Abstract
Laminins play a fundamental role in basement membrane architecture and function in human skin. The C-terminal laminin G domain-like (LG) modules of laminin α chains are modified by proteolysis to generate LG1-3 and secreted LG4-5 tandem modules. In this study, we provide evidence that skin-derived cells process and secrete biologically active peptides from the LG4-5 module of the laminin α3, α4 and α5 chain in vitro and in vivo. We show enhanced expression and processing of the LG4-5 module of laminin α3 in keratinocytes after infection and in chronic wounds in which the level of expression and further processing of the LG4-5 module correlated with the speed of wound healing. Furthermore, bacterial or host-derived proteases promote processing of laminin α3 LG4-5. On a functional level, we show that LG4-5-derived peptides play a role in wound healing. Moreover, we demonstrate that LG4-derived peptides from the α3, α4 and α5 chains have broad antimicrobial activity and possess strong chemotactic activity to mononuclear cells. Thus, the data strongly suggest a novel multifunctional role for laminin LG4-5-derived peptides in human skin and its involvement in physiological processes and pathological conditions such as inflammation, chronic wounds and skin infection.
Key Words: Extracellular matrix, Laminin, Antimicrobial peptides, Wound healing, Chemotaxis, Host defense
Introduction
The basement membrane (BM) of epithelial tissues is a specialized extracellular matrix (ECM) which separates the epidermis from the dermis. Classically, the BM provides mechanical and structural support for epithelial cells and can directly interact with cells via outside-in signaling. Laminins are major components of the BM with fundamental roles in architecture and function [1]. Laminins are heterotrimeric glycoproteins consisting of α, β and γ chains. In vertebrates, five α, three β and three γ chains have been identified, which are linked together by disulfide bonds to form either a cruciform, Y-shaped or rod-shaped structure. The laminin αβγ heterotrimers are assembled into at least 18 different laminin isoforms [2, 3]. Laminin α chains are modified at the C-terminal globular (G) domain divided into five globular modules, termed LG1-5, each with a molecular weight of approximately 20 kDa [4, 5, 6, 7]. These LG modules mainly mediate the interaction of laminins with cell surface receptors such as integrins or with heparan sulfate proteoglycans, whereas the N-terminal region is mainly responsible for the self-assembly and incorporation of laminins into the BM. The LG4-5 domains are separated from LG1-3 by a linker region, which is sensitive to proteolytic processing. For several α chains (α3, α4 and α5), a proteolytic cleavage of the LG4-5 modules has been described, thus generating a secreted LG4-5 module which harbors binding sites for heparin [8] and which seems to play a role during the epithelialization phase of wound repair [9, 10].
In human skin, three laminin α chains are expressed; laminin α3 and α5 chains mainly by keratinocytes and the laminin α4 chain primarily by fibroblasts in the dermis [6, 11, 12]. Our group has previously reported that peptides derived from the LG4 module of human laminin α4 and α5 chains exhibit a dose-dependent antimicrobial activity against Gram-positive and Gram-negative bacteria by the induction of pore formation [13]. These data suggest that processed peptides from the ECM component laminin are able to protect epithelial tissues from invasion by pathogens and are part of the host innate defense mechanism. Similarly, it has been shown that proteolytically released ECM fragments affect inflammatory immune responses, chemotaxis and wound repair. Therefore, ECM fragments not only provide structural support for tissue architecture, but also have bioactive properties and were therefore designated as ‘matrikines’ [14, 15].
Laminin-332 mutations cause epidermolysis bullosa, a severe and usually lethal skin-blistering disease. Most forms of epidermolysis bullosa show severe chronic wounds and skin infections which contribute to impaired wound healing [16]. In this study, we investigated if the LG4-5 modules of laminin α chains in human skin are further processed and whether peptides derived from these modules affect innate immune responses. We show that (1) human skin and skin-derived cells process and secrete biologically active peptides from the LG4-5 module of the laminin α3, α4 and α5 chains in vitro and in vivo, (2) expression of laminin α3 is increased in human keratinocytes by proinflammatory cytokines and growth factors, (3) expression and processing of laminin α3 are increased in chronic wounds and in human keratinocytes after bacterial infection, (4) bacterial and neutrophil-derived proteases are able to further process the laminin α3 LG4-5 module and (5) laminin LG4 module-derived peptides from the α3, α4 and α5 chains display antimicrobial activity and potent chemotactic activity on immune cells and further promote wound healing by increasing keratinocyte proliferation and migration. In summary, our data indicate for the first time that the expression and processing of laminins are tightly regulated in human skin and that processed peptides from laminin α chain LG4-5 modules have critical functions in innate skin immune response and wound healing.
Materials and Methods
Peptide Synthesis
Peptides were synthesized utilizing the Fmoc/tBu chemistry using a multiple peptide synthesizer (Syro II, MultiSynTech). After final deprotection, the crude peptide was purified by high-performance liquid chromatography (HPLC) on a reversed-phase Nucleosil 100-5 C18 column to a purity of >95% using a linear gradient of 5–80% acetonitrile in 0.05% trifluoroacetic acid for 45 min. Peptide mass was checked by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry and by electrospray ionization mass spectrometry. Peptides were lyophilized and dissolved in ddH2O to a concentration of 1 mg/ml and stored at 4°C. The LG4 modules of laminin α3-α5 were digested in silico with staphylococcal peptidase I using the ExPASy PeptideCutter program (http://ca.expasy.org/tools/peptidecutter) and were synthesized as described above. To further ensure the specificity of the responses, peptides were tested and confirmed to be free of endotoxin contamination using Limulus Amebocyte Lysate Chromogenic Endotoxin quantitation kit (Pierce®, Thermo Scientific) according to the manufacturer's protocol.
Cell Culture
HaCaT keratinocytes (immortalized human skin keratinocyte cell line) and primary human fibroblasts (isolated from human foreskin) were cultivated in Dulbecco's modified Eagle medium (DMEM) or RPMI-1640 (Lonza; PAA) supplemented with 10% fetal calf serum (FCS; Biochrom AG). Isolation and cultivation of primary human keratinocytes were described elsewhere [17]. Primary keratinocytes from different donors were used up to passage 4. For the enrichment of secreted proteins from HaCaT keratinocyte and primary fibroblast supernatant, cells were grown to confluence in roller bottles with a growth area of 850 cm2 (BD Falcon™, Schubert and Weiss OMNILAB). Serum was withdrawn and confluent cells were cultivated for 96 h. Cell supernatants (from 1-2 liters of culture) were collected and kept frozen at −20°C for heparin-affinity chromatography. The use and culturing of human skin tissues or skin-derived cells in this study was approved by the Medical Ethical Committee of the University of Tübingen (43/2008B01; 43/2009B02) and was performed in accordance with the principles of the Declaration of Helsinki.
Western Blot
To detect laminin α3, α4 and α5, cell lysates (approx. 20 µg) were loaded onto 3–8% NuPAGE gel and probed with the following antibodies: laminin α3 (HPA009309, Prestige Antibodies®, Sigma), laminin α5 (ab55413, Abcam) and laminin α4 (ab69634, Abcam).
Protein extracts from freshly operated human skin were isolated from approximately 3 g of tissue homogenized under liquid nitrogen using a pestle. The homogenized tissue was resuspended in phosphate buffer and was cleared using 100-µm and 40-µm filters.
The filtrated homogenate was diluted to a volume of 500 ml and applied to heparin-affinity chromatography as described later. Three eluted fractions were collected and 5 µg of protein were separated by electrophoresis on 15% SDS-polyacrylamide gels and blotted onto PVDF membranes (Roche). Recombinant laminin α3 LG4-5 and polyclonal antibody against the native LG4-5 fragment have been described elsewhere [18]. Proteins were visualized with secondary peroxidase-conjugated antibodies (Cell Signaling) and the ECL system (Amersham).
Debridements were collected from patients with chronic wounds under informed consent according to the Ethical Committee of the University of Tübingen (Project No. 634/2012) together with the skin specimens of healthy patients, and were lysed immediately. Equal amounts of protein per sample, determined before using Bradford colorimetric assay, were analyzed. The proteins were separated on polyacrylamide gradient gel 4–20% (NuSep, PEQLAB) and blotted onto PVDF membranes according to the manufacturer's instructions. Polyclonal antibody anti-α3 LG4-5 and β-actin (Cell Signaling) were used and visualized with the corresponding anti-rabbit secondary antibody coupled to horseradish peroxidase (Cell Signaling) and the ECL system (Amersham).
Microbial Strains and Antimicrobial Assay
For the infection studies of HaCaT keratinocytes, overnight bacterial or yeast cultures were diluted 1:100 (v/v) in LB (Miller) broth or YPD medium, respectively. Mid-logarithmic bacterial and yeast suspensions were then resuspended and diluted to a final concentration of approximately 106 CFU/ml in DMEM supplemented with 2% FCS which served as the growth medium during host cell infection. The following pathogens were used: Staphylococcus aureus strain (S. aureus 113) and a clinical isolate (S. aureus 6), Escherichia coli K12, Pseudomonas aeruginosa ATCC 27853 and the yeast Candida albicans ATCC 9028. Inhibitory concentrations were evaluated by CFU assay as previously described.
Proteolytic Cleavage of Recombinant Laminin α3 LG4-5
Alkaline protease and elastase B were purified from P. aeruginosa as described previously [19]. The S. aureus metalloproteinase aureolysin (Axxora GmbH) and S. aureus V8 protease (Perbio Science) were used at indicated amounts and incubated for 1 h at 37°C in PBS, each with 15 ng of recombinant laminin α3 LG4-5. Neutrophil elastase (NE; 1-10 mU) and cathepsin G (0.1-0.5 mU) were obtained from Lee Biosolutions. Additionally, a protease inhibitor cocktail (cOmplete, Mini Protease Inhibitor Cocktail Tablet, Roche) was used to abolish proteolytic activity.
Heparin-Binding Proteins
Cell culture supernatant was passed through a heparin sepharose chromatography column (HiTrap™ Heparin HP 1-ml column) using a peristaltic pump at a flow rate of 1-0.5 ml/min according to the manufacturer's instructions (GE Healthcare). The flow-through was continuously reloaded onto the column overnight and after washing with 5 column volumes of 10 mM sodium phosphate (pH 7.0), it was eluted with a continuous gradient using 10 mM sodium phosphate and 1.5 M NaCl (pH 7.0). The eluted proteins (4-7 ml) were desalted using a cellulose tubular membrane (Cellu·Sep H1 MWCO 1 kDa; Bioron) overnight at 4°C, concentrated by speed-vac and resuspended in 1-2 ml of ddH2O. The concentrated solution was then fractioned by injecting it into the HPLC system. Separations were performed with a linear gradient system from 5 to 80% of solvent B (80% acetonitrile in 0.050% aqueous trifluoroacetic acid) for 60 min. From 2 min after the start of elution, fractions were collected every 2 min. The 30 fractions obtained from each cell line were concentrated in a vacuum concentrator and each was resuspended in 20 µl of ddH2O to measure the protein content (NanoPhotometer™, Pearl Implen). Heparin-binding proteins were screened for antimicrobial activity using both RDA and CFU assays. Only heparin-binding protein fractions with antimicrobial activity were considered for subsequent analysis.
MALDI-TOF and Liquid Chromatography-Mass Spectrometry
Prior to MALDI-TOF, secreted heparin-binding protein fractions from primary fibroblasts and HaCaT keratinocytes were analyzed by SDS-PAGE Tris/glycine (10%) and silver-gel staining. Bands of interest (range 10-100 kDa) were cut out of the gel, destained and analyzed by in-gel trypsin digestion after reduction and alkylation and MALDI-TOF mass spectrometry with 2,5-dihydroxybenzoic acid (DHB) as the matrix (Reflex IV, Bruker Daltonics). Peptides from the mass spectra of in-gel digest samples were matched against the SWISS-PROT database using the MASCOT search engine for peptide-mass-fingerprinting.
Samples of interest were dissolved in solution A (2% acetonitrile and 0.1% formic acid), and 1 pmol of each sample was separated by reversed-phase liquid chromatography (nano HPLC system, Eksigent NanoLC-2D) with a gradient ranging from 0 to 55% solution B (80% acetonitrile and 0.1% formic acid) within 120 min. The eluted peptides were analyzed in a LTQ Orbitrap hybrid mass spectrometer (Thermo Fischer Scientific). The liquid chromatography-mass spectrometry data were automatically processed against the SWISS-PROT database using a Proteome Discoverer (Thermo Scientific). Identified sequences were manually evaluated (and then validated by comparison with their respective synthetic counterpart).
Keratinocyte Viability and Migration
For the viability assay, HaCaT keratinocytes (2.5 – 5 × 103 cells) were seeded into 96-well plates, cultured for 24 h and then, for an additional 24 h, under serum starvation conditions with increasing concentrations of LG4-derived peptides or LL-37 (10-8-10-6M) in RPMI + 1% FCS. After washing, 4-methylumbelliferyl heptanoate in PBS (100 µg/ml) was added and incubated for 1 h at 37°C. The assay is based on the hydrolysis of 4-methylumbelliferyl heptanoate by intracellular esterases of viable/proliferating cells resulting in the production of highly fluorescent 4-methylumbelliferone that can be measured. Microplates were measured in a fluorescence microplate reader (Berthold) at 355 nm excitation and 460 nm emission. Measurement of the number of cells compared to the control was determined as percentage of viable cells.
Cell migration was monitored using the xCELLigence system (Roche Applied Science). For determination of cell migration, 160 µl of media (RPMI, 1% FCS) containing laminin LG4-derived peptides (3-SFM29, 4-TLF20 and 5-PGR12), LL-37 (each 10-6M) or an appropriate solvent as a negative control and 10% FCS as a positive control were added to each well of a CIM plate 16 (Roche Applied Science; 8-μm pore size). The upper chambers were filled with medium (RPMI + 1% FCS, 30 μl/well), and the plate was incubated at 37°C in 5% CO2 for 1 h. The background was measured using an RTCA DP analyzer. The cells were added to each well (40,000 cells/well) and the plate was incubated at 25°C. After 30 min, the CIM plate was assembled onto the RTCA DP analyzer, and cell migration was assessed at 15-min intervals for up to 48 h at 37°C in 5% CO2. The obtained data were analyzed using the provided RTCA software.
Real-Time RT-PCR Gene Expression Analysis
A real-time RT-PCR analysis was used to analyze the expressions of laminin α3 (forward primer: tgc cca tgt cct cac act aa; reverse primer: cac gtc tcc ccc att cac) and β-actin (forward primer: ttg tta cag gaa gtc cct tgc c; reverse primer: atg cta tca cct ccc ctg tgt g) in primary and HaCaT keratinocytes following infection with live bacteria and yeast for 6 h in RPMI + 2% FCS. HaCaT keratinocytes were stimulated with chemokines, cytokines and growth factors for 24 h, at 37°C, 5% CO2 in RPMI + 10% FCS. Stimulated or control keratinocytes were harvested and total RNA was isolated with the NucleoSpin® RNA II kit (Macherey-Nagel) according to the manufacturer's protocol. One microgram RNA was reverse-transcribed (Invitrogen) using random hexamer primers. cDNA corresponding to 25 ng RNA served as a template for real-time PCR. Gene expression was analyzed using SYBR Green I mix (Roche) in a LightCycler® 480 System. All experiments were performed in triplicate and the differential expression was detected by using the comparative 2-ΔΔCT method, using β-actin as a reference gene.
Immunofluorescence Staining
Glass coverslips containing either infected or uninfected HaCaT monolayer cells were fixed in 4% paraformaldehyde for 2 min and then washed and incubated for 10 min with 0.5% Triton X100 in PBS. Coverslips were washed and incubated in blocking buffer (goat serum) at a 1:20 dilution for 20 min. Cells were then incubated overnight with an antibody against laminin α3 (anti-LAMA3; HPA009309, Sigma-Aldrich) at a 1:50 dilution. Cells were washed 3 times and then incubated at room temperature for 1 h with secondary goat anti-rabbit immunoglobulin G antibody conjugated with Cy™3 (Invitrogen) at a dilution of 1:100. Cells were washed another 3 times and incubated for 5 min with Yo-Pro-1 (Molecular Probes, Invitrogen). All washing steps and antibody dilutions were performed with BSA (0.05%) and Tween 20 (0.05%) in PBS. Fluorescent signals were photographed with a confocal laser scanning microscope (Leica TCS SP; Leica Microsystems) at a magnification of ×600.
Monocyte Chemotaxis
Peripheral blood was obtained from healthy volunteers after informed consent according to the Ethics Committee of the University of Tübingen (Project No. 204/2012). Briefly, EDTA anticoagulated whole blood was diluted with an equal volume of PBS. The diluted sample was layered on top of a Biocoll separating solution (Biochrom) and centrifuged without brakes. Enriched cells were washed with PBS. Monocytes were purified from human PBMCs with the use of a MACS™ CD14 monocyte isolation kit (Miltenyi Biotec) according to the manufacturer's recommendation. Purity of each monocyte population was >95% as determined after MACS by flow-cytometric assessment with anti-CD14 antibody (Miltenyi Biotech). Migration of monocytes was assessed using a 96-well MultiScreen™ filtration plate with a 5-µm pore size (Merck Millipore). In brief, chemotactic factors or peptide solvent (control; ddH2O) diluted in chemotaxis medium (RPMI 1640 + 1% FCS) was placed in the wells of the lower compartment (100 µl) and 75 µl of monocytes, suspended in chemotaxis medium (1 × 106 cells/ml), were added to the upper compartment. After incubation at 37°C in humidified air with 5% CO2 for 90 min, the filters were removed and the migrated monocytes in the lower compartment were harvested and counted using a flow cytometer (LSR II, BD Biosciences). The results are presented as a percentage of migrated monocytes compared to the input monocytes.
The viability of monocytes after peptide treatment was determined by propidium iodide (PI) uptake. In brief, monocytes were incubated for 90 min at 37°C in 5% CO2 with the appropriate peptide concentrations (10-5M for peptides and 10-8M for fMLP) or 50% ethanol as a positive control. Viable or dead cells were estimated by flow cytometry (LSR II) after staining with 5 μM PI.
Intracellular Calcium Flux
Red blood cells were removed from EDTA-anti-coagulated blood using RBC lysing buffer (BioLegend). Leukocytes (107/ml) were stained with anti-CD14 PE-Cy7 (BioLegend) in a loading medium of 2-µM fluo-4 AM (Invitrogen) and 0.02% Pluronic F127 (Molecular Probes) in RPMI without phenol red (Gibco) for 30 min at 37°C. Cells were washed and incubated for another 30 min at 37°C to allow complete ester hydrolysis. Cells were aliquoted and stored on ice until calcium measurement. For stimulation, cells were warmed to 37°C and baseline was measured for 40-50 s before stimulant was added for measurement. Fluo-4 fluorescence was detected with an excitation of 488 nm and a 530/30 emission filter on an LSR II flow cytometer.
Statistical Analysis
Statistical significance for differences between groups was determined using the Kruskal-Wallis nonparametric test, followed by the Dunn post hoc test using Graphpad Prism (Graphpad Software; http://www.graphpad.com).
Results
Identification of Secreted Peptides from the LG4-5 Module of Laminin α Chains in Human Skin Cells in vitro
We could show that primary human fibroblasts produce laminin α4 and that primary keratinocytes as well as the immortalized human keratinocyte cell line HaCaT synthesize laminin α3 and α5 chains (online suppl. fig. S1; for all online supplementary material, see www.karger.com/doi/10.1159/000357032) confirming previous studies [20]. In order to identify naturally processed and functionally active laminin α chain-derived peptides in human skin-derived cells, we first enriched heparin-binding proteins and peptides from the supernatants of human primary fibroblasts and the keratinocyte cell line HaCaT. Subsequently, we enriched proteins and peptides with antimicrobial activity against Gram-positive (S. aureus 113) and Gram-negative (E. coli K12) bacteria by HPLC fractionation of heparin-binding proteins. Fractions which showed antimicrobial activity were then digested by trypsin and analyzed by MALDI-TOF or liquid chromatography-mass spectrometry. Peptides were identified using database information (NCBI, MASCOT search engine). Interestingly, as a proof of principle, we identified laminin α3- and α5-derived peptides from the LG4-5 module in the supernatant of HaCaT keratinocytes and laminin α4-derived peptides from the LG4-5 module in the supernatant of primary fibroblasts. Furthermore, we could also detect proteins with a known role in antimicrobial action like kallikrein 7 [21] and other uncharacterized, secreted proteins with heparin-binding affinity, which are potentially new antimicrobial factors (table 1). We repeated the procedure with non-trypsin-digested samples to identify naturally processed laminin-derived peptides in HaCaT keratinocytes and primary fibroblasts. Without trypsin processing, we were able to identify one 10-mer peptide derived from the LG4 module of the laminin α4 chain with the sequence ENDFMTLFLAH (named 4-END11) in the supernatant of primary fibroblasts (online suppl. fig. 2). Using this technique, we could identify secreted LG4-5-derived peptides in human skin-derived cells in vitro.
Table 1.
List of identified secreted proteins enriched by heparin chromatography and screened for antimicrobial activity after MALDI-TOF and liquid chromatography-mass spectrometry analysis
| HaCaT keratinocytes | Primary fibroblasts |
|---|---|
| Nucleolin | Secreted frizzled-related protein 2 |
| Kallikrein 7 | Cathepsin K |
| AN1-type zinc finger protein 3 | Complement C1r subcomponent |
| Heparin-binding and integrin-binding | Leucine-rich repeat-containing protein 15 |
| segment of human fibronectin | Spondin-1/thrombospondin |
| Tenascin | Follistatin |
| Laminin subunit α3, LG4 – 5 | Laminin subunit α4, LG4 – 5 |
| Laminin subunit α5, LG4 – 5 |
In bold type: identified laminin LG4 – 5-derived fragments.
Proteolytic Processing of the LG4-5 Module of the Laminin α3 Chain Is Induced in Wounded Human Skin in vivo
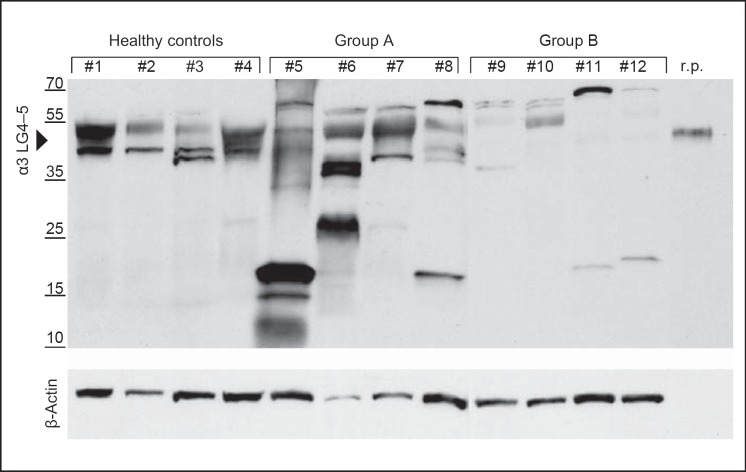
The laminin α3 subunit, which is part of laminin-332, is a major component of epithelial cells. Therefore, we searched for peptides derived from the laminin α3 LG4-5 module in human keratinocytes. Using an antibody-based approach, we analyzed the processing of the laminin α3 LG4-5 module in HaCaT and primary human keratinocytes. However, no smaller peptides were detected as a result of further processing (data not shown). We then asked whether the laminin α3 chain LG4-5 module is further processed in healthy human skin in vivo. We isolated proteins and peptides secreted and deposited within the dermis and epidermis from healthy human skin explants and separated heparin-binding proteins to enrich for laminin α chain LG4-derived peptides and proteins. Three heparin-binding fractions were collected from the skin explants of healthy individuals (online suppl. fig. 3). Only fraction 3 was positive for the full-length LG4-5 module (approx. 40 kDa) processed from the laminin α3 chain, which was detected by Western blot analysis using a polyclonal antibody specific for the α3 LG4-5 module. However, we did not detect immunoreactive LG4-5-derived peptides <40 kDa in any of the fractions. Subsequently, we asked whether expression and further processing of the laminin α3 LG4-5 module is induced in wounded skin. For this purpose, we analyzed wounded human skin and searched for α3 LG4-5-derived peptides. Samples from 8 patients with chronic wounds in the absence of apparent infections (wound debridement of chronic leg ulcers) and skin explants from 4 healthy donors were analyzed for laminin α3 LG4-5 protein profile by Western blot using an antibody against the LG4-5 module of the laminin α3 chain (fig. 1). The patient population was subdivided into group A patients with good to moderate wound healing and group B patients with poor wound healing. Group A, in contrast to group B, displayed accelerated reepithelialization, less erythema and shrinking of the ulcer over time. Interestingly, we found - besides deposition of the processed laminin α3 LG4-5 module protein (approx. 40 kDa) - smaller fragments from the LG4-5 module ranging from 35 to 12 kDa in most of the patients. The patients in both groups A and B featured a fragment of approximately 18 kDa in size more often than those in the healthy control group. However, in group B patients (No. 9-12) with poor wound healing, the amount of deposited LG4-5 was lower than in group A patients (No. 5-8) and healthy donors (fig. 1). Furthermore, samples from group A patients displayed increased amounts of deposited and processed LG4-5 peptides compared to those from healthy skin. In the skin samples of the healthy donors (No. 1-4), we failed to detect smaller peptides processed from the LG4-5 module as shown previously. These data suggest that in healthy human skin, the laminin α3 LG4-5 module is processed in vivo and that further processing of the α3 LG4-5 module generating smaller peptides is promoted during wound healing.
Fig. 1.
Expression and proteolytic processing of the laminin α3 LG4-5 module are increased in wounded skin. Western blots of freshly prepared whole lysates from skin specimens of healthy controls and samples from patients with chronic wounds (debridement) are shown. Group A exhibited good wound healing and group B displayed poor and impaired wound healing. Recombinant laminin α3 LG4-5 (r.p.) was applied (20 ng) to probe full-length α3 LG4-5 (approx. 40 kDa). The reproducibility of the Western blot assay was tested in at least 3 separate runs.
Cytokines and Growth Factors Induce Laminin α3 Expression in Human Keratinocytes
Wound healing is a highly dynamic process and involves complex interactions of the ECM, growth factors, chemokines and proinflammatory cytokines, pathogens and infiltrating leukocytes. We first tested whether regulatory cytokines and growth factors known to be involved in wound healing (TGF-β, TNF, IFN-γ, IL-6, IL-1β, IL-10, EGF and IL-4) could stimulate the expression of laminin α3 and the deposition of laminin α3LG4-5 modules.
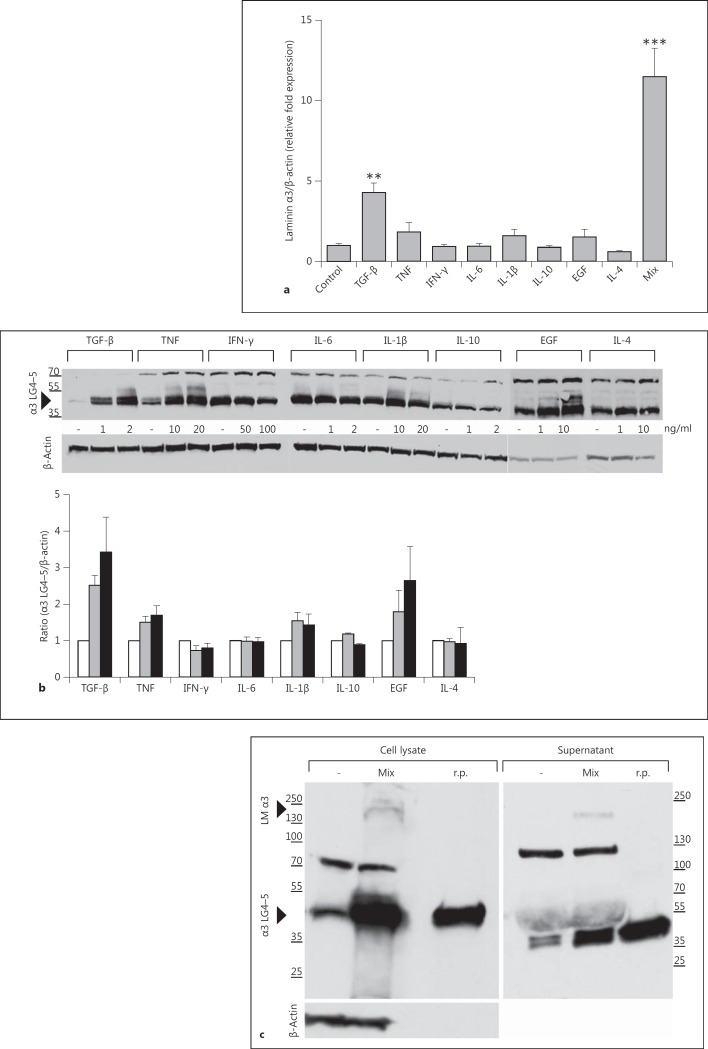
We stimulated HaCaT keratinocytes for 24 h with increasing doses of these cytokines or growth factors and performed either real-time PCR for laminin α3 (fig. 2a) or Western blot analyses with an antibody specific for the laminin α3 LG4-5 module (fig. 2b). Interestingly, TGF-β, TNF, IL-1β, EGF and, more extensively, a combination of the latter compounds induced laminin α3 RNA (fig. 2a) and protein expression and a dose-dependent upregulation of the laminin α3 LG4-5 module compared to untreated cells (fig. 2b). In contrast, the anti-inflammatory Th2 cytokine IL-4 inhibited laminin α3 production in HaCaT keratinocytes. In addition, no further processing of the laminin α3 LG4-5 module was detected. In wounds, a combined sensing and interdependence of different cytokines and growth factors regulate the process of healing.
Fig. 2.
Proinflammatory cytokines and growth factors increase laminin α3 expression in keratinocytes. a Real-time PCR analysis of induction of RNA expression (laminin α3) by stimulation of HaCaT keratinocytes with TGF-β, TNF, IFN-γ, IL-6, IL-1β, IL-10, EGF or IL-4 for 24 h with the highest concentrations or with a mix (TGF-β 1 ng/ml, TNF 10 ng/ml, IL-1β 10 ng/ml and EGF 10 ng/ml). The graph shows means ± SD from at least 3 independent experiments. ** p < 0.01, *** p < 0.001. b Western blot analysis and corresponding densitometric quantification of at least 3 independently performed experiments of α3 LG4-5 production in HaCaT keratinocytes 24 h after exposure to several cytokines and growth factors. Unstimulated cells (white bars), first concentrations (grey bars) and second concentrations (black bars) were: 0, 1 and 2 ng/ml of TGF-β; 0, 10 and 20 ng/ml of TNF; 0, 50 and 100 ng/ml of IFN-γ; 0, 1 and 2 ng/ml of IL-6; 0, 10 and 20 ng/ml of IL-1β; 0, 1 and 2 ng/ml of IL-10; 0, 1 and 10 ng/ml of EGF, and 0, 1 and 10 ng/ml of IL-4, respectively, in comparison to untreated cells. β-Actin served as a loading control. c Western blot analysis of α3 LG4-5 (approx. 40 kDa) and laminin α3 (approx. 200 kDa) in cell lysate and supernatant of HaCaT cells stimulated (Mix) with a cytokine mix (TGF 1 ng/ml, TNF 10 ng/ml, IL-1β 10 ng/ml and EGF 10 ng/ml) or unstimulated (-). Recombinant α3 LG4-5 (r.p.; 20 ng) served as a control. The results are representative of at least 3 independently performed Western blot experiments.
Next, we analyzed the effect of a combination of TGF-β, TNF, IL-1β and EGF in HaCaT in more detail. As shown in figure 2c, this combination of cytokines and growth factors was able to induce increased levels of laminin α3 (approx. 200 kDa) and the α3 LG4-5 module (approx. 40 kDa) in cell lysates and also its secretion to culture supernatant. Surprisingly, further processing of the laminin α3 LG4-5 module was still not found or only to a limited extent. Western blot analyses revealed the 40 kDa LG4-5 module as well as an additional product of 70-80 kDa in the cell lysates and a product of approximately 120 kDa in the supernatant of HaCaT keratinocytes. Since we used a polyclonal antibody to the laminin α3 LG4-5 module for immunodetection, we were able to detect uncleaved laminin α3 with intact LG4-5 (approx. 200 kDa). Detected bands of 70-80 kDa and approximately 120 kDa seem to be either dimers/trimers formed by laminin α3 LG4-5 or N-terminally cleaved products from the laminin α3 chain.
Laminin α3 Chain Expression and Processing of Its LG4-5 Module Are Increased after Infection
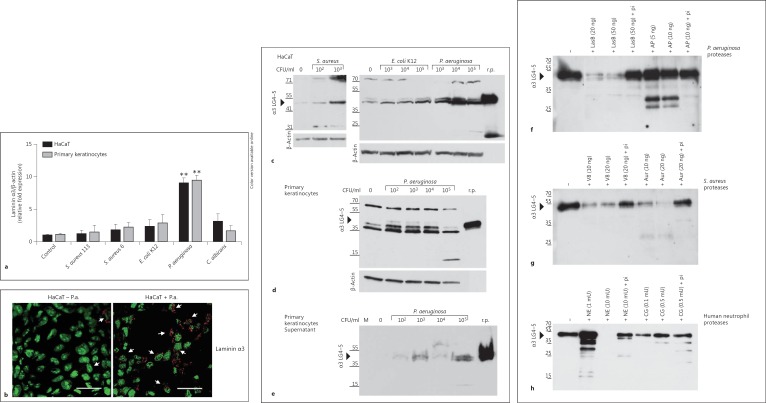
Wounds can be easily infected by pathogens. To analyze whether laminin α3 chain expression is induced by pathogens, we determined expression of laminin α3 in primary keratinocytes and HaCaT cells 6 h after infection with various pathogens using real-time PCR (fig. 3a).
Fig. 3.
Laminin α3 chain and α3 LG4-5 expression in keratinocytes is upregulated following pathogenic challenge and bacterial/neutrophil proteases are able to cleave α3 LG4-5. a HaCaT keratinocytes and primary human keratinocyteswere analyzed by real-time-PCR 6 h after infection with various bacterial pathogens [S. aureus 113, S. aureus 6 (clinical isolate 6), E. coli K12 and P. aeruginosa] and the yeast C. albicans. The fold induction of human laminin α3 expression was normalized to β-actin, compared to the uninfected cells (control) set as 1. Means ± SD of 3 independent experiments are shown. ** p < 0.01. b HaCaT cells were analyzed by confocal microscopy for laminin α3 expression 6 h after infection with 1 × 106 CFU/ml P. aeruginosa (HaCaT + P.a.) compared to the uninfected HaCaT cells (HaCaT - P.a.). The cell nuclei are green and the laminin α3 is red. Arrows indicate slight expression of laminin α3 in uninfected cells and increased expression in infected cells. Bar: 30 μm. Colors refer to the online version only. c-h One gel, representative of >3 experiments performed. c-e Recombinant α3 LG4-5 (r.p.; 20 ng) served as a control. c Western blot of confluent HaCaT cells infected with S. aureus 113 (0, 102 and 103 CFU/ml), E. coli K12 (0, 103, 104 and 105 CFU/ml) or P. aeruginosa (103, 104 and 105 CFU/ml) for 6 h indicating expression of laminin α3 LG4-5 (approx. 40 kDa) in cell lysates. β-Actin served as a loading control. d Laminin α3 LG4-5 expression in cell lysates of primary keratinocytes in response to P. aeruginosa (102, 103, 104 and 105 CFU/ml) infection for 6 h compared to uninfected cells (0 CFU/ml). β-Actin served as a loading control. e Secretion of α3 LG4-5 into supernatant shown by immunoblot of cell culture supernatants from primary keratinocytes infected with increasing concentrations of P. aeruginosa compared to uninfected cells (0). Control media alone (M) was applied. f, g Recombinant α3 LG4-5 (approx. 15 ng) was incubated with purified proteases from P.aeruginosa, i.e. elastase B (LasB) or alkaline protease (AP) (f), or the metalloprotease aureolysin (Aur) and the serine proteinase V8 from S. aureus (g) for 60 min at 37°C. h Recombinant α3 LG4-5 (approx. 15 ng) was incubated with increasing concentrations of NE (1, 10 mU) or cathepsin G (CG; 0.1, 0.5 mU) in PBS for 60 min at 37°C. Addition of protease inhibitor cocktail (pi) abolished proteolytic activity. Proteolytic processing was analyzed by Western blot using a polyclonal antibody to probe α3 LG4-5.
Interestingly, expression of laminin α3 was significantly induced by the Gram-negative pathogen P. aeruginosa, whereas induction of laminin α3 expression remained modest following exposure to Gram-positive bacteria, two S. aureus strains (S. aureus 113 and S. aureus 6) and the yeast C. albicans indicating specific pathways of induction. Immunofluorescence analyses of laminin α3 (fig. 3b) confirmed the strong induction of laminin α3 expression in HaCaT keratinocytes after pseudomonal infection also on the protein level.
To examine if processing of the laminin α3 LG4-5 module in human keratinocytes is affected after exposure to pathogens, we harvested the cell lysates and supernatants of HaCaT keratinocytes and primary keratinocytes 6 h after bacterial infection. Following exposure to several bacteria including S. aureus, E. coli and P. aeruginosa, more of the laminin α3 LG4-5 module (approx. 40 kDa) was detected, with P. aeruginosa again being the most prominent (fig. 3c). These investigations, conducted with HaCaT keratinocytes, could also be confirmed using primary human keratinocytes (fig. 3d). Using a polyclonal antibody targeting the laminin α3 LG4-5 module, we observed additional higher molecular weight products, potentially representing dimerizaton of the laminin α3 LG4-5 module (70-80 kDa) similar to previous results. To analyze whether infection also increasesthe secretion of the laminin α3 LG4-5 module, we analyzed the culture supernatants of primary human keratinocytes 6 h after infection with increasing concentrations (CFU/ml) of P. aeruginosa. Increased secretion was observed in supernatants from infected keratinocytes when compared to noninfected ones (fig. 3e). Next, we asked whether bacterial proteases are able to further process the laminin α3 LG4-5 module. For this purpose, recombinant laminin α3 LG4-5 was incubated with (1) purified elastase B or alkaline protease - both exoproteases derived from P. aeruginosa - (fig. 3f) or (2) the metalloprotease aureolysin and the serine proteinase V8 - both from S. aureus (fig. 3g). We show that purified proteases from P. aeruginosa or S. aureus cleave laminin α3 LG4-5 in a dose-dependent manner. The susceptibility of laminin α3 LG4-5 to proteolytic cleavage by pseudomonal and staphylococcal proteases was abolished when adding protease inhibitor cocktail.
Wounds are often infiltrated by neutrophils. Secreted neutrophil proteases could have important consequences for epithelial repair during wound healing. Thus, we analyzed the ability of NE and cathepsin G to cleave laminin α3 LG4-5 (fig. 3h). The band intensity of intact laminin α3 LG4-5 was reduced dose-dependently when compared to the control, indicating that both serine proteases are able to further degrade the laminin α3 LG4-5 module.
Functional Analyses of Laminin LG4 Peptides Generated in silico
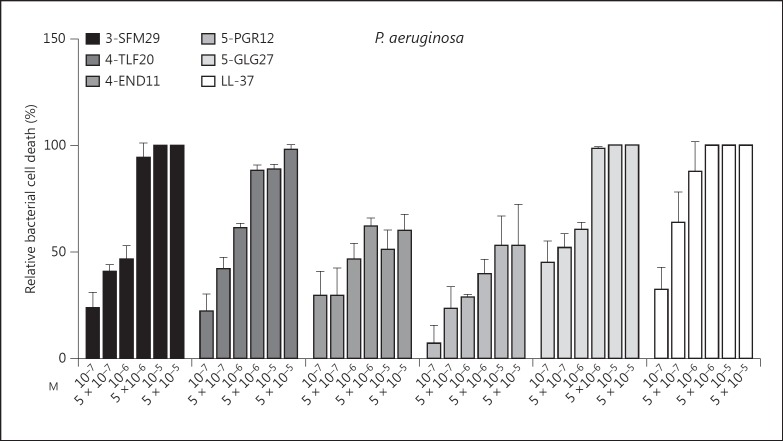
Since processing of laminin α3 LG4-5 occurs in chronic wounds and can be induced by bacterial and neutrophil proteases, we asked whether α3 LG4-5-derived peptides are functionally involved in host defense. To this end, four peptides from the laminin α3 and α5 LG4 module and three peptides from the α4 chain LG4 module predicted to be generated by staphylococcal peptidase I using the ExPASy PeptideCutter were synthesized and screened for heparin-binding activity. We identified one heparin-binding peptide per α chain LG4 module with strong antimicrobial activity against S. aureus, E. coli, P. aeruginosa and the yeast C. albicans (table 2). The identified peptides are termed as 3-SFM29, 4-END31 and 5-GLG27 (the first number indicates the origin of the laminin α chain, followed by the first 3 amino acid compositions and total numbers of amino acids). Furthermore, we observed that shortened peptides from the LG4 module of laminin α4 and α5 (4-TLF20 and 5-PGR12) had equal or even higher antimicrobial activity (table 2). Therefore, in the following functional assays, we concentrated on the effects of the three laminin α chain LG4 module-derived peptides 3-SFM29, 4-TLF20 and 5-PGR12. The 11-mer fragment ENDFMTLFLAH (4-END11) derived from laminin α4 LG4 and formerly identified in primary human fibroblasts (online suppl. fig. 2) showed partially overlapping sequences with the predicted peptides 4-END31 and 4-TLF20. Laminin LG4-derived peptides showed antibacterial activity against P. aeruginosa in a dose-dependent manner similar to the known antimicrobial peptide LL-37 (fig. 4).
Table 2.
Antimicrobial active peptides derived from the LG4 modules of laminin α3, α4 and α5 chains
| Chain | Peptide | Sequence | IC901, µM |
|||
|---|---|---|---|---|---|---|
| S. aureus 113 | E. coli K12 | P. aeruginosa 27853 | C. albicans 9028 | |||
| α3 | 3-SFM29 | SFMALYLSKGRLVFALGTDGKKLRIKSKE | ~2 | ~2 | ~1 | ~3 |
| α4 | 4-END31 | ENDFMTLFLAHGRLVYMFNVGHKKLKIRSQE | >10 | ~3 | n.d. | >10 |
| 4-TLF20 | TLFLAHGRLVYMFNVGHKKL | ~4 | ~3 | ~4 | >20 | |
| 4-END11 | ENDFMTLFLAH | >50 | >50 | >50 | >50 | |
| α5 | 5-GLG27 | GLGTRLRAQSRQRSRPGRWHKVSVRWE | ~2 | ~5 | ~2 | ~2 |
| 5-PGR12 | PGRWHKVSVRWE | ~3 | ~3 | >50 | >50 | |
Sequences of peptides are given in the single-letter code and are named with the number presenting the corresponding α chain and the first 3 amino acids followed by the total number of amino acids. Data are the mean ± SD of 3 independent experiments.
The inhibitory concentration which leads to at least 90% cell death in the CFU assay.
Fig. 4.
Laminin α chain LG4 peptides show potent dose-dependent antimicrobial activity against P. aeruginosa. The dose-dependent antibacterial effects were tested in a CFU assay against P. aeruginosa ATCC 27853 versus no peptide control (solvent; ddH2O). Relative bacterial cell death was calculated from the mean reduction in the number of CFU after peptide treatment compared to control. The graph shows means ± SD from at least 4 independent experiments.
Laminin LG4 Peptides Support Keratinocyte Migration
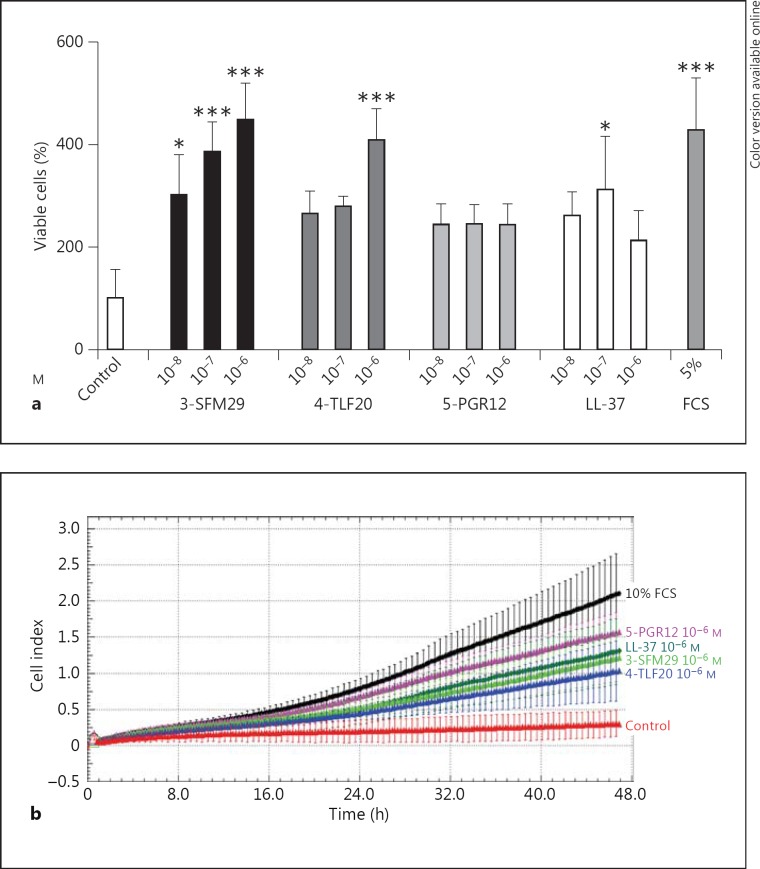
Laminin-332 and laminin-551 play a key role in regulating keratinocyte migration in wound healing [12]. Based on our studies of patients with chronic wounds showing increased levels of laminin α3 LG4-5 and fragments thereof, we next investigated the functional consequences on keratinocyte viability and migration. HaCaT cells following serum starvation (RPMI + 1% FCS) were incubated with increasing concentrations of 3-SFM29, 4-TLF 20 and 5-PGR12 for 24 h, and the increase of viable cells was determined indicative for keratinocyte proliferation (fig. 5a). Notably, the α3 LG4 peptide 3-SFM29 and the α4 LG4 peptide 4-TLF20 induced a 3- to 4-fold increase of viable cells in a concentration-dependent manner (10-8-10-6M), with the α5 LG4 peptide 5-PGR12 leading to an approximately 2-fold increase of viable cells similar to the host defense peptide LL-37. As a positive control, we used RPMI supplemented with 5% FCS. Proproliferative effects of LG4-derived peptides were further confirmed by BrdU incorporation assay (data not shown). Next, we monitored if laminin LG4 peptides regulate the migratory ability of HaCaT keratinocytes in real time, using the xCELLigence system. As expected, LG4 peptides significantly promoted the migration of keratinocytes compared to the nontreated control over time (p < 0.0001; fig. 5b). In addition, media containing 10% FCS was included as a positive control. Therefore, LG4 peptides have effects similar to LL-37, which is known to induce keratinocyte migration.
Fig. 5.
Effect of laminin LG4 peptides on keratinocyte viability, proliferation and migration. a Cell viability expressed as % viable cells was assayed in a fluorescence cell viability assay 24 h after treatment with increasing peptide concentrations (10-8-10-6M in RPMI + 1% FCS) of 3-SFM29, 4-TLF20, 5-PGR12 and LL-37 and as a negative control peptide solvent (control; ddH2O), respectively, and RPMI supplemented with 5% FCS as a positive control. The results (means ± SD) compared to controls are representative of at least 3 independently performed experiments. * p < 0.05, *** p < 0.001. b Keratinocyte cell migration was examined for up to 48 h using the xCELLigence real-time cell analyzer DP (Roche Diagnostics). HaCaT keratinocytes were treated with indicated peptides (10-6M), solvent without peptide as a negative control or with RPMI + 10 % FCS as a positive control. The results are expressed as cell (migration) index ± SD. The data are representative of at least 3 independent experiments.
Together, our data suggest that laminin LG4 peptides enhance the survival and proliferation of keratinocytes and significantly induce keratinocyte migration over time.
Laminin LG4 Peptides Are Potent Chemotactic and Activating Factors for Human Monocytes
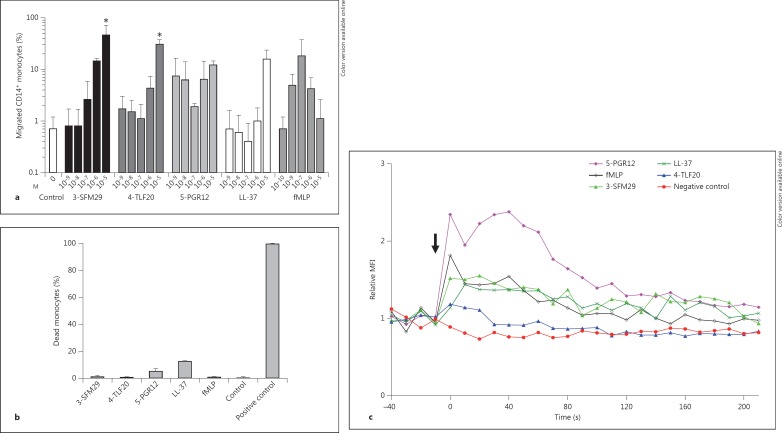
Monocytes/macrophages are essential for wound healing, where they dominate the cellular infiltrate and participate until the reparative process is completed. To test whether the laminin α chain LG4-derived peptides 3-SFM29, 4-TLF20 and 5-PGR12 contribute to the recruitment of monocytes, we performed chemotaxis assays with human whole-blood leukocytes isolated by density-gradient centrifugation. Above all, monocytes showed high chemotactic activity compared to other peripheral blood mononuclear cells in response to laminin LG4 peptides (online suppl. fig. S4). Therefore, we evaluated the ability of these peptides to induce chemotaxis of purified monocytes using a modified Boyden chamber assay. As shown in figure 6a, the laminin peptides 3-SFM29 and 4-TLF20 exhibited potent and significant chemotactic activity towards monocytes in a dose-dependent manner (approx. 46% migration of monocytes). Importantly, laminin peptides were even more efficient than cathelicidin LL-37 at the same concentrations (10-9- 10-5M), which was already reported to have strong chemotactic activity [22]. 5-PGR12 also induced chemotaxis, but this was concentration-independent. fMLP served as an additional positive control (10-10-10-6M), which led to approximately 18% chemotactic migration; random migration resulted in approximately 1% migrated cells. To exclude the cytotoxic effects of peptides (10-5M), the viability of monocytes was evaluated by PI staining after incubation under the same conditions. Flow cytometric analysis indicated that the peptides did not induce monocyte cell death at all concentrations tested (fig. 6b). Only 5-PGR12 and LL-37 showed a slight cytotoxic effect (<15%) at the maximum concentration when compared to the untreated control.
Fig. 6.
Laminin LG4 peptides induced chemotaxis of human peripheral blood monocytes. a The migration of human monocytes in response to 3-SFM29, 4-TLF20, 5-PGR12, LL-37, fMLP and peptide solvent (ddH2O; control) was evaluated in a chemotaxis assay after 90 min. Transmigrated monocytes in the lower wells were harvested, stained for anti-CD14 and quantified by counting CD14+ cells using flow cytometry. Percent migrated CD14+ monocytes are expressed as the means ± SD of 3 independent experiments. * p < 0.05. b The effect of peptide treatment (each 10-5M), fMLP (10-8M) or with 50% ethanol as a positive control on the monocyte cell membrane permeability was measured by PI exclusion (presented as % dead monocytes), using flow cytometry expressed as the means ± SD from 2 independent experiments. c Calcium mobilization induced by laminin α chain LG4 peptides (3-SFM29, 4-TLF20 and 5-PGR12; each 10-5M) or fMLP (10-9M). Leukocytes were loaded with fluo-4 AM and stimulated. The arrow indicates the time point when peptides were added. The median fluo-4 fluorescence intensity (MFI) of the CD14+ subpopulation was determined at 10-second intervals. One representative measurement out of 3 independent experiments is shown.
The addition of peptides to both chambers (checkerboard analysis) in equal concentrations efficiently reduced the migration of CD14+ monocytes. Thus, the migration of monocytes induced by 3-SFM29, 4-TLF20 and 5-PGR12 were based predominantly on chemotaxis rather than on chemokinesis, similar to the effects of LL-37 and fMLP (table 3).
Table 3.
Checkerboard analysis of monocyte migration in response to laminin LG4 peptides 3-SFM29, 4-TLF20, 5-DGR12, LL-37 and fMLP with indicated molar concentrations relative to the control set as 1
| Upper chamber (M) |
|||
|---|---|---|---|
| 0 | 10–7 | 10–5 | |
| Lower chamber (M) | |||
| 3-SFM29 | |||
| 0 | 1.0 ±0.3 | 4.8 ± 2.7 | 10.0± 2.4 |
| 10–7 | 1.1 ±1.5 | 0.2 ± 0.1 | 10.0± 4.0 |
| 10– 5 | 14.0 ±3.7 | 9.9 ± 1.2 | 7.8± 0.9 |
| 4-TLF20 | |||
| 0 | 1.0 ±0.3 | 0.4 ± 0.3 | 4.2± 1.4 |
| 10–7 | 3.5 ±1.3 | 1.1 ± 1.4 | 3.3± 1.0 |
| 10– 5 | 34.8 ±3.9 | 21.7 ± 2.3 | 11.9± 0.4 |
| 5-PGR12 | |||
| 0 | 1.0 ±0.5 | 5.2 ± 2.0 | 0.5± 0.02 |
| 10–7 | 28.6 ±2.6 | 13.6 ± 5.3 | 0.6± 0.1 |
| 10–5 | 1.7 ±0.1 | 1.1 ± 0.1 | 0.8± 0.3 |
| LL-37 | |||
| 0 | 1.0 ±0.2 | 2.8 ± 0.4 | 3.0± 0.8 |
| 10–7 | 2.0 ±0.7 | 0.4 ± 0.2 | 2.5± 0.9 |
| 10–5 | 10.0 ±1.3 | 6.5 ± 1.4 | 1.5± 0.4 |
| fMLP | |||
| 0 | 1.0 ±0.3 | 1.0 ± 0.6 | 3.7± 1.3 |
| 10– 9 | 0.4 ±0.5 | 1.4 ± 1.1 | 3.1± 2.3 |
| 10–8 | 17.4 ±5.1 | 15.5 ± 4.8 | 4.0± 2.0 |
The means ± SD of 3 independent experiments are shown. The concentrations in the upper chamber for fMLP are equal to those in the lower chamber: 0, 10–9 and 10–8 M, respectively.
Classical chemoattractants bind and activate G protein-coupled, 7-transmembrane cell receptors, which promote cellular calcium mobilization. We then analyzed intracellular calcium mobilization in monocytes following exposure to the laminin LG4 peptides 3-SFM29, 4-TLF20 and 5-PGR12. Figure 6c shows changes in intracellular free calcium in monocytes after the addition of the indicated peptides. Calcium flux was strongly increased in monocytes after stimulation with 5-PGR12 and 3-SFM29, and a slight effect to 4-TLF20 was observed within seconds (each at 10-5M). These data indicate that laminin LG4 peptides derived from the laminin α3, α4 and α5 chains induce a transient calcium flux and attract monocytes.
Discussion
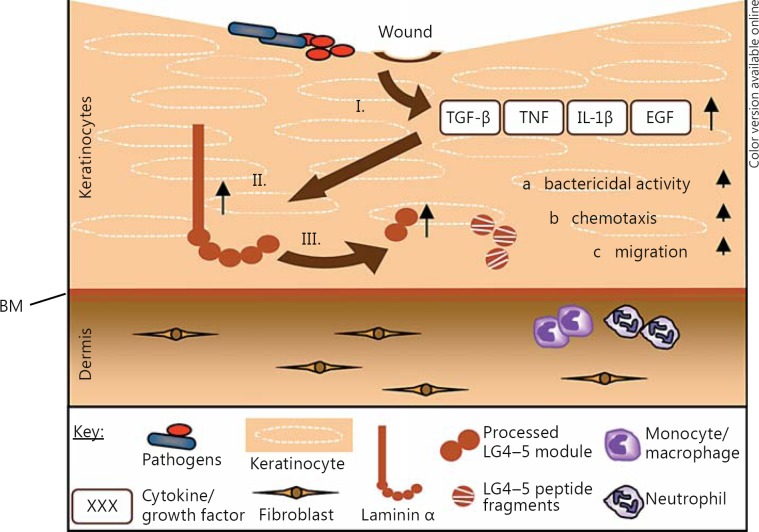
In this study, we show that (1) human skin and skin-derived cells process and secrete biologically active peptides derived from the LG4-5 module of the laminin α3, α4 and α5 chains in vitro and in vivo, (2) expression of laminin α3 is increased in human keratinocytes by proinflammatory cytokines and growth factors, (3) expression and processing of laminin α3 are increased in chronic wounds and in human keratinocytes after bacterial infection, (4) bacterial and neutrophil-derived proteases are able to further process the laminin α3 LG4-5 module and (5) laminin LG4 module-derived peptides from the α3, α4 and α5 chains display antimicrobial activity and potent chemotactic activity on immune cells and further promote wound healing by increasing keratinocyte vitality, proliferation and, notably, migration. In summary, our data indicate for the first time that the expression and processing of laminins are tightly regulated in human skin and that processed peptides from the laminin α chain LG4-5 modules have critical functions in innate skin immune response and wound healing. These data suggest an important role in keratinocyte reepithelialization during wound healing by stimulating the proliferation and, potentially, the adhesion of keratinocytes on the wound bed and facilitating BM formation at the dermal-epidermal junction. Our results support the hypothesis that laminin LG4-5 mediates host defense in impaired skin (fig. 7).
Fig. 7.
Proposed model for laminin LG4-5-mediated host defense. Most pathogens cannot penetrate the skin unless the integrity of the barrier has been compromised by injury, i.e. in wounded skin. Immediately after infection/injury, macrophages and other cells like keratinocytes at the site of tissue damage release proinflammatory cytokines and growth factors such as TNF or TGF-β (I), which lead to increased synthesis of the laminin α chain (II) and also to increased release of its processed LG4-5 module (III). Via bacterial or endogenous proteases, the LG4-5 module can be further processed to generate smaller fragments. Released LG4 peptide fragments, in turn, can mediate the host defense through their antimicrobial properties (a) and chemotactic activities (b) and promote wound healing by induction of keratinocyte proliferation and migration (c).
Our work indicates that laminin LG4 peptides from the laminin α3, α4 and α5 chains exhibit potent antimicrobial activity towards Gram-positive and Gram-negative bacteria through membrane perturbation as part of their mode of action (data not shown), consistent with other findings for laminin α1, α4 and α5 [23, 24]. Many antimicrobial peptides interact with membranes as part of their direct antibacterial mechanism leading to membrane perturbation, disruption of membrane-associated physiological events and/or translocation across membranes to interact with cytoplasmic targets [25].
Several antimicrobial peptides are inducible by both infections and injuries [26]. We show that laminin α3 chain expression is upregulated after pathogenic stimuli. The primary site for infection allows bacteria to encounter an exposed BM, where laminins, fibronectin and collagen IV are important components [27]. Laminin also seems to have a distinct affinity for bacterial surface components which facilitates entry, representing an important initial step in pathogenesis [28]. In addition, it was demonstrated that serine proteases derived from Aspergillus fumigatus are capable of degrading laminin, indicating that secreted pathogen-derived proteases use the degradation of laminins to invade host tissues [29, 30]. Here, we describe that secreted proteases from P. aeruginosa and S. aureus are able to cleave laminin α3 LG4-5 to generate smaller peptide fragments which can modulate host defense.
Beyond that, we found that laminin α3, α4 and α5 LG4 peptides promote keratinocyte proliferation and migration, all key parameters during reepithelialization after wounding. It is known that laminins can influence cell adhesion, migration, differentiation, proliferation, angiogenesis and tumor invasion [31]; these are partially mediated by binding of the LG4-5 modules to integrins and syndecans [11]. Reepithelialization involves multiple processes including the formation of a provisional wound bed matrix, the migration and proliferation of epidermal keratinocytes that maintain the differentiation of new epithelium and the reformation of the BM zone [32]. In a previous work, a synthetic peptide within the α3 LG4 module (NSFMALYLSKGR) with heparin-binding activity, whichincreased the expression of matrix metalloproteinase (MMP)-1 in keratinocytes and fibroblasts, was described, suggesting that the α3 LG4-5 module plays an important role in tissue remodeling during wound healing [33]. Carulli et al. [34] identified a syndecan-1- and syndecan-4-binding site within laminin α3 LG4, crucial for keratinocyte migration. A recently published work describes a laminin α3 LG4 peptide able to promote epidermal repair in vivo and in vitro [10]. Another work concerning laminin α4 LG4 module suggested that cell adhesion to the laminin α4 LG4 module through syndecans may be important in angiogenesis in wounded skin [11].
Most interestingly, we could show in this report that the laminin α3, α4 and α5 LG4 peptides exert potent chemotactic activity for monocytes, demonstrating the multifunctionality of the laminin LG4-derived peptides. Monocytes/macrophages are essential for wound healing, where they dominate the cellular infiltrate and participate until the reparative process is complete. They secrete growth factors and chemotactic factors and mediate further distinct biological functions. It is known that monocytes and neutrophils can adhere to fibronectin, laminin, collagen and vitronectin and as a result exhibit enhanced phagocytic/bactericidal activity [35, 36]. Proteolytic fragments of ECM molecules like thrombospondin or elastin also exhibit chemotactic activity for monocytes [37, 38].
NE seems to play a key role in generating peptide fragments from ECM or other skin-derived proteins with inflammatory properties. NE cleaves the hemidesmosomal protein bullous pemphigoid antigen 180 (BP180) and generates peptides with neutrophil chemotactic activity to amplify the inflammatory cascade in mice [39]. Other studies showed that NE was able to cleave laminin-332 and generate laminin-γ2-derived peptides with chemotactic activity, recruiting more neutrophils to sites of infection [40]. Several observations suggest that laminin α4 plays a role in leukocyte immune cell recruitment and participates in the production of inflammatory mediators [41]. A synthetic mouse-laminin α5-derived peptide was able to increase MMP-9 and MMP-14 production by macrophages and induce macrophage and neutrophil chemotactic migration both in vitro and in vivo [42]. In addition, our observation showed that human NE and cathepsin G were able to digest laminin α3 LG4-5 to generate smaller fragments. These fragments could have distinct functions in the recruitment of immune cells. Moreover, LG4-5 fragments generated by bacteria or NE were similar in molecular weight to fragments found in patients suffering from chronic wounds (35-12 kDa).
In recent years, a series of studies has described ECM proteins, which became active peptide fragments (matrikines) after cleavage, effecting different cellular events. Spenlé et al. [43] proposed that the invasion of the intestine by pathogens may require an interaction of pathogens with laminin as an intestinal BM component and that processed laminin α LG4-5 module peptides together with other host-defense peptides might provide protection of intestinal crypts.
Similar observations have been made for other ECM components such as collagen type VI, characterizing its antibacterial activity by membrane disruption and describing its previously unrecognized role as an innate host-defense effector molecule in connective tissues [44].
Taken together, our data demonstrate that the peptide fragments of the LG4-5 module from the laminin α3, α4 and α5 chains play a role during innate host defense, wound healing and reepithelialization, together with proinflammatory cytokines and growth factors such as TNF or TGF-β. Laminin LG4-5-derived peptides show antimicrobial activity and support the proliferation and migration of keratinocytes and the recruitment of immune cells and other types of cells that participate in skin host defense during wounding/infection.
Supplementary Material
Supplementary data
Acknowledgements
This work was supported by the ‘Deutsche Forschungsgemeinschaft’ (SE 2146/2-1), SFB766, SFB773 and grants from the Medical Faculty of the University of Tübingen (Fortüne program: Nos. 1847-0-0 and 2027-0-0) and by ‘Agence Nationale de la Recherche’ (grant ANR-08-PCVI-0031). We are also grateful to Prof. Anke Strölin who provided access to chronic-wound patient material. We thank Sabine Schmidt and Sarina Kühnlein for their expert technical help with peptide purification and chromatography, and especially Birgit Fehrenbacher for her assistance in confocal microscopy.
References
- 1.Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997;9:651–658. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- 2.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 3.Marinkovich MP. Laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 4.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JCR, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 6.Talts JF, Sasaki T, Miosge N, Gohring W, Mann K, Mayne R, Timpl R. Structural and functional analysis of the recombinant G domain of the laminin alpha 4 chain and its proteolytic processing in tissues. J Biol Chem. 2000;275:35192–35199. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- 7.Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19:309–317. doi: 10.1016/s0945-053x(00)00072-x. [DOI] [PubMed] [Google Scholar]
- 8.Tisi D, Talts JF, Timpl R, Hohenester E. Structure of the C-terminal laminin G-like domain pair of the laminin alpha 2 chain harbouring binding sites for alpha-dystroglycan and heparin. EMBO J. 2000;19:1432–1440. doi: 10.1093/emboj/19.7.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rousselle P, Beck K. Laminin 332 processing impacts cellular behavior. Cell Adh Migr. 2013;7:122–134. doi: 10.4161/cam.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousselle P, Carulli S, Chajra H, Dayan G, Pin D, Herbage B. The syndecan binding sequence KKLRIKSKEK in laminin alpha3 LG4 domain promotes epidermal repair. Eur J Dermatol. 2013 doi: 10.1684/ejd.2013.1974. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Matsuura H, Momota Y, Murata K, Matsushima H, Suzuki N, Nomizu M, Shinkai H, Utani A. Localization of the laminin alpha4 chain in the skin and identification of a heparin-dependent cell adhesion site within the laminin alpha4 chain C-terminal LG4 module. J Invest Dermatol. 2004;122:614–620. doi: 10.1111/j.0022-202X.2004.22325.x. [DOI] [PubMed] [Google Scholar]
- 12.Sugawara K, Tsuruta D, Ishii M, Jones JC, Kobayashi H. Laminin-332 and −511 in skin. Exp Dermatol. 2008;17:473–480. doi: 10.1111/j.1600-0625.2008.00721.x. [DOI] [PubMed] [Google Scholar]
- 13.Senyurek I, Klein G, Kalbacher H, Deeg M, Schittek B. Peptides derived from the human laminin alpha 4 and alpha 5 chains exhibit antimicrobial activity. Peptides. 2010;31:1468–1472. doi: 10.1016/j.peptides.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran KT, Lamb P, Deng JS. Matrikines and matricryptins: implications for cutaneous cancers and skin repair. J Dermatol Sci. 2005;40:11–20. doi: 10.1016/j.jdermsci.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Tolar J, Xia L, Lees CJ, Riddle M, McElroy A, Keene DR, Lund TC, Osborn MJ, Marinkovich MP, Blazar BR, Wagner JE. Keratinocytes from induced pluripotent stem cells in junctional epidermolysis bullosa. J Invest Dermatol. 2013;133:562–565. doi: 10.1038/jid.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanke I, Steffen H, Christ C, Krismer B, Gotz F, Peschel A, Schaller M, Schittek B. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J Invest Dermatol. 2011;131:382–390. doi: 10.1038/jid.2010.328. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto O, Bachy S, Odenthal U, Bernaud J, Rigal D, Lortat-Jacob H, Smyth N, Rousselle P. Normal human keratinocytes bind to the alpha3LG4/5 domain of unprocessed laminin-5 through the receptor syndecan-1. J Biol Chem. 2003;278:44168–44177. doi: 10.1074/jbc.M300726200. [DOI] [PubMed] [Google Scholar]
- 19.Obernesser HJ, Doring G, Botzenhart K. Extracellular toxins of Pseudomonas aeruginosa. I. Purification and characterization of two exoproteases. Zentralbl Bakteriol A. 1981;249:76–88. [PubMed] [Google Scholar]
- 20.Fleischmajer R, Kuroda K, Utani A, Douglas ME, Perlish JS, Arikawa-Hirasawa E, Sekiguchi K, Sanzen N, Timpl R, Yamada Y. Differential expression of laminin alpha chains during proliferative and differentiation stages in a model for skin morphogenesis. Matrix Biol. 2000;19:637–647. doi: 10.1016/s0945-053x(00)00092-5. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 22.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson GH. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 23.Andersson E; Rydengard V, Sonesson A, Morgelin M, Bjorck L, Schmidtchen A. Antimicrobial activities of heparin-binding peptides. Eur J Biochem. 2004;271:1219–1226. doi: 10.1111/j.1432-1033.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- 24.Malmsten M, Davoudi M, Schmidtchen A. Bacterial killing by heparin-binding peptides from PRELP and thrombospondin. Matrix Biol. 2006;25:294–300. doi: 10.1016/j.matbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Fjell CD, Hiss JA, Hancock RE, Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2012;11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 26.Menzies BE, Kenoyer A. Staphylococcus aureus infection of epidermal keratinocytes promotes expression of innate antimicrobial peptides. Infect Immun. 2005;73:5241–5244. doi: 10.1128/IAI.73.8.5241-5244.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink DL, Green BA, St Geme., JW III The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV. Infect Immun. 2002;70:4902–4907. doi: 10.1128/IAI.70.9.4902-4907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rambukkana A, Salzer JL, Yurchenco PD, Tuomanen EI. Neural targeting of Mycobacterium leprae mediated by the G domain of the laminin-alpha2 chain. Cell. 1997;88:811–821. doi: 10.1016/s0092-8674(00)81927-3. [DOI] [PubMed] [Google Scholar]
- 29.Singh B, Fleury C, Jalalvand F, Riesbeck K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol Rev. 2012;36:1122–1180. doi: 10.1111/j.1574-6976.2012.00340.x. [DOI] [PubMed] [Google Scholar]
- 30.Tronchin G, Bouchara JP, Larcher G, Lissitzky JC, Chabasse D. Interaction between Aspergillus fumigatus and basement membrane laminin: binding and substrate degradation. Biol Cell. 1993;77:201–208. doi: 10.1016/s0248-4900(05)80189-3. [DOI] [PubMed] [Google Scholar]
- 31.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 32.O'Toole EA. Extracellular matrix and keratinocyte migration. Clin Exp Dermatol. 2001;26:525–530. doi: 10.1046/j.1365-2230.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 33.Momota Y, Suzuki N, Kasuya Y, Kobayashi T, Mizoguchi M, Yokoyama F, Nomizu M, Shinkai H, Iwasaki T, Utani A. Laminin alpha3 LG4 module induces keratinocyte migration: involvement of matrix metalloproteinase-9. J Recept Signal Transduct Res. 2005;25:1–17. doi: 10.1081/rrs-200047870. [DOI] [PubMed] [Google Scholar]
- 34.Carulli S, Beck K, Dayan G, Boulesteix S, Lortat-Jacob H, Rousselle P. Cell surface proteoglycans syndecan-1 and −4 bind overlapping but distinct sites in laminin alpha3 LG45 protein domain. J Biol Chem. 2012;287:12204–12216. doi: 10.1074/jbc.M111.300061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermann M, Jaconi ME, Dahlgren C, Waldvogel FA, Stendahl O, Lew DP. Neutrophil bactericidal activity against Staphylococcus aureus adherent on biological surfaces. Surface-bound extracellular matrix proteins activate intracellular killing by oxygen-dependent and -independent mechanisms. J Clin Invest. 1990;86:942–951. doi: 10.1172/JCI114796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman SL, Tucci MA. Regulation of human monocyte/macrophage function by extracellular matrix. Adherence of monocytes to collagen matrices enhances phagocytosis of opsonized bacteria by activation of complement receptors and enhancement of Fc receptor function. J Clin Invest. 1990;86:703–714. doi: 10.1172/JCI114766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansfield PJ, Suchard SJ. Thrombospondin promotes chemotaxis and haptotaxis of human peripheral blood monocytes. J Immunol. 1994;153:4219–4229. [PubMed] [Google Scholar]
- 38.Hunninghake GW, Davidson JM, Rennard S, Szapiel S, Gadek JE, Crystal RG. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science. 1981;212:925–927. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- 39.Lin L, Heimbach L, Li N, Rubenstein D, Shapiro SD, An L, Giudice GJ, Diaz LA, Senior RM, Liu Z. Neutrophil elastase cleaves the murine hemidesmosomal protein BP180/type XVII collagen and generates degradation products that modulate experimental bullous pemphigoid. Matrix Biol. 2012;31:38–44. doi: 10.1016/j.matbio.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mydel P, Shipley JM, Adair-Kirk TL, Kelley DG, Broekelmann TJ, Mecham RP, Senior RM. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J Biol Chem. 2008;283:9513–9522. doi: 10.1074/jbc.M706239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenne E, Soehnlein O, Genove G, Rotzius P, Eriksson EE, Lindbom L. Immune cell recruitment to inflammatory loci is impaired in mice deficient in basement membrane protein laminin alpha4. J Leukoc Biol. 2010;88:523–528. doi: 10.1189/jlb.0110043. [DOI] [PubMed] [Google Scholar]
- 42.Adair-Kirk TL, Atkinson JJ, Broekelmann TJ, Doi M, Tryggvason K, Miner JH, Mecham RP, Senior RM. A site on laminin alpha 5, AQARSAASKVKVSMKF, induces inflammatory cell production of matrix metalloproteinase-9 and chemotaxis. J Immunol. 2003;171:398–406. doi: 10.4049/jimmunol.171.1.398. [DOI] [PubMed] [Google Scholar]
- 43.Spenlé C, Hussenet T, Lacroute J, Lefebvre O, Kedinger M, Orend G, Simon-Assmann P. Dysregulation of laminins in intestinal inflammation. Pathol Biol (Paris) 2012;60:41–47. doi: 10.1016/j.patbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Abdillahi SM, Balvanovic S, Baumgarten M, Morgelin M. Collagen VI encodes antimicrobial activity: novel innate host defense properties of the extracellular matrix. J Innate Immun. 2012;4:371–376. doi: 10.1159/000335239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data