Abstract
Cathelicidin LL-37 is a multifunctional immunomodulatory and antimicrobial host defense peptide that has an important role in the immune defenses of the skin and other epithelial barriers. We have previously demonstrated that at physiological concentrations LL-37 synergistically augments the production of immune mediators in response to microbial compounds in human primary keratinocytes. Here we define the signaling mechanisms responsible for this activity. We demonstrate that inhibition of Src family kinases (SFKs) strongly inhibited the synergistic chemokine production in response to LL-37 and flagellin in keratinocytes. SFK activation was induced by LL-37 stimulation and was required for the downstream activation of Akt (protein kinase B) and the transcription factors CREB and ATF1. In cells stimulated with LL-37 and flagellin together, Akt activation was primarily induced by LL-37, while both flagellin and LL-37 contributed to the activation of CREB and ATF1 and consequently chemokine induction. The purinergic receptor P2X7 was identified as the receptor upstream of SFK activation in LL-37-stimulated keratinocytes. Overall, these findings established the P2X7–SFK–Akt–CREB/ATF1 signaling pathway activated by LL-37 in primary keratinocytes. These signaling mechanisms mediated the synergistic effects of LL-37 on chemokine production in flagellin-stimulated keratinocytes, and thus might have a role in the immune defenses of the skin and possibly other epithelial barriers.
Key Words: Cathelicidin, Chemokine, Flagellin, Keratinocyte, LL-37, Src family kinase
Introduction
LL-37 is a human cathelicidin family host defense peptide with a cationic charge and an ability to fold into an amphipathic α-helical structure. It is produced primarily by leukocytes and epithelial cells through proteolytic cleavage from its precursor human cathelicidin antimicrobial protein 18 [1]. LL-37 is found at mucosal surfaces and in tissues at estimated concentrations of 2–5 µg/ml [2,3] and its levels can increase dramatically during acute inflammation, for example in the skin lesions of psoriasis patients [4]. Under physiological conditions LL-37 is weakly antimicrobial but has a broad spectrum of immunomodulatory properties. In particular, LL-37 activities on epithelial cells and keratinocytes include modulation of cell survival [5,6], induction of migration [7,8] and promotion of wound healing [7,9]. We and others have previously demonstrated that LL-37 stimulates the secretion of immune mediators by keratinocytes and other epithelial cells, including chemokine CXCL8/IL-8 and cytokines IL-6 and IL-18 [10,11,12,13], and at low physiological LL-37 concentrations it strongly augments chemokine production in response to microbial stimuli [14], potentially promoting immune defenses of the skin.
The importance of LL-37 in the immune defenses of the skin and other epithelial barriers is demonstrated by the moderately increased susceptibility to infection in knockout mice for the LL-37 orthologue cathelicidin antimicrobial peptide [15,16]. Reduced LL-37 levels are also linked to increased susceptibility to skin infections in patients with atopic dermatitis [4,17,18]. At the same time, LL-37 and cathelicidin antimicrobial peptide are also implicated in the pathologies of inflammatory and autoimmune disorders of the skin, such as in rosacea and psoriasis [19,20]. Overall, this emphasizes the importance of further understanding the immunomodulatory activities of LL-37 in the skin and its role in modulating keratinocyte responses to microbial stimuli.
The signaling mechanisms mediating LL-37 activities in keratinocytes and other epithelial cells are complex. Stimulation of keratinocyte migration and wound healing by LL-37 is associated with activation of Akt, mitogen-activated protein kinases, focal adhesion kinase and STAT3, and these responses are mediated by the epidermal growth factor receptor and to a lesser extent formyl peptide receptor-like 1 [7,8,9]. Production of immune mediators in response to LL-37 in epithelial cells depends on the activities of mitogen-activated protein kinases and NFĸB downstream of epidermal growth factor receptor and Gi-protein-coupled receptors [10,11,13]. However, the components of LL-37-activated signaling pathways are incompletely defined and in particular the mechanisms of synergy between LL-37 and microbial compounds in keratinocyte stimulation are poorly understood.
In this study, the signaling mechanisms leading to chemokine induction in keratinocytes in response to LL-37 and flagellin were investigated. We demonstrated the role of the Src family kinases (SFKs) in LL-37 activity and established that the LL-37-activated signaling pathway consisted of the P2X7 receptor, SFKs, Akt and the transcription factors CREB and ATF1. We further showed that LL-37 significantly enhanced the flagellin-induced activation of CREB and ATF1, suggesting this may be in part responsible for the synergistic effects of the peptide on chemokine induction by flagellin in keratinocytes.
Materials and Methods
Reagents
LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-NH2) was synthesized using F-Moc chemistry at the Nucleic Acid/Protein Synthesis Unit or at the Brain Research Centre (University of British Columbia, Vancouver, B.C., Canada), purified to >90% purity using reverse-phase HPLC and confirmed by mass spectrometry. Salmonella typhimurium flagellin was obtained from InvivoGen. Inhibitors (SU6656, PP2, and KN-62) were purchased from Biosource, Calbiochem or Sigma-Aldrich. PP2 and SU6656 were resuspended in DMSO (Sigma-Aldrich), and KN-62 in methanol. All experiments included DMSO or methanol vehicle controls. The inhibitors and peptides were checked against possible cytotoxic effects using the WST-1 cell viability reagent (Roche). The effects of the treatments on cell viability were calculated relative to untreated cells (defined as 100% viability) and cells exposed to 1% Triton-X (defined as 0% viability), using the formula [(OD treated – OD Triton-X)/(OD untreated – OD Triton-X)] × 100%.
Cell Culture
Primary human adult epidermal keratinocytes (Cascade Biologics) were maintained in Epilife media (Cascade Biologics) with the addition of Human Keratinocyte Growth Supplement, containing bovine pituitary extract, bovine insulin, hydrocortisone, bovine transferrin and human epidermal growth factor (Cascade Biologics). Keratinocytes were seeded at 7,000 cells/cm2, grown to 70% confluence and rested for 1 h in fresh unsupplemented media prior to stimulation. The cells were maintained at 37°C and 5% CO2, and used between passages 2 and 5.
Western Blotting
Cells were washed with ice-cold PBS with 1 mM vanadate (Sigma-Aldrich) and lysed in TNE – 1% Triton-X buffer (10 mM Tris, 150 mM NaCl, 2 mM EDTA, 1% (v/v) Triton X-100, pH 7.4), supplemented with 1 mM PMSF and protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Protein concentration in the lysates was quantified using the BCA Protein Assay Kit (Pierce). 5 × SDS sample buffer, containing 250 mM Tris pH 6.8, 10% (w/v) SDS, 30% (v/v) glycerol, 0.5 M DTT and 0.1% bromophenol blue, was added and the lysates were denatured at 97°C for 7 min. The lysates were resolved on an 8% SDS-PAGE gel, followed by transfer at 95 V for 1 h to polyvinylidene fluoride membranes (BioRad). The membranes were blocked for 1 h in 5% (w/v) BSA in TBST (10 mM Tris-HCl pH 8.0, 150 mM NaCl and 0.1% v/v Tween-20) and then incubated overnight at 4°C with antibodies against phospho-Src (Y416, rabbit polyclonal; the product note indicates ‘may cross-react with other SFKs when phosphorylated at equivalent sites’), phospho-CREB (S133, clone 87G3; the product note indicates that ‘also recognizes the phosphorylated form of CREB-related factor ATF1’), phospho-Akt (S473, clone 193H12), β-actin (all from Cell Signaling) or GAPDH (RDI/Fitzgerald), in TBST with either 1% (w/v) BSA or 5% (w/v) nonfat milk according to the manufacturers’ instructions. The membranes were washed in TBST and developed with HRP-conjugated goat anti-rabbit IgG (Cell Signaling) or HRP-conjugated sheep anti-mouse IgG (GE Healthcare) and the Chemiluminescence Detection System (Amersham Biotech).
Quantitative Real-Time PCR
RNA was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer's protocols. Gene expression was analyzed by qRT-PCR using a SuperScript III Platinum Two-Step qRT-PCR Kit with SYBR Green (Invitrogen) in the PRISM 7000 sequence detection system (Applied Biosystems). Fold changes were determined by the comparative cycle threshold method and normalized against mRNA levels of the β2-microglobulin housekeeping gene. The primers were designed using the Roche online primer design tool available at qpcr.probefinder.com.
ELISA
Cytokine ELISA was performed using the anti-human CXCL8/IL-8 antibody clones 893A6G8 and biotinylated 790A28G2 (Biosource), followed by avidin-HRP (eBioscience) as per the manufacturers’ protocols. The ELISAs were developed with the TMB Liquid Substrate System (Sigma-Aldrich) and imaged with the PowerWave 340 plate reader (BioTek Instruments). Cytokine quantification was done against serial dilutions of recombinant CXCL8/IL-8 (R&D Systems).
Statistical Analyses
Statistical analyses used Prism 4.0 software (GraphPad Inc.), with two-tailed Student's t test for comparisons of two datasets and ANOVA with the Bonferroni or Dunnett post hoc tests for multiple comparisons.
Results
SFK-Inhibitors Suppressed Chemokine Induction in Response to LL-37 and Flagellin in Keratinocytes
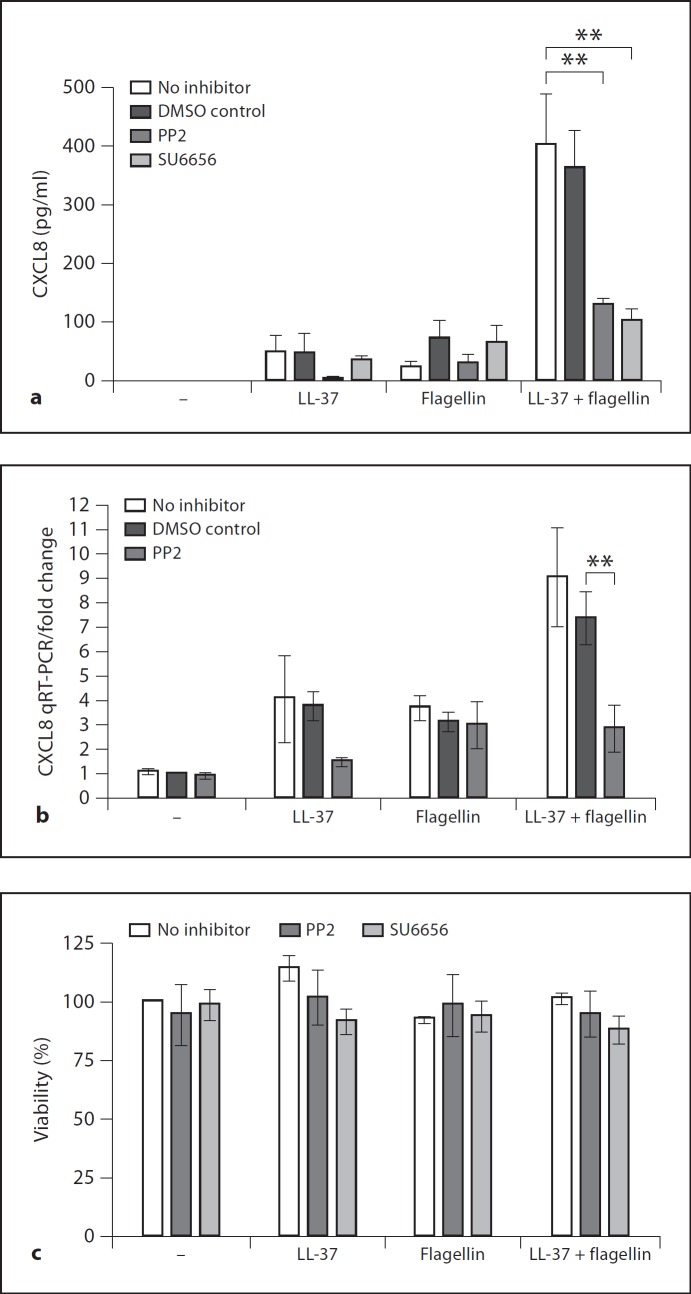
To analyze the role of SFKs in the chemokine induction activities of LL-37 on human primary keratinocytes, the cells were pretreated with SFK-specific chemical inhibitors SU6656 or PP2 [21,22,23], and stimulated with LL-37 alone (3 µg/ml) or together with flagellin (0.5 µg/ml) for 24 h. Synergistic induction of chemokine CXCL8/IL-8 by the combined treatment with LL-37 and flagellin was observed (fig. 1a). Importantly, this was strongly and significantly suppressed by the SFK inhibitors, indicating the essential role of SFK-signaling in this response (fig. 1a). A dose titration of SFK inhibitor PP2 was performed and demonstrated significant inhibitory activity at the concentrations of 5–10 µM but not in the lower concentration range (online suppl. fig. 1a; see www.karger.com?doi=10.1159/000335901 for all online suppl.material). The negative control compound PP3 (chemically related to PP2 but known not to inhibit SFKs) had no inhibitory activity (online suppl. fig. 1b). We further tested the effect of SFK inhibition on the production of CXCL8 transcript by qRT-PCR at the 6-hour time point and demonstrated a significant reduction in CXCL8 gene expression in response to LL-37 and flagellin stimulation in the SFK-inhibitor PP2-treated cells (fig. 1b). The residual low levels of CXCL8 induction in the presence of the inhibitor may be either due to incomplete SFK inhibition or alternatively due to the presence of a minor SFK-independent pathway also mediating CXCL8 induction in response to LL-37 and flagellin. Neither the peptide nor the inhibitors showed significant cytotoxicity at the concentrations used, as demonstrated with the WST-1 cell viability assay (fig. 1c). The experiments included DMSO vehicle controls at levels identical to the inhibitor treatments, and no DMSO toxicity or effects on chemokine production were detected (fig. 1a, b).
Fig. 1.
The role of SFKs in the LL-37- and flagellin-mediated chemokine induction in human primary keratinocytes. a, b CXCL8/IL-8 chemokine production by human primary keratinocytes stimulated with LL-37 (3 µg/ml) and flagellin (0.5 µg/ml) in the presence of SFK inhibitors PP2 (10 µM) and SU6656 (5 µM), or DMSO vehicle control. CXCL8 production measured by ELISA at 24 h of stimulation (a) and by qRT-PCR at 6 h of stimulation (b). All data from 3 to 4 independent experiments, statistical analysis by ANOVA with Bonferroni's multiple comparison post hoc test, ** p < 0.01. c WST1 cell viability assay indicating minimal cytotoxicity of the peptide and inhibitors over 24 h. The effects of the treatments on cell viability are calculated relative to untreated cells (defined as 100% viability) and cells exposed to 1% Triton-X (defined as 0% viability), using the formula [(OD treated – OD Triton-X)/(OD untreated – OD Triton-X)] × 100%; data from 3 independent experiments.
LL-37-Induced Activating Phosphorylation of SFKs in Human Primary Keratinocytes
To confirm the role of SFKs in LL-37 signaling in human primary keratinocytes, the cells were stimulated with LL-37 at physiological 3 µg/ml concentration and analyzed for SFK phosphorylation by Western blotting. Increased activating SFK phosphorylation at the residue equivalent to Y416 of Src was detected at 15 min of stimulation (fig. 2a; the manufacturer's note indicates that the antibody cross-reacts with other Src family members when phosphorylated at the equivalent site). The effect was statistically significant as shown by quantification of the blots from three independent experiments using ImageJ 1.43u software (fig. 2b). There were no changes in the levels of total Src protein in the cells over 15–30 min of LL-37 stimulation (online suppl. fig. 2). This further confirmed that LL-37 stimulation induced activation of SFKs in primary keratinocytes. To explore the involvement of different SFK family members in LL-37 activity we performed an experiment involving a transient siRNA knockdown of Src and Yes in keratinocytes and observed a mild reduction in CXCL8 production in response to LL-37 and flagellin stimulation in both the Src and Yes knockdown cells (online suppl. fig. 3), suggesting that multiple SFKs may be involved in mediating the response. Similar redundancies and overlaps in function between SFKs have been reported in other pathways, for example in macrophage phagocytosis and signaling downstream of cytokine receptors [24,25,26,27].
Fig. 2.
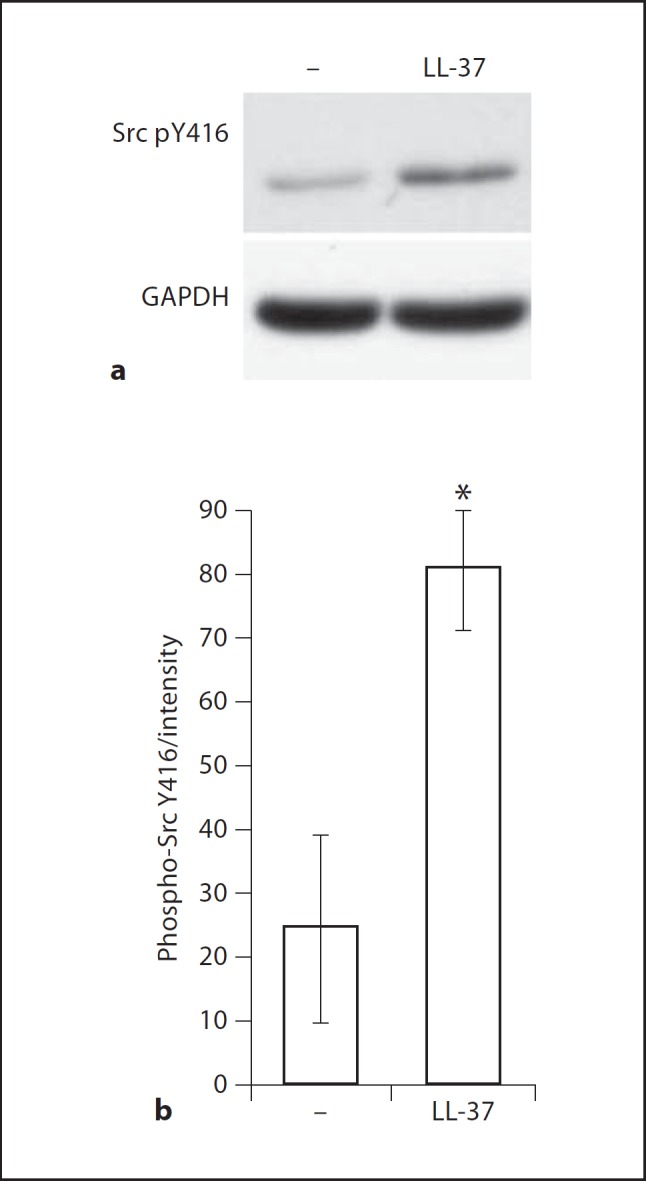
LL-37-induced SFK phosphorylation in primary keratinocytes. The cells were stimulated with LL-37 at 3 µg/ml and analyzed for Src Y416 phosphorylation by Western blotting; note that the antibody cross-reacts with other SFKs when phosphorylated at the equivalent site (Cell Signaling Technology). a Representative blots at 15 min of stimulation. b Blot intensities from 3 independent experiments quantified using ImageJ 1.43u software and normalized against the intensity of the loading control. Whiskers show means ± SEM from 3 independent experiments. * p ≤ 0.05 using paired t test.
SFK Activation in LL-37-Stimulated Keratinocytes Was Mediated by the P2X7 Receptor
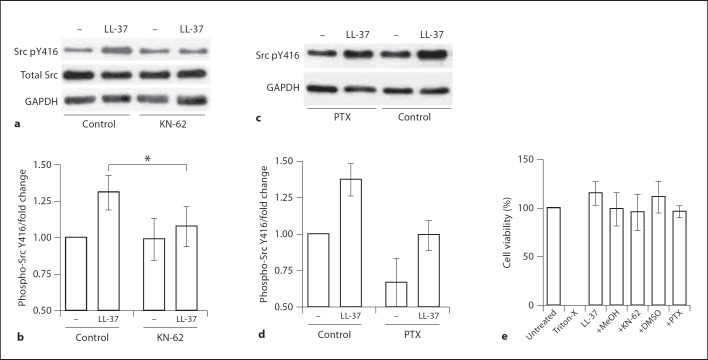
To identify the receptor upstream of SFK activation in keratinocytes we examined the role of the purinergic receptor P2X7, one of the known LL-37 receptors. Primary keratinocytes were pretreated with the P2X7 inhibitor KN-62 or vehicle control methanol, stimulated with LL-37 at 5 µg/ml and analyzed by Western blotting for the activating phosphorylation of SFKs at the tyrosine residue equivalent to Y416 of Src. As was the case previously, significant SFK activation was detected at 15 min of stimulation, and the P2X7 inhibitor KN-62 significantly inhibited this response (fig. 3a, b). Inhibition of Gi-protein-coupled receptors with pertussis toxin (PTX) resulted in a reduction in the background levels of phospho-Src in keratinocytes, however responsiveness to LL-37 was still apparent after PTX pretreatment (fig. 3c, d). The peptide and the inhibitors were nontoxic to the cells at the concentration used in this study (fig. 3e). This indicated that the LL-37-induced SFK activation in keratinocytes was mediated by the P2X7 receptor.
Fig. 3.
The role of P2X7 upstream of SFKs in the signaling pathway activated by LL-37 in primary keratinocytes. a, b The cells were pretreated with P2X7 inhibitor KN-62 (5 µM) or an equivalent concentration of methanol vehicle control for 1 h and stimulated with LL-37 (5 µg/ml). a Western blots of cell lysates at 15 min of stimulation analyzed for phospho-Src Y416 (Src pY416), total Src and GAPDH loading control. b Quantification of the phospho-Src Y416 blots using ImageJ 1.43u software, band intensities normalized against GAPDH loading control and shown as fold change relative to the intensity of the untreated sample. All whiskers show means ± SEM from 4 independent experiments; * p < 0.05 using paired t test; analysis by ANOVA with Dunnett's post hoc test showed significant induction of phospho-Src Y416 levels in the control but not the inhibitor-treated samples. c, d The cells were pretreated with the Gi-protein coupled receptor inhibitor PTX (100 ng/ml) or an equivalent concentration of DMSO vehicle control for 1 h and stimulated with LL-37 (5 µg/ml). c Western blots of cell lysates at 15 min of stimulation analyzed for phospho-Src Y416 and GAPDH loading control. d Quantification of the phospho-Src Y416 blots, band intensities normalized against GAPDH loading control and shown as fold change relative to the intensity of the untreated sample, data from 5 independent experiments. e Cell viability at 24 h of stimulation with LL-37 (5 µg/ml) alone or in combination with KN-62 (5 µM), PTX (100 ng/ml), or vehicle controls, measured using the WST1 assay and expressed relative to the viability of untreated cells (defined as 100%) and cells treated with 1% Triton-X (defined as 0%).
SFK-Dependent Downstream Activation of Akt, CREB and ATF1 in LL-37-Stimulated Keratinocytes
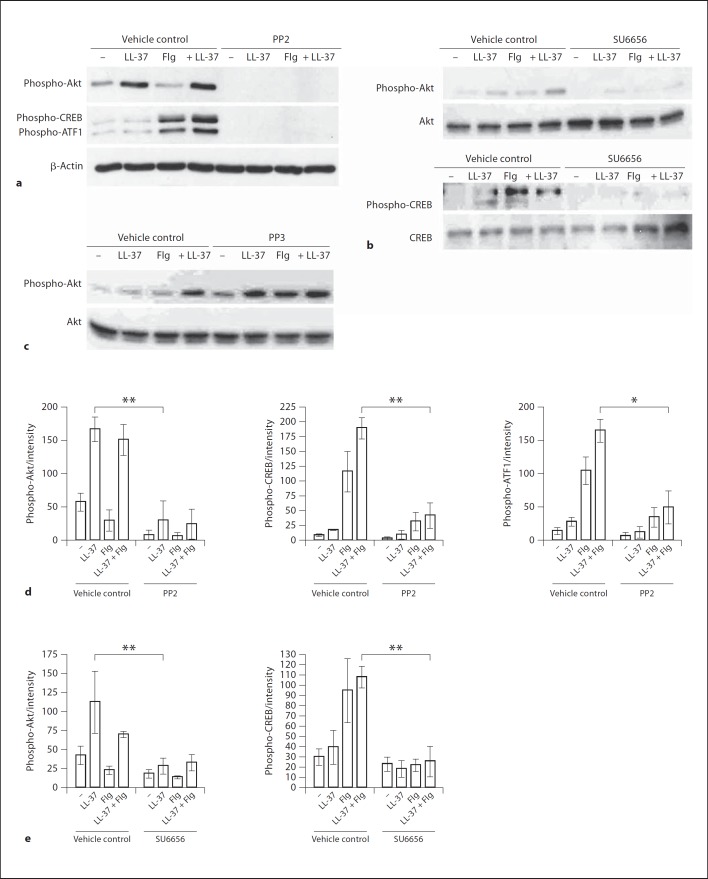
To analyze the signaling pathways downstream of SFKs in LL-37-stimulated keratinocytes and to explore the mechanisms of LL-37 and flagellin synergistic stimulation, human primary keratinocytes were pretreated with the SFK-inhibitor PP2 or vehicle control DMSO and analyzed by Western blotting for activating phosphorylation of Akt and its downstream target transcription factors CREB and ATF1 [28]. Significant activation of Akt was observed at 30 min of LL-37 stimulation, while flagellin stimulation resulted in the activation of CREB and ATF1 (fig. 4a, b). The combined stimulation with LL-37 and flagellin significantly augmented CREB and ATF1 activation (fig. 4a), suggesting that this signaling response might be one of the mechanisms responsible for the enhanced chemokine induction by the combined stimulation.
Fig. 4.
The role of SFKs upstream of Akt, CREB and ATF1 in the signaling pathway activated by LL-37 and flagellin (Flg) in primary keratinocytes. The cells were pretreated with the SFK inhibitors PP2 or SU6656 at 10 µM or an equivalent concentration of DMSO vehicle control for 1 h, and stimulated with LL-37 (3 µg/ml) and/or flagellin (0.5 µg/ml) for 30 min. Cell lysates were analyzed for phospho-Akt S473, phospho-CREB S133, phospho-ATF1 or β-actin loading control by Western blotting. Western blots showing the inhibitory activity of the SFK inhibitor PP2 on the LL-37- and flagellin-induced activation of Akt, CREB and ATF1 in keratinocytes (a); the inhibitory effect of the SFK inhibitor SU6656 on the LL-37- and flagellin-induced activation of Akt and CREB in keratinocytes (b); blots representative of 3 independent experiments. c Control compound PP3 (10 µM) did not inhibit Akt activation in response to LL-37 and flagellin stimulation in keratinocytes; representative of 2 experiments. d, e Blot intensities were quantified using ImageJ 1.43u software and standardized against the intensities of the loading control for each lane. Whiskers show means ± SEM. * p < 0.05, ** p < 0.01 using ANOVA with Bonferroni's post hoc test.
Importantly, when the cells were pretreated with the SFK inhibitor PP2, the induction of Akt, CREB and ATF1 phosphorylation was almost completely stopped in response to LL-37 stimulation, and also in the response to combined stimulation with LL-37 and flagellin (fig. 4a). The effects of SFK inhibition on the activation of Akt and CREB were further confirmed using the alternative SFK inhibitor SU6656 (fig. 4b). In contrast, the control compound PP3 (a chemical mimic of PP2 specifically modified to lack the inhibitory activity) did not suppress Akt activation (fig. 4c), showing that the inhibitory activity of PP2 was not due to off-target effects. Blots from three independent experiments were quantified to confirm the statistical significance of the data (fig. 4d, e). Levels of total Akt and CREB proteins were also quantified confirming that reduced phospho-Akt and CREB levels in the inhibitor-treated cells were due to reduced protein phosphorylation, rather than due to the loss of the total Akt and CREB proteins (online suppl. fig. 4). Overall, this demonstrated an essential role of SFKs in the downstream activation of Akt and the transcription factors CREB and ATF1, in response to LL-37 and LL-37 plus flagellin in keratinocytes.
Discussion
In this work a signaling pathway essential for mediating the chemokine induction activity of LL-37 in human keratinocytes was established, consisting of the P2X7 receptor, SFKs, Akt and transcription factors CREB and ATF1 (fig. 5). We further demonstrated the critical role of SFK activity in the synergistic effects of LL-37 on keratinocyte responses to flagellin (fig. 5), suggesting that this may contribute to the sensing of microbial compounds in the human skin and the immune defenses of epithelial barriers.
Fig. 5.
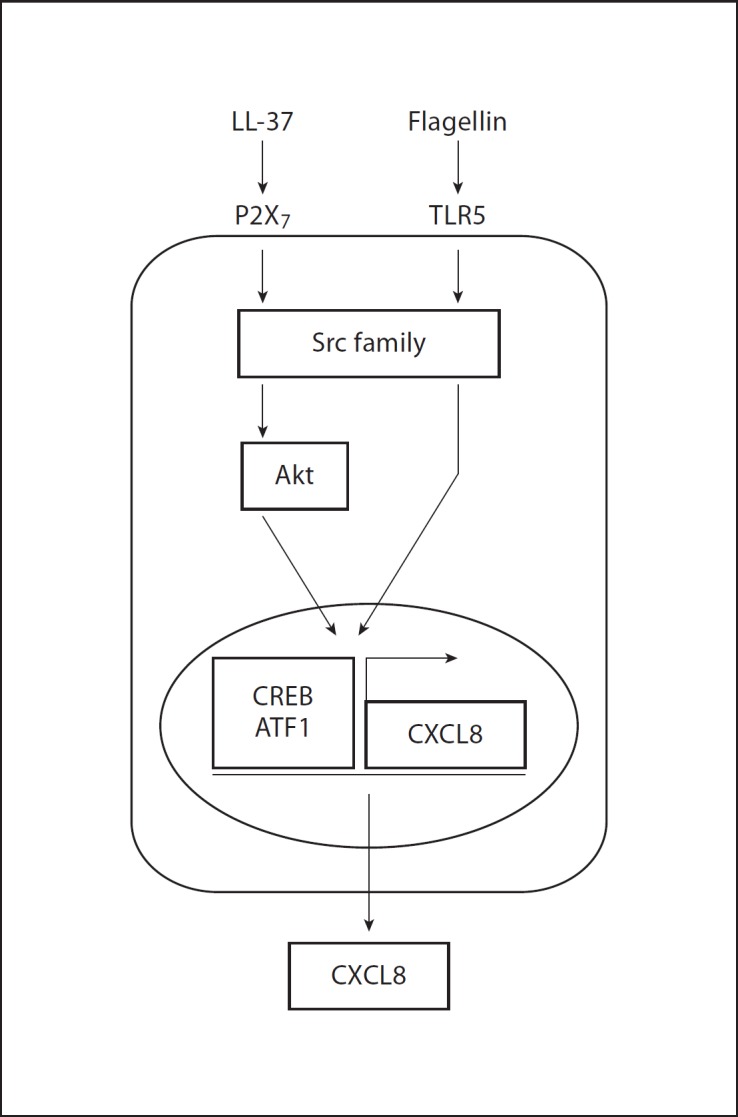
Signaling pathways mediating chemokine induction in response to LL-37 and flagellin stimulation in human primary keratinocytes.
The role of SFK tyrosine kinases in LL-37 signaling and biological activity was established in human keratinocytes. Increased tyrosine phosphorylation had been previously reported in LL-37-stimulated keratinocytes and was associated with the induction of keratinocyte migration [7], while pharmacological inhibition of SFKs had been shown to repress LL-37-induced chemokine secretion in smooth muscle cells [29]. However, to our knowledge the role of SFKs in LL-37 activity had not been explored further. The SFK family includes nine members, characterized by their conserved modular structure and a common mechanism of regulation through reversible phosphorylation of two conserved tyrosine residues [30]. Partial redundancy and overlap in functions between different SFK members occurs in many SFK-dependent biological processes, such as macrophage phagocytosis, cytokine receptor signaling and others [24,25,26,27]. Similarly, redundancy and overlap in function between SFKs may also mediate the response to LL-37 stimulation in keratinocytes.
The role of the P2X7 receptor in the downstream activation of SFKs was demonstrated in LL-37-stimulated keratinocytes. P2X7 had previously been shown to mediate chemokine induction by LL-37 in smooth muscle cells [29] and gingival fibroblasts [31], LL-37 stimulation of fibroblast proliferation [32] and IL-1β processing in monocytes [33]. Stimulation of purinergic receptors was also linked to cytokine production in keratinocytes [34], but this report is the first demonstration of their role in the LL-37-mediated production of immune mediators in this cell type. Previous studies suggested that IL-8 induction in response to LL-37 in keratinocytes is mediated by Gi-protein-coupled receptors [12], and the discrepancies between the findings may be due to the differences in the cells used (adult vs. neonatal cells in the study by Braff et al. [12]) and the concentration of LL-37 (3 µg/ml in our work vs. 45 µg/ml in the previous study). While the concentration used in this work more closely reflects physiological LL-37 levels under noninflammatory conditions, the higher levels of peptide used in previous studies may model pathological conditions such as the skin of psoriasis patients.
This work further established the role of Akt and CREB in mediating LL-37 chemokine induction activities in keratinocytes downstream of SFKs. The activation of Akt and CREB downstream of SFKs has been previously demonstrated in other signaling pathways, with class I phosphoinositide 3-kinases (PI3Ks) typically playing an intermediate role in the SFK-dependent Akt activation. Thus SFK-phosphorylated ITAM motifs (on various receptor and adaptor molecules) can act as binding sites for the SH2 domain of PI3K regulatory subunits, mediating PI3K recruitment and activation [35]. In turn, PI3K catalyzes the formation of the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3) that binds the pleckstrin homology domain of Akt, promoting Akt activation [35,36]. The transcription factor CREB can be phosphorylated and activated directly by Akt as well as by other kinases [28]. In response to LL-37 stimulation, the activation of Akt has been previously shown in keratinocytes [7], as well as other cell types [37].
Activation of the SFK-dependent signaling pathway was also shown to be essential for the synergistic induction of immune mediators in keratinocytes in response to physiological LL-37 concentrations in conjunction with the bacterial TLR5 agonist flagellin. Although this study used commercially available flagellin from S. typhimurium, the flagellin interaction with TLR5 is known to be mediated by a conserved region of the flagellin molecule found across many bacterial species [38]. Flagellin from other bacteria, for example Pseudomonas aeruginosa, which causes opportunistic skin infections in immunocompromised individuals, signals through TLR5 in epithelial cells and mouse models via common signaling pathways [39,40]. Therefore, the data in this study is relevant to other bacterial species including known skin pathogens. The immunostimulatory activities of LL-37 in the skin demonstrated here are likely to be important for immune defenses; however, their enhanced or inappropriate activation may also contribute to inflammatory skin disorders, such as rosacea [19]. This suggests that components of the characterized signaling pathway are potential targets for therapeutic intervention. Inhibitors of SFKs have been widely investigated as anticancer drugs due to their inhibitory effects on cell proliferation and survival, and a number of small molecules are in clinical trials [41]. Their potential immunosuppressive properties are also being addressed [42]. However, the use of such treatments in inflammatory skin diseases needs to be undertaken with caution, as the role of cathelicidins in disease pathology remains controversial. Indeed LL-37 is known to regulate many aspects of immune response, for example by antagonizing inflammatory activities of endotoxin and IFNγ [43,44,45], and its inhibition could exacerbate inflammatory disease pathology. Reduction in LL-37 immunomodulatory activity is also likely to compromise the antimicrobial defenses of the skin [4,15,18].
Overall, this study has established the P2X7–SFK–Akt–CREB/ATF1 signaling pathway mediating the chemokine induction activity of LL-37 in human primary keratinocytes and the synergistic effects of LL-37 on keratinocyte responses to microbial compound flagellin. These pathways likely contribute to the activities of cathelicidin LL-37 in the immune defenses of the skin and epithelial barriers, while components of such pathways are potential targets for intervention in skin disorders associated with altered peptide function.
Disclosure Statement
The authors declare no conflicts of interest.
Supplementary Material
Supplementary data
Acknowledgements
We gratefully acknowledge the financial support from the Foundation for the National Institutes of Health and the Bill and Melinda Gates Foundation, through two separate program grants from the Grand Challenges in Global Health Initiative, and from the Canadian Institutes for Health Research (CIHR). A.N. was a CIHR and Michael Smith Foundation for Health Research (MSFHR) postdoctoral research fellow. N.C.J.F. was the recipient of studentships from the Natural Sciences and Engineering Research Council and the MSFHR. R.E.W.H. is a Canada Research Chair in Microbiology.
References
- 1.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 2.Bals R, Weiner DJ, Meegalla RL, Wilson JM. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest. 1999;103:1113–1117. doi: 10.1172/JCI6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med. 2002;165:992–995. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- 4.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 5.Barlow PG, Li Y, Wilkinson TS, Bowdish DM, Lau YE, Cosseau C, Haslett C, Simpson AJ, Hancock RE, Davidson DJ. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol. 2006;80:509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamorro CI, Weber G, Gronberg A, Pivarcsi A, Stahle M. The human antimicrobial peptide LL-37 suppresses apoptosis in keratinocytes. J Invest Dermatol. 2009;129:937–944. doi: 10.1038/jid.2008.321. [DOI] [PubMed] [Google Scholar]
- 7.Carretero M, Escamez MJ, Garcia M, Duarte B, Holguin A, Retamosa L, Jorcano JL, Rio MD, Larcher F. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128:223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 8.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H, Yang L, Higashiyama S, Yoshimura A, Sugai M, Hashimoto K. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 9.Yin J, Yu FS. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Invest Ophthalmol Vis Sci. 2010;51:1891–1897. doi: 10.1167/iovs.09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 11.Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, Yu LL, Pistolic J, Falsafi R, Tagg J, Hancock RE. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun. 2008;76:4163–4175. doi: 10.1128/IAI.00188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, Lin K, Streib JE, Dorschner R, Leung DY, Gallo RL. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005;174:4271–4278. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 13.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human beta-defensins (-1, −2, −3, −4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005;175:1776–1784. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 14.Filewod NC, Pistolic J, Hancock RE. Low concentrations of LL-37 alter IL-8 production by keratinocytes and bronchial epithelial cells in response to proinflammatory stimuli. FEMS Immunol Med Microbiol. 2009;56:233–240. doi: 10.1111/j.1574-695X.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- 15.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 16.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 17.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DY. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–348. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, Morhenn VB, Gallo RL. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 20.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, Cao W, Wang YH, Su B, Nestle FO, Zal T, Mellman I, Schroder JM, Liu YJ, Gilliet M. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 21.Geahlen RL, Handley MD, Harrison ML. Molecular interdiction of Src-family kinase signaling in hematopoietic cells. Oncogene. 2004;23:8024–8032. doi: 10.1038/sj.onc.1208078. [DOI] [PubMed] [Google Scholar]
- 22.Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA. SU6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 24.Lowell CA, Soriano P, Varmus HE. Functional overlap in the Src gene family: inactivation of Hck and Fgr impairs natural immunity. Genes Dev. 1994;8:387–398. doi: 10.1101/gad.8.4.387. [DOI] [PubMed] [Google Scholar]
- 25.Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng F, DeFranco AL, Lowell CA. Fcγ receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669–682. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi N, Kono T, Hatakeyama M, Minami Y, Miyazaki T, Perlmutter RM, Taniguchi T. Functional coupling of the src-family protein tyrosine kinases p59fyn and p53/56lyn with the interleukin 2 receptor: implications for redundancy and pleiotropism in cytokine signal transduction. Proc Natl Acad Sci USA. 1993;90:4201–4205. doi: 10.1073/pnas.90.9.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 28.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 29.Zuyderduyn S, Ninaber DK, Hiemstra PS, Rabe KF. The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J Allergy Clin Immunol. 2006;117:1328–1335. doi: 10.1016/j.jaci.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 30.Boggon TJ, Eck MJ. Structure and regulation of Src family kinases. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 31.Montreekachon P, Chotjumlong P, Bolscher JG, Nazmi K, Reutrakul V, Krisanaprakornkit S. Involvement of P2X7 purinergic receptor and MEK1/2 in interleukin-8 up-regulation by LL-37 in human gingival fibroblasts. J Periodontal Res. 2011;46:327–337. doi: 10.1111/j.1600-0765.2011.01346.x. [DOI] [PubMed] [Google Scholar]
- 32.Tomasinsig L, Pizzirani C, Skerlavaj B, Pellegatti P, Gulinelli S, Tossi A, Di Virgilio F, Zanetti M. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J Biol Chem. 2008;283:30471–30481. doi: 10.1074/jbc.M802185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1β processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 34.Inoue K, Hosoi J, Denda M. Extracellular ATP has stimulatory effects on the expression and release of IL-6 via purinergic receptors in normal human epidermal keratinocytes. J Invest Dermatol. 2007;127:362–371. doi: 10.1038/sj.jid.5700526. [DOI] [PubMed] [Google Scholar]
- 35.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 36.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 37.Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, Rehaume L, Hancock RE. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 38.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 39.Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun. 2005;73:7151–7160. doi: 10.1128/IAI.73.11.7151-7160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benati D, Baldari CT. Src family kinases as potential therapeutic targets for malignancies and immunological disorders. Curr Med Chem. 2008;15:1154–1165. doi: 10.2174/092986708784310404. [DOI] [PubMed] [Google Scholar]
- 42.Wong WS. Inhibitors of the tyrosine kinase signaling cascade for asthma. Curr Opin Pharmacol. 2005;5:264–271. doi: 10.1016/j.coph.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, Powers JP, Bryan J, Brinkman FS, Hancock RE. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 44.Kandler K, Shaykhiev R, Kleemann P, Klescz F, Lohoff M, Vogelmeier C, Bals R. The antimicrobial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol. 2006;18:1729–1736. doi: 10.1093/intimm/dxl107. [DOI] [PubMed] [Google Scholar]
- 45.Nijnik A, Pistolic J, Wyatt A, Tam S, Hancock RE. Human cathelicidin peptide LL-37 modulates the effects of IFN-gamma on APCs. J Immunol. 2009;183:5788–5798. doi: 10.4049/jimmunol.0901491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data