Abstract
Acute kidney injury (AKI) is a common complication in critically ill patients and is associated with high mortality. Recruitment of neutrophils is a hallmark in the pathogenesis of AKI. Although ischemia-reperfusion injury (IRI) is a frequently used research model of AKI, the clinical relevance of IRI-induced AKI is limited. Epidemiologically, sepsis is the prevailing cause of kidney injury. However, it is still unknown whether these distinct entities of AKI share the same pathophysiological mechanisms. This study was initiated to investigate the molecular mechanisms of neutrophil recruitment into the kidney in a murine model of sepsis-induced AKI. By using a flow cytometry-based method, we show that the two β2-integrins Mac-1 and LFA-1 as well as E-selectin and P-selectin are involved in neutrophil recruitment into the kidney after induction of sepsis. The molecular mechanisms of neutrophil recruitment were further investigated using intravital microscopy, demonstrating that blocking one of these four molecules reduces the number of adherent leukocytes. This was accompanied by a renal upregulation of E-selectin, P-selectin and ICAM-1 (the counter-receptor of β2-integrins on endothelial cells) after sepsis induction. We conclude that blocking P-selectin, E-selectin, Mac-1 or LFA-1 protects mice from sepsis-induced AKI.
Key Words: Neutrophil recruitment, Acute kidney injury, Adhesion molecules, Selectins, Integrins
Introduction
Acute kidney injury (AKI) is a common complication in critically ill patients and is associated with high morbidity and mortality [1, 2, 3]. AKI is an independent predictor of increased mortality in an intensive-care setting [4]. Even patients that are discharged from the ICU and recover from AKI have an increased risk of developing chronic kidney disease [5]. Despite this clinical significance and advances in renal replacement therapy, little change in the prognosis of patients with AKI could be observed over the last 50 years [6]. A more sophisticated understanding of the molecular pathophysiology may enable clinicians to finally overcome these obstacles [7].
AKI can be due to different causes including ischemia-reperfusion injury (IRI) and sepsis. Sepsis is the leading cause and accounts for up to 50% of all cases [8].
Previous studies have revealed leukocyte recruitment as a hallmark of AKI [9]. In particular, neutrophil infiltration of the renal tissue is directly involved in the development of IRI-induced AKI. The deterioration of organ failure is proportional to the degree of neutrophil recruitment into the kidney [10, 11]. Leukocyte recruitment into inflamed or damaged tissues proceeds in a cascade-like fashion [12], in which different adhesion molecules are required for each step. E-selectin and P-selectin expressed on endothelial cells mediate the first contact between leukocytes and endothelial cells in a process termed ‘leukocyte tethering’ [13, 14]. This enables leukocytes to roll on the endothelium and pick up inflammatory signals such as chemokines and cytokines that are presented on the endothelial surface. The engagement of chemokine receptors on leukocytes triggers the activation of the two β2-integrins LFA-1 and Mac-1 on the leukocyte surface; they subsequently bind to their counter-receptors on endothelial cells and mediate arrest [15]. The last step in this cascade is transmigration, i.e. the leukocytes leave the vessel and migrate into the inflamed tissue [14]. Recent studies demonstrated that leukocyte recruitment into the kidney occurs at two different sites [16]: Leukocyte rolling and recruitment in the postcapillary venules in the cortex are E-selectin-dependent [17], whereas glomerular inflammation results in an increase in the duration of retention of static and migrating leukocytes [18].
However, it is unknown whether the molecular mechanisms of neutrophil recruitment into the kidney are different following different insults, i.e. in this case IRI versus sepsis induced by cecal ligation and puncture (CLP). Although previous studies have revealed roles for E-selectin, P-selectin and LFA-1 in the frequently used IRI model of AKI [10, 19, 20], blockade of LFA-1 and its ligand ICAM-1 did not protect kidney function in toxic-induced (mercuric chloride-induced) AKI [21]. It is well accepted that the molecular mechanisms of neutrophil recruitment are organ- and stimulus-specific [16, 18, 22]. Although the molecules involved in neutrophil recruitment into the kidney after IRI have been extensively investigated, the molecular basis of neutrophil recruitment during sepsis-induced AKI is still unknown.
This study investigated the molecular mechanisms of neutrophil recruitment into the kidney in a sepsis-induced model of AKI. To achieve this, we used function-blocking monoclonal antibodies against different adhesion molecules (P-selectin, E-selectin, LFA-1 and Mac-1) in wild-type mice. To validate our model, we determined the impact of neutrophils on renal function. A newly developed FACS method was used to investigate the total number of neutrophils in the kidney, whereas impairment of the kidney function was monitored by measuring creatinine levels in the blood. Furthermore, we used intravital microscopy of the postcapillary venules in the cortex of the kidney in order to directly visualize the different steps of the leukocyte recruitment cascade and investigate which adhesion molecules are involved in these processes in our model of sepsis-induced AKI. Immunohistochemistry was used to show the expression pattern of adhesion molecules in the kidney after inducing sepsis.
Material and Methods
Animals
8- to 12-week-old C57BL/6 mice (Janvier, Le Genest St Isle, France) and LysM-GFP+ mice [23] were housed in the SPF facility. The Animal Care and Use Committees of the University of Münster, Germany approved all animal experiments.
Reagents
If not stated otherwise, all reagents were obtained from Sigma-Aldrich (Taufkirchen, Germany).
Sepsis Induction by CLP
CLP was performed as described previously [24]. Briefly, after induction of anesthesia via intraperitoneal injection (i.p.) of ketamine (100 mg/kg) and xylazine (10 mg/kg), animals were placed on a heating pad to maintain body temperature. A skin incision of approximately 5 mm in the left abdominal area was made, the peritoneal sheath was incised and the cecum was carefully exteriorized. After 12 mm of cecum had been ligated using polyglactin sutures, two transmural injuries were made to the ligated section using a 20-gauge needle to allow peritoneal dissemination of bacteria. The permeability of the cecum contents was checked by light, manual palpitation of the cecum which was then returned to the abdominal cavity. Sham animals underwent the same procedure, but cecal puncture and ligation was omitted. The peritoneal sheath and skin were sutured in 2 layers with polyglactin sutures and the animals then received fluid resuscitation with 200 μl saline i.p. and were left to recover. Fluid therapy was continued at 6, 12, 24 and 36 h after surgery.
Therapeutic Intervention Experiments
Twelve groups of wild-type and LysM-GFP+ mice received 100 µg of blocking antibody against Mac-1 (Clone M1/70), LFA-1 (Clone M17/4), P-selectin (Clone RB40.34) or E-selectin (Clone 9A9) separately or in combination, or isotype-matched control antibodies i.p. after surgical closure of the peritoneal sheath. In other experiments, mice received 50 μg of anti-Ly6G antibody (clone 1A8) i.p. or a matched isotype control antibody (Rat IgG2a, κ) immediately after closure of the abdomen. This has previously been shown to cause neutrophil depletion without affecting the monocyte population [25].
Functional Analysis of Kidney Failure
Animals were euthanized after 48 h and blood samples were taken by cardiac puncture. Kidneys were flushed extensively with PBS in situ and were removed for the determination of neutrophil infiltration and histology, respectively. Serum creatinine levels were determined using a creatinine ELISA assay (Diazyme, Poway, Calif., USA) according to the manufacturer's protocol. Neutrophil recruitment into the kidneys was determined by flow cytometry [17]. Briefly, kidneys were homogenized and incubated with collagenase IX, hyaluronidase and DNAse for 45 min at 37°C. Tissue suspension were filtered through a 70-µm cell strainer and the resulting cells were subsequently stained for Ly-6B.2 (Abcam), Ly-6G and CD45 (Biolegend) and quantified using FACS counting beads (Molecular Probes, Eugene, Oreg., USA).
Morphologic Evaluation of Kidneys
Kidney specimens were fixed in formalin solution and embedded in paraffin. HE staining was performed using standard protocols. Histologic morphology was scored for loss of brush borders (0-3), tubular vaculolization (0-3), cell recruitment (0-3) and apoptosis (0-3) by a blinded investigator.
Immunhistochemistry
Staining for E-selectin, P-selectin and ICAM-1 was performed as previously described [26]. Briefly, after heat-mediated antigen retrieval (E- and P-selectin) or proteinase K retrieval (ICAM-1), kidneys were incubated with anti-P-selectin, anti-E-selectin or anti-ICAM-1 primary antibody (M-19, Santa Cruz Biotechnology, Calif., USA) and then incubated with 1:250 corresponding biotinylated secondary antibody and finally developed with avidin-biotin-peroxidase. Pictures were analyzed using ImageJ software according to the guidelines for analysis of histologic staining by the University of Auckland [27]. Briefly, histologic images were split into color channels and the channel containing histologic staining was processed. Then, staining was renormalized with the threshold tool. The area of interest (glomerulus or peritubular vessel) was selected with the region-of-interest tool and was evaluated for fraction of stained area.
Intravital Microscopy of the Kidney
Intravital microscopy of the kidney was performed 8 h after CLP or sham surgery as described previously [17]. Briefly, kidneys were exteriorized and subjected to epifluorescence microscopy with an AxioScopeA1 (Carl Zeiss, Göttingen, Germany) and a CCD-camera (Sensicam QE, Cooke Corp., Bayreuth, Germany). Epifluorescence was generated using a Lambda DG-4 fast wavelength switcher (Sutter, Novato, Calif., USA) and videos were recorded, renormalized and analyzed using SlideBook 5.0 (Intelligent Imaging Innovations, Denver, Colo., USA). At the end of the experiment, fluospheres (Molecular Probes) were injected to determine the blood flow in the postcapillary venules of the cortex.
Leukocyte Blood Counts
To determine the effect of anti-Ly6G treatment, blood from mice was drawn into a heparinized syringe and erythrocytes were lysed with Phram Lyse (BD Biosciences, San Jose, Calif., USA). The remaining cells were stained with CD45 PerCP and Gr-1 PE (Clone RB6-8C5, Biolegend, San Diego, Calif., USA) and quantified via FACS counting beads.
Statistics
Statistical analysis was performed with SPSS (version 20.0, Chicago, Ill., USA). Differences between groups were evaluated by one-way analysis of variance, the Newman-Keuls test and the Student t test where appropriate. Normal distribution was assessed by means of the Shapiro-Wilk test and consecutive Q-Q plotting. Data are presented as mean ± SEM, and p < 0.05 was considered statistically significant.
Results
CLP-Induced Kidney Injury Is Dependent on Neutrophil Infiltration
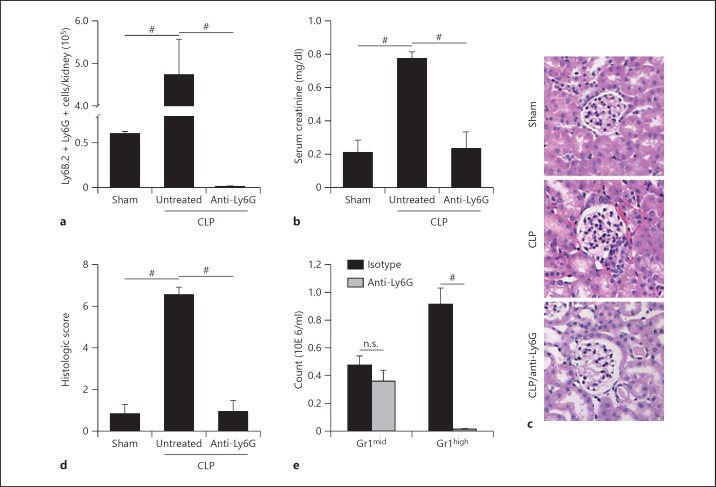
Animals were found to be septic after 48 h despite fluid resuscitation and mortality was found to be as high as 50% in untreated animals (data not shown). Following sepsis induction, the number of neutrophils in the kidney was significantly elevated compared to sham animals (fig. 1a). In addition, serum creatinine levels in septic mice were significantly increased (fig. 1b), showing that mice developed AKI within 48 h after the induction of sepsis. To assess whether neutrophil infiltration is critical for the decline of organ function, animals were injected with a depleting dose of an anti-Ly6G antibody after surgery. In animals treated with anti-Ly6G antibody, there were almost no neutrophils detectable in the kidney 48 h after the induction of sepsis (fig. 1a, c). These antibody treated mice were completely protected from CLP-induced kidney injury as the serum creatinine did not rise over values seen in sham-operated animals (fig. 1b) and histologic evaluation revealed preserved kidneys in anti-Ly6G treated animals (fig. 1d). To exclude a monocytic component to our results, we stained leukocytes isolated from isotype-treated or anti-Ly6G-treated animals with Gr1 and found no significant difference in the monocyte (Gr1mid) population (fig. 1e).
Fig. 1.
CLP-induced kidney injury is dependent on neutrophil infiltration. a Neutrophils in the kidney 48 h after CLP or sham surgery. Neutrophil recruitment is almost absent in mice injected with a neutrophil-depleting anti-Ly6G antibody. b Serum creatinine levels of sham mice and septic animals (48 h after inducing sepsis). Neutrophil depletion preserves organ function in septic animals. c Representative HE stainings of the cortex of CLP or sham operated mice that were injected with either an isotype control or anti-Ly6G antibody. d Histologic scoring of (c). e Blood counts of Gr1mid (monocytes) and Gr1high (neutrophils) 48 h after injection of an isotype control or anti-Ly6G antibody (n = 3-5). # p < 0.05.
Blockade of P-Selectin, E-Selectin, Mac-1 or LFA-1 Decreases Neutrophil Recruitment into the Kidney and Preserves Organ Function during CLP-Induced AKI
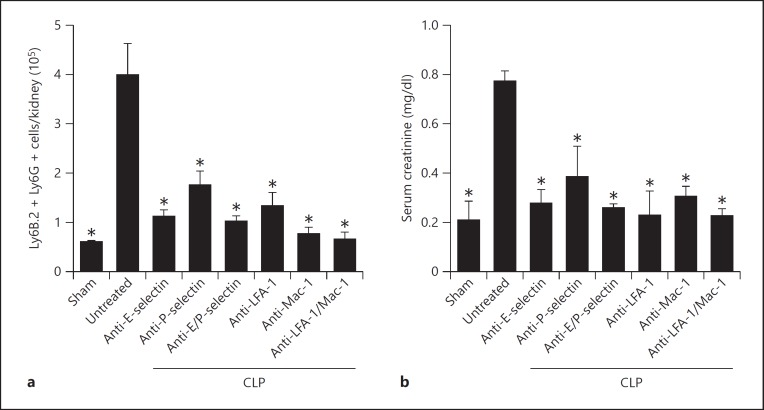
Animals treated with blocking antibodies appeared to have decreased mortality, but due to low case numbers this observation did not reach statistical significance (data not shown). The number of neutrophils as determined by flow cytometry was 4.00 × 105 cells/kidney in untreated septic animals and 0.89 × 105 cells/kidney in sham animals (fig. 2a). Blocking one individual adhesion molecule alone (E-selectin, P-selectin, Mac-1 or LFA-1) or the combination of different adhesion molecules (E-selectin and P-selectin or Mac-1 and LFA-1) significantly decreased the number of neutrophils when compared to untreated septic mice (fig. 2a). However, no differences were detected among the different intervention groups (fig. 2a). In septic mice, serum creatinine levels were significantly increased compared to sham-operated mice (fig. 2b). Blocking one individual adhesion molecule alone or the combination of different adhesion molecules significantly decreased the serum creatinine levels when compared to untreated septic mice (fig. 2b).
Fig. 2.
Blockade of P-selectin, E-selectin, Mac-1 or LFA-1 decreases neutrophil recruitment into the kidney and preserves organ function during CLP-induced AKI. a Neutrophil infiltration into the kidney was determined by FACS analysis 48 h after sham operation or after inducing sepsis. b Serum creatinine levels of sham mice, septic mice and septic mice pretreated with blocking antibodies (48 h after inducing sepsis; n = 3-5). * p < 0.05 to CLP alone.
Blockade of Adhesion Molecules Preserves Kidney Morphology
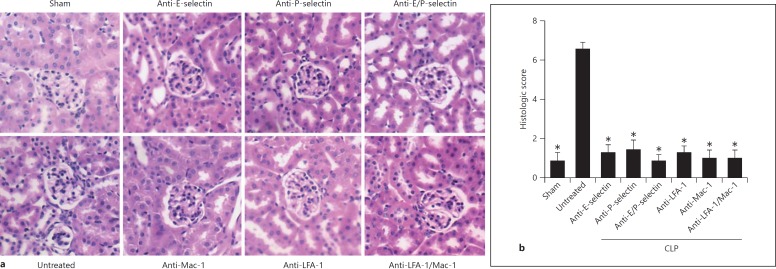
Tubular edema, cell recruitment and loss of tubular epithelial cells are histological features of AKI. Therefore, we performed HE staining of the kidneys after sham operation or CLP to investigate these histological features. Kidneys from septic mice had massive tubular edema, cell recruitment and loss of tubular epithelial cells (fig. 3a). These findings were ameliorated in mice injected with blocking antibodies against E-selectin, P-selectin, Mac-1, LFA-1, combined E-selectin and P-selectin and combined LFA-1 and Mac-1. To quantify these changes, we used a previously published histological score, optimized according to histologic features reported in a recent study evaluating septic kidneys in humans [28, 34]. With the help of this score, it could be demonstrated that blocking one of the adhesion molecules or the combination of different molecules abolished the morphological changes accompanied by AKI (fig. 3b).
Fig. 3.
Blockade of adhesion molecules preserves kidney morphology. a Representative HE staining of the cortex of the kidney after CLP-induced kidney injury or sham operation. CLP-induced AKI causes leukocyte infiltration, loss of brush borders in the proximal tubule, vacuolization of tubular endothelium and apoptosis. Leukocyte infiltration and morphologic changes were less severe in kidney sections from animals that were injected with blocking antibodies against different the adhesion molecules. b Histologic scoring of kidneys from sham mice, septic mice and septic mice treated with blocking antibodies (n = 3-5). * p < 0.05 to CLP alone.
Neutrophil Rolling and Adhesion in Postcapillary Venules of the Cortex of the Kidney Is Regulated by Different Adhesion Molecules
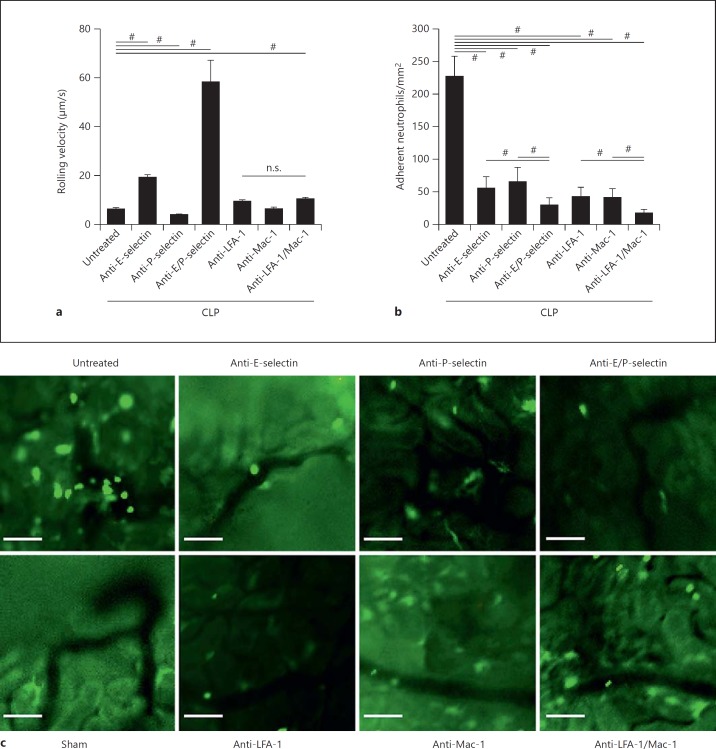
Rolling velocity and adhesion of leukocytes in postcapillary venules of the cortex of the kidney were investigated by using intravital microscopy of the kidney as described previously [17, 29]. In sham-operated mice, no rolling and adherent leukocytes were observed (data not shown). However, intravital microscopy of mice 8 h after the induction of sepsis showed an abundant number of rolling and adherent neutrophils in the postcapillary venules of the kidney cortex (fig. 4a-c). Neutrophils in untreated septic animals rolled at a velocity of 6.34 µm/s (fig. 4a). Injection of isotype control antibodies did not change the rolling velocity or the number of adherent neutrophils when compared to untreated animals (data not shown). Blocking E-selectin using a monoclonal antibody elevated the rolling velocity to 19.27 µm/s whereas blocking of P-selectin by a monoclonal antibody reduced the mean rolling velocity to 4.4 µm/s (fig. 4a). Blocking E-selectin and P-selectin significantly elevated the rolling velocity of neutrophils compared to in untreated septic mice (fig. 4a). Blockade of Mac-1 had no impact on rolling velocity (6.39 µm/s). However, blockade of LFA-1 significantly increased the mean rolling velocity of neutrophils to 9.52 µm/s (fig. 4a).
Fig. 4.
Neutrophil rolling and adhesion in postcapillary venules of the cortex of the kidney is regulated by different adhesion molecules. Rolling velocities (a) and number of adherent neutrophils (b) in postcapillary venules of the cortex of the kidney 8 h after sepsis induction. c Representative micrographs of cortex venules of sham animals, CLP animals and CLP animals following treatment with anti-E-selectin, anti-P-selectin, anti-E-selectin/anti-P-selectin, anti-LFA-1, anti-Mac-1 and anti-Mac-1/anti-LFA-1 (n = 3-5). Bars: 50 µm. # p < 0.05.
CLP animals revealed 227.84 ± 30.45 adherent neutrophils per mm2 in the postcapillary venules of the cortex of the kidney (fig. 4b). Antibody blocking of either E-selectin or P-selectin significantly decreased the number of adherent neutrophils by a similar amount (fig. 4b, c). Blocking both selectins had a synergistic effect (fig. 4b, c). Similarly, injection of a blocking anti-LFA-1 antibody decreased the number of adherent neutrophils (fig. 4b, c). A significant decrease was also seen after injection of a blocking anti-Mac-1 antibody (fig. 4b, c). Blocking both integrins also had an additive effect on the number of adherent cells (fig. 4b, c).
The shear stress and blood flow in the vessels was similar in all animals as measured by the velocity of microspheres injected into the tail vein of the mice. This excluded confounding hemodynamic contributions to our findings (data not shown).
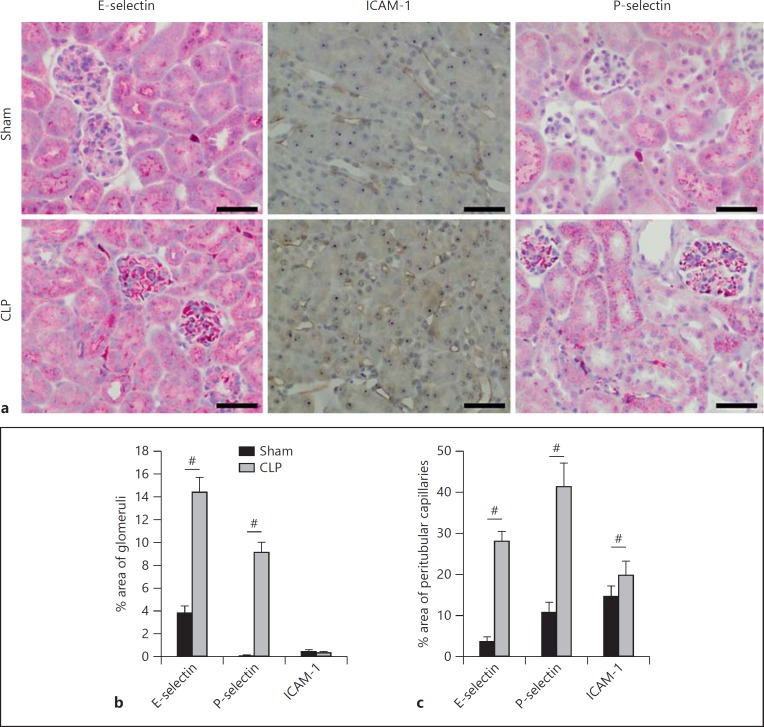
Endothelial E-Selectin, P-Selectin and ICAM-1 Are Upregulated in CLP-Induced AKI
Immunohistochemistry revealed that CLP induction dramatically increased the expression of E-selectin and P-selectin in the glomeruli and peritubular capillaries (fig. 5). Sham-treated animals stained only very weakly for E-selectin and P-selectin at both sites (fig. 5b, c). Interestingly, ICAM-1 was not detectable in the glomeruli (fig. 5b) and its expression in sham animals was focal and very minor. Following CLP, a diffuse but moderate upregulation of ICAM-1 was observed in the pertubular capillaries of the renal cortex, but not in the glomeruli (fig. 5a, c).
Fig. 5.
CLP-induced sepsis causes upregulation of selectins and integrin ligands in the kidney vessels. a Representative images of kidney immunohistochemistry staining for E-selectin, P-selectin and ICAM-1. Bars: 50 µm. b Quantitative evaluation of adhesion molecule expression in the glomeruli. c Quantitative evaluation of adhesion molecule expression in peritubular capillaries (n = 3-5). # p < 0.05.
Discussion
Neutrophil recruitment into the kidney is a crucial event in acute kidney failure [11]. In the IRI model of kidney failure, neutrophil depletion protectes mice from developing AKI [10]. Similarly, hampering neutrophil recruitment by injection of blocking anti-E-selectin or anti-P-selectin antibodies or preventing activation of the β2-integrin LFA-1 using mice deficient for molecules in selectin-signaling has been found to ameliorate organ failure [10, 17, 19]. Current data lead to the hypothesis that the decline in organ function is a consequence of leukocyte influx and is thus linearly dependent on neutrophils infiltrating kidney tissue [11]. However, the molecular mechanisms of neutrophil recruitment into the kidney in the context of sepsis were unknown, as such mechanisms have been shown to be stimulus-specific. We here demonstrate that CLP-induced AKI is dependent on neutrophil recruitment similar to IRI-induced AKI [10], but is different from toxic kidney injury [21]. This finding is consistent with the recent advances in the pathophysiological understanding of sepsis-induced AKI in humans: the classic paradigm is that decreased blood flow in septic patients is the principal pathophysiological cause of AKI. However, volume resuscitation and renal vasodilators have little effect [30] and renal blood flow was found to be normal or even increased in resuscitated sepsis patients [31, 32]. In addition, acute tubular necrosis is rarely found in septic kidneys [33]. In contrast, a histologic feature commonly seen in septic kidneys is leukocyte infiltration [34]. Furthermore, proinflammatory cytokines such as IL-1 and TNF-α were found to induce AKI in sepsis [35]. These data and similar findings have led to the hypothesis that AKI in sepsis is driven by inflammation rather than by relative ischemia [36].
Neutrophils and monocytes are crucial for the elimination of pathogens [37]. Depletion of neutrophils, as clinically seen in patients with neutropenia, greatly impairs the host defense against pathogens. Even the degradation of extracellular neutrophil traps alone causes hypersusceptibility to sepsis following CLP in mice [38], highlighting the importance of neutrophils in combating pathogens in the early stages of infection. However, mortality in mice was not different after the depletion of neutrophils following CLP-induced sepsis, but was increased if both monocytes and neutrophils were depleted [37]. In fact, removal of neutrophils via leukofiltration in patients with systemic inflammatory shock syndrome improved renal and pulmonary functions [39]. Based on these results, targeting neutrophils in septic patients is a matter of ongoing discussion [40, 41]. Neutrophils are essential for pathogen clearance in untreated infection, so targeting neutrophils (and thereby collateral organ damage) has been suggested to potentially improve outcome in patients who are treated with antibiotics [40, 41]. However, while it seems safe to assume that the functions of neutrophils in sepsis may cause severe collateral end-organ damage, further clinical studies are needed to answer the question if the inhibition or reduction of neutrophil extravasation can be beneficial for a subpopulation of septic patients.
In previous studies, assessing neutrophil rolling in the kidney cortex was considered feasible at 4 h after reperfusion [42]. However, in our study, no rolling was apparent in CLP animals at this point (data not shown). This was most likely due to the more direct damage to the kidney after IRI. Similarly, we did not observe a significant increase in serum creatinine levels 24 h after CLP (data not shown) despite different findings reported in CD-1 mice [24]. Furthermore, we also found that antibiotic treatment with low-dose piperacillin and tazobactam (10 mg/1.25 mg/kg) 12 h after surgery protected mice from kidney damage and that 24 h later, the animals appeared to have recovered from sepsis (data not shown). However, Doi et al. [43] pointed out the existence of profound differences between mouse strains in this model. The mortality observed in our experiments without antibiotic treatment after 48 h does correlate with the findings in CD-1 mice following sublethal CLP with antibiotic treatment, which supports the hypothesis that CD-1 mice are indeed more susceptible to CLP-induced sepsis [24].
Previous studies from our group have analyzed neutrophil rolling velocities and adherence in mice after blockade of different adhesion molecules following IRI-induced AKI [17]. Rolling velocities were higher after IRI in untreated animals (9.4 µm/s) and also after the injection of blocking antibodies (e.g. 17.82 µm/s after anti-LFA-1 and 5.69 µm/s after anti-E-selectin). However, neutrophil rolling in the kidney cortex was measured after 4 h in these experiments. A likely explanation for these overall lower rolling velocities in animals with sepsis is a higher concentration of the adhesion molecules P-selectin and E-selectin and the β2-integrin ligand ICAM-1 on the renal endothelium due to the longer incubation time. However, blockade of Mac-1 with monoclonal antibodies did not impact rolling velocity in both models.
The number of adherent neutrophils after 8 h in the postcapillary venules of the cortex of the kidney was similar to the IRI-induced AKI model. Blockade of each adhesion molecule resulted in comparable decreases in the number of adherent cells, including blockade of Mac-1 in this model. The fact that inhibiting Mac-1 did not influence the rolling velocity but did significantly decrease the number of adherent neutrophils is very interesting and suggests that Mac-1 is either involved in postadhesion strengthening or crawling. Unfortunately, these two steps can currently not be investigated by intravital microscopy of the postcapillary venules of the cortex of the kidney. Future studies using more sophisticated analysis methods have to address the question of how Mac-1 blockade leads to a reduced number of adherent cells. In addition, the simultaneous blockade of Mac-1 and LFA-1 or P-selectin and E-selectin, respectively, had a synergistic impact on the mean number of adherent neutrophils after IRI-induced kidney injury.
Even though the number of recruited neutrophils was found to be similar in kidneys 48 h after the induction of sepsis when compared to 24 h after reperfusion, serum creatinine levels after IRI-induced AKI were almost twice as high as in sepsis animals. This finding is likely explained by differences in these models, e.g. a more direct damage to the kidney is observed in the IRI-induced AKI model.
Histopathologic characteristics of sepsis-induced AKI constitutes a field of ongoing research. A recently published study on human samples suggested that acute tubular necrosis is an uncommon feature in septic kidneys [33]. We developed a histopathology score based on the findings described by Leelahavanichkul et al. [28] in the CLP-induced AKI model in CD-1 mice and another recent clinical study [34].
Our data demonstrate that the adhesion molecules E-selectin, P-selectin, LFA-1 and Mac-1 are involved in the recruitment of neutrophils into the kidney and organ function in sepsis-induced AKI.
Acknowledgements
We would like to thank Bernadette Gelschefrath for technical assistance. This work was supported by grants from the German Research Foundation (AZ 428/3-1, AZ 428/6-1 and SFB 1009/A5 to A.Z. and HE-6810/1-1 to J.H.) and the Interdisciplinary Center of Clinical Research (to A.Z.).
References
- 1.Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clec'h C, Gonzalez F, Lautrette A, Nguile-Makao M, Garrouste-Orgeas M, Jamali S, Golgran-Toledano D, Descorps-Declere A, Chemouni F, Hamidfar-Roy R, Azoulay E, Timsit JF. Multiple-center evaluation of mortality associated with acute kidney injury in critically ill patients: a competing risks analysis. Crit Care. 2011;15:R128. doi: 10.1186/cc10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murugan R, Kellum JA. Acute kidney injury: what's the prognosis? Nat Rev Nephrol. 2011;7:209–217. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078–F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ympa YP, Sakr Y, Reinhart K, Vincent JL. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med. 2005;118:827–832. doi: 10.1016/j.amjmed.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 7.Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet. 2005;365:417–430. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 8.Uchino S. The epidemiology of acute renal failure in the world. Curr Opin Crit Care. 2006;12:538–543. doi: 10.1097/01.ccx.0000247448.94252.5a. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 10.Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 2000;14:48–54. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- 11.Singbartl K, Ley K. Leukocyte recruitment and acute renal failure. J Mol Med (Berl) 2004;82:91–101. doi: 10.1007/s00109-003-0498-8. [DOI] [PubMed] [Google Scholar]
- 12.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 13.Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herter J, Zarbock A. Integrin regulation during leukocyte recruitment. J Immunol. 2013;190:4451–4457. doi: 10.4049/jimmunol.1203179. [DOI] [PubMed] [Google Scholar]
- 15.Zarbock A, Deem TL, Burcin TL, Ley K. Galphai2 is required for chemokine-induced neutrophil arrest. Blood. 2007;110:3773–3779. doi: 10.1182/blood-2007-06-094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossaint J, Zarbock A. Tissue-specific neutrophil recruitment into the lung, liver, and kidney. J Innate Immun. 2013;5:348–357. doi: 10.1159/000345943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block H, Herter JM, Rossaint J, Stadtmann A, Kliche S, Lowell CA, Zarbock A. Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury. J Exp Med. 2012;209:407–421. doi: 10.1084/jem.20111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devi S, Li A, Westhorpe CL, Lo CY, Abeynaike LD, Snelgrove SL, Hall P, Ooi JD, Sobey CG, Kitching AR, Hickey MJ. Multiphoton imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19:107–112. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 19.Singbartl K, Ley K. Protection from ischemia-reperfusion induced severe acute renal failure by blocking E-selectin. Crit Care Med. 2000;28:2507–2514. doi: 10.1097/00003246-200007000-00053. [DOI] [PubMed] [Google Scholar]
- 20.Audoy-Remus J, Richard JF, Soulet D, Zhou H, Kubes P, Vallieres L. Rod-shaped monocytes patrol the brain vasculature and give rise to perivascular macrophages under the influence of proinflammatory cytokines and angiopoietin-2. J Neurosci. 2008;28:10187–10199. doi: 10.1523/JNEUROSCI.3510-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghielli M, Verstrepen WA, De Greef KE, Helbert MH, Ysebaert DK, Nouwen EJ, de Broe ME. Antibodies to both ICAM-1 and LFA-1 do not protect the kidney against toxic (HgCl2) injury. Kidney Int. 2000;58:1121–1134. doi: 10.1046/j.1523-1755.2000.00269.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Kubes P. Molecular mechanisms of leukocyte recruitment: organ-specific mechanisms of action. Thromb Haemost. 2003;89:213–220. [PubMed] [Google Scholar]
- 23.Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–726. [PubMed] [Google Scholar]
- 24.Doi K, Hu X, Yuen PS, Leelahavanichkul A, Yasuda H, Kim SM, Schnermann J, Jonassen TE, Frokiaer J, Nielsen S, Star RA. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73:1266–1274. doi: 10.1038/ki.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 26.Singbartl K, Forlow SB, Ley K. Platelet, but not endothelial, P-selectin is critical for neutrophil-mediated acute postischemic renal failure. FASEB J. 2001;15:2337–2344. doi: 10.1096/fj.01-0199com. [DOI] [PubMed] [Google Scholar]
- 27.Jensen EC. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec (Hoboken) 2013;296:378–381. doi: 10.1002/ar.22641. [DOI] [PubMed] [Google Scholar]
- 28.Leelahavanichkul A, Yasuda H, Doi K, Hu X, Zhou H, Yuen PS, Star RA. Methyl-2-acetamidoacrylate, an ethyl pyruvate analog, decreases sepsis-induced acute kidney injury in mice. Am J Physiol Renal Physiol. 2008;295:F1825–F1835. doi: 10.1152/ajprenal.90442.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herter JM, Rossaint J, Block H, Welch H, Zarbock A. Integrin activation by P-Rex1 is required for selectin-mediated slow leukocyte rolling and intravascular crawling. Blood. 2013;121:2301–2310. doi: 10.1182/blood-2012-09-457085. [DOI] [PubMed] [Google Scholar]
- 30.Heyman SN, Lieberthal W, Rogiers P, Bonventre JV. Animal models of acute tubular necrosis. Curr Opin Crit Care. 2002;8:526–534. doi: 10.1097/00075198-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Langenberg C, Bellomo R, May C, Wan L, Egi M, Morgera S. Renal blood flow in sepsis. Crit Care. 2005;9:R363–R374. doi: 10.1186/cc3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69:1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 33.Langenberg C, Bagshaw SM, May CN, Bellomo R. The histopathology of septic acute kidney injury: a systematic review. Crit Care. 2008;12:R38. doi: 10.1186/cc6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lerolle N, Nochy D, Guerot E, Bruneval P, Fagon JY, Diehl JL, Hill G. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol. 2002;168:5817–5823. doi: 10.4049/jimmunol.168.11.5817. [DOI] [PubMed] [Google Scholar]
- 36.Regueira T, Andresen M, Mercado M, Downey P. Physiopathology of acute renal failure during sepsis. Med Intensiva. 2011;35:424–432. doi: 10.1016/j.medin.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Ocuin LM, Bamboat ZM, Balachandran VP, Cavnar MJ, Obaid H, Plitas G, DeMatteo RP. Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J Leukoc Biol. 2011;89:423–432. doi: 10.1189/jlb.0810479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng W, Paunel-Gorgulu A, Flohe S, Hoffmann A, Witte I, Mackenzie C, Baldus SE, Windolf J, Logters TT. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit Care. 2012;16:R137. doi: 10.1186/cc11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treacher DF, Sabbato M, Brown KA, Gant V. The effects of leucodepletion in patients who develop the systemic inflammatory response syndrome following cardiopulmonary bypass. Perfusion. 2001;16((suppl)):67–73. doi: 10.1177/026765910101600i110. [DOI] [PubMed] [Google Scholar]
- 40.Brown KA, Treacher DF. Neutrophils as potential therapeutic targets in sepsis. Discov Med. 2006;6:118–122. [PubMed] [Google Scholar]
- 41.Lewis SM, Khan N, Beale R, Treacher DF, Brown KA. Depletion of blood neutrophils from patients with sepsis: treatment for the future? Int Immunopharmacol. 2013;17:1226–1232. doi: 10.1016/j.intimp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Sun D, Ben-Nun A, Wekerle H. Regulatory circuits in autoimmunity: recruitment of counter-regulatory CD8+ T cells by encephalitogenic CD4+ T line cells. Eur J Immunol. 1988;18:1993–1999. doi: 10.1002/eji.1830181219. [DOI] [PubMed] [Google Scholar]
- 43.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]