parameter in differentiating patients with sepsis from those with the systemic inflammatory response syndrome [14]. Furthermore, Gibot et al. [15] reported that the concentrations of sTREM-1 remained stable or even higher in nonsurviving septic patients whereas they decreased in survivors, suggesting that the followed-up of plasma levels of sTREM-1 may have prognostic value during sepsis. Other studies have, however, questioned the prognostic value of sTREM-1 in sepsis [16, 17, 18], particularly since the causative microorganism can influence the pattern of the immune response elicited [19]. The prognostic and therapeutic value of TREM-1 may therefore require careful validation for each particular situation.
Streptococcus pyogenes is a common human pathogen responsible for mild infections such as pharyngitis, impetigo or scarlet fever [20] but it can also cause severe invasive infections such as bacteremia, sepsis and septic shock, which are associated with high mortality rates [21]. In recent years, there has been an increase in the variety and severity of S. pyogenes infections, including those that are invasive. The rapid progression and death in patients with severe S. pyogenes infections underscores the importance of early diagnosis and prompt initiation of antimicrobial and supportive therapy [22]. In this regard, the aim of the present study was to investigate the relevance of TREM-1 for the progression of streptococcal sepsis. We found a significant correlation between the levels of sTREM-1 in the plasma of S. pyogenes-infected septic patients and disease severity. This correlation was confirmed using an experimental murine model of S. pyogenes sepsis. We further demonstrated that by modulating TREM-1 signaling using the fusion protein rTREM-1/Fc or the synthetically produced conserved extracellular domain LP17, disease outcome in septic mice was significantly improved. Our results underscore the potential prognostic as well as therapeutic value of TREM-1 in patients affected by streptococcal sepsis.
Materials and Methods
Ethical Issues
The study involving human subjects was approved by the regional ethical committee and drug agency authority in each country [23]. Written informed consent was obtained from all subjects or their legal guardian. The study was performed in accordance with the Declaration of Helsinki. All experiments involving animals were performed in strict accordance with the guidelines of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. All experiments were approved by the Ethical Board of the Lower Saxony State Office for Consumer Protection and Food Safety in Oldenburg, Germany.
Patient Material
Plasma samples collected from patients with the streptococcal toxic shock syndrome (STSS; n = 18) were available from a placebo-controlled trial of intravenous immunoglobulin G [8]. STSS was defined according to established criteria [24]. Infections were caused by S. pyogenes of varying serotypes with T1 strains dominating. Only samples collected at baseline (prior to the administration of the study drug) were used to assess the correlation between plasma concentrations of sTREM-1 and disease severity. Only patients that had received placebo (n = 10) were included in the study of sTREM over the first 3 days of observation.
Bacteria
S. pyogenes strain A20 is a clinical isolate obtained from DSMZ (the German Collection of Microorganisms and Cell Cultures; DSM 2071). Stocks were cultured in Todd-Hewitt broth (Oxoid), supplemented with 1% yeast extract and collected in mid log phase for experimental infections. When required, S. pyogenes was labeled with carboxyfluorescein (Molecular Probes, Göttingen, Germany) as previously described [25].
Experimental Model of S. pyogenes Sepsis
C3H/HeN female mice (Harlan-Winkelmann, Borchen, Germany) were intravenously inoculated with 105 CFU of S. pyogenes via a lateral tail vein. Infected mice were sacrificed by CO2 asphyxiation at progressive times of infection and bacteria were assessed in the blood, liver and spleen by preparing homogenates of these organs in PBS and plating 10-fold serial dilutions on blood agar.
To modulate TREM-1 in vivo, mice were intravenously injected with 5 µg (0.2 mg/kg body weight) of rTREM-1/Fc, a fusion protein consisting of the extracellular domain of mouse TREM-1 and the Fc portion of human IgG1 (R&D Systems, Minneapolis, Minn., USA), 2 h prior and 2 h after bacterial inoculation. Alternatively, mice were injected every 24 h intravenously with 200 µl of a 300 µM solution of the synthetically produced conserved extracellular domain LP17 (117 µg) [11] or with the inactive LP17 scrambled peptide (LP17-CTR) starting 4 h after the onset of infection (peptide sequence of LP17: LQVTDSGLYRCVIYHPP and LP17-CTR: TDSRCVIGLYHPPLQVY).
Measurement of sTREM-1 in Serum
sTREM in plasma samples was measured by Quantikine human or murine TREM-1 ELISA (R&D Systems) according to the manufacturer's instructions.
Cytokine ELISA
Cytokine levels were determined in serum by ELISA according to the recommendations of the manufacturer (BD Pharmingen, San Diego, Calif., USA) using matched antibody pairs and recombinant cytokines as standards.
RT-PCR
Total RNA was prepared using the RNeasy RNA extraction kit (Qiagen, Hilden, Germany). The PCR primer sequences were for the murine trem-1: 5′-CGG AAT TCG AGC TTG AAG GAT GAG GAA GGC-3′ and 5′-GGA TCA ATC CAG AGT CTG TCA CTT GAA GGT CAG TC-3′, and for the murine housekeeping gene β-actin: 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′ and 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′. The resultant PCR products were electrophoresed on 2% agarose gel, stained with ethidium bromide and visualized under UV. Quantitative real-time PCR was carried out using the SensiFAST SYBER No-ROX kit (Bioline, Luckenwalde, Germany) and RotorGene Q (Quiagen).
Isolation and Infection of Bone Marrow-Derived Macrophages
Macrophages were derived from bone marrow extruded from the femur and tibia of C3H/HeN mice and in vitro differentiated as previously described [26]. Differentiated bone marrow-derived macrophages (BMDM) were infected with S. pyogenes at a multiplicity of infection (MOI) ranging from 1:1 to 100:1 bacteria per macrophage for the indicated time period at 37°C. Cells were then either fixed with 3.7% paraformaldehyde and used for microscopy analysis or lysed with RLT buffer and used for RNA extraction, or further incubated in medium containing 100 µg/ml of gentamicin to kill all extracellular bacteria.
In some experiments, BMDM were treated with 50 µg/ml of anti-TREM-1 antibodies (R&D Systems) or rTREM-1/Fc (R&D Systems) 1 h prior to infection. Control BMDM received the same amount of human IgG1.
Immunofluorescence Microscopy
BMDM were seeded on glass coverslips and infected with carboxyfluorescein-labeled S. pyogenes for 1 h at 37°C and fixed with 3.7% formaldehyde overnight at 4°C. BMDM were then washed, permeabilized with 200 µl of 0.1% Triton X-100 for 5 min at room temperature, blocked with PBS supplemented with 10% FCS and incubated for 45 min at room temperature with FITC-conjugated rabbit anti-TREM-1 antibodies (BD Pharmingen). Coverslips were washed with PBS, mounted on glass slides with Mowiol containing DAPI (Prolong Gold, Promega, Madison, Wisc., USA) and analyzed by fluorescence microscopy using an AxioVision microscope (Zeiss, Oberkochen, Germany).
Histopathology
Liver tissue was removed and fixed in 10% formalin for 24 h and subsequently embedded in paraffin wax. Three-micrometer sections were stained with hematoxylin and eosin and examined light microscopically using an Olympus BX51 microscope.
Statistical Analysis
Statistical calculations were performed using GraphPad PRISM software (San Diego, Calif., USA). Comparisons between two groups were made by use of two-tailed t tests and those of more than two groups using analysis of variance followed by a Tukey's HSD test. Survival time curves were compared using the log rank test. The nonparametric Spearman test was used to determine correlations between groups. All data are presented as means ± SD; p < 0.05 was considered significant.
Results
S. pyogenes Induces Expression of TREM-1
First, we determined if S. pyogenes could induce upregulation of TREM-1 on phagocytic cells. For this purpose, trem-1 mRNA expression was assessed in S. pyogenes-infected BMDM at progressive times of infection. The semiquantitative PCR analysis displayed in figure 1a and the quantitative RT-PCR data displayed in figure 1b show that trem-1 mRNA was induced on BMDM after exposure to S. pyogenes and that the degree of trem-1 upregulation was highly dependent on the infectious dose. The dose dependency of TREM-1 induction was further evidenced at the protein level using fluorescence microscopy (fig. 1c). TREM-1 induction was also increased on bone marrow-derived murine neutrophils after exposure to S. pyogenes (online suppl. fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000348283).
Fig. 1.
S. pyogenes induces expression of TREM-1 in BMDM. a Expression of trem-1 mRNA on BMDM after infection with S. pyogenes at MOI of 1:1, 10:1 and 100:1 bacteria per macrophage. BMDM were exposed to S. pyogenes for 1 h, washed and further incubated in the presence of gentamicin for 4 h. Total RNA was isolated followed by RT-PCR analysis of trem-1 and β-actin gene expression. b Quantitative expression of trem-1 mRNA on BMDM after infection with S. pyogenes at MOI of 1:1, 10:1 and 100:1 bacteria per macrophage measured by real-time PCR. c Fluorescence microscope photographs of TREM-1 in BMDM infected with S. pyogenes at MOI of 1:1 (cii), 10:1 (ciii) and 100:1 (civ) bacteria per macrophage. TREM-1 expression on uninfected macrophages is also shown (ci). TREM-1 appears in red and S. pyogenes in green. Macrophage nuclei are stained by DAPI (blue). Bar = 25 µm.
The expression of TREM-1 during S. pyogenes in vivo infection was then investigated using a previously described murine infection model [27]. In this model, intravenous inoculation of C3H/HeN mice with 105 CFU of S. pyogenes strain A20 resulted in severe sepsis characterized by progressive bacterial growth in the blood and liver (fig. 2a), high levels of serum IL-6 (fig. 2b), which is an important mediator of septic shock [28, 29], and 100% mortality by day 4 of infection (fig. 2c). The quantitative PCR analysis shown in fig. 2d demonstrated a progressive increase in the upregulation of TREM-1 in the liver of mice during the course of S. pyogenes infection. TREM-1 was also highly upregulated on peritoneal cells (macrophages and neutrophils) isolated from the peritoneal cavity of C3H/HeN mice after intraperitoneal injection of S. pyogenes (online suppl. fig. 2).
Fig. 2.
S. pyogenes induces expression of TREM-1 during in vivo infection. a, b Means ± SD of 5 mice. One of 3 representative experiments are shown. a Kinetics of bacterial growth in the blood (●) and liver (■) of C3H/HeN mice after intravenous inoculation with 105 CFU of S. pyogenes. b Serum levels of IL-6 in C3H/HeN mice after intravenous inoculation with 105 CFU of S. pyogenes. Sera were collected at the indicated time points and the levels of IL-6 in the serum were determined by ELISA. * p < 0.05; ** p < 0.01, *** p < 0.001. c Survival of C3H/HeN mice after intravenous infection with 105 CFU of S. pyogenes. d Expression of trem-1 mRNA in the liver of C3H/HeN mice at progressive times after intravenous inoculation with 105 CFU of S. pyogenes. Induction of trem-1 was determined by RT-PCR. Expression of β-actin serves as an internal control.
Plasma Levels of sTREM-1 Correlate with Disease Severity in S. pyogenes-Infected Mice
TREM-1 can be cleaved off the membrane of activated neutrophils and monocytes to release sTREM-1 [13]. High levels of sTREM-1 have been detected in the peritoneal lavage fluid of septic mice subjected to cecal ligation and puncture [13] as well as in the plasma of patients at risk of sepsis [6, 14, 15]. Therefore, we investigated if sTREM-1 was released into the serum of S. pyogenes-infected C3H/HeN mice during the course of infection. Serum sTREM-1 was readily detectable 24 h after bacterial inoculation and increased significantly at 48 h of infection, the point of time when the mice undergo overwhelming sepsis and begin to die (fig. 3a). We next plotted the levels of sTREM-1 in the serum of infected mice against the amount of bacteria in the liver and blood. A strong positive correlation was found between the serum levels of sTREM-1 and the bacterial loads in the liver (fig. 3b; r = 0.951, p < 0.001) and blood (fig. 3c; r = 0.9423, p < 0.001). Therefore, serum levels of sTREM-1 appear to be a suitable marker of the severity of S. pyogenes infection.
Fig. 3.
sTREM-1 is shed in the plasma of S. pyogenes-infected mice. a Kinetics of sTREM-1 serum levels in S. pyogenes-infected C3H/HeN mice. b Correlation of sTREM-1 in serum samples with the amount of bacteria in the liver of S. pyogenes-infected mice. Spearman's correlation coefficient r = 0.951, p < 0.001. c Correlation of sTREM-1 in serum samples with the amount of bacteria in the blood of S. pyogenes-infected mice. Spearman's correlation coefficient r = 0.9423, p < 0.001. Each point represents 1 individual animal. ** p < 0.01, *** p < 0.001.
Plasma Levels of sTREM-1 Correlate with Disease Severity in Patients with Streptococcal Toxic Shock
Having demonstrated the potential prognostic value of serum sTREM-1 in experimental streptococcal sepsis, we next sought to validate these results in patients with streptococcal toxic shock. For this purpose, sTREM-1 levels were assessed in the plasma collected from patients with streptococcal toxic shock during the acute phase of infection. At baseline, all patients had elevated sTREM-1 levels with concentrations ranging from 257 to 3,075 pg/ml (mean = 1,011 pg/ml; fig. 4a). Importantly, there was a significant correlation (r = 0.73, p < 0.0009) between the plasma concentration of sTREM-1 and the severity of disease defined by the patient's baseline SAPS II (Simplified Acute Physiology Score; fig. 4a).
Fig. 4.
sTREM-1 in the plasma of STSS patients. a Spearman's test correlation between plasma sTREM-1 levels and disease severity determined by the SAPS II score in patients with streptococcal septic shock. Each point represents the correlation value for 1 patient (n = 18). Spearman's correlation coefficient r = 0.73, p < 0.0009. b Line plot diagram showing plasma sTREM-1 concentration detected on 3 consecutive days. Each patient is presented by a specific symbol. _ = Survivors; - - - = patients with a fatal outcome.
Longitudinally, plasma sTREM-1 concentrations declined over time in most cases (fig. 4b; ----) with the exception of 3 patients, who exhibited a completely different profile (fig. 4b; - - -). One of these patients had extremely high values of sTREM-1 (fig. 4b; white squares) and the other 2 patients demonstrated a sharp increase in plasma sTREM-1 during days 1-3 (fig. 4b; white diamond and white squares). These patients were the only ones with a fatal outcome in this cohort. These observations indicate that serum sTREM-1 levels can be used as a predictor of outcome in patients with streptococcal sepsis. According to the specification of the sTREM kit used, plasma levels of healthy controls showed a mean serum level of sTREM-1 of 134 pg/ml.
TREM-1 Modulates the Inflammatory Response of Macrophages to S. pyogenes in vitro
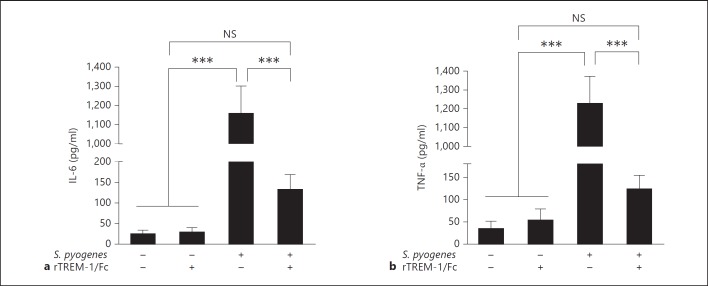
It has previously been shown that TREM-1 synergizes with lipopolysaccharides and amplifies the synthesis of proinflammatory cytokines by monocytes/macrophages [8, 9] and that rTREM-1/Fc is able to modulate and attenuate the inflammatory response to bacterial infections in vivo by trapping bacterial ligands and therefore avoiding TREM-1 signaling on the cellular surface of monocytes/macrophages [11]. Hence, we investigated the potential contribution of TREM-1 to the production of inflammatory cytokines by macrophages after exposure to S. pyogenes. For this purpose, IL-6 and TNF-α levels were determined in the supernatant of macrophages infected with S. pyogenes in the presence or absence of rTREM-1/Fc, a fusion protein containing the extracellular domain of murine TREM-1 and the Fc portion of human IgG1 that acts as decoy receptor to compete with cell-associated TREM-1 [8]. We found a significant attenuation of IL-6 (fig. 5a) and TNF-α (fig. 5b) production by macrophages treated with rTREM-1/Fc. Treatment with rTREM-1/Fc did not influence the capacity of BMDM to phagocyte or kill S. pyogenes (data not shown).
Fig. 5.
Effect of rTREM-1/Fc treatment in the production of inflammatory cytokines by BMDM. Production of IL-6 (a) and TNF-α (b) by uninfected or S. pyogenes-infected BMDM in the presence or absence of rTREM-1/Fc. BMDM were treated with 50 ng/ml of either rTREM-1/Fc or human IgG1 isotype control 1 h prior to infection with S. pyogenes at an MOI of 10:1 bacteria per macrophage. After a 1-hour incubation, the culture medium was replaced by medium supplemented with 100 µg/ml of gentamicin to kill all extracellular bacteria; 50 ng/ml of either rTREM-1/Fc or human IgG1 isotype control were retained in the medium and the levels of IL-6 and TNF-α measured in the supernatant 4 h thereafter. Each bar represents the mean ± SD of 10 different values obtained from two independent experiments. * p < 0.05, ** p < 0.01. *** p < 0.001.
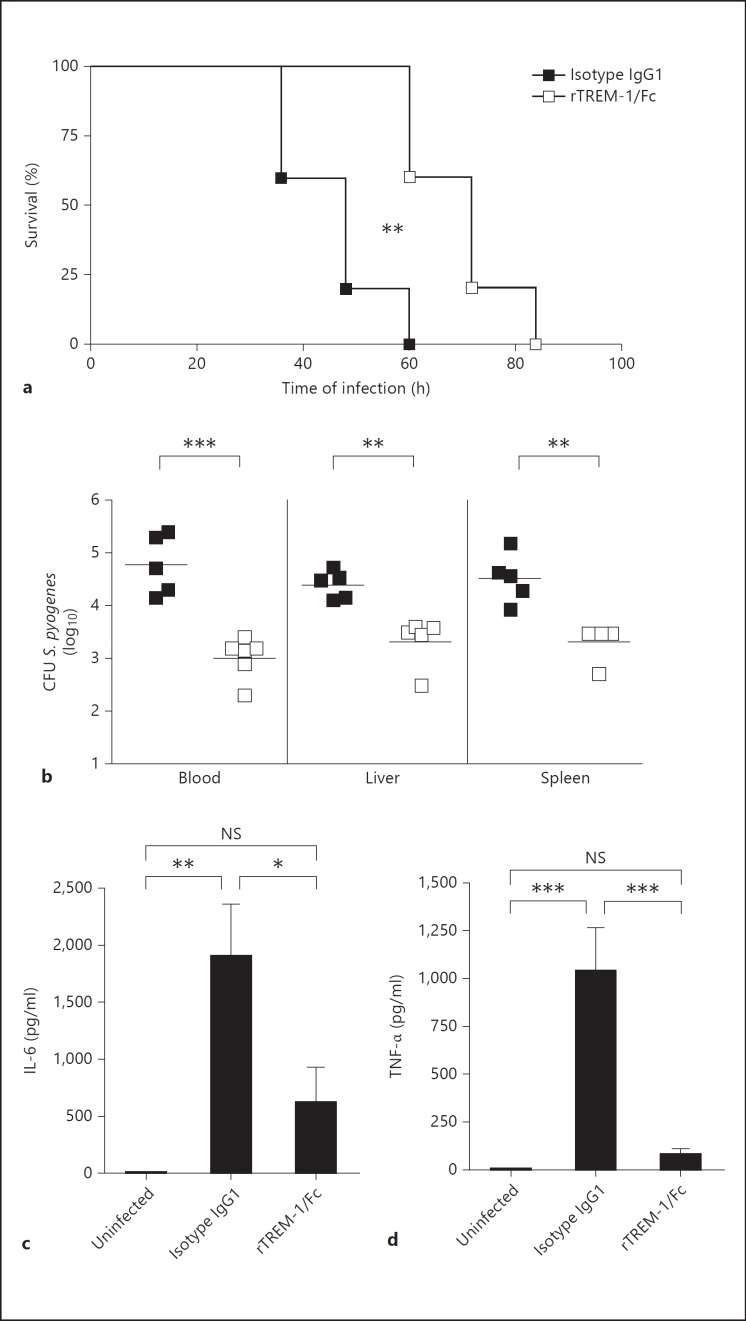
Modulation of TREM-1 in vivo Has a Beneficial Effect during S. pyogenes-Induced Sepsis
It has been reported that treatment with TREM-1-blocking agents exerts a beneficial effect in septic mice undergoing microbial sepsis caused by cecal ligation and puncture [8, 10] and in rats affected by severe pneumonia caused by Pseudomonas aeruginosa[11]. Therefore, our next step was to address the therapeutic effect of TREM-1 modulation in the course of S. pyogenes-induced sepsis. For this purpose, C3H/HeN mice were intravenously infected with S. pyogenes and treated with the TREM-1-modulating agent rTREM-1/Fc or with human IgG1 as a control. The effect of rTREM-1/Fc in S. pyogenes-infected animals was evaluated by assessing survival times, bacterial growth and systemic inflammatory cytokines. As shown in figure 6a, treatment with rTREM-1/Fc significantly improved the survival of infected mice with respect to isotype IgG1-treated animals (p < 0.01). Bacterial loads were significantly lower in the blood, liver and spleen of rTREM-1/Fc-treated mice (fig. 6b) and systemic levels of IL-6 (fig. 6c) and TNF-α (fig. 6d) were also significantly decreased compared with the control group. Importantly, the modulation of TREM-1 significantly attenuated but did not abolish IL-6 and TNF-α production.
Fig. 6.
Effect of rTREM-1/Fc treatment in the course of S. pyogenes infection in mice. a Survival curves of S. pyogenes-infected mice treated with either isotype control IgG1 (■) or rTREM-1/Fc (□) 2 h before and 2 h after intravenous inoculation with 105 CFU of S. pyogenes; log rank test. b Bacterial loads in the blood, liver and spleen of S. pyogenes-infected mice treated with either isotype control IgG1 (■) or rTREM-1/Fc (□) at 48 h after intravenous inoculation with 105 CFU of S. pyogenes. Each symbol represents the bacterial load of 1 animal. One of 3 representative experiments are shown. IL-6 (c) and TNF-α (d) levels in the serum of uninfected or S. pyogenes-infected mice treated with either isotype control IgG1 or rTREM-1/Fc at 48 h of infection. Means ± SD of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001.
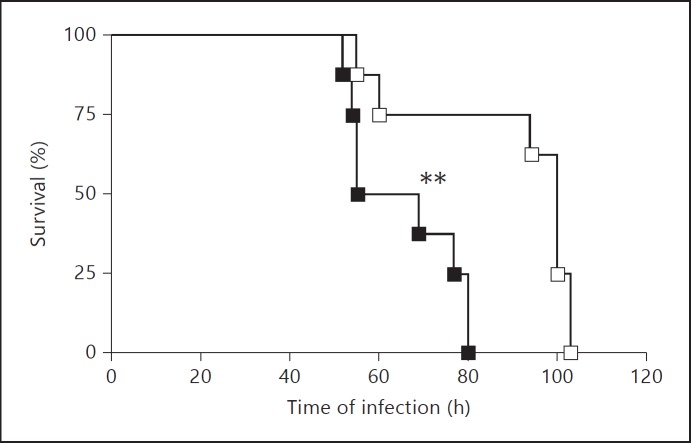
Similar results were obtained when S. pyogenes-infected mice were treated with the synthetically produced conserved extracellular domain LP17. This domain contains the highly conserved extracellular binding region of the surface TREM-1 protein based on the TREM-1 sequence in GenBank/EMBL/DDBJ (accession No. AF241219). Thus, mice treated with LP17 survived longer than those treated with the inactive scrambled LP17 control peptide (fig. 7). In addition, LP17-treated mice (online suppl. fig. 3A) exhibited lower levels of infection-associated pathology in the liver than scrambled LP17 control peptide-treated animals (online suppl. fig. 3B), which is reflected by the reduced thrombus and decreased necrotic tissue formation.
Fig. 7.
Effect of LP17 treatment in the course of S. pyogenes infection in mice. Survival curves of S. pyogenes-infected mice treated with either LP17 peptide (□) or scrambled LP17-control peptide (■) after intravenous inoculation with 105 CFU of S. pyogenes; log rank test. ** p < 0.01.
Discussion
One of the most severe clinical manifestations of S. pyogenes infections are STSS [20]. Early diagnosis and timely therapeutic intervention is crucial for the successful treatment of this syndrome. In this study, we have investigated the role of TREM-1, a promising candidate for the diagnosis and prognosis of septic patients [5, 6], in the pathogenesis of sepsis induced by S. pyogenes. We found that TREM-1 expression was dramatically upregulated on macrophages and neutrophils after in vitro exposure to S. pyogenes. More importantly, we found a significant positive correlation between the serum sTREM-1 levels and the bacterial loads in the liver and blood of S. pyogenes-infected mice. We extended this investigation to patients with STSS and found a similar positive correlation between the plasma sTREM-1 levels and the severity of sepsis. Furthermore, sequential measurement of plasma concentrations of sTREM-1 in 10 of these septic patients showed that plasma sTREM-1 concentrations declined over time in survivors (7 patients) but sharply increased in 2 patients who succumbed. Together, these observations highlight the value of plasma sTREM-1 as a surrogate biomarker in patients with streptococcal sepsis.
Regarding the physiological function of TREM-1, it has been proposed that TREM-1 is an amplifier of the inflammatory response to bacterial infection [7, 8, 9]. Thus, evidence indicates that simultaneous triggering of TREM-1, and Toll- or Nod-like receptors results in a synergistic effect on the production of proinflammatory cytokines [7, 8, 30, 31]. Therefore, TREM-1 seems to potentiate the signal transmitted by the pattern recognition receptors to ensure a rapid host response upon pathogen recognition. In this study, we demonstrated that TREM-1 is involved in the immune response of phagocytic cells to S. pyogenes since attenuation of TREM-1 signaling using rTREM-1/Fc significantly decreased the production of proinflammatory cytokines such as IL-6 and TNF-α. The potential molecular mechanism underlying this protective effect might be the capturing of bacterial ligands by the rTREM-1/Fc protein, thus reducing TREM-1 signaling on the surface of immune cells and production of inflammatory cytokines by these cells. However, the nature of TREM-1 ligands is still a mystery. It has been reported that apart from bacterial molecules like lipopolysaccharides, a ligand on human platelets [32], which enhances the activation of neutrophils and monocytes/macrophages, might also exists. It was shown that sTREM-1 binds to human platelets and that this interaction can be blocked by LP17 [32]. HMGB1 (high-mobility group box 1) and Hsp70 (heat shock protein 70) could be potential ligands of TREM-1 [33]. Nevertheless, the specific ligands involved in TREM-1 signaling of Gram-positive bacteria and those which might be involved in S. pyogenes infection remain to be elucidated.
Despite its important role in the initiation of a rapid immune response after pathogen recognition, activation of TREM-1 has been shown to contribute to the harmful amplification of the inflammatory reaction in situations of generalized inflammatory response to infection such as sepsis and septic shock [9, 34]. Thus, interfering with TREM-1 signaling has been shown to attenuate systemic inflammation and promotes survival during experimental sepsis [8] as well as pulmonary inflammation during experimental P. aeruginosa pneumonia in rats [11]. Together, these studies suggested that modulating TREM-1 activity could be a viable treatment for sepsis. In contrast, Lagler et al. [12] reported that modulation of TREM-1 did not affect the inflammatory response or severity of infection in mice infected with Streptococcus pneumoniae. In our model of experimental S. pyogenes-induced sepsis, mitigation of TREM-1 signaling using rTREM-1/Fc as well as the synthetically produced conserved extracellular domain LP17 significantly improved disease outcome in septic mice. This was demonstrated by the attenuated production of inflammatory cytokines, decreased bacterial loads and extended survival times observed in LP17-treated mice. Our results are therefore in agreement with previous studies showing the detrimental effect of TREM-1 activation in the pathology of sepsis and imply that modulation of TREM-1 may help to optimize the efficacy of existing treatments in patients with streptococcal septic shock.
More importantly, we found that treatment of S. pyogenes-infected septic mice with rTREM-1/Fc signalling resulted in significantly decreased IL-6 and TNF-α levels but not in the complete inhibition of cytokine production. Inflammatory cytokines, in particular IL-6, are considered to be deleterious [28, 29], yet they also have beneficial effects during severe S. pyogenes infections, as shown by the more severe outcome of S. pyogenes invasive infection in mice deficient in IL-6 production [35].
In summary, the results of our study underscore the potential value of sTREM-1 as a surrogate marker for monitoring patients with streptococcal sepsis as well as a potential therapeutic agent for the treatment of this syndrome.
Disclosure Statement
The authors have no conflicts of interest to declare.
Supplementary Material
Supplementary data
Acknowledgments
Financial support for this study was provided by internal funding of the Helmholtz Center for Infection Research.
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 4.Rivers EP, McIntyre L, Morro DC, Rivers KK. Early and innovative interventions for severe sepsis and septic shock: taking advantage of a window of opportunity. CMAJ. 2005;173:1054–1065. doi: 10.1503/cmaj.050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventetuolo CE, Levy MM. Biomarkers: diagnosis and risk assessment in sepsis. Clin Chest Med. 2008;29:591–603. doi: 10.1016/j.ccm.2008.07.001. vii. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, She D, Feng D, Jia Y, Xie L. Dynamic changes of serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) reflect sepsis severity and can predict prognosis: a prospective study. BMC Infect Dis. 2011;11:53. doi: 10.1186/1471-2334-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000;164:4991–4995. doi: 10.4049/jimmunol.164.10.4991. [DOI] [PubMed] [Google Scholar]
- 8.Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410:1103–1107. doi: 10.1038/35074114. [DOI] [PubMed] [Google Scholar]
- 9.Colonna M, Facchetti F. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J Infect Dis. 2003;187((suppl 2)):S397–S401. doi: 10.1086/374754. [DOI] [PubMed] [Google Scholar]
- 10.Gibot S, Alauzet C, Massin F, Sennoune N, Faure GC, Bene MC, Lozniewski A, Bollaert PE, Levy B. Modulation of the triggering receptor expressed on myeloid cells-1 pathway during pneumonia in rats. J Infect Dis. 2006;194:975–983. doi: 10.1086/506950. [DOI] [PubMed] [Google Scholar]
- 11.Gibot S, Buonsanti C, Massin F, Romano M, Kolopp-Sarda MN, Benigni F, Faure GC, Bene MC, Panina-Bordignon P, Passini N, Levy B. Modulation of the triggering receptor expressed on the myeloid cell type 1 pathway in murine septic shock. Infect Immun. 2006;74:2823–2830. doi: 10.1128/IAI.74.5.2823-2830.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagler H, Sharif O, Haslinger I, Matt U, Stich K, Furtner T, Doninger B, Schmid K, Gattringer R, de Vos AF, Knapp S. TREM-1 activation alters the dynamics of pulmonary IRAK-M expression in vivo and improves host defense during pneumococcal pneumonia. J Immunol. 2009;183:2027–2036. doi: 10.4049/jimmunol.0803862. [DOI] [PubMed] [Google Scholar]
- 13.Gibot S, Cravoisy A, Kolopp-Sarda MN, Bene MC, Faure G, Bollaert PE, Levy B. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med. 2005;33:792–796. doi: 10.1097/01.ccm.0000159089.16462.4a. [DOI] [PubMed] [Google Scholar]
- 14.Gibot S, Kolopp-Sarda MN, Bene MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200:1419–1426. doi: 10.1084/jem.20040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibot S, Kolopp-Sarda MN, Bene MC, Cravoisy A, Levy B, Faure GC, Bollaert PE. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004;141:9–15. doi: 10.7326/0003-4819-141-1-200407060-00009. [DOI] [PubMed] [Google Scholar]
- 16.Barati M, Bashar FR, Shahrami R, Zadeh MH, Taher MT, Nojomi M. Soluble triggering receptor expressed on myeloid cells 1 and the diagnosis of sepsis. J Crit Care. 2010;25:362e1–362e6. doi: 10.1016/j.jcrc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Bopp C, Hofer S, Bouchon A, Zimmermann JB, Martin E, Weigand MA. Soluble TREM-1 is not suitable for distinguishing between systemic inflammatory response syndrome and sepsis survivors and nonsurvivors in the early stage of acute inflammation. Eur J Anaesthesiol. 2009;26:504–507. doi: 10.1097/EJA.0b013e328329afca. [DOI] [PubMed] [Google Scholar]
- 18.Phua J, Koay ES, Zhang D, Lee KH. How well do serum sTREM-1 measurements prognosticate in septic shock? Anaesth Intensive Care. 2008;36:654–658. doi: 10.1177/0310057X0803600504. [DOI] [PubMed] [Google Scholar]
- 19.Opal SM. Concept of PIRO as a new conceptual framework to understand sepsis. Pediatr Crit Care Med. 2005;6:S55–S60. doi: 10.1097/01.PCC.0000161580.79526.4C. [DOI] [PubMed] [Google Scholar]
- 20.Jaggi P, Shulman ST. Group A streptococcal infections. Pediatr Rev. 2006;27:99–105. doi: 10.1542/pir.27-3-99. [DOI] [PubMed] [Google Scholar]
- 21.Lamagni TL, Darenberg J, Luca-Harari B, Siljander T, Efstratiou A, Henriques-Normark B, Vuopio-Varkila J, Bouvet A, Creti R, Ekelund K, Koliou M, Reinert RR, Stathi A, Strakova L, Ungureanu V, Schalen C, Jasir A. Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol. 2008;46:2359–2367. doi: 10.1128/JCM.00422-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamagni TL, Neal S, Keshishian C, Powell D, Potz N, Pebody R, George R, Duckworth G, Vuopio-Varkila J, Efstratiou A. Predictors of death after severe Streptococcus pyogenes infection. Emerg Infect Dis. 2009;15:1304–1307. doi: 10.3201/eid1508.090264. [DOI] [PubMed] [Google Scholar]
- 23.Darenberg J, Ihendyane N, Sjolin J, Aufwerber E, Haidl S, Follin P, Andersson J, Norrby-Teglund A. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003;37:333–340. doi: 10.1086/376630. [DOI] [PubMed] [Google Scholar]
- 24.Defining the group A streptococcal toxic shock syndrome Rationale and consensus definition. The Working Group on Severe Streptococcal Infections. JAMA. 1993;269:390–391. [PubMed] [Google Scholar]
- 25.Goldmann O, Hertzen E, Hecht A, Schmidt H, Lehne S, Norrby-Teglund A, Medina E. Inducible cyclooxygenase released prostaglandin E2 modulates the severity of infection caused by Streptococcus pyogenes. J Immunol. 2010;185:2372–2381. doi: 10.4049/jimmunol.1000838. [DOI] [PubMed] [Google Scholar]
- 26.Goldmann O, Sastalla I, Wos-Oxley M, Rohde M, Medina E. Streptococcus pyogenes induces oncosis in macrophages through the activation of an inflammatory programmed cell death pathway. Cell Microbiol. 2009;11:138–155. doi: 10.1111/j.1462-5822.2008.01245.x. [DOI] [PubMed] [Google Scholar]
- 27.Medina E, Goldmann O, Rohde M, Lengeling A, Chhatwal GS. Genetic control of susceptibility to group A streptococcal infection in mice. J Infect Dis. 2001;184:846–852. doi: 10.1086/323292. [DOI] [PubMed] [Google Scholar]
- 28.Hack CE, De Groot ER, Felt-Bersma RJ, Nuijens JH, Strack Van Schijndel RJ, Eerenberg-Belmer AJ, Thijs LG, Aarden LA. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- 29.Pettila V, Hynninen M, Takkunen O, Kuusela P, Valtonen M. Predictive value of procalcitonin and interleukin 6 in critically ill patients with suspected sepsis. Intensive Care Med. 2002;28:1220–1225. doi: 10.1007/s00134-002-1416-1. [DOI] [PubMed] [Google Scholar]
- 30.Bleharski JR, Kiessler V, Buonsanti C, Sieling PA, Stenger S, Colonna M, Modlin RL. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170:3812–3818. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 31.Netea MG, Azam T, Ferwerda G, Girardin SE, Kim SH, Dinarello CA. Triggering receptor expressed on myeloid cells-1 (TREM-1) amplifies the signals induced by the NACHT-LRR (NLR) pattern recognition receptors. J Leukoc Biol. 2006;80:1454–1461. doi: 10.1189/jlb.1205758. [DOI] [PubMed] [Google Scholar]
- 32.Haselmayer P, Grosse-Hovest L, von Landenberg P, Schild H, Radsak MP. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood. 2007;110:1029–1035. doi: 10.1182/blood-2007-01-069195. [DOI] [PubMed] [Google Scholar]
- 33.El Mezayen R, El Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett. 2007;111:36–44. doi: 10.1016/j.imlet.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibot S, Le Renard PE, Bollaert PE, Kolopp-Sarda MN, Bene MC, Faure GC, Levy B. Surface triggering receptor expressed on myeloid cells 1 expression patterns in septic shock. Intensive Care Med. 2005;31:594–597. doi: 10.1007/s00134-005-2572-x. [DOI] [PubMed] [Google Scholar]
- 35.Diao H, Kohanawa M. Endogenous interleukin-6 plays a crucial protective role in streptococcal toxic shock syndrome via suppression of tumor necrosis factor alpha production. Infect Immun. 2005;73:3745–3748. doi: 10.1128/IAI.73.6.3745-3748.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data