Abstract
Polyinosinic:polycytidylic acid (poly I:C), a synthetic double-stranded RNA, acts on myeloid cells and induces potent antitumor immune responses including natural killer (NK) cell activation. Myeloid-derived suppressor cells (MDSCs) systemically exist in tumor-bearing hosts and have strong immunosuppressive activity against antitumor effector cells, thereby dampening the efficacy of cancer immunotherapy. Here we tested what happened in MDSCs in poly I:C-treated mice. NK-sensitive syngenic tumor (B16)-bearing C57BL/6 mice were employed for this study. Intraperitoneal poly I:C treatment induced MDSC activation, driving CD69 expression and interferon (IFN)-γ production in NK cells. IFN-γ directly inhibited proliferation of B16 cells. This NK cell priming led to growth retardation of B16 tumors, although no direct tumoricidal activity was induced in NK cells. Mechanistic analysis using KO mice and function-blocking monclonal antibody revealed that MDSCs produced IFN-α via the mitochondrial antiviral signaling protein (MAVS) pathway after in vivo administration of poly I:C, and activated NK cells through the IFNAR pathway. MDSC-mediated NK cell priming was reconstituted by IFN-α in a coculture system. Either the MAVS or IFNAR signaling pathway was required for activation of MDSCs that led to growth retardation of B16 tumor in vivo. The results infer that MDSC is a target of poly I:C to prime NK cells, which exert antitumor activity to NK-sensitive tumor cells.
Key Words: Myeloid-derived suppressor cells, Mitochondrial antiviral signaling protein, Tumor immunotherapy, Double-stranded RNA
Introduction
The innate sensing of microbial molecular patterns results in the modulation of the cellular immune system [1, 2, 3]. This innate-adaptive linkage closely associates with suppression of infection and tumorigenesis. Many reports showed that polyinosinic:polycytidylic acid (poly I:C), a synthetic pattern of double-stranded RNA, has potent stimulatory effects on immune responses to viral infection and cancer [4, 5, 6, 7, 8]. Poly I:C is an agonist for pattern-recognition receptors (PRRs), Toll-like receptor 3 (TLR3) and melanoma differentiation-associated protein 5 (MDA5), which transduce signals to the adaptor molecules TICAM-1 (also known as TRIF) and mitochondrial antiviral signaling protein (MAVS; IPS-1, Cardif, VISA) [9, 10, 11, 12]. They differentially modulate the functions of myeloid dendritic cells (DCs) and macrophages, including cytokine/IFN production and expression of surface molecules that drive effector cell activation.
TLR3/TICAM-1 and MDA5/MAVS activate the transcription factors, NF-κB and interferon (IFN) regulatory factor 3 (IRF-3), to typically induce type-I IFN. Type-I IFN evokes subsequent activation of the IFNAR pathway, which participates in the induction of IFN-stimulated genes (ISGs) including IRF-7 [13, 14]. IRF-7 further modifies the function of poly I:C by upregulating PRRs. Thus, the activity of poly I:C immediately affects IRF-3-derived genes and secondarily upregulates genes by activation of the IFNAR pathway. These pathways are crucial for driving the effector functions of NK cells and cytotoxic T cells that result in tumor regression after poly I:C treatment [6, 15].
NK cells are important for antitumor effects not only through direct cytotoxic activity, but also indirectly, through the production of cytokines including IFN-γ [16, 17, 18, 19, 20]. DX5+ or NK1.1+ cells have been used as conventional NK cells, which have features distinct from other lymphoid cells. Optimal NK cell responses require the presence of accessory cells such as DCs or macrophages [21]. NK cells are essential for poly I:C-induced growth retardation of NK-sensitive tumors such as B16 melanomas since poly I:C treatment does not induce antitumor activity in NK cell-depleted mice [4, 5]. IFN-γ production and cytotoxic activity by NK cells are potentiated by stimulating mice in vivo with poly I:C. NK cell activation appears to have many modes and myeloid NK cell contact serves a critical factor for antitumor NK cell activation.
Myeloid-derived suppressor cells (MDSCs) belongs to myeloid lineages with potent immunosuppressive activity against antitumor immune responses in mice and humans [22, 23]. MDSCs are widely distributed at tumor sites and in the peripheral organs, spleen and lymph nodes. Defined as a CD11b+Gr1+ subset in mice, they are heterogeneous populations of early myeloid progenitors that arise in bone marrow. Recently, they have also been found to originate from hematopoietic stem and progenitor cells accumulated in the spleen under tumor-bearing conditions [24]. The immunoregulatory functions of MDSCs in cancer have been studied extensively [22, 25]. MDSCs inhibit antigen-dependent T cell proliferation through the production of immunosuppressive factors including arginase-1, reactive oxygen species and reactive nitrogen species, and the release of immunosuppressive cytokines. However, the effect of MDSCs on NK cell function in tumor-bearing hosts is controversial. The anergy of NK cells is reportedly induced by MDSCs through membrane-bound TGF-β in a tumor-implant model using 3LL, B16 and EG7 cells [26]. MDSCs derived from patients with hepatocellular carcinoma inhibit autologous NK cell activity when cocultured in vitro [27]. Splenic MDSCs in TS/A tumor-bearing mice repress NK cell cytotoxicity [28]. A subset of MDSCs expresses NKG2D ligand on the cell surface and activates NK cells through NKG2D-NKG2D ligand interaction [29]. Although MDSCs express PRRs, their contribution to the MDSC function in poly I:C-induced growth retardation of tumors has not been fully understood.
Recent studies have demonstrated that TLR stimulation could modulate the function of immunosuppressive myeloid-derived cells as well as myeloid DCs in cancer. Tumor-associated macrophages and MDSCs were converted from tumor supporters to tumoricidal effectors after treatment with TLR agonists [7, 30, 31]. It was demonstrated that CpG treatment blocks MDSC-mediated T cell suppression associated with the maturation and differentiation of MDSCs [30, 31]. In this study, we revealed that poly I:C treatment allows cancer-expanded MDSCs to prime NK cells through the MAVS and the type-I IFN signaling pathway in vivo, leading to retardation of tumor growth.
Materials and Methods
Mice and Tumor Cells
Inbred C57BL/6 wild-type (WT) mice were purchased from Clea, Japan. TICAM-1−/− and MAVS−/− mice were generated in our laboratory. IFNAR1−/− mice were kindly provided by T. Taniguchi (University of Tokyo). Mice of 6- to 10-weeks of age were used in all experiments that were performed according to animal experimental ethics committee guidelines of Hokkaido University. B16D8 cells were developed in our laboratory [4]. B16D8 cells were cultured at 37°C under 5% CO2 in RPMI containing 10% FBS, penicillin and streptomycin. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (USA). The protocol was approved by the Committee on the Ethics of Animal Experiments in the Animal Safety Center, Hokkaido University, Japan. All mice were used according to the guidelines of the institutional animal care and use committee of Hokkaido University, who approved this study as ID number 08-0290, ‘Analysis of Anti-Tumor Immune Response Induced by the Activation of Innate Immunity’.
Tumor Challenge and Poly I:C Treatment
Mice were shaved at the back and injected s.c with B16D8 cells (6 × 105), 3LL cells (3 × 106) or EL4 cells (1 × 106) suspended in 200 µl PBS(-). Tumor size was measured using a caliper. Tumor volume was calculated using the following formula: tumor volume (cm3) = (long diameter) × (short diameter)2 × 0.4. Poly I:C (GE Bioscience) (200 µg/head) with no detectable LPS was injected i.p. as indicated. In some cases, polymixin B-treated poly I:C was used. When an average tumor volume of 0.4-0.6 cm3 was reached, the treatment was started and was repeated every 4 days.
Cell Isolation and Culture
When tumor volume reached 1-2 cm3, i.e. 14-18 days after tumor challenge, mice were injected i.p. with 200 µg poly I:C or PBS(-). After 4 h, CD11b+Gr1+ MDSC-like cells were isolated from splenocyte suspension or single-cell suspension from the collagenase-treated tumor of poly I:C-injected or PBS-injected mice by using biotin-conjugated anti-Gr1 monoclonal antibody (RB6-8C5) and Streptavidin Microbeads (Miltenyi) as described previously [7]. NK cells were purified from splenocytes of naïve mice by using DX5 Microbeads (Miltenyi). In these purification steps, two rounds of positive selection were performed. We routinely prepared Gr1+ cells at more than 95% purity and almost 100% of Gr1+ cells expressed CD11b. The purity of DX5+ cells was more than 90%. Isolated CD11b+Gr1+ cells and DX5+ cells were cocultured for 20-24 h. In some experiments, anti-IFNAR1 monoclonal antibody (MAR1-5A3) was added to the culture for neutralization of IFNAR1. Recombinant mouse IFN-α (R&D systems) was used for stimulation of CD11b+Gr1+ cells and NK cells.
Cells isolated from mouse spleen were incubated for 24 h and the conditioned medium was collected. Concentrations of IFN-α and IFN-γ were determined by ELISA according to manufacturer's instructions (PBL Interferon Source and eBioscience). NK cytotoxicity was determined by standard 51Cr release assay as described previously [32].
Flow Cytometric Analysis
Mononuclear cells prepared from mouse spleen or tumor were treated with anti-CD16/32 (no. 93) and stained with FITC- or APC-anti-CD45.2 (no. 104), FITC- or PE-anti-CD11b (M1/70), APC- or PE-anti-GR1 (RB6-8C5), PE- or APC-anti-NK1.1 (PK136), PE-anti-CD49b (DX5), FITC-, PE- or APC-anti-CD3ε (145-2C11), FITC- or PE-anti-CD69 (H1.2F3), PE-anti-CD80 (16-10A1), PE-anti-CD86 (GL-1), PE-anti-CD40 (1C10), PE-anti-CD155 (TX56), PE-anti-CD70 (FR70), PE-anti-IL-15Ra (DNT15Ra), FITC-anti-CD150 [A12 (7D4)], and anti-RAE-1 (eBioscience and Biolegend). Samples were analyzed with a FACSCalibur instrument or FACS Aria instrument (BD Bioscience) and data analysis was performed by FlowJo software (Tree Star).
T Cell Proliferation Assay
T cell proliferation was measured by changes in fluorescence intensity using carboxyfluorescein diacetate succinimidyl ester (CFSE). Splenocytes from OT-I transgenic mice were labeled with 1 µM CFSE, placed into a round bottom 96-well plate containing CD11b+Gr1+ cells as indicated. Splenocytes were cultured in the presence of 100 nM OVA-derived peptide SIINFEKL. After 3 days, cells were harvested, stained with APC-anti-CD8α (53-6.7) and PE-anti-TCR vβ 5.1, 5.2 (MR9-4) or PE-anti-CD3ε (145-2C11), and the CFSE signal of gated lymphocytes was analyzed by flow cytometry. The extent of cell proliferation was quantified by FlowJo software (Tree Star).
Quantitative PCR Analysis
RNA was prepared with RNeasy kit (QIAGEN) or TRIZOL reagent (Invitrogen) according to the manufacturer's instruction. Reverse transcription was performed using High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). Real-time PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems) with StepOne™ Real-time PCR system (Applied Biosystems). Expression of the cytokine gene was normalized to the expression of glyceraldehyde phosphate dehydrogenase (GAPDH). The following primers were used for PCR: IFNα4 forward, 5′-CTGCTGGCTGTGAGGACATACT-3′, IFNα4 reverse, 5′-AGGCACAGAGGCTGTGTTTCTT-3′, IL-15 forward, 5′-TTAACTGAGGCTGGCATTCATG-3′, IL-15 reverse, 5′-ACCTACACTGACACAGCCCAAA-3′, IL-18 forward, 5′-GACAAAGAAAGCCGCCTCAA-3′, IL-18 reverse, 5′-ATGGCAGCCATTGTTCCTG-3′, INAM forward, 5′-CAACTGCAATGCCACGCTA-3′, INAM reverse, 5′-TCCAACCGAACACCTGAGACT-3′, GAPDH forward, 5′-GCCTGGAGAAACCTGCCA-3′, GAPDH reverse, 5′-CCCTCAGATGCCTGCTTCA-3′. Data was analyzed by the ΔΔCt method.
Statistics
If not otherwise stated, data were expressed as arithmetic means ± SD, and statistical analyses were made by 2-tailed Student's t test. p < 0.05 was considered statistically significant.
Results
CD11b+Gr1+ Cells Expanded in B16 Tumor-Bearing Mice Are Immunosuppressive
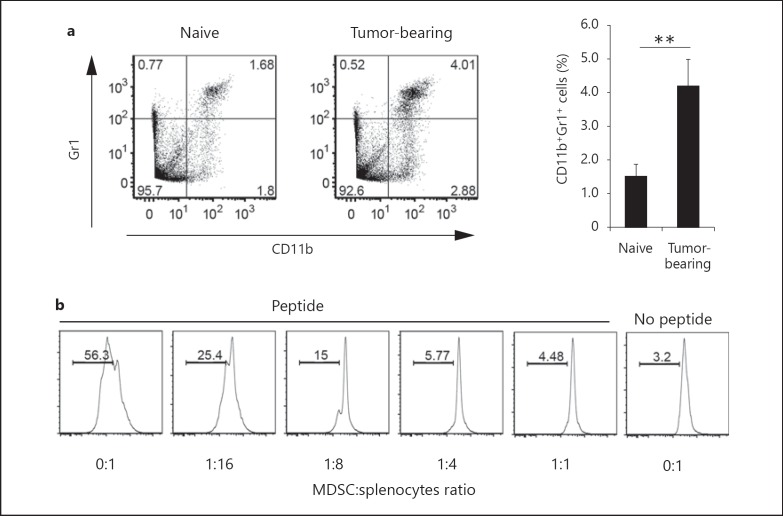
CD11b+Gr1+ cells representing MDSCs accumulate in large numbers in the lymphoid tissues of tumor-bearing mice [22, 23]. We therefore investigated the spleens of mice bearing syngenic tumor cells. B16 melanoma cells, 3LL lung cancer cells or EL4 thymoma cells were s.c. injected into WT mice and, 16 days later, splenic populations of immune cells were examined in the tumor-bearing mice. The proportion of CD11b+Gr1+ cells in the spleens of B16-implanted mice was higher than that in tumor-free naïve mice, consistent with previous reports (fig. 1a). Similar profiles were obtained with the 3LL and EL4 cell lines (data not shown).
Fig. 1.
Immunosuppressive activity of CD11b+Gr1+ cells in the spleen of B16 tumor-bearing mice. a WT mice were injected s.c. with B16D8 melanoma cells. The percentage of CD11b+Gr1+ cells in the spleen was determined on day 16 by flow cytometry (n = 6). Cells were gated on CD45+ cells. b CD11b+Gr1+ cells were isolated from spleens of B16 tumor-bearing mice, and cultured with CFSE-labeled OT-I splenocytes (1 × 106) at the indicated ratios. After 3 days, proliferation of CD8α+TCRvβ+ cells was measured. Data shown are representative of at least 2 independent experiments. ** p < 0.01.
To examine whether CD11b+Gr1+ cells had immunosuppressive activity, we harvested CD11b+Gr1+ cells from the spleens of B16 tumor-implanted mice, and cocultured CD11b+Gr1+ cells with OT-I splenocytes in the presence of OVA peptide. CD11b+Gr1+ cells from tumor-bearing mice efficiently inhibited antigen-specific proliferation of CD8+ OT-I T cells (fig. 1b). Therefore, CD11b+Gr1+ cells accumulated in the spleen of B16 tumor-bearing mice and had immunosuppressive functions.
We also assessed the immunosuppressive activity of CD11b+Gr1+ cells against NK cells activated by PMA/ionomycin and tested activation as level of IFN-γ production. No inhibitory effect of CD11b+Gr1+ cells on the production of IFN-γ by NK cells was observed (online suppl. fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000355126). Therefore, CD11b+Gr1+ cells expanded in B16 tumor-bearing mice exhibited immunosuppressive activity toward CD8+ T cells but not NK cells.
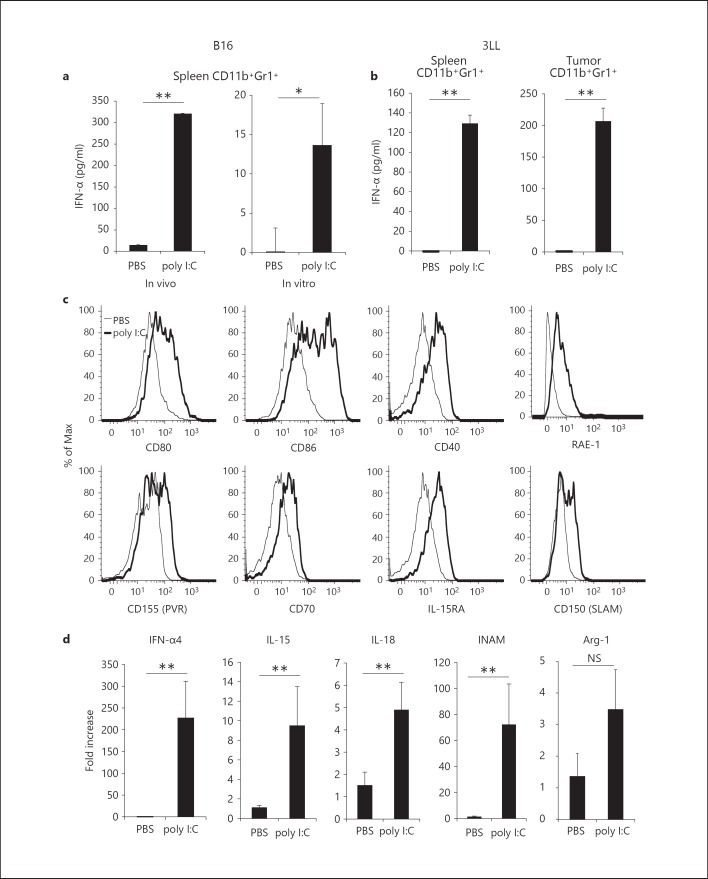
In vivo Poly I:C Induces Cytokine Production and Maturation of CD11b+Gr1+ Cells
Type-I IFNs are systemically produced in tumor-bearing mice by i.p. injection of poly I:C. Poly I:C usually acts on TLR3 in myeloid/epithelial cells and MDA5 in systemic cells, leading to type-I IFN production [33]. Since CD11b+Gr1+ cells expressed both TLR3 and MDA5, we examined whether type-I IFNs were produced by CD11b+Gr1+ cells in B16 tumor-bearing mice in response to poly I:C injection. Interestingly, we found that IFN-α is produced in splenic CD11b+Gr1+ cells harvested from poly I:C-treated B16 tumor-bearing mice, but not in CD11b+Gr1+ cells unexposed to poly I:C (fig. 2a, left panel). The results were also confirmed in vitro: type-I IFNs was minimally produced in poly I:C-untreated CD11b+Gr1+ cells but robustly in poly I:C-treated cells from the spleen or tumor in direct response to poly I:C (fig. 2a, right panel). The results were reproducible with different tumor cell lines, specifically 3LL and EL4, and different sources of CD11b+Gr1+ cells (fig. 2b; online suppl. fig. 2a). In addition, the costimulatory molecules CD80 and CD86 on these cells were upregulated in response to poly I:C (fig. 2c). To further investigate the effect of poly I:C treatment on the function of CD11b+Gr1+ cells, we analyzed the gene expression of CD11b+Gr1+ cells isolated from B16 tumor-bearing mice, 4 h after injection with poly I:C or PBS. We found an increase in mRNA for IFN-α4, IL-15, IL-18 and INAM (fig. 2d) [21, 32]. Furthermore, in vivo poly I:C treatment for 8 h resulted in upregulation of RAE-1, PVR (CD155), CD70, IL-15RA, SLAM (CD150) and CD40 on CD11b+Gr1+ cell surface (fig. 2c). These molecules are involved in DC-mediated NK cell activation [18, 34, 35]. However, mRNA for arginase-1, which is involved in MDSC-mediated inhibition of T cell proliferation, was not increased in CD11b+Gr1+ cells (fig. 2d). These results suggest that in vivo pretreatment of mice with poly I:C effectively induces the maturation of CD11b+Gr1+ cells, resulting in enhanced expression of NK cell-activating molecules.
Fig. 2.
Effect of poly I:C treatment on CD11b+Gr1+ cells. a B16 tumor-bearing mice were injected i.p. with 200 µg poly I:C or PBS as a negative control. After 4 h, CD11b+Gr1+ cells were purified from spleens and incubated for 24 h (left panel). CD11b+Gr1+ cells isolated from spleens of B16 tumor-bearing mice were treated with 50 µg/ml poly I:C or PBS for 24 h (right panel). The concentration of IFN-α in conditioned medium was determined. b 3LL cells (3 × 106) were implanted into B6 WT mice and CD11b+Gr1+ cells were isolated from spleen (left panel) or tumor (right panel) after poly I:C injection as described in a. c Spleen cells were prepared from B16 tumor-bearing mice treated with poly I:C or PBS for 8 h as described in a and surface expression of CD80, CD86, CD40, RAE-1, CD155, CD70, IL-15RA and CD150 on CD11b+Gr1+ cells was determined. d CD11b+Gr1+ cells were isolated from B16 tumor-bearing mice treated with poly I:C or PBS for 4 h as described in a and mRNA for IFN-α4, IL-15, IL-18, INAM and arginase-1 was measured (n = 3). Data shown are representative of at least 2 independent experiments. ** p < 0.01, * p < 0.05. NS = Not significant.
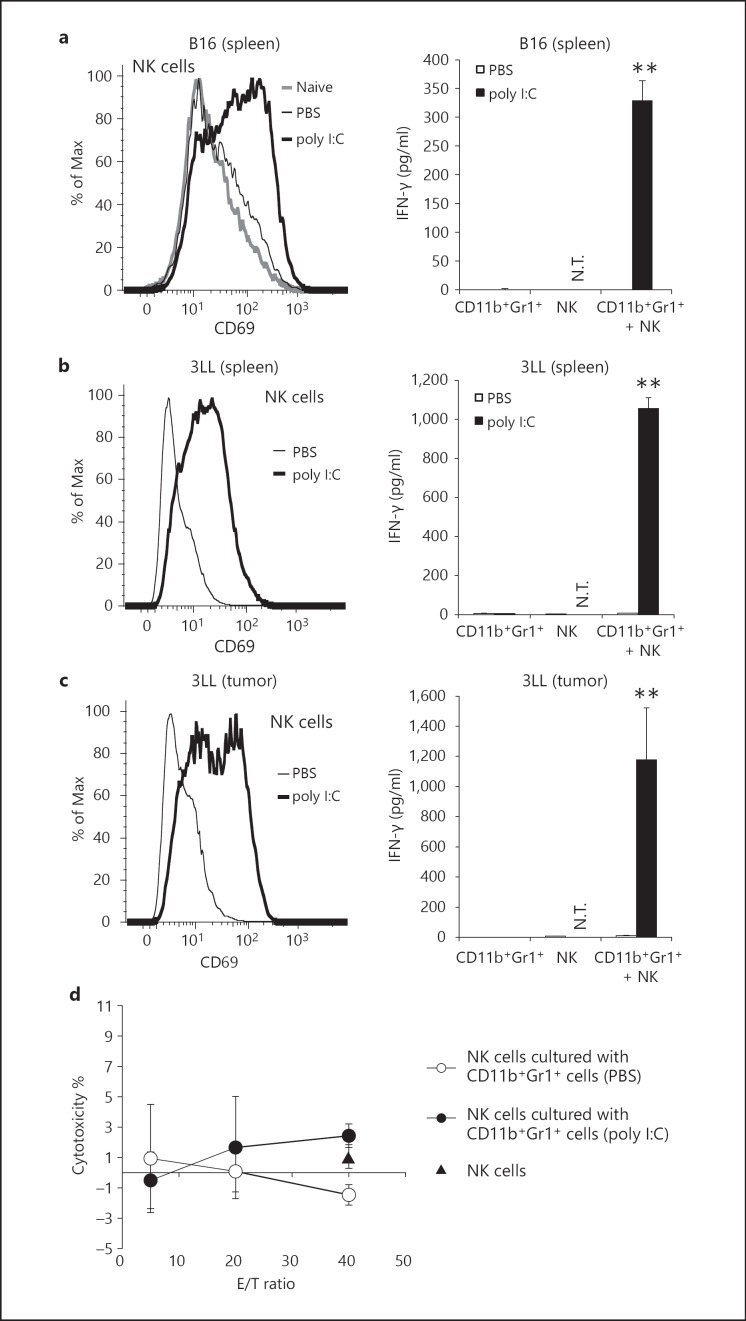
CD11b+Gr1+ Cells from Poly I:C-Treated Tumor-Bearing Mice Activate NK Cells
To investigate whether CD11b+Gr1+ cells from poly I:C-injected tumor-bearing mice are capable of activating NK cells, we isolated CD11b+Gr1+ cells from the spleens of tumor-bearing mice after poly I:C administration and cocultured the cells with NK cells from naïve mice. NK cells upregulated CD69 on their surface in response to the CD11b+Gr1+ cells from poly I:C-injected B16 tumor-bearing mice. However, the level of CD69 on NK cells was not changed when the cells were mixed with CD11b+Gr1+ cells from PBS-injected tumor-bearing mice (fig. 3a, left panel). CD11b+Gr1+ cells from poly I:C-injected tumor-bearing mice also induced NK cell IFN-γ production (fig. 3a, right panel). Similar results were obtained with NK cells cocultured with CD11b+Gr1+ cells from mice bearing 3LL- or EL4 cell tumors after poly I:C treatment (fig. 3b, c; online suppl. fig. 2b, c). Furthermore, CD11b+Gr1+ cells from tumors of 3LL-implant mice had a similar ability to induce IFN-γ production and CD69 expression in NK cells after poly I:C treatment (fig. 3c). IFN-γ inhibits proliferation of B16 cells in vitro without affecting the cell viability (online suppl. fig. 3; data not shown). In contrast, CD11b+Gr1+ cells did not drive a cytotoxic phenotype from NK cells (fig. 3d). In vitro stimulation of CD11b+Gr1+ cells with poly I:C did not induce NK cytotoxicity in coculture (data not shown). These results demonstrated that when poly I:C was injected into tumor-bearing mice, CD11b+Gr1+ cells acquired the ability to prime NK cells as measured by CD69 expression and IFN-γ production, but did not induce cytotoxic activity.
Fig. 3.
NK cells are primed by CD11b+Gr1+ cells isolated from poly I:C-injected tumor-bearing mice. a-c CD11b+Gr1+ cells were isolated from spleens or tumors of B16 (a), 3LL (b, c) tumor-bearing mice pretreated with 200 µg poly I:C or PBS for 4 h and cultured with NK cells from naïve WT mice. After 24 h, CD69 expression on NK cells (a-c, left panels) and IFN-γ concentration in conditioned medium (a-c, right panels) were determined. CD69 expression of NK1.1+CD3ε- cells is indicated (a-c). N.T. = Not tested. d Cytotoxic activity of NK cells cocultured with or without CD11b+Gr1+ cells isolated from poly I:C- or PBS-treated tumor-bearing mice was determined by standard 51Cr release assay (n = 3). Triangle: NK cells not cultured with CD11b+Gr1+ cells. Data shown are representative of at least 3 independent experiments. ** p < 0.01.
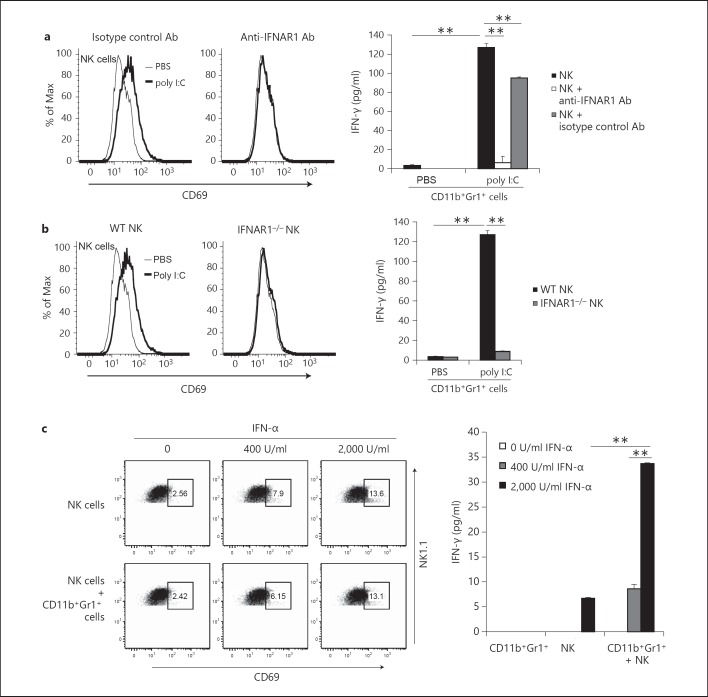
Type-I IFN Signaling Is Essential for NK Cell Priming by CD11b+Gr1+ Cells
Next, we investigated the mechanisms by which CD11b+Gr1+ cells primed NK cells through the in vivo administration of poly I:C. Soluble factors and membrane-associated molecules induced by poly I:C are reportedly involved in in vivo NK cell activation [15, 18]. As shown in figure 2a, CD11b+Gr1+ cells from poly I:C-treated tumor-bearing mice produced IFN-α. To examine whether type-I IFN signaling through IFNAR was involved in NK cell activation, we added anti-IFNAR1 antibodies to cultures to inhibit type-I IFN signaling by both CD11b+Gr1+ and NK cells that express IFNAR1. CD69 upregulation on NK cells and IFN-γ production induced by activated CD11b+Gr1+ cells were completely abrogated by anti-IFNAR1 antibodies (fig. 4a). These results suggest that type-I IFN signaling is essential for NK cell priming by CD11b+Gr1+ cells.
Fig. 4.
Type-I IFNs from CD11b+Gr1+ cells and IFNAR in NK cells are indispensable for NK cell activation. a CD11b+Gr1+ cells were isolated from spleens of B16 tumor-bearing mice treated with 200 µg poly I:C or PBS for 4 h and cultured with NK cells from naïve WT mice in the presence or absence of 10 µg/ml anti-IFNAR1 antibody (Ab). After 24 h, CD69 expression on NK cells (left panels) and IFN-γ concentration in conditioned medium (right panel) was determined (n = 3). b CD11b+Gr1+ cells isolated as described in a were cultured for 24 h with NK cells from naïve WT mice or IFNAR1−/− mice, and CD69 expression (left panels) and IFN-γ production were measured (n = 3) (right panel). c Recombinant IFN-α was added to cultures of naïve NK cells with or without CD11b+Gr1+ cells from nontreated tumor-bearing mice. After incubation for 24 h, CD69 expression on NK cells (left panels) and IFN-γ concentration in conditioned medium (right panel) were determined (n = 3). CD69 expression of NK1.1+CD3ε- cells is indicated (a-c). Data shown are representative of two independent experiments. ** p < 0.01.
To investigate type-I IFN signaling in NK cell priming, we prepared NK cells from IFNAR1−/− mice. IFNAR1−/− NK cells were cocultured with CD11b+Gr1+ cells. CD11b+Gr1+ cells from poly I:C-stimulated WT mice stimulated CD69 expression and IFN-γ production by WT NK cells but not IFNAR1−/− NK cells (fig. 4b). Therefore, type-I IFN signaling in NK cells is essential for NK priming by CD11b+Gr1+ cells.
To examine whether IFNAR signaling is the only route for the induction of CD11b+Gr1+ cell-mediated NK priming, we added recombinant mouse IFN-α to cultures of NK cells or to cocultures of untreated CD11b+Gr1+ cells and WT NK cells. Recombinant mouse IFN-α in NK cell cultures resulted in induction of CD69 expression on the NK cells (fig. 4c, left panels). However, CD69 expression was minimally augmented in the NK cells cocultured with CD11b+Gr1+ cells. In contrast, NK cell IFN-γ production was clearly induced at high concentrations of IFN-α (2,000 IU/ml) and augmented by CD11b+Gr1+ cells (fig. 4c, right panel).
We investigated if cell-cell contact is involved in NK cell activation in cocultures of CD11b+Gr1+ cells and naïve NK cells using the Transwell system. Sufficient NK cell priming was detected when NK cells were cocultured with in vivo poly I:C-activated CD11b+Gr1+ cells. However, expression of CD69 and production of IFN-γ by NK cells was abrogated by separation of the cells by the Transwell membrane (online suppl. fig. 4a, b). These results, together with the results in figure 4, suggest that two modes of NK priming occur simultaneously in CD11b+Gr1+ cells: one mode is through type-I IFN production and the other is via cell-cell contact.
MAVS and IFNAR Are Required to Activate MDSCs with in vivo Poly I:C Treatment
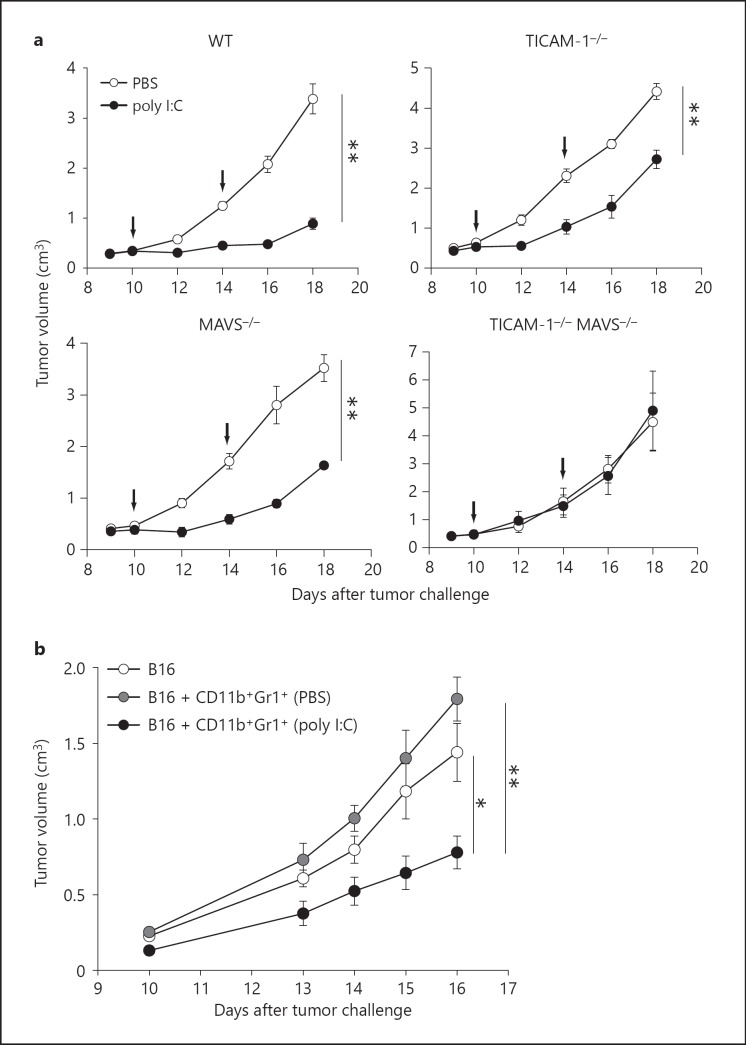
Poly I:C induces growth retardation of B16 tumors implanted in WT mice [4, 5, 6, 36]. To determine the signaling pathway that is essential for the retardation of B16 tumor growth in vivo, we implanted B16 melanoma cells s.c. into TICAM-1−/− and MAVS−/− mice. B16 tumor growth was monitored after poly I:C injection. Marked tumor growth retardation was observed in poly I:C-treated mice (fig. 5a). The poly I:C antitumor effect was only partly abrogated in either TICAM-1−/− or MAVS−/− mice and was completely abolished in TICAM-1−/−/MAVS−/− mice (fig. 5a). Therefore, both TICAM-1 and MAVS signals are involved in the antitumor activity of poly I:C, consistent with earlier reports [4, 5, 33].
Fig. 5.
Retardation of B16 tumor growth by poly I:C treatment in mouse models. a Both TICAM-1 and MAVS signals are involved in B16 tumor growth retardation after poly I:C therapy. B16 cells (6 × 105) were implanted s.c. into WT, TICAM-1−/−, MAVS−/− and TICAM-1 and MAVS double-knockout mice. Tumor-bearing mice were treated with 200 µg poly I:C or PBS on days 10 and 14 (arrows) (n = 3-5 per group). Data are average ± SEM. b In vivo poly I:C-activated CD11b+Gr1+ cells inhibit B16 tumor growth. B16 cells (6 × 105) were mixed with or without CD11b+Gr1+ cells (1 × 106) from spleens of B16 tumor-bearing mice treated with 200 µg poly I:C or PBS for 4 h. Cell mixtures were implanted s.c. into WT mice on day 0 (n = 4 per group). Data are average ± SEM and are representative of 2 independent experiments. ** p < 0.01, * p < 0.05.
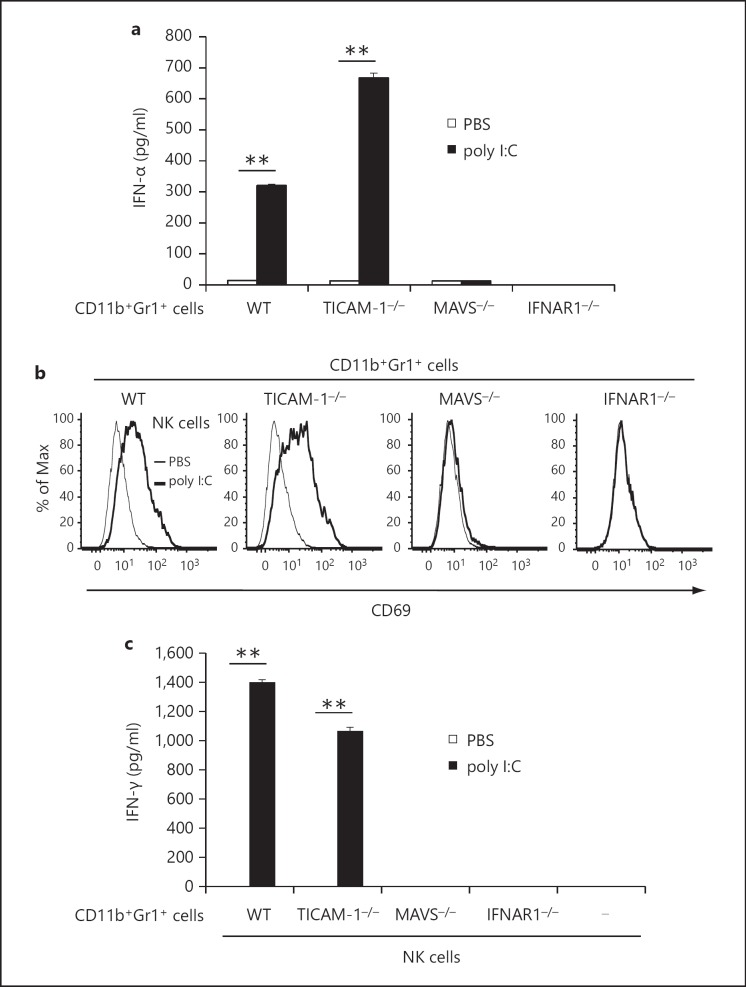
Next, we determined the mechanisms involved in the in vivo activation of CD11b+Gr1+ cells by poly I:C. To investigate the signaling pathway that was important for poly I:C-induced activation of CD11b+Gr1+ cells in vivo, we challenged TICAM-1−/− and MAVS−/− mice with B16 melanoma cells. After tumor formation, poly I:C was injected i.p. into the mice and CD11b+Gr1+ cells were isolated from the spleen and were cocultured with naïve WT NK cells. CD11b+Gr1+ cells from tumor-bearing TICAM-1−/− mice produced IFN-α at levels comparable to cells from WT mice (fig. 6a). In parallel, CD69 expression on NK cells and IFN-γ production was observed in conditioned medium from mixed cultures of TICAM-1−/− CD11b+Gr1+ cells and naïve WT NK cells. The results suggest that in vivo TICAM-1 signaling is not mandatory for CD11b+Gr1+ cell activation to induce NK cell priming (fig. 6a-c). CD11b+Gr1+ cells from tumor-bearing MAVS-/- mice treated with poly I:C did not produce IFN-α or induce CD69 expression and IFN-γ production in NK cells (fig. 6a-c). Similar results were obtained with CD11b+Gr1+ cells from B16 tumor-bearing IFNAR1−/− mice (fig. 6a-c). These results suggest that MAVS as well as type-I IFN signaling is crucial for poly I:C-dependent NK cell priming in CD11b+Gr1+ cells of tumor-bearing mice.
Fig. 6.
MAVS and type-I IFN signaling pathways are critical for CD11b+Gr1+ cell activation in vivo. a B16 cells (6 × 105) were implanted into WT, TICAM-1−/−, MAVS-/- or IFNAR1−/− mice. Tumor-bearing mice were treated with 200 µg poly I:C or PBS for 4 h and CD11b+Gr1+ cells were isolated from spleens, and allowed to stand for 24 h. IFN-α concentration in conditioned medium was determined (n = 3). b, c CD11b+Gr1+ cells were isolated from KO mouse lines as described in a, and were cultured with naïve WT NK cells for 24 h. CD69 expression on NK cells (b) and IFN-γ concentration in conditioned medium (c) were determined (n = 3). Data shown are representative of 3 independent experiments. ** p < 0.01.
The MAVS pathway is conserved in most cell types in mice. We examined whether NK cell priming induced by poly I:C (i.e. MAVS signal)-activated CD11b+Gr1+ cells was involved in retardation of B16 tumor growth. NK-sensitive B16 tumor cells were mixed with CD11b+Gr1+ cells isolated from poly I:C (or control PBS)-injected tumor-bearing mice, and inoculated s.c. into WT mice (fig. 5b). Significant B16 growth retardation was detected only in those tumors containing poly I:C-treated CD11b+Gr1+ cells (fig. 5b). The B16 tumors with intact CD11b+Gr1+ cells showed higher growth rates than B16 tumor cells only, which might reflect the previously-reported tumor-supporting activity of MDSCs [37]. Thus, MDSC-like CD11b+Gr1+ cells can be converted to cells with an NK-priming activity that induces growth retardation of NK-sensitive tumors in mice. NK-priming would be a condition prior to full activation of antitumor NK cells.
Discussion
We demonstrated that in vivo poly I:C treatment led to CD11b+Gr1+ MDSC maturation and cytokine production in tumor-bearing mice. Poly I:C treatment rendered tumor and spleen MDSCs competent for DX5+ NK cell priming as measured by CD69 expression and IFN-γ production. Poly I:C-dependent NK priming raises through the MAVS pathway (fig. 6). Among a number of proteins that were upregulated after poly I:C treatment, type-I IFN produced by activated MDSCs was critical for NK cell priming since it activated the IFNAR pathway in NK cells. However, NK cells barely exerted direct cytotoxic activity to B16 cells in response to poly I:C-matured MDSCs. This NK activation profile resembles that of IFN-γ-producing innate lymphoid cells. Some populations of these innate lymphocytes produce IFN-γ but exhibit little cytotoxic activity [38], similar to the NK cells affected by MDSC. These findings would allow us to speculate that the production of IFN-γ without cytotoxic activity is an activation state of NK cells or innate lymphocytes where MDSCs contribute.
In tumor-bearing hosts, poly I:C treatment resulted in tumor regression. Poly I:C induces direct killing of 3LL tumor cells by M2-M1 conversion of tumor-associated macrophages [7]. The TICAM-1 signal facilitates tumor-associated macrophage conversion as well as cross-presentation by DCs leading to antigen-specific CTL induction, which is also evoked by poly I:C [8]. The action of CD11b+Gr1+ MDSCs on implant B16 tumor was tumor-supporting when MDSCs were embedded into the tumor (fig. 5b). However, once MDSCs were pretreated with poly I:C and mixed with B16 cells, tumor growth was prohibited (fig. 5b). The result suggests that MDSC has plasticity to change the function from tumor-supporting to tumor-suppressing even in vivo. Here, we highlight the first evidence of MDSCs to evoke NK cell priming, which ultimately associates with retardation of tumor growth.
Although the exact mechanism of tumor regression by MDSC-NK activation remains to be elucidated, we speculate that IFN-γ produced by the primed NK cells could evoke antitumor activity. One possibility is that IFN-γ directly inhibits the growth of a certain tumor line including B16 melanoma by inducing cell cycle arrest. IFN-γ has a synergistic effect on type-I IFNs, arresting cell cycle to cell death in some tumor cell lines independent of p53 [39]. IFN-γ also induces angiostasis, which prevents rapid tumor progression [19], and inhibits metastasis and proliferation of B16 melanoma [17, 20]. In fact, we observed that IFN-γ directly inhibits proliferation of B16 cells in vitro, suggesting that NK cell-derived IFN-γ might inhibit B16 tumor growth during poly I:C treatment (online suppl. fig. 3).
Retardation of B16 growth was partially abrogated in TICAM-1−/− or MAVS−/− mice and completely abrogated in TICAM-1−/−, MAVS−/− double KO mice (fig. 5a), the two pathways contributing to in vivo poly I:C-derived tumor suppression. In addition, MDSC activation is completely abrogated in IFNAR1−/− mice and IFNAR in NK cells is involved in efficient IFN-γ production induced by activated MDSCs. Type-I IFN receptor signaling is crucial for growth retardation of B16 tumor in poly I:C therapy. Therefore, a variety of situations result in NK activation/priming in the therapeutic use of poly I:C in tumor-bearing mice, although IFNAR is the common factor.
Miyake et al. [5], reported that MAVS is responsible for NK cell-dependent tumor regression using MAVS−/− mice with poly I:C stimulation; however, the cell types for the poly I:C response and the mechanism of induction of NK-sensitive tumor regression remain undefined. NK-activating ligands on the DC surface as well as soluble factors induced by IRF-3 and IFNAR stimulation are crucial for DC-mediated NK cell activation [15, 32]. In addition, MDSC is a cell type that specifically drives NK priming through the MAVS pathway (fig. 6). MAVS-dependent IRF-3 activation occurs through stromal cells other than DCs, and these cells including MDSCs participate in NK-sensitive tumor regression. The result of this MDSC function is in contrast to that of DCs where the TLR3/TICAM-1 pathway preferentially promotes NK cell activation [4]. Specific depletion of MDSCs in preformed tumors, if possible, would enable us to confirm the functional importance of MAVS in tumor regression reported by Miyake et al. [5], who postulated that stromal cells were a source of the cell type that induces MAVS-mediated NK priming. However, the function of poly I:C via the MDSC-NK cell pathway is only a part of the total antitumor activity of poly I:C, which would be difficult to detect by tumor-size reduction. MDSCs are expanded in a late phase of implant tumor, where poly I:C may act on MDSCs and exert antitumor activity via poly I:C-stimulated MDSCs.
A recent report suggested that type-I IFNs act on accessory cells such as DCs, leading to the production of IL-15, IL-12, IL-18 and NKG2D ligands such as RAE-1. IFN-α acted on NK cells and slightly induced IFN-γ production, which was augmented in the presence of MDSCs. Cell-cell interaction between MDSCs and NK cells appears to be required for robust IFN-γ production. Induction of NK-activating ligands in association with natural NK cytotoxicity is involved in cell-cell contact-mediated NK cell activation. We found mRNA for downstream genes of IRF-3, especially IL-15, IL-18, INAM and RAE-1 elevated in MDSCs after treatment with poly I:C. However, IL-15 and RAE-1 appear not to participate in IFN-γ production by NK cells with MDSCs because neutralizing antibodies to IL-15, RAE-1 or NKG2D did not inhibit IFN-γ production by NK cells. Therefore, other molecules should be involved in NK cell activation by MDSCs. In fact, INAM or other molecules expressed on the MDSC surface sustain NK cell activation following poly I:C treatment (fig. 2; data not shown) as in bone marrow-derived cells [32].
MDSCs that have expanded in tumor-bearing hosts strongly suppress antitumor immune responses [22, 23]. Reduction of MDSC population or function is achieved by treatment with reagents that are related to improvement in tumor-specific immunity [40]. Furthermore, maturation of MDSCs can be accomplished through IFN-α production by plasmacytoid DCs or direct TLR9 stimulation, which contributes to tumor regression [30, 31]. Direct administration of IFN-α, i.e. IFN therapy, however, has not been successful as a universal therapy in cancer patients. Serious side effects are associated with high therapeutic doses of type-I IFN as well as poly I:C. Development of less toxic reagents with sufficient IRF-3/7 activation would be important for anti-MDSC cancer therapy.
Supplementary Material
Supplementary data
Acknowledgements
We thank Drs. H. Takaki, J. Kasamatsu, K. Funami, M. Tatematsu and M. Azuma in our laboratory for their fruitful discussions. This work was supported in part by Grants-in-Aids from the Ministry of Education, Science and Culture (specified project for advanced research) and the Ministry of Health, Labor and Welfare of Japan as well as by the Akiyama Life Science Foundation (H.S.), the Takeda Science Foundation (H.S.), the Yasuda Cancer Foundation (T.S.) and the Ono Foundation (T.S.). Financial support by a MEXT Grant-in-Project ‘the Carcinogenic Spiral’, ‘the National Cancer Center Research and Development Fund (23-A-44)’, and the Japan Initiative for Global Research Network on Infectious Diseases is gratefully acknowledged.
References
- 1.Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, et al. Trial watch: experimental Toll-like receptor agonists for cancer therapy. Oncoimmunol. 2012;1:699–716. doi: 10.4161/onci.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 3.Seya T, Shime H, Ebihara T, Oshiumi H, Matsumoto M. Pattern recognition receptors of innate immunity and their application to tumor immunotherapy. Cancer Sci. 2010;101:313–320. doi: 10.1111/j.1349-7006.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akazawa T, Ebihara T, Okuno M, Okuda Y, Shingai M, Tsujimura K, et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci USA. 2007;104:252–257. doi: 10.1073/pnas.0605978104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyake T, Kumagai Y, Kato H, Guo Z, Matsushita K, Satoh T, et al. Poly I:C-induced activation of NK cells by CD8 alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J Immunol. 2009;183:2522–2528. doi: 10.4049/jimmunol.0901500. [DOI] [PubMed] [Google Scholar]
- 6.Salem ML, El-Naggar SA, Kadima A, Gillanders WE, Cole DJ. The adjuvant effects of the Toll-like receptor 3 ligand polyinosinic-cytidylic acid poly (I:C) on antigen-specific CD8+ T cell responses are partially dependent on NK cells with the induction of a beneficial cytokine milieu. Vaccine. 2006;24:5119–5132. doi: 10.1016/j.vaccine.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Shime H, Matsumoto M, Oshiumi H, Tanaka S, Nakane A, Iwakura Y, et al. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc Natl Acad Sci USA. 2012;109:2066–2071. doi: 10.1073/pnas.1113099109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azuma M, Ebihara T, Oshiumi H, Matsumoto M, Seya T. Cross-priming for antitumor CTL induced by soluble Ag + poly I:C depends on the TICAM-1 pathway in mouse CD11c+/CD8α+ dendritic cells. Oncoimmunol. 2012;1:581–592. doi: 10.4161/onci.19893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauzzi MC, Del Corno M, Gessani S. Dissecting TLR3 signalling in dendritic cells. Immunobiology. 2010;215:713–723. doi: 10.1016/j.imbio.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-β induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 12.Seth RB, Sun L, Ea C-K, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Honda K, Takaoka A, Taniguchi T. Type I inteferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 15.Seya T, Kasamatsu J, Azuma M, Shime H, Matsumoto M. Natural killer cell activation secondary to innate pattern sensing. J Innate Immun. 2011;3:264–273. doi: 10.1159/000326891. [DOI] [PubMed] [Google Scholar]
- 16.Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, et al. NK cells and cancer. J Immunol. 2007;178:4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- 17.Takeda K, Nakayama M, Sakaki M, Hayakawa Y, Imawari M, Ogasawara K, et al. IFN-γ production by lung NK cells is critical for the natural resistance to pulmonary metastasis of B16 melanoma in mice. J Leukoc Biol. 2011;90:777–785. doi: 10.1189/jlb.0411208. [DOI] [PubMed] [Google Scholar]
- 18.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, et al. IFN-γ-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, α-galactosylceramide. Blood. 2002;100:1728–1733. [PubMed] [Google Scholar]
- 20.Kakuta S, Tagawa Y-I, Shibata S, Nanno M, Iwakura Y. Inhibition of B16 melanoma experimental metastasis by interferon-gamma through direct inhibition of cell proliferation and activation of antitumour host mechanisms. Immunology. 2002;105:92–100. doi: 10.1046/j.0019-2805.2001.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman K, Riley E. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, et al. Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci USA. 2012;109:2491–2496. doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-β1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 27.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived ‘suppressor’ cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoglmeier C, Bauer H, Nörenberg D, Wedekind G, Bittner P, Sandholzer N, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17:1765–1775. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 31.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K, et al. Identification of a poly(I:C)-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J Exp Med. 2010;207:2675–2687. doi: 10.1084/jem.20091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, et al. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009;206:2967–2976. doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K, Oshima H, Hayakawa Y, Akiba H, Atsuta M, Kobata T, et al. CD27-mediated activation of murine NK cells. J Immunol. 2000;164:1741–1745. doi: 10.4049/jimmunol.164.4.1741. [DOI] [PubMed] [Google Scholar]
- 35.Chan CJ, Andrews DM, Mclaughlin NM, Yagita H, Gilfillan S, Colonna M, et al. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol. 2010;184:902–911. doi: 10.4049/jimmunol.0903225. [DOI] [PubMed] [Google Scholar]
- 36.Navabi H, Jasani B, Reece A, Clayton A, Tabi Z, Donninger C, et al. A clinical grade poly I:C-analogue (Ampligen) promotes optimal DC maturation and Th1-type T cell responses of healthy donors and cancer patients in vitro. Vaccine. 2009;27:107–115. doi: 10.1016/j.vaccine.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 38.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells - a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 39.Arany I, Fleischmann CM, Tyring SK, Fleischmann WR. Interferon regulates expression of mda-6/WAF1/CIP1 and cyclin-dependent kinases independently from p53 in B16 murine melanoma cells. Biochem Biophys Res Commun. 1997;233:678–680. doi: 10.1006/bbrc.1997.6516. [DOI] [PubMed] [Google Scholar]
- 40.Ugel S, Delpozzo F, Desantis G, Papalini F, Simonato F, Sonda N, et al. Therapeutic targeting of myeloid-derived suppressor cells. Curr Opin Pharmacol. 2009;9:470–481. doi: 10.1016/j.coph.2009.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data