Abstract
Polymorphonuclear neutrophils (PMN) are the most abundant circulating leukocytes. They represent a first line of innate immunity against a large panel of microbial pathogens, pending development of specific immune responses. The role of PMN in human immunodeficiency virus type 1 (HIV-1) disease has mainly been investigated from the point of view of the increased susceptibility of HIV-1-infected patients to bacterial and fungal infections. However, it is now clear that the relationship between PMN and HIV-1 is far more complex. This review examines both the beneficial and the detrimental effects of PMN during HIV infection.
Key Words: Human immunodeficiency virus, Neutrophils, Virus-host cell interactions, Inflammatory diseases
Introduction
Polymorphonuclear neutrophils (PMN) are the most abundant blood leukocytes and are key components of the early innate response to bacterial and fungal pathogens. Several lines of evidence also suggest a key role of PMN in controlling viruses [1, 2, 3]. In response to pathogens, PMN rapidly migrate from the blood to inflamed tissues, where their activation triggers microbicidal mechanisms such as the release of proteolytic enzymes and antimicrobial peptides, and rapid production of reactive oxygen species (ROS) in an ‘oxidative burst’. PMN are usually short-lived cells, dying spontaneously by necrosis or apoptosis, and apoptotic PMN are recognized and phagocytosed by tissular macrophages. PMN activation by circulating microbial products, endogenous cytokines, and other proinflammatory mediators increases their lifespan and is critical for their antimicrobial efficacy. A newly identified form of ROS-dependent death, distinct from necrosis and apoptosis, leads to the generation of neutrophil extracellular traps (NETs), which are also crucial for PMN antimicrobial activity [4].
Human immunodeficiency virus type 1 (HIV-1) establishes persistent infection in humans. The pathogenesis of HIV infection is highly complex and involves numerous components of the immune system. In particular, the virus targets and replicates inside CD4+ T cells. Infection of CD4+ T cells and their resulting depletion is the main mechanism of HIV pathogenesis, as the immunodeficiency it causes exposes patients to an escalating risk of opportunistic infections. Antiretroviral therapy (ART) prevents AIDS-related complications and prolongs patients' life expectancy. However, despite sustained viral suppression by ART, HIV-infected patients still have a higher basal level of immune activation than do healthy individuals [5], an abnormality implicated in non-AIDS-defining comorbidities such as osteoporosis, atherosclerosis, neurocognitive decline, and premature aging [6, 7, 8]. B cells, T cells, and natural killer cells also play a key role in controlling HIV replication.
The role of PMN in HIV-1 disease has mainly been examined from the point of view of patients' increased susceptibility to bacterial and fungal infections. HIV-1 does not infect PMN but leads to impaired PMN responses (phagocytosis, oxidative burst, and bacterial killing) and a higher rate of apoptosis [9, 10, 11, 12, 13, 14, 15]. The immune defects associated with HIV infection, and especially CD4 cell depletion, are largely corrected by ART [11].
It is now becoming clear that PMN can have both beneficial and detrimental effects in HIV infection. For example, PMN play an active role in controlling HIV-1, whereas PMN to which HIV particles have bound may provoke further mucosal inflammation and thereby enhance HIV-1 transmission [16].
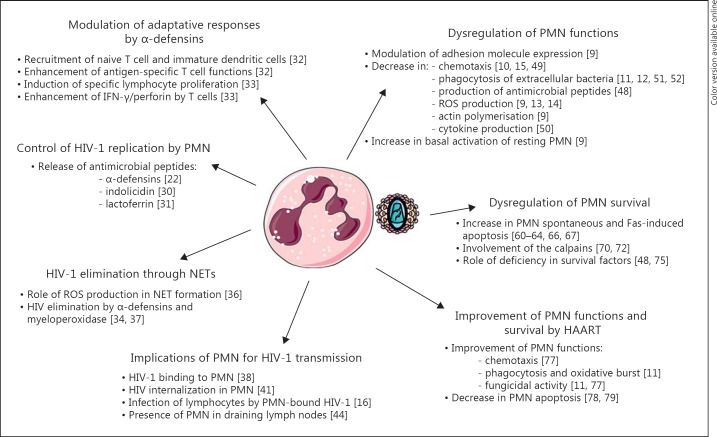
This article reviews recent findings on the beneficial and detrimental consequences of PMN interactions with HIV (fig. 1).
Fig. 1.
Summary of the different facets of the interactions between PMN and HIV-1, i.e. the role of PMN in the control of HIV-1 infection, the role of PMN in HIV-1 transmission, the dysregulation of PMN functions and survival in HIV-infected patients, and the effects of HAART on PMN functions and survival.
Active Role of PMN in the Control of HIV-1 Infection
Involvement of PMN Products in the Control of HIV Infection
Control of HIV Replication. Although PMN-derived mediators such as ROS, TNF-α, and IL-8 have been reported to enhance HIV-1 replication [17, 18], an active role of PMN in controlling HIV-1 replication through the release of defensins has also emerged. Human α-defensins are cysteine-rich cationic peptides with antimicrobial activity. Humans express 6 α-defensins: proteins 1-4 are produced by PMN, while defensins 5 and 6 are produced by Paneth cells. The anti-HIV-1 activity of α-defensins has been actively studied since Zhang et al. [19] first reported that HNP-1, HNP-2, and HNP-3 were the main components of the CD8+ cell-derived soluble antiviral factor. However, subsequent studies showed that HNP-1-3 are not produced by CD8 cells and that their presence is due to contamination with PMN [20, 21]. These α-defensins have been implicated in several steps of the HIV-1 replication cycle, notably blocking both virus entry into target cells and HIV-mediated fusion by inhibiting viral envelope glycoprotein gp120 binding to CD4 receptors and coreceptors [22, 23, 24], and by downregulating CD4 and CXCR4 expression [22, 23, 25]; α-defensins also inhibit HIV-1 replication at the reverse transcription and integration steps [23, 24, 25]. α-Defensins have been reported to inhibit HIV-1 infection by upregulating chemokine expression and secretion [26] and to directly inactivate HIV-1 virions in serum-free medium [24].
Increased α-defensin levels have been reported in HIV-infected patients [27], notably in breast milk, and play a role in preventing HIV transmission [28]. This α-defensin upregulation in PMN, which is not modified by ART, has been linked to the chronic immune activation seen in HIV infection [27].
No human θ-defensins have been isolated to date, but humans have 3 θ-defensin pseudogenes that contain premature stop codons. In nonhuman primates, θ-defensins have been isolated from PMN and from bone marrow. Naturally occurring and synthetic θ-defensins have anti-HIV-1 activity. θ-Defensins interact with viral gp41, thus preventing HIV env-mediated fusion with the cytoplasmic membrane [25, 29].
Finally, other antimicrobial peptides stored in PMN granules, such as indolicidin and lactoferrin, have been reported to exhibit potent inhibitory activities against HIV-1 [30, 31].
Modulation of Adaptive Responses. In addition to their direct role in host defense against microbial infection, α-defensins are thought to contribute to adaptive immunity. Indeed, α-defensins exhibit immunostimulatory activities, including recruitment of naive T cells and immature dendritic cells to sites of infection, as well as enhancement of antigen-specific T cell functions [32]. A recent study showed that defensins induce specific lymphocyte proliferation to HIV antigens and enhance IFN-γ/perforin secretion by CD4+/CD8+ T cells [33].
Involvement of PMN in HIV-1 Elimination through NETs
Activated PMN produce NETs - extracellular structures composed of genomic DNA and histones/chromatin released during PMN death - that capture and kill invading bacteria and fungi [4]. Saitoh et al. [34] recently demonstrated that NETs can also capture HIV-1 and promote HIV-1 elimination. PMN detect HIV-1 via Toll-like receptor (TLR)-7 and TLR-8, which recognize viral nucleic acids [35]. Engagement of TLR-7 and TLR-8 in PMN generates ROS production that triggers NET formation and promotes HIV-1 elimination through the actions of α-defensins and myeloperoxidase [4, 36, 37]. However, HIV has developed mechanisms to counteract this PMN antiviral response. For example, HIV-1 stimulates IL-10 production by dendritic cells via DC-SIGN and thereby suppresses TLR-mediated NET formation [34].
HIV-1 Binds to PMN: Implications for HIV-1 Transmission
Binding of the HIV envelope to CD4 is followed by its binding to other coreceptors, namely the chemokine receptors CCR5 and CXCR4, followed by membrane fusion and cell entry. One study has shown unconventional CD4 expression on the surface of peripheral blood PMN in some HIV-1-infected patients and uninfected individuals. The molecular conformation of PMN-expressed CD4 is very similar to that of CD4 expressed on the surface of lymphocytes, and CD4+ PMN have been shown to bind HIV-1 gp120 [38]. HIV-1 binding has also been reported on the surface of CD4-negative PMN, independently of gp120 [16]. In addition, PMN constitutively express CXCR4 and may selectively bind X4 strains of HIV [39]. FPLRI, a formyl peptide receptor belonging to the 7-transmembrane G protein-coupled receptor family, has also been reported to be expressed on the PMN surface and to be an efficient coreceptor for HIV-1 primary isolates [40]. HIV internalization by PMN can also occur through phagocytosis after opsonization with specific anti-HIV antibodies present after the seroconversion phase, and through binding to Fcγ receptors on the PMN surface [41].
As PMN are the most abundant peripheral leukocytes, HIV binding to the PMN surface might favor the spread of HIV. PMN-bound HIV-1 virions have been shown to infect activated PBMC and to transfer the infection to lymphocytes more efficiently than free HIV-1 [16, 42]. Furthermore, HIV binding to PMN is enhanced by inflammatory stimuli produced by T lymphocytes, such as TNF-α, in turn increasing the rate of PBMC infection [16]. Interestingly, sustained contact between viable HIV-1-bearing PMN and CD4 lymphocytes may be facilitated through GM-CSF production by HIV-1-infected PBMC, thus prolonging PMN survival and increasing the percentage of HLA-DR+ PMN [43]. PMN are abundant in the inflamed oral and genital mucosae and have also been observed in draining lymph nodes of HIV-1-infected patients [44]. Thus, HIV-1 binding to human PMN and the ability of PMN-bound HIV-1 to infect activated PBMC represent an additional mechanism by which mucosal inflammation may enhance HIV-1 transmission [45].
Dysregulation of PMN Functions and Apoptosis in HIV-Infected Patients
PMN Functional Abnormalities in HIV-Infected Patients
Although some authors have reported an increase in PMN phagocytosis, ROS production, and intracellular bactericidal activity in HIV-infected patients as compared to healthy individuals [46, 47], there is general agreement that PMN from HIV-infected patients, and particularly those with AIDS, exhibit a variety of functional defects resulting in impaired bacterial and fungal killing [9, 10, 11, 12, 13, 14, 15]. These defects are not always accompanied by neutropenia or other leukopenias. They result in increased susceptibility to gram-negative and gram-positive bacterial infections and mycoses, and are likely to be a major contributor to the increased morbidity/mortality associated with HIV infection.
PMN activities negatively affected by HIV infection include the regulation of adhesion molecule expression [9], production of antimicrobial peptides such as leukotrienes [48], chemotaxis [10, 15, 49], phagocytosis of extracellular bacteria [11, 12], ROS production [9, 13, 14], actin polymerization [9], and cytokine production [50].
The mechanisms underlying PMN functional defects in HIV-infected patients are not fully understood but could be related to a direct effect of HIV or HIV proteins on PMN. HIV alters FcyR-mediated phagocytosis by downmodulating the γ signaling chain of FcγR [51] and HIV inhibits the formation of the phagosome through Nef-dependent alteration of recycling endosomal compartment membranes [52]. Continued exposure to HIV or viral products such as Tat protein reduces the expression of chemotactic receptors on the PMN surface [53]. An inappropriate cellular distribution of the PMN NADPH oxidase complex, which is the main source of superoxide anion, has also been described in HIV-infected patients [14], although the mechanism remains unknown.
Defective PMN responses could also be related to developmental defects during hematopoiesis. This has been implicated in the profound alteration of PMN chemotaxis observed in HIV-infected patients, a defect independent of neutropenia and associated with decreased expression of chemotactic receptors [15]. Potential mechanisms include an altered bone marrow cytokine environment, direct suppression of hematopoiesis by viral proteins, myelosuppressive effects of antiretroviral drugs, bone marrow infection by viruses, bacteria, or fungi, and, possibly, HIV infection of hematopoietic stem cells.
An altered plasma cytokine environment might play a role in some PMN abnormalities. In particular, a decrease in IL-15 production has been reported in untreated HIV-infected patients and in patients with ART failure as compared to healthy subjects [54]. Interestingly, IL-15 has been shown to enhance PMN functions in vitro [55] and to induce monocytes to produce IL-8, a chemokine specifically involved in PMN recruitment [54].
Finally, untreated HIV-infected patients exhibit increased basal activation of resting PMN, as reflected by increased CD11b and decreased L-selectin expression, increased basal actin polymerization, and increased ROS production, which could explain the reduced capacity of PMN to respond to stimulation [9]. Basal PMN activation may be related at least in part to increased plasma levels of extracellular mitochondrial DNA released from damaged or dead cells [56]. In addition, a significant loss of Th17 cells and an increase in regulatory T cells (Tregs) has been reported in patients with progressive HIV disease, while ART treatment partially normalizes the Th17/Treg ratio [57]. Perturbations of Th17 cells during HIV infection could compromise mucosal defenses against resident and pathogenic microbes. The loss of Th17 cells observed during HIV infection might also contribute to microbial translocation of bacterial products from mucosal tissues, resulting in systemic immune activation [5, 58, 59].
Increased PMN Apoptosis in HIV-Infected Patients
PMN are unique in their susceptibility to undergo rapid spontaneous apoptosis once released from the bone marrow, resulting in their clearance from the circulation within a few hours. Two major pathways that regulate apoptosis have been documented in various cell types, including PMN. The first depends on so-called death receptors such as TNFR and Fas (CD95) that can directly trigger a caspase cascade via activation of caspase-8, an initiator caspase. The second, called the intrinsic apoptosis pathway, involves mitochondria and Bcl-2 family members and results in caspase-9 activation.
PMN apoptosis is accelerated as soon as the early stages of untreated HIV infection [60, 61, 62, 63, 64]. Downregulation of proinflammatory capacity has also been reported during PMN apoptosis [65]. This increased PMN apoptosis might be involved in the PMN functional impairment observed in HIV-infected patients. Shortened PMN survival due to apoptosis could also contribute to the neutropenia observed in the later stages of HIV infection [66, 67].
Some authors observed increased spontaneous PMN apoptosis in HIV-infected patients but found no correlation with viral load [60, 61, 64]. This spontaneous PMN death is dependent on caspase-3 but independent of caspase-8, suggesting that the intrinsic pathway is involved in PMN death. Spontaneous death of PMN from HIV-infected patients was also reduced upon incubation with catalase/SOD, which is known to reduce ROS levels, suggesting that the underlying mechanism may be related to increased basal ROS production [63]. Several studies suggest that ROS affect the intrinsic apoptotic pathway, probably by targeting mitochondria. ROS might also promote apoptosis by interfering with the activation of survival pathways mediated by NF-κB and MAPKs [68]. This process might affect the ERK [61] and P38 MAPK [63] pathways, resulting in a PMN apoptosis/survival imbalance.
Salmen et al. [61] reported increased PMN susceptibility to apoptosis following Fas cross-linking and found that this correlated with both viral load and coexpression of Fas/FasL surface molecules. HIV is not known to infect PMN but, like other bystander cells, PMN may be targeted by HIV proteins secreted by infected cells, and this could enhance their susceptibility to Fas-induced apoptosis. It has been reported that incubation of the cell line HL60 (PMN promyelocytic leukemia) with purified Nef induces apoptosis [69].
PMN apoptosis in untreated AIDS patients has also been correlated with increased activity of calpains [70], a family of noncaspase cysteine proteases involved in PMN apoptosis [71]. Increased PMN death during SIV infection is prevented by inhibiting calpain activation but not caspase activation [72].
Apoptosis is an intrinsic cellular process that can be regulated by external factors such as endogenous cytokines. Increased PMN apoptosis during HIV infection might thus be related to changes in circulating levels of various cytokines, some of which have a crucial role in PMN survival. In particular, immune activation in HIV infection is associated with increased levels of various proinflammatory mediators such as TNF-α. Although TNF-α, at low concentrations, has been shown to have an antiapoptotic effect on PMN [73], it can also trigger the death-receptor-dependent pathway via TRADD recruitment and subsequent caspase-8 activation. In addition, TNFRI signaling can result in sequential activation of p38 MAPK and class IA PI3Ks, leading to ROS production and subsequent activation of effector caspases via a novel caspase-8- and mitochondria-independent apoptotic pathway [74].
A deficiency in survival factors such as proinflammatory cytokines or colony-stimulating factors could also contribute to the accelerated PMN apoptosis observed in HIV infection. Indeed, G-CSF and GM-CSF administration can promote PMN survival in this setting [75] and also reverses neutropenia [48]. We found that PMN death was increased during the chronic phase of SIV infection in Asian rhesus macaques (RMs) and was significantly more frequent in RMs that progressed rapidly to AIDS [72]. Interestingly, levels of the inflammatory cytokines IL-8 and IL-1β, which prevent PMN death in vitro, were lower in RMs progressing towards AIDS than in nonprogressors.
SIV-infected RMs have been used to investigate the relationship between PMN susceptibility to death during the acute phase of infection and subsequent disease severity. We found that PMN death increased early during the acute phase of SIV infection in RMs, and that it was significantly more severe in RMs that progressed rapidly to AIDS and also coincided with neutropenia. In contrast, PMN death and PMN counts were not modified in African green monkeys, a nonpathogenic model of SIV infection, despite similar high-level viral replication [76]. These findings suggest that increased PMN apoptosis may play a key role in early viral replication and dissemination within the host.
Effects of Highly Active Antiretroviral Therapy on PMN Functions and Survival
The advent of highly active ART (HAART) has led to a significant decline in HIV-related morbidity and mortality. This standard treatment of HIV infection consists of various combinations of nucleoside reverse transcriptase inhibitors, nonnucleoside reverse transcriptase inhibitors, protease inhibitors, integrase inhibitors, and fusion inhibitors.
HAART significantly improves PMN functions, including phagocytosis, the oxidative burst [11], chemotaxis, and fungicidal activity against Candida albicans [77]. In addition, the larger the fall in viral load, the better the restoration of PMN functions. HAART also markedly reduces PMN apoptosis, possibly contributing to the observed recovery of PMN counts during treatment [78, 79]. In particular, treatment with protease inhibitors induces a rapid and significant decrease in PMN apoptosis, which correlates with improved chemotactic function [62]. These results were obtained in patients with both an immunological and a virological response, and also in patients with an isolated immunological response [62]. Several lines of evidence suggest that this can be attributed to a direct effect of protease inhibitors via calpaine inhibition [70, 80]. Nevertheless, PMN defects are still observed in some patients who respond positively to HAART [81].
Basal immune activation persists despite effective HAART and is a critical factor in HIV pathogenesis [5]. Indeed, this immune activation is postulated to be the leading cause of non-AIDS-defining comorbidities such as atherosclerosis, osteoporosis, and cognitive impairment [6], accelerating the replicative senescence of T cells that would otherwise accumulate [82]. These abnormalities are associated with increased levels of inflammatory mediators such as high-sensitivity C-reactive protein, IL-6, and D-dimer [83], which can be referred to as inflammaging [6].
PMN can also contribute to the tissue injury associated with autoimmune and inflammatory diseases. Indeed, excessive or inappropriate PMN stimulation can trigger excessive ROS production and thereby amplify the inflammatory response [84], damaging lipids, proteins, and DNA. In addition, inappropriate PMN survival and persistence at sites of inflammation are thought to contribute to the pathology of chronic inflammatory diseases through the release of cytotoxic contents into the extracellular environment. α-Defensins exhibit potent antimicrobial activities but also exert immunomodulatory effects by inducing cytokine and chemokine production, inflammatory and immune cell activation, and prolongation of PMN survival [85].
We recently demonstrated that basal activation of circulating PMN persists in HIV-1-infected patients despite effective HAART, along with a decrease in spontaneous PMN apoptosis [pers. data]. Such excessive PMN activation might play a key role in the chronic systemic proinflammatory state observed in HIV-infected patients despite sustained ART-mediated viral suppression [6] and could participate in T lymphocyte senescence [86].
Conclusion
ART significantly improves PMN functions and survival, but basal immune activation and inflammation nonetheless persist. We recently observed persistent PMN activation in HIV-infected patients on effective HAART, a phenomenon that might play a key role in the chronic systemic proinflammatory state and also participate in T lymphocyte senescence. We are currently investigating PMN activation status in HIV-infected patients during effective ART, together with the activation and senescence status of CD4+ and CD8+ T lymphocytes and monocytes. If these parameters correlate with the onset of inflammatory disorders, it may be possible to identify new predictive markers.
References
- 1.Bastian A, Schäfer H. Human α-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul Pept. 2001;101:157–161. doi: 10.1016/s0167-0115(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 2.Fujisawa H. Inhibitory role of neutrophils on influenza virus multiplication in the lungs of mice. Microbiol Immunol. 2001;45:679–688. doi: 10.1111/j.1348-0421.2001.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 3.Yasin B, Wang W, Pang M, Cheshenko N, Hong T, Waring AJ, et al. Beta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J Virol. 2004;78:5147–5156. doi: 10.1128/JVI.78.10.5147-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 5.D'Ettorre G, Paiardini M, Ceccarelli G, Silvestri G, Vullo V. HIV-associated immune activation: from bench to bedside. AIDS Res Hum Retroviruses. 2011;27:355–364. doi: 10.1089/aid.2010.0342. [DOI] [PubMed] [Google Scholar]
- 6.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 8.Mogensen TH, Melchjorsen J, Larsen CS, Paludan SR. Innate immune recognition and activation during HIV infection. Retrovirology. 2010;7:54. doi: 10.1186/1742-4690-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbim C, Prevot M, Bouscarat F, Franzini E, Chollet-Martin S, Hakim J, et al. Polymorphonuclear neutrophils from human immunodeficiency virus-infected patients show enhanced activation, diminished fMLP-induced L-selectin shedding, and an impaired oxidative burst after cytokine priming. Blood. 1994;84:2759–2766. [PubMed] [Google Scholar]
- 10.Roilides E, Mertins S, Eddy J, Walsh TJ, Pizzo PA, Rubin M. Impairment of neutrophil chemotactic and bactericidal function in children infected with human immunodeficiency virus type 1 and partial reversal after in vitro exposure to granulocyte-macrophage colony-stimulating factor. J Pediatr. 1990;117:531–540. doi: 10.1016/s0022-3476(05)80684-5. [DOI] [PubMed] [Google Scholar]
- 11.Michailidis C, Giannopoulos G, Vigklis V, Armenis K, Tsakris A, Gargalianos P. Impaired phagocytosis among patients infected by the human immunodeficiency virus: implication for a role of highly active anti-retroviral therapy. Clin Exp Immunol. 2012;167:499–504. doi: 10.1111/j.1365-2249.2011.04526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pos O, Stevenhagen A, Meenhorst PL, Kroon FP, Furth R. Impaired phagocytosis of Staphylococcus aureus by granulocytes and monocytes of AIDS patients. Clin Exp Immunol. 2008;88:23–28. doi: 10.1111/j.1365-2249.1992.tb03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitrak DL, Bak PM, DeMarais P, Novak RM, Andersen BR. Depressed neutrophil superoxide production in human immunodeficiency virus infection. J Infect Dis. 1993;167:1406–1410. doi: 10.1093/infdis/167.6.1406. [DOI] [PubMed] [Google Scholar]
- 14.Salmen S, Montilla D, London M, Velázquez D, Berrueta L. Analysis of p22-phox and p47-phox subcellular localization and distribution in neutrophils from human immunodeficiency virus (HIV) infected patients (in Spanish) Rev Invest Clin. 2012;64:40–51. [PubMed] [Google Scholar]
- 15.Heit B, Jones G, Knight D, Antony JM, Gill MJ, Brown C, et al. HIV and other lentiviral infections cause defects in neutrophil chemotaxis, recruitment, and cell structure: immunorestorative effects of granulocyte-macrophage colony-stimulating factor. J Immunol. 2006;177:6405–6414. doi: 10.4049/jimmunol.177.9.6405. [DOI] [PubMed] [Google Scholar]
- 16.Gabali AM, Anzinger JJ, Spear GT, Thomas LL. Activation by inflammatory stimuli increases neutrophil binding of human immunodeficiency virus type 1 and subsequent infection of lymphocytes. J Virol. 2004;78:10833. doi: 10.1128/JVI.78.19.10833-10836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho JL, He S, Hu A, Geng J, Basile FG, Almeida MG, et al. Neutrophils from human immunodeficiency virus (HIV)-seronegative donors induce HIV replication from HIV-infected patients' mononuclear cells and cell lines: an in vitro model of HIV transmission facilitated by Chlamydia trachomatis. J Exp Med. 1995;181:1493–1505. [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida T, Jones VC, Kobayashi M, Li XD, Pollard RB, Suzuki F. Acceleration of R5 HIV replication by polymorphonuclear neutrophils in cultures of macrophages. Immunol Cell Biol. 2007;85:215–219. doi: 10.1038/sj.icb.7100024. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L. Contribution of human alpha-defensin 1, 2, and 3 to the anti-HIV-1 activity of CD8 antiviral factor. Science. 2002;298:995–1000. doi: 10.1126/science.1076185. [DOI] [PubMed] [Google Scholar]
- 20.Mackewicz CE, Yuan J, Tran P, Diaz L, Mack E, Selsted ME, et al. Alpha-defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS. 2003;17:F23–F32. doi: 10.1097/00002030-200309260-00001. [DOI] [PubMed] [Google Scholar]
- 21.Zaharatos GJ, He T, Lopez P, Yu W, Yu J, Zhang L. Alpha-defensins released into stimulated CD8+ T-cell supernatants are likely derived from residual granulocytes within the irradiated allogeneic peripheral blood mononuclear cells used as feeders. J Acquir Immune Defic Syndr 1999. 2004;36:993–1005. doi: 10.1097/00126334-200408150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Furci L, Sironi F, Tolazzi M, Vassena L, Lusso P. Alpha-defensins block the early steps of HIV-1 infection: interference with the binding of gp120 to CD4. Blood. 2007;109:2928–2936. doi: 10.1182/blood-2006-05-024489. [DOI] [PubMed] [Google Scholar]
- 23.Demirkhanyan LH, Marin M, Padilla-Parra S, Zhan C, Miyauchi K, Jean-Baptiste M, et al. Multifaceted mechanisms of HIV-1 entry inhibition by human α-defensin. J Biol Chem. 2012;287:28821–28838. doi: 10.1074/jbc.M112.375949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang TL, Vargas J, DelPortillo A, Klotman ME. Dual role of α-defensin-1 in anti-HIV-1 innate immunity. J Clin Invest. 2005;115:765–773. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidel A, Ye Y, de Armas LR, Soto M, Yarosh W, Marcsisin RA, et al. Cyclic and acyclic defensins inhibit human immunodeficiency virus type-1 replication by different mechanisms. PLoS One. 2010;5:e9737. doi: 10.1371/journal.pone.0009737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo CJ, Tan N, Song L, Douglas SD, Ho WZ. Alpha-defensins inhibit HIV infection of macrophages through upregulation of CC-chemokines. AIDS. 2004;18:1217–1218. doi: 10.1097/00002030-200405210-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Agostino C, Lichtner M, Mastroianni CM, Ceccarelli G, Iannetta M, Antonucci S, et al. In vivo release of alpha-defensins in plasma, neutrophils and CD8 T-lymphocytes of patients with HIV infection. Curr HIV Res. 2009;7:650–655. doi: 10.2174/157016209789973600. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn L, Trabattoni D, Kankasa C, Semrau K, Kasonde P, Lissoni F, et al. α-Defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr 1999. 2005;39:138–142. [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo SA, Wang W, Rawat SS, Jung G, Waring AJ, Cole AM, et al. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J Biol Chem. 2006;281:18787–18792. doi: 10.1074/jbc.M602422200. [DOI] [PubMed] [Google Scholar]
- 30.Robinson WE, McDougall B, Tran D, Selsted ME. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J Leukoc Biol. 1998;63:94–100. doi: 10.1002/jlb.63.1.94. [DOI] [PubMed] [Google Scholar]
- 31.Carthagena L, Becquart P, Hocini H, Kazatchkine MD, Bouhlal H, Belec L. Modulation of HIV binding to epithelial cells and HIV transfer from immature dendritic cells to CD4 T lymphocytes by human lactoferrin and its major exposed LF-33 peptide. Open Virol J. 2011;5:27–34. doi: 10.2174/1874357901105010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 33.Mohan T, Sharma C, Bhat AA, Rao DN. Modulation of HIV peptide antigen specific cellular immune response by synthetic α- and β-defensin peptides. Vaccine. 2013;31:1707–1716. doi: 10.1016/j.vaccine.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 34.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. Toll-like receptor and RIG-1-like receptor signaling. Ann NY Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 36.Moguilevsky N, Steens M, Thiriart C, Prieels JP, Thiry L, Bollen A. Lethal oxidative damage to human immunodeficiency virus by human recombinant myeloperoxidase. FEBS Lett. 1992;302:209–212. doi: 10.1016/0014-5793(92)80442-j. [DOI] [PubMed] [Google Scholar]
- 37.Klebanoff SJ, Coombs RW. Viricidal effect of polymorphonuclear leukocytes on human immunodeficiency virus-1: role of the myeloperoxidase system. J Clin Invest. 1992;89:2014. doi: 10.1172/JCI115810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas P. Expression of CD4 on human peripheral blood neutrophils. Blood. 2003;101:4452–4456. doi: 10.1182/blood-2002-10-3056. [DOI] [PubMed] [Google Scholar]
- 39.Förster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, et al. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160:1522–1531. [PubMed] [Google Scholar]
- 40.Shimizu N, Tanaka A, Mori T, Ohtsuki T, Hoque A, Jinno-Oue A, et al. A formylpeptide receptor, FPRL1, acts as an efficient coreceptor for primary isolates of human immunodeficiency virus. Retrovirology. 2008;5:52. doi: 10.1186/1742-4690-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, et al. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J Virol. 2013;87:5468–5476. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olinger GG, Saifuddin M, Spear GT. CD4-negative cells bind human immunodeficiency virus type 1 and efficiently transfer virus to T cells. J Virol. 2000;74:8550–8557. doi: 10.1128/jvi.74.18.8550-8557.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu J, Sha BE, Thomas LL. HIV-1-infected peripheral blood mononuclear cells enhance neutrophil survival and HLA-DR expression via increased production of GM-CSF: implications for HIV-1 infection. J Acquir Immune Defic Syndr. 2011;56:16–25. doi: 10.1097/QAI.0b013e3181fa1fa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Folkvord JM, McCarter MD, Ryder J, Meditz AL, Forster JE, Connick E. Alpha-defensins 1, 2, and 3 are expressed by granulocytes in lymphoid tissues of HIV-1-seropositive and -seronegative individuals. J Acquir Immune Defic Syndr. 2006;42:529–536. doi: 10.1097/01.qai.0000225010.68815.1b. [DOI] [PubMed] [Google Scholar]
- 45.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 46.Bandres JC, Trial J, Musher DM, Rossen RD. Increased phagocytosis and generation of reactive oxygen products by neutrophils and monocytes of men with stage 1 human immunodeficiency virus infection. J Infect Dis. 1993;168:75–83. doi: 10.1093/infdis/168.1.75. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz R, Lu Y, Villines D, Sroussi HY. Effect of human immunodeficiency virus infection on S100A8/A9 inhibition of peripheral neutrophils oxidative metabolism. Biomed Pharmacother. 2010;64:572–575. doi: 10.1016/j.biopha.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH. Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J Clin Invest. 1998;102:663–670. doi: 10.1172/JCI2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubes P, Heit B, van Marle G, Johnston JB, Knight D, Khan A, et al. In vivo impairment of neutrophil recruitment during lentivirus infection. J Immunol. 2003;171:4801–4808. doi: 10.4049/jimmunol.171.9.4801. [DOI] [PubMed] [Google Scholar]
- 50.Gasperini S, Zambello R, Agostini C, Trentin L, Tassinari C, Cadrobbi P, et al. Impaired cytokine production by neutrophils isolated from patients with AIDS. AIDS. 1998;12:373–379. doi: 10.1097/00002030-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Kedzierska K, Ellery P, Mak J, Lewin SR, Crowe SM, Jaworowski A. HIV-1 downmodulates gamma signaling chain of Fc gamma R in human macrophages: a possible mechanism for inhibition of phagocytosis. J Immunol. 2002;168:2895–2903. doi: 10.4049/jimmunol.168.6.2895. [DOI] [PubMed] [Google Scholar]
- 52.Mazzolini J, Herit F, Bouchet J, Benmerah A, Benichou S, Niedergang F. Inhibition of phagocytosis in HIV-1-infected macrophages relies on Nef-dependent alteration of focal delivery of recycling compartments. Blood. 2010;115:4226–4236. doi: 10.1182/blood-2009-12-259473. [DOI] [PubMed] [Google Scholar]
- 53.Meddows-Taylor S, Martin DJ, Tiemessen CT. Altered expression of Fc gammaRIII (CD16) on polymorphonuclear neutrophils from individuals with human immunodeficiency virus type 1 disease and pulmonary tuberculosis. Clin Diagn Lab Immunol. 1997;4:789–791. doi: 10.1128/cdli.4.6.789-791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Ettorre G, Forcina G, Lichtner M, Mengoni F, D'Agostino C, Massetti AP, et al. Interleukin-15 in HIV infection: immunological and virological interactions in antiretroviral-naive and -treated patients. AIDS. 2002;16:181. doi: 10.1097/00002030-200201250-00006. [DOI] [PubMed] [Google Scholar]
- 55.Mastroianni CM, d'Ettorre G, Forcina G, Lichtner M, Mengoni F, D'Agostino C, et al. Interleukin-15 enhances neutrophil functional activity in patients with human immunodeficiency virus infection. Blood. 2000;96:1979–1984. [PubMed] [Google Scholar]
- 56.Cossarizza A, Pinti M, Nasi M, Gibellini L, Manzini S, Roat E, et al. Increased plasma levels of extracellular mitochondrial DNA during HIV infection: a new role for mitochondrial damage-associated molecular patterns during inflammation. Mitochondrion. 2011;11:750–755. doi: 10.1016/j.mito.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 57.He Y, Li J, Zheng Y, Luo Y, Zhou H, Yao Y, et al. A randomized case-control study of dynamic changes in peripheral blood Th17/Treg cell balance and interleukin-17 levels in highly active antiretroviral-treated HIV type 1/AIDS patients. AIDS Res Hum Retroviruses. 2012;28:339–345. doi: 10.1089/aid.2011.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 59.Marchetti G, Bellistrì GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 60.Pitrak DL, Tsai HC, Mullane KM, Sutton SH, Stevens P. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. J Clin Invest. 1996;98:2714. doi: 10.1172/JCI119096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salmen S, Teran G, Borges L, Goncalves L, Albarran B, Urdaneta H, et al. Increased Fas-mediated apoptosis in polymorphonuclear cells from HIV-infected patients. Clin Exp Immunol. 2004;137:166–172. doi: 10.1111/j.1365-2249.2004.02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mastroianni CM, Mengoni F, Lichtner M, D'Agostino C, d'Ettorre G, Forcina G, et al. Ex vivo and in vitro effect of human immunodeficiency virus protease inhibitors on neutrophil apoptosis. J Infect Dis. 2000;182:1536–1539. doi: 10.1086/315858. [DOI] [PubMed] [Google Scholar]
- 63.Salmen S, Montes H, Soyano A, Hernández D, Berrueta L. Mechanisms of neutrophil death in human immunodeficiency virus-infected patients: role of reactive oxygen species, caspases and map kinase pathways. Clin Exp Immunol. 2007;150:539–545. doi: 10.1111/j.1365-2249.2007.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baldelli F, Preziosi R, Francisci D, Tascini C, Bistoni F, Nicoletti I. Programmed granulocyte neutrophil death in patients at different stages of HIV infection. AIDS. 2000;14:1067–1069. doi: 10.1097/00002030-200005260-00024. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi SD. An apoptosis-differentiation program in human polymorphonuclear leukocytes facilitates resolution of inflammation. J Leukoc Biol. 2003;73:315–322. doi: 10.1189/jlb.1002481. [DOI] [PubMed] [Google Scholar]
- 66.Moses A, Nelson J, Bagby GC., Jr The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91:1479–1495. [PubMed] [Google Scholar]
- 67.Firnhaber C, Smeaton L, Saukila N, Flanigan T, Gangakhedkar R, Kumwenda J, et al. Comparisons of anemia, thrombocytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the Americas. Int J Infect Dis. 2010;14:e1088–e1092. doi: 10.1016/j.ijid.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang B. Elucidation of molecular events leading to neutrophil apoptosis following phagocytosis: cross-talk between caspase 8, reactive oxygen species, and MAPK/ERK activation. J Biol Chem. 2003;278:28443–28454. doi: 10.1074/jbc.M210727200. [DOI] [PubMed] [Google Scholar]
- 69.Okada H, Takei R, Tashiro M. HIV-1 Nef protein-induced apoptotic cytolysis of a broad spectrum of uninfected human blood cells independently of CD95 (Fas) FEBS Lett. 1997;414:603–606. doi: 10.1016/s0014-5793(97)01080-6. [DOI] [PubMed] [Google Scholar]
- 70.Lichtner M, Mengoni F, Mastroianni CM, Sauzullo I, Rossi R, De Nicola M, et al. HIV protease inhibitor therapy reverses neutrophil apoptosis in AIDS patients by direct calpain inhibition. Apoptosis. 2006;11:781–787. doi: 10.1007/s10495-006-5699-5. [DOI] [PubMed] [Google Scholar]
- 71.Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 72.Elbim C, Monceaux V, François S, Hurtrel B, Gougerot-Pocidalo MA, Estaquier J, et al. Increased neutrophil apoptosis in chronically SIV-infected macaques. Retrovirology. 2009;29:1. doi: 10.1186/1742-4690-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van den Berg JM, Weyer S, Weening JJ, Roos D, Kuijpers TW. Divergent effects of tumor necrosis factor alpha on apoptosis of human neutrophils. J Leukoc Biol. 2001;69:467–473. [PubMed] [Google Scholar]
- 74.Geering B, Gurzeler U, Federzoni E, Kaufmann T, Simon HU. A novel TNFR1-triggered apoptosis pathway mediated by class IA PI3Ks in neutrophils. Blood. 2011;117:5953–5962. doi: 10.1182/blood-2010-11-322206. [DOI] [PubMed] [Google Scholar]
- 75.Pitrak DL. Filgrastim treatment of HIV-infected patients improves neutrophil function. AIDS. 1999;13((suppl 2)):S25–S30. [PubMed] [Google Scholar]
- 76.Elbim C, Monceaux V, Mueller YM, Lewis MG, Francois S, Diop O, et al. Early divergence in neutrophil apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J Immunol. 2008;181:8613–8623. doi: 10.4049/jimmunol.181.12.8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mastroianni CM, Lichtner M, Mengoni F, D'Agostino C, Forcina G, d'Ettorre G, et al. Improvement in neutrophil and monocyte function during highly active antiretroviral treatment of HIV-1-infected patients. AIDS. 1999;13:883–890. doi: 10.1097/00002030-199905280-00003. [DOI] [PubMed] [Google Scholar]
- 78.Matsushita S, Yoshimura K, Kimura T, Kamihira A, Takano M, Eto K, et al. Spontaneous recovery of hemoglobin and neutrophil levels in Japanese patients on a long-term Combivir containing regimen. J Clin Virol. 2005;33:188–193. doi: 10.1016/j.jcv.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Levine AM, Karim R, Mack W, Gravink DJ, Anastos K, Young M, et al. Neutropenia in human immunodeficiency virus infection: data from the women's interagency HIV study. Arch Intern Med. 2006;166:405. doi: 10.1001/archinte.166.4.405. [DOI] [PubMed] [Google Scholar]
- 80.Hadad N, Levy R, Schlaeffer F, Riesenberg K. Direct effect of human immunodeficiency virus protease inhibitors on neutrophil function and apoptosis via calpain inhibition. Clin Vaccine Immunol. 2007;14:1515–1521. doi: 10.1128/CVI.00130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore DA, Benepal T, Portsmouth S, Gill J, Gazzard BG. Etiology and natural history of neutropenia in human immunodeficiency virus disease: a prospective study. Clin Infect Dis. 2001;32:469–475. doi: 10.1086/318495. [DOI] [PubMed] [Google Scholar]
- 82.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 83.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ricevuti G. Host tissue damage by phagocytes. Ann NY Acad Sci. 1997;832:426–448. doi: 10.1111/j.1749-6632.1997.tb46269.x. [DOI] [PubMed] [Google Scholar]
- 85.Nagaoka I, Suzuki K, Murakami T, Niyonsaba F, Tamura H, Hirata M. Evaluation of the effect of α-defensin human neutrophil peptides on neutrophil apoptosis. Int J Mol Med. 2010;26:925–934. doi: 10.3892/ijmm_00000544. [DOI] [PubMed] [Google Scholar]
- 86.Oliveira BF, Nogueira-Machado JA, Chaves MM. The role of oxidative stress in the aging process. SciWorld J. 2010;10:1121–1128. doi: 10.1100/tsw.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]