Abstract
The pattern of activation of dopamine neurotransmission in the nucleus accumbens (NAc) of rats produced by H1 histamine antagonists which have behavioral effects like those of psychostimulant drugs was examined. Diphenhydramine and (+)-chlorpheniramine were compared to triprolidine, a potent and selective H1 antagonist, and (−)-chlorpheniramine, which is less active than its enantiomer at H1 receptors. Affinities of the drugs at DA, serotonin, and norepinephrine transporters, at H1 receptors, and potencies for DA uptake inhibition in striatal synaptosomes were determined to assess mechanisms by which the compounds increased DA levels. Intravenous diphenhydramine (1.0–3.0 mg/kg), (+)- and (−)-chlorpheniramine (1.0–5.6 mg/kg), but not triprolidine (1.0–3.0 mg/kg) elicited a cocaine-like pattern of stimulation of DA transmission, with larger effects in the NAc shell than core. The absence of stereospecific effects with chlorpheniramine enantiomers, along with the lack of an effect with triprolidine suggest that the effects on DA transmission were not related to H1 receptor antagonism. Although in vivo potencies were not directly related to DA transporter affinities, it is hypothesized that actions at that site, modulated by other actions, possibly those at the serotonin transporter, are primarily responsible for the neurochemical actions of the drugs on DA neurotransmission, and might underlie the occasional misuse of these medications.
Keywords: Histamine H1 antagonists, dopamine, Nucleus Accumbens shell, diphenhydramine, chlorpheniramine, cocaine
INTRODUCTION
In general, antihistamines, or preparations containing antihistaminic compounds are safe and reliable medications. However, there have been occasional case reports or epidemiological surveys indicating instances of abuse of these over-the-counter (OTC) drugs themselves, as well as preparations containing them. The reports of abuse include those of the drugs or preparations alone or in combination with other drugs (see for example, Hughes et al. 1999). One abused combination with considerable notoriety involved the antihistaminic compound, tripelennamine, which was taken with the opioid compound pentazocine, and had the street name of “T’s and blues” (Schnoll et al. 1985). This combination was taken by opioid dependent individuals, apparently as a heroin substitute, when heroin was not readily available. Abuse, or misuse, of other antihistaminic compounds, mainly antagonists at histaminic H1 receptors, like diphenhydramine or (+)-chlorpheniramine, have also been reported (Banerji & Anderson 2001, Barsoum et al. 2000, Cox et al. 2001, Dinndorf et al. 1998). A recent Community Epidemiology Work Group report (NIDA/NIH October 2006) indicated an emerging trend in Texas of abuse of a combination of heroin and diphenhydramine, with the street name “cheese,” which was also reported in the news media.
Preclinical studies have also shown that some H1 histaminic antagonists have behavioral stimulant and reinforcing effects in different animal species. For example, (+)-chlorpheniramine and diphenhydramine, induced conditioned place preference in rats (Halpert et al. 2003, Suzuki et al. 1999) and maintained self administration behavior in monkeys (Beardsley & Balster 1992, Bergman & Spealman 1986, Wang & Woolverton 2007). Some antagonists at H1 receptors show also cocaine-like effects in drug-discrimination studies in pigeons and rats (Suzuki et al. 1997, Zacny 1989), and in schedule-controlled behavior in monkeys (McKearney 1982, Bergman 1990, Bergman & Spealman 1986, Bergman & Spealman 1988). Although the main pharmacological target of these drugs is the H1 receptor, some of the older antihistaminic medications can bind with high to moderate affinity to other sites (Kulkarni et al. 2006, Young et al. 1988). The reinforcing effects of these compounds have been attributed to mechanisms other than H1 receptor antagonism, and especially to interactions with brain monoamine systems (see for example (Bergman 1990, Campbell et al. 2005, Dringenberg et al. 1998)). Some antihistaminic compounds have been suggested to increase dopamine (DA) and serotonin turnover in rodent brains (Shishido et al. 1991), and to stimulate DA neurotransmission in DA terminal areas in anesthetized rats (Dringenberg et al. 1998).
Virtually all drugs abused by humans share the ability to increase DA neurotransmission in experimental animals (Di Chiara & Imperato 1988, Di Chiara et al. 1993), and the increase occurs preferentially in the shell as compared to the core subdivision of the NAc (Pontieri et al. 1995, Pontieri et al. 1996, Tanda et al. 1997). In addition, preferential effects in these subdivisions are observed with local cerebral metabolism, and it has been suggested that these selective or preferential effects underlie the reinforcing effects of abused drugs (Di Chiara et al. 1999, Koob 2003). Thus, the goal of the present study was to investigate the effects on DA neurotransmission of selected H1 antagonist drugs contained in OTC preparations. To this end the present study examined the pattern of DA stimulation after acute administration of the histamine H1 antagonists, diphenhydramine and (+)-chlorpheniramine, and compared those effects to those of a highly selective H1 antagonist, triprolidine, and the less active (−)-enantiomer of chlorpheniramine. The in vitro effects of these drugs at sites involved in the actions of the psychostimulant cocaine were also assessed.
METHODS
Subjects
Male Sprague Dawley rats (Taconic, Germantown, NY), experimentally naive at the start of the study and weighing 300 to 350g, were doubly housed and had free access to food and water. All rats were housed in a temperature- and humidity-controlled room and were maintained on a 12-h light/dark cycle (lights were on from 0700–1900h). Experiments were conducted during the light phase.
Animals used in this study were maintained in facilities fully accredited by AAALAC International, and all procedures were conducted in accordance with the guidelines of the Institutional Care and Use Committee of the NIDA Intramural Research Program, and the National Research Council, Guide for care and use of laboratory animals.
In vivo microdialysis
Surgery
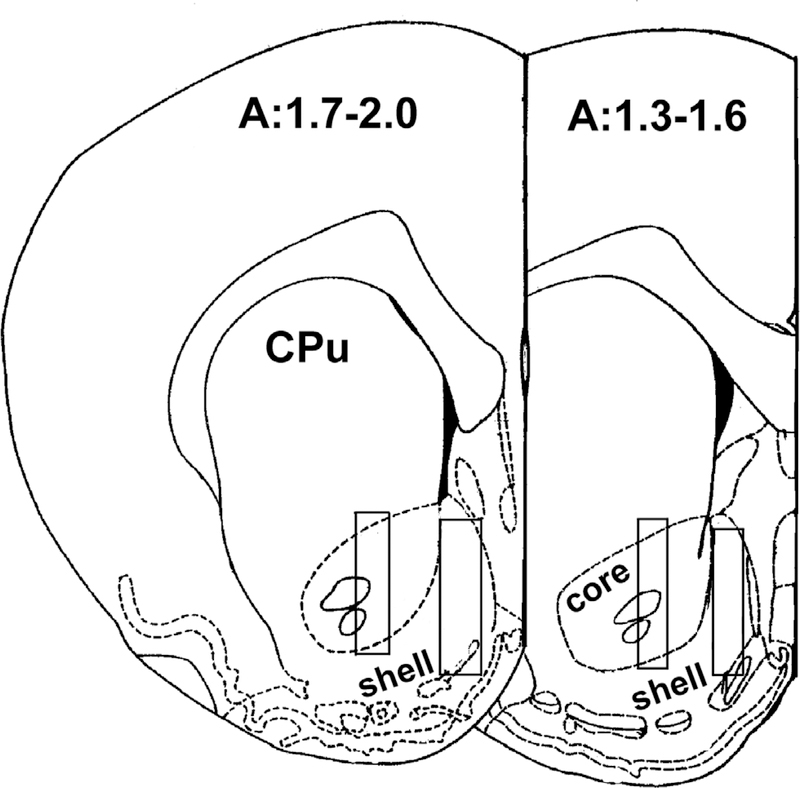
Under a mixture of ketamine and xylazine (60.0 and 12.0 mg/kg i.p., respectively) anesthesia, rats were implanted with an intravenous catheter and microdialysis probes. Rats were first prepared with a silastic catheter implanted into the external jugular vein, with the catheter exiting the skin at the back between the shoulders, as described previously (Solinas et al. 2006). Rats were then placed in a stereotaxic apparatus, the skull was exposed, and two small holes were drilled to expose the dura. Rats were then implanted in the right and left brain side with concentric dialysis probes (see below) aimed at the NAc shell or core, as described previously (Tanda et al. 2005, Tanda et al. 2007) (see Figure 1 for placements). Coordinates were from the rat brain atlas Paxinos and Watson (1987) (uncorrected coordinates: shell, A= +2.0, L=±1.1, V=7.9; core, A=+1.6, L=±1.9, V=7.7; Anterior (A), mm from bregma; Lateral (L) mm from bregma; Vertical (V) mm from dura). After the surgery rats were allowed to recover overnight in hemispherical CMA-120 cages (CMA/Microdialysis AB, Solna, Sweden).
Figure 1.
Forebrain sections, redrawn from Paxinos and Watson, 1987, showing the limits of the positions of the dialyzing portions of the microdialysis probes (superimposed rectangles) resulting from histological analysis of brain sections. On each section the anterior coordinate (A) (measured from Bregma) is indicated. CPU = caudate putamen; core = nucleus accumbens core; shell = nucleus accumbens shell.
Concentric dialysis probes were prepared with AN69 fibers (Hospal Dasco, Bologna, Italy). Briefly, two 4-cm pieces of silica-fused capillary tubes (the inlet and outlet tubing of the probes) were inserted into a 6-mm capillary (0.25 mm external diameter) dialysing fiber (closed by a drop of glue on the other side), with the inlet tubing set at about 0.1 mm from the closed end of the fiber, and the outlet set at 1.5 mm from the inlet tip. The open end of the dialysis membrane was then glued and the protruding two silica-fused tubings were inserted and glued into a 22-G stainless steel needle (2.4 mm length). The needle was attached to a CMA/10 clip (CMA/Microdialysis AB, Solna, Sweden) and mounted in a stereotaxic holder. The exposed dialysing surface of the fibers, i.e. not covered by glue, was limited to the lowest 1.5 mm portion of the probes.
Analytical and experimental procedure
Experiments were performed on freely-moving rats in the same hemispherical cages in which they recovered from surgery. About 20–22 h after implant, probes were connected to fluid swivels (375/D/22QM, Instech, Plymouth Meeting, PA, USA), and Ringer’s solution (147.0 mM NaCl, 2.2 mM CaCl2 and 4.0 mM KCl) was delivered by a 1.0 ml syringe, operated by a BAS Bee Syringe Pump Controller (BAS West Lafayette, IN, USA), through the dialysis probes at a constant flow rate of 1 μl/min. Collection of dialysate samples started after 30 min, and 10 μl samples were taken every 10 min and immediately analyzed, as detailed below.
After stable DA values (less than 10% variability) were obtained for at least three consecutive samples (typically after about 1–2 hours), rats were treated with the test drugs or saline. Rats were used only once and received only one drug dose/treatment. Dialysate samples were injected without purification into a high-performance liquid chromatography apparatus equipped with an MD 150 mm X 3.2 mm column, particle size 3.0 μm (ESA, Chelmsford, MA) and a coulometric detector (5200a Coulochem II, ESA, Chelmsford, MA) to quantify DA. The oxidation and reduction electrodes of the analytical cell (5014B; ESA, Chelmsford, MA) were set at +125 mV and −125 mV, respectively. The mobile phase, containing 100 mM NaH2PO4, 0.1 mM Na2EDTA, 0.5 mM n-octyl sulfate, and 18% (v/v) methanol (pH adjusted to 5.5 with Na2HPO4), was pumped by an ESA 582 (ESA, Chelmsford, MA) solvent delivery module at 0.60 ml/min. Assay sensitivity for DA was 2 fmoles per sample.
Histology
At the end of the experiment, rats were euthanatized by pentobarbital overdose, brains were removed and left to fix in 4% formaldehyde in saline solution. Brains were then cut on a vibratome 1000 Plus (The Vibratome Company, St. Louis, MO, USA) in serial coronal slices (orientation as per Paxinos and Watson, 1987) in order to identify the location of the probes. In all the experiments the locations of the probes were verified. Figure 1 schematically shows typical locations of the dialyzing portion of the probes implanted in each region. The sections are redrawn from Paxinos and Watson (1987) and the anterior coordinates measured from bregma for each brain area are indicated. Only the experiments in which the probes were located in these areas have been used in the present paper.
Behavioral observations
Behavior was observed every 10 minutes during the course of the microdialysis experiments. The following behavioral signs were noted: wakefulness (eyes were open and animals were responsive to external stimuli, but not ambulating); locomotor activity (intermittent ambulation was observed); digging (episodes of manipulation of bedding with forepaws); tremors (continuous shaking of the head and/or the body). For each animal the appearance of a particular behavioral sign during the 30 minutes after drug administration was noted, and incidence of signs is reported in table 1. Because only the presence of a particular behavior was scored and the sample size was relatively small, we did not perform a statistical analysis of data reported on table 1.
Table 1.
Behavioral effects (behavioral score) elicited by intravenous H1 antagonists administration during microdialysis experiments.
| Drug, dose (mg/kg) | Wakefulness | Locomotor activity | Digging | Tremors | Maximum Shell DA (% of basal) |
|---|---|---|---|---|---|
| Diphenhydramine | |||||
| 1 | 4/4 | 4/4 | 0/4 | 0/4 | 143 ± 4 |
| 3 | 4/4 | 4/4 | 2/4 | 0/4 | 339 ± 43 |
| (+)-Chlorpheniramine | |||||
| 1 | 4/5 | 4/5 | 0/5 | 0/5 | 147 ± 9 |
| 3 | 4/4 | 4/4 | 1/4 | 0/4 | 188 ± 11 |
| 5.6 | 4/4 | 4/4 | 4/4 | 3/4 | 285 ± 39 |
| (−)-Chlorpheniramine | |||||
| 1 | 4/4 | 2/4 | 0/4 | 0/4 | 126 ± 12 |
| 3 | 5/5 | 5/5 | 1/5 | 1/5 | 178 ± 17 |
| 5.6 | 5/5 | 5/5 | 1/5 | 4/5 | 216 ± 7 |
| Triprolidine | |||||
| 1 | 0/4 | 0/4 | 0/4 | 0/4 | 111 ± 5 |
| 3 | 2/4 | 0/4 | 0/4 | 1/4 | 115 ± 7 |
Values represent the incidence of each behavioral sign, i.e. the number of animals displaying a particular sign during the 30 minutes after drug administration over the total number of animals tested. The last column on the right shows the maximum increase in DA levels obtained for each dose of H1 antagonist tested.
In Vitro Studies
Histamine H1 Receptor Binding
Frozen rat brains including cerebellum were thawed on ice. Membranes were prepared by homogenizing thawed brain in 20 volumes (w/v) of ice cold 50 mM Na-K buffer (37.8 mM Na2HPO4, 12.2mM KH2PO4, pH 7.5 at 25° C) using a Brinkman Polytron (setting 6 for 20 sec.) and centrifuged at 20,000 × g for 10 min at 4°C. The resulting pellet was resuspended in buffer, recentrifuged and resuspended in buffer to a concentration of 200 mg/ml. Ligand binding experiments were conducted in polystyrene assay tubes containing 0.5 ml 50 mM Na-K buffer for 60 min at room temperature. Each tube contained 2.0 nM [3H]mepyramine (PerkinElmer Life Sciences; specific activity 20 Ci/mmol) and 20 mg tissue (original wet weight). Nonspecific binding was determined using 0.1 mM promethazine (Sigma Aldrich). Incubations were terminated by rapid filtration through Whatman GF/B filters, presoaked in 0.3% polyethyleneimine (PEI), using a Brandel R48 filtering manifold (Brandel Instruments, Gaithersburg, MD). The filters were washed twice with 5 ml of cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0 ml) was added and the vials were counted the next day using a Beckman 6000 liquid scintillation counter (Beckman Coulter Instruments, Fullerton, California).
Dopamine Transporter (DAT) Binding
Brains from male Sprague-Dawley rats, weighing 200–225 g, were removed, the striatum was dissected and quickly frozen. Membranes were prepared by homogenizing tissues in 20 volumes (w/v) of ice cold modified sucrose phosphate buffer (0.32 M sucrose, 7.74 mM Na2HPO4, 2.26 mM NaH2PO4, pH adjusted to 7.4) using a Brinkman Polytron (setting 6 for 20 sec) and centrifuged at 20,000 × g for 10 min at 4° C. The resulting pellet was resuspended in buffer, recentrifuged and resuspended in buffer to a concentration of 10 mg/ml. Ligand binding experiments were conducted in assay tubes containing 0.5 ml sucrose phosphate buffer for 120 min on ice. Each tube contained 0.5 nM [3H]WIN 35,428 (PerkinElmer Life Sciences; specific activity 84 Ci/mmol) and 1.0 mg striatal tissue (original wet weight). Nonspecific binding was determined using 0.1 mM cocaine HCl. Incubations were terminated by rapid filtration through Whatman GF/B filters, presoaked in 0.05% PEI, using a Brandel R48 filtering manifold. The filters were washed twice with 5ml cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0ml) was added and the vials were counted the next day using a Beckman 6000 liquid scintillation counter.
In vitro DA uptake protocol
Freshly harvested rat brain striatal tissue was homogenized in ice cold buffer (5 mM HEPES, 0.32 M sucrose) using 10 strokes with a Teflon glass homogenizer followed by centrifugation at 1000 g for 10 min at 4° C. The supernatant was saved and recentrifuged at 10,000 × g for 20 min at 4 °C. The supernatant was then discarded and the pellet was gently resuspended in ice cold incubation buffer (127 mM NaCl, 5 mM KCl, 1.3 mM NaH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, 1.498 mM HEPES acid, 10 mM D-Glucose, 1.14 mM L-ascorbic acid, pH 7.4) and placed on ice for 15 minutes.
The synaptosomal tissue preparation was incubated in buffer in assay test tubes at 37° C to which 10 uM pargyline (Sigma) and either the drug being tested or no drug was added, as appropriate. After a 10 minute pre-incubation in the presence of drug, [3H]dopamine (Amersham Biosciences, specific activity 48 Ci/mMol) was added to each tube (final concentration 0.5 nM) and the incubation was carried on for 5 minutes. The reaction was terminated by the addition of 3 mL ice-cold buffer to each tube and rapid filtration through Whatman GF/B glass fiber filter paper (presoaked in 0.1% PEI in water) using a Brandel cell harvester. After filtration, the filters were washed with two additional 3 ml washes and transferred into scintillation vials. Beckman Ready Value (Beckman-Coulter) was added and the vials were counted the next day using a Beckman 6000 LS counter. The reported values represent specific uptake from which nonspecific uptake was subtracted (defined as uptake in the presence of 100 μM (−)-cocaine HCl).
Serotonin Transporter (SERT) Binding
Brains from male Sprague-Dawley rats weighing 200–225 g were removed; the midbrain was rapidly dissected and frozen. Membranes were prepared by homogenizing tissues in 20 volumes (w/v) of 50 mM Tris containing 120mM NaCl and 5 mM KCl, (pH 7.4 at 25° C), using a Brinkman Polytron (setting 6 for 20 sec) and centrifuged at 20,000 × g for 10 min at 4° C. The resulting pellet was resuspended in buffer, recentrifuged and resuspended in buffer to a concentration of 15 mg/ml. Ligand binding experiments were conducted in assay tubes containing 0.5 ml buffer for 60 min at room temperature. Each tube contained 1.4 nM [3H]citalopram (Amersham Biosciences, specific activity 83 Ci/mMol) and 1.5 mg midbrain tissue (original wet weight). Nonspecific binding was determined using 10 μM fluoxetine (Sigma). Incubations were terminated by rapid filtration through Whatman GF/B filters, presoaked in 0.3% PEI, using a Brandel R48 filtering manifold. The filters were washed twice with 5 ml cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0 ml) was added and the vials were counted the next day using a Beckman 6000 liquid scintillation counter.
Norepinephrine Transporter (NET) Binding
Brains from male Sprague-Dawley rats weighing 200–225 g were removed; the frontal cortex was rapidly dissected and frozen. Membranes were prepared by homogenizing tissues in 20 volumes (w/v) of 50 mM Tris containing 120 mM NaCl and 5 mM KCl, (pH 7.4 at 25° C), using a Brinkman Polytron (setting 6 for 20 sec) and centrifuged at 20,000 × g for 10 min at 4° C. The resulting pellet was resuspended in buffer, recentrifuged and resuspended in buffer to a concentration of 80 mg/ml. Ligand binding experiments were conducted in assay tubes containing 0.5 ml buffer for 180 min at 0–4° C. Each tube contained 0.5 nM [3H]nisoxetine (PerkinElmer Life Science, specific activity 87Ci/mMol) and 8 mg frontal cortex tissue (original wet weight). Nonspecific binding was determined using 1 μM desipramine (Sigma). Incubations were terminated by rapid filtration through Whatman GF/B filters, presoaked in 0.05% PEI, using a Brandel R48 filtering manifold. The filters were washed twice with 5 ml cold buffer and transferred to scintillation vials. Beckman Ready Safe (3.0 ml) was added and the vials were counted using a Beckman 6000 liquid scintillation counter.
Drugs
The drugs tested were: diphenhydramine HCl, (+)-chlorpheniramine HCl, and trans-triprolidine HCl (Sigma RBI, St. Louis, MO); (−)-chlorpheniramine (Wako Chemicals, Richmond, VA, USA); and (−)-cocaine HCl (Sigma RBI, St. Louis, MO, and NIDA). For in vivo studies drugs were dissolved in 0.9% NaCl and were injected i.v. in a volume of 1.0 ml/kg.
The doses of diphenhydramine used in the present experiments (1 and 3 mg/kg i.v.) were selected based on a recent report (Jun et al. 2004) of synergy of action between diphenhydramine and dextromethorphan. Doses of the other drugs were chosen in a range (1.0 to 5.6 mg/kg) similar to that used for diphenhydramine, in order to make meaningful comparison of the dose-effect curves of the different drugs on dopamine transmission. Higher doses of these compounds could not be tested due to the appearance of tremors and convulsions in some of the animals.
Data analysis
All in vitro data were analyzed using a non-linear, least squares regression analysis (GraphPad Prism software, San Diego, CA), with Ki values calculated from IC50 values using the equation of Cheng and Prusoff (1973) and historical KD values of the radioligand.
In the microdialysis study, results were expressed as a percentage of basal DA values, which were calculated as means of the three consecutive samples immediately preceding the test drug or saline injection. These results are not shown in the figures. All results are presented as group means (±SEM). Statistical analysis (Statistica software, Stat Soft, Tulsa, OK) was carried out using one-, two-, or three-way ANOVA for repeated measures over time applied to the data obtained from serial assays of dialysate DA normalized as percentage of basal values of each group. Significant results were subjected to post-hoc Tukey’s tests. Statistical analysis of differences in basal DA values (fmol/10 μl sample ± SEM) between different experimental groups and brain areas was carried out with one-way ANOVA. Changes were considered to be significant when p<0.05. Sample size and basal DA values, expressed as fmoles/sample ± SEM, for each microdialysis experimental group are indicated in the corresponding figure legends.
RESULTS
In Vivo Microdialysis Studies
Basal values of DA from the same brain area (reported in the figure captions) did not significantly differ between various experimental groups. In addition, there were no significant differences between basal DA levels in the shell as compared to the core of the NAc within the same or between different treatment groups (Two-way ANOVA, main effect brain area: F(1,66) = 3.376, P> 0.05, NS; main effect treatment group: F(9,66) = 0.400, p> 0.05, NS; effect of interaction of brain area and treatment: F(9,66) = 0.336, p>0.05). Further, intravenous saline administration did not significantly modify extracellular DA levels in dialysates from the NAC shell or core (data not shown).
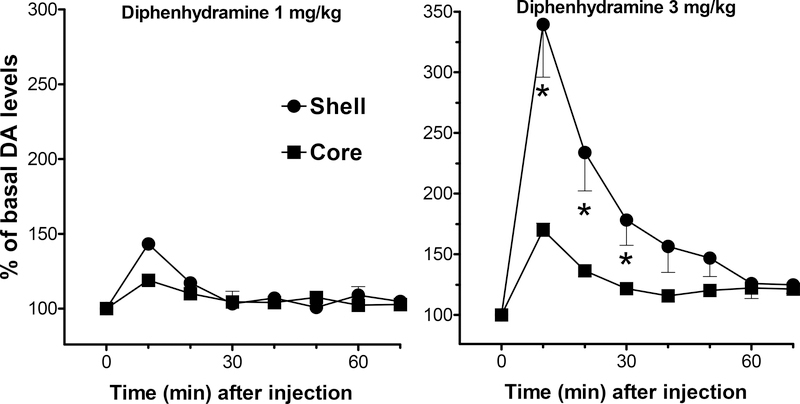
Diphenhydramine (1.0 and 3.0 mg/kg) produced a significant and dose-dependent stimulation of extracellular levels of DA in the NAc shell and in the NAc core (Fig. 2). A three-way ANOVA showed significant main effects of NAc subdivision (F(1,12) = 9.480, p<0.01), drug dose (F(1,12) = 23.225, p<0.001), and time (F(7,84) = 45.923, p<0.0001); the analysis also showed significant interactions between NAc area and drug dose (F(1,12) = 6.647, p<0.05), area and time (F(7,84) = 14.701, p<0.0001), drug dose and time (F(7,84) = 20.562, p<0.0001), and area, drug dose, and time (F(7,84) = 8.987, p<0.0001). In the NAc shell the stimulation of DA levels reached the maximum obtained, 340 % at the 3 mg/kg dose, at 10 min after injection. As showed in Fig. 2, the effect in the NAc core was less than that elicited in the shell. The stimulation of DA reached the maximum obtained, 170 %, during the first 10 min after the injection of 3.0 mg/kg in the NAc core, and rapidly returned to basal value levels within 40 min.
Figure 2.
Effects of intravenous diphenhydramine (1.0 and 3.0 mg/kg) administration on extracellular DA levels in dialysates from rats implanted with microdialysis probes in the NAc shell (filled circles) and core (filled squares). Note that the effect of diphenhydramine on dialysate DA from the shell is significantly greater than its effect on DA levels in the core. Results are means, with vertical bars representing SEM, of the amount of DA in 10-min dialysate samples, expressed as percentage of basal values. Basal DA values (fmoles/sample) and group size (n) were: 38.3 ± 8.5 (4), and 36.6 ± 2.8 (4) for 1 and 3 mg/kg groups in the NAc shell, and 57.13 ± 7.3 (4), and 49.8 ± 2.1 (4) for 1 and 3 mg/kg in the NAc core. *= P<0.05 compared to the corresponding time point in the core.
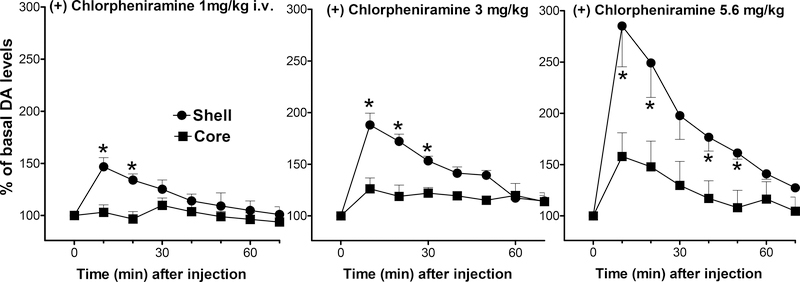
Administration of (+)-chlorpheniramine (1.0 – 5.6 mg/kg) also elicited a dose-dependent and significant stimulation of extracellular DA levels in the NAc shell and core (Fig. 3). A three-way ANOVA showed significant main effects of NAc area (F(1,20) = 26.149, p<0.001), drug dose (F(2,20) = 12.328, p<0.001), and time (F(7,140) = 43.822, p<0.0001); also shown were significant interactions between area and drug dose (F(2,20) = 3.614, p<0.05), area and time (F(7,140) = 12.228, p<0.0001), and drug dose and time (F(14,140) = 7.755, p<0.0001), but not a significant interaction between area, drug dose, and time (F(14,140) = 1.169, p=0.305). DA levels reached the maximum obtained (270 %) in the first 10 min after injection of 5.6 mg/kg of (+)-chlorpheniramine in the NAc shell, and returned to basal, non-stimulated levels after about 60 min (Fig. 3).
Figure 3.
Effects of intravenous (+)-chlorpheniramine (1.0, 3.0, and 5.6 mg/kg) administration on extracellular DA levels in dialysates from rats implanted with microdialysis probes in the NAc shell (filled circles) and core (filled squares). Note that the effect of (+)-chlorpheniramine on dialysate DA from the shell is significantly greater than its effect on DA levels in the core. Results are means, with vertical bars representing SEM, of the amount of DA in 10-min dialysate samples, expressed as percentage of basal values. Basal DA values (fmoles/sample) and group size (n) were: 37.3 ± 6.0 (5), 43.3 ± 7.5 (4), and 47.0 ± 3.0 (4) for 1, 3, and 5.6 mg /kg groups in the NAc shell, and 48.8 ± 8.9 (5), 49.6 ± 5.4 (4), and 47.3 ± 4.7 (4) for 1, 3, and 5.6 mg/kg in the NAc core. *= P<0.05 compared to the corresponding time point in the core.
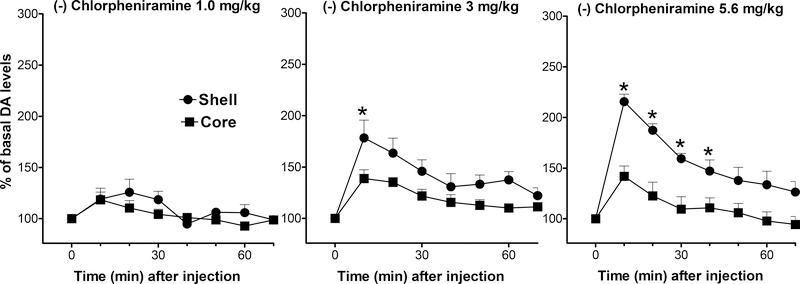
The (−)-enantiomer of chlorpheniramine, at doses from 1.0 to 5.6 mg/kg, stimulated extracellular DA levels in dialysates from the NAc shell and core in a dose-dependent manner (Fig. 4). A three-way ANOVA showed significant main effects of NAc area (F(1,22) = 20.602, p<0.001), drug dose (F(2,22) = 9.265, p<0.005), and time (F(7,154) = 39.792, p<0.0001); also shown were significant interactions between area and drug dose (F(2,22) = 3.858, p<0.05), area and time (F(7,154) = 5.209, p<0.0001), and drug dose and time (F(14,154) = 3.977, p<0.0001), without a significant interaction between area, drug dose, and time (F(14,154) = 1.403, p=0.157). The stimulation of DA levels in the NAc shell was rapid and transient. The maximal effect obtained (210%) was reached at 10 min after injection, with DA levels returning to basal values after about 1 hour.
Figure 4.
Dose-dependent effects of intravenous (−)-Chlorpheniramine (1.0, 3.0, and 5.6 mg/kg) administration on extracellular DA levels in dialysates from rats implanted with microdialysis probes in the NAc shell (filled circles) and core (filled squares). Data are expressed as % of basal DA values. *= P<0.05 compared to the corresponding time point in the core. Note that though (−)-Chlorpheniramine has a 16 fold lower affinity for H1 receptors compared to the (+) enantiomer, it shows a significant stimulation of DA levels, and significantly greater effects on DA levels in the shell compared to the core. Results are means, with vertical bars representing SEM, of the amount of DA in 10-min dialysate samples, expressed as percentage of basal values. Basal DA values (fmoles/sample) and group size (n) were: 49.3 ± 7.5 (4), 43.2 ± 5.0 (5), and 39.5 ± 5.1 (5) for 1, 3, and 5.6 mg /kg groups in the NAc shell, and 50.6 ± 2.7 (4), 53.6 ± 5.5 (5), and 40.5 ± 6.7 (5) for 1, 3, and 5.6 mg/kg in the NAc core. *= P<0.05 compared to the corresponding time point in the core.
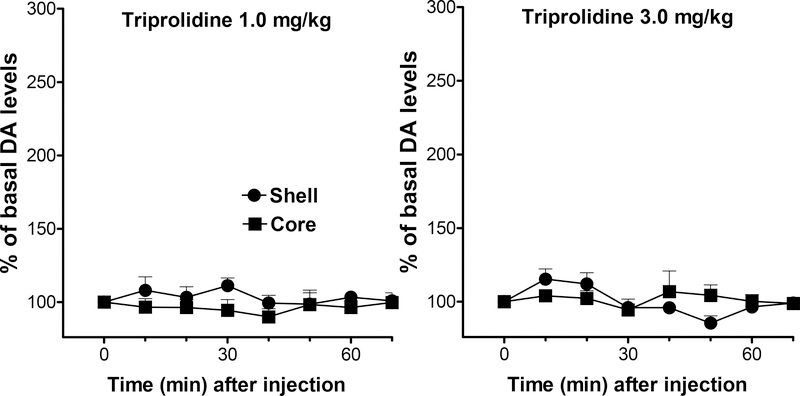
Triprolidine administration (1 and 3 mg/kg) did not elicit any significant modification of extracellular DA levels in dialysates from the NAc shell and core at any time after injection, as shown in Fig. 5. A three-way ANOVA did not show any significant main effect, nor any significant interactions of the analyzed factors: drug dose, time, and brain area.
Figure 5.
Effects of intravenous Triprolidine (1.0 and 3.0 mg/kg) administration on extracellular DA levels in dialysates from rats implanted with microdialysis probes in the NAc shell (filled circles) and core (filled squares). Note that though triprolidine shows very high affinity and selectivity for H1 receptors it does not modify dialysate DA levels from the shell or from the core. Results are means, with vertical bars representing SEM, of the amount of DA in 10-min dialysate samples, expressed as percentage of basal values. Basal DA values (fmoles/sample) and group size (n) were: 43.0 ± 2.3 (4), and 39.0 ± 7.7 (4) for 1 and 3 mg/kg groups in the NAc shell, and 43.3 ± 3.5 (4), and 41.0 ± 5.1 (4) for 1 and 3 mg/kg in the NAc core.
Behavioral observations
All drugs, except triprolidine, increased the incidence of wakefulness and locomotor activity at all doses (see Table 1). Digging under the bedding was observed with higher doses of drugs, especially with (+)-chlorpheniramine. The largest doses of chlorpheniramine enantiomers produced tremors in almost all animals.
In Vitro Studies
As previously reported (e.g. Haaksma et al. 1990) triprolidine had a high affinity for histamine H1 receptors, that was greater than that for any of the other antagonists. In addition, the (+)-enantiomer of chlorpheniramine had an affinity for H1 receptors that was about 13-fold greater than that for the (−)-enantiomer (Table 2). In contrast, triprolidine had a micromolar affinity for the DAT, at which, among the H1 antagonists, (+)-chlorpheniramine had the highest affinity, which was less than 2-fold greater than that for (−)-chlorpheniramine, and less than 3-fold lower than the affinity of cocaine for the DAT. The Ki values at the DAT correlated with potencies for inhibition of DA uptake in synaptosomes (R2 = 0.998; p < 0.0001), and the correlation remained significant with the exclusion of the values for triprolidine (R2 = 0.939; p = 0.0312). The highest affinity obtained at the SERT was for (+)-chlorpheniramine, which was greater than its affinity for the DAT and more than 10-fold greater than that for any of the other compounds. Affinities of the compounds at the NET were uniformly in the micromolar range.
Table 2:
Affinities of H1 antagonists compared to cocaine at H1 histamine receptors, monoamine transporters and potency as inhibitors of DA uptakea.
| Compound | H1 Ki Value (± SEM) | DAT Ki Value (± SEM) | SERT Ki Value (± SEM) | NET Ki Value (± SEM) | DA Uptake Inhibition IC50 Value (± SEM) |
|---|---|---|---|---|---|
| Triprolidine | 27.7 ± 2.06 | 6010 ± 475 | 3340 ± 134 | 12800 ± 487 | 19000 ± 2520 |
| (+)-Chlorpheniramine | 37.9 ± 1.08 | 203 ± 20.0 | 25.4 ± 2.03 | 6140 ± 845 | 669 ± 54.9 |
| Diphenhydramine | 96.0 ± 7.43 | 851 ± 72.2 | 9050 ± 699 | 2070 ± 159 | 2080 ± 115 |
| (−)-Chlorpheniramine | 506 ± 69 | 383 ± 15.5 | 355 ± 47.8 | 5390 ± 782 | 1420 ± 144 |
| Cocaine | 1040 ± 43.0 | 71.8 ± 4.56 | 293 ± 30 | 2120 ± 314 | 293 ± 30 |
All values are in nM.
DISCUSSION
In the present experiments the effects of the histamine H1 receptor antagonists diphenhydramine, chlorpheniramine, and triprolidine on DA neurotransmission in the NAc shell and core were assessed by microdialysis coupled with HPLC and electrochemical detection, in awake freely-moving rats. Diphenhydramine and both enantiomers of chlorpheniramine dose-dependently increased DA levels in the two sub-regions of the NAc, with the effects greater in the shell as compared to the core of the NAc. Previous microdialysis studies with diphenhydramine in anesthetized rats suggested a similar pattern of activation, though subregions of the NAc were not investigated (Dringenberg et al. 1998). A study by Galosi et al. (2001) suggests that the effects of histaminergic drugs on DA may be initiated by actions outside the NAc, in urethane-anesthetized rats. The present experiments employed experimental conditions in which different classes of drugs abused by humans, including psychostimulants (e.g., cocaine, amphetamine) and other abused drugs (e.g., opioids, nicotine, cannabinoids), show a similar pattern of greater activation of DA transmission in the shell compared to the core of the NAc (Pontieri et al. 1995, Pontieri et al. 1996, Tanda et al. 1997). Under these conditions, triprolidine, a high affinity and selective histamine H1 receptor antagonist that has not been reported to be misused, did not elicit any significant change in extracellular DA levels in dialysates from the NAc shell or core at any dose tested.
All of the H1 antagonists that significantly increased DA levels in the present experiments also elicited behavioral effects characterized primarily by wakefulness and ambulation. These behavioral effects are similar to those previously reported after intravenous administration of psychostimulants at doses that produced comparable increases in DA levels (Pontieri et al. 1995).
Results from the in vitro experiments confirmed the high affinity of triprolidine for H1 receptors (Haaksma et al. 1990) which was the highest affinity among the H1 antagonists tested in this study. The obtained rank-order of affinities was triprolidine having the highest affinity, followed in order by (+)-chlorpheniramine, diphenhydramine, and (−)-chlorpheniramine confirmed potency relations previously reported (e.g. Chang et al., 1979). The lower affinity of the (−)-enantiomer of chlorpheniramine confirmed the stereoselectivity of chlorpheniramine at H1 receptors (Chang et al. 1979, Roth & Govier 1958).
If the increase in DA neurotransmission elicited by intravenous administration of selected H1 receptor antagonists was the result simply of an action of these drugs at H1 histamine receptors, triprolidine, which has the highest affinity for H1 receptors would have had the greatest potency for increasing DA levels in the microdialysis experiments. However, triprolidine, which has been shown to rapidly penetrate the blood brain barrier (Chen et al. 2003, Estelle & Simons 1999), did not elicit significant increases in DA levels, whereas (−)-chlorpheniramine, which had the lowest affinity for H1 receptors (about 18 times lower than triprolidine) significantly increased DA levels in the NAc. In this respect, the maximum effect on DA transmission of 5.6 mg/kg of each of the chlorpheniramine enantiomers (about 270% and 210% increases, respectively for (+)- and (−)-enantiomers) was more in agreement with their less than 2-fold difference in affinities for the DAT, than with their more than a 13-fold difference in H1 receptor affinity.. Further, diphenhydramine showed an affinity for H1 receptors that was lower than that for triprolidine and (+)-chlorpheniramine, but increased DA transmission in the NAc shell significantly, and to the greatest extent among the H1 antagonists tested. The rank order of affinities at H1 receptors did not correlate with the order of potency to stimulate DA levels in vivo. Thus, the data suggest that the effect of these compounds on DA levels is not due to actions at H1 histamine receptors.
Previous studies have suggested that the behavioral effects of diphenhydramine and chlorpheniramine are not mediated by H1 receptors and have implicated dopaminergic mechanisms (e.g. Bergman 1990, Bergman & Spealman 1986, Suzuki et al. 1997). In addition, displacement of [3H]cocaine from mouse cerebral cortex membranes has been reported with (+)- and (−)-chlorpheniramine, and these enantiomers were equally potent in doing so (Reith et al. 1984), and in stimulating rates of operant behavior in squirrel monkeys (Bergman 1990). The present studies of displacement of [3H]WIN 35,428 from the DAT are consistent with the lack of stereoselectivity of the chlorpheniramine enantiomers and extend the binding results to diphenhydramine demonstrating dopamine transporter affinities in the sub-micromolar range. The highest affinity was shown by (+)-chlorpheniramine, which was less than three-fold lower in affinity than cocaine. The (+)-enantiomer was less than two-fold higher in affinity than its (−)-enantiomer and about four-fold higher in affinity than diphenhydramine. Finally triprolidine had the lowest affinity for the DAT, which was greater than 80-fold lower in affinity than cocaine. The same drugs were also tested on DA uptake in vitro and the potencies in this functional assay were highly correlated with the affinities obtained in the DA transporter binding assay.
It is significant that, though (−)-chlorpheniramine had a 13-fold lower affinity than the (+)-enantiomer at H1 receptors, the difference between the two enantiomers was less than two-fold in the functional assay for DA uptake, and in DAT binding. These effects of chlorpheniramine enantiomers in vitro likely reflect the small differences obtained in the in vivo DA microdialysis experiments. Bergman (1990) made the same observation for the behavioral stimulant effects of chlorpheniramine enantiomers. Thus, the behavioral and neurochemical effects of chlorpheniramine enantiomers and the other H1 antagonists on behavior may be due to their abilities to bind to the DAT and to block DA uptake, as shown in the present in vitro experiments. However, effects at the DAT alone do not account for the effects of diphenhydramine. This compound had lower affinity for the DAT and higher IC50 values in DA uptake assays as compared to chlorpheniramine enantiomers, but was the H1 receptor antagonist with the largest in vivo effect on DA.
The present experiments also showed that (+)-chlorpheniramine binds to the SERT with high affinity, comparable to its affinity for H1 receptors, and about 8-fold higher than its affinity for DAT sites. The rank order of potency for SERT was (+)-chlorpheniramine > (−)-chlorpheniramine >> triprolidine >diphenhydramine. On the other hand, binding results for the NET showed a very low affinity of the H1 antagonists, with a potency rank order of: diphenhydramine > (−)-chlorpheniramine > (+)-chlorpheniramine > triprolidine. While the in vitro experiments do not immediately suggest a site of action for diphenhydramine that could facilitate its ability to stimulate DA neurotransmission, activity at the SERT by (+)-chlorpheniramine could attenuate its effects mediated by the DAT, as has been suggested for some behavioral effects of various psychostimulants (Ritz & Kuhar 1989). Thus, the combined actions at the DAT and SERT may reconcile the relative potencies observed in vivo and the in vitro effects of these H1 antagonists.
It is interesting to note that diphenhydramine shares with cocaine the ability to block sodium channels (Kim et al. 2000, Kuo et al. 2000), and that both diphenhydramine and (+)-chlorpheniramine have been tested as local anesthetics (Green et al. 1994, Orhan et al. 2007). It has also been shown that some drugs sharing with cocaine the ability to block sodium channels and to function as local anesthetics, show effects similar to those of cocaine in some behavioral tests (e.g. Graham & Balster 1993, Wilcox et al. 1999). However, it has also been reported that the cocaine-like reinforcing effects of local anesthetics, and the stimulation of DA transmission in the nucleus accumbens and striatum, correlate better with the affinity of these drugs for the DAT than with their affinity for sodium channels (Hernandez et al. 1991, Wilcox et al. 2005, Wilcox et al. 1999, Woodward et al. 1995). Indeed lidocaine, a local anesthetic without significant DAT affinity did not stimulate dopamine transmission (Hernandez et al. 1991, Woodward et al. 1995), and was not self-administered (Wilcox et al. 1999). Thus, it is unlikely that actions at sodium channels and local anesthetic effects induced by the histamine antagonists played a significant role in the outcome of the present microdialysis studies.
Recent studies have shown a potentiation of some behavioral effects of psychostimulant drugs by histamine H3 receptor blockade (Munzar et al., 2004; Campbell et al., 2005), which is correlated with a potentiation of dopamine transmission in the NAc shell (Munzar et al. 2004). An effect of chlorpheniramine and diphenhydramine at H3 receptors, which are largely expressed in limbic areas of the brain, could explain the present results. However, there currently is no information on the affinities of the present H1 antagonists at H3 receptors. Thus an action of these drugs mediated by central H3 histamine receptors remains a possibility.
Abuse of over the counter H1 antagonists in combination with opioids has been reported, and the abuse of diphenhydramine and heroin combinations has been implicated as an emerging, potential deadly, trend in a recent Community Epidemiology Work Group report (NIDA/NIH October 2006). Poly-drug use is not uncommon among drug abusers. For example, the abuse of combinations of drugs such as cocaine and heroin (with the street name “Speedball”) has been repeatedly reported (for review see: Leri et al. 2003). It is noteworthy that in the present experiments, the time course of the stimulation of DA in the mesolimbic system produced by H1 antagonists more closely resembles that previously shown for indirect DA agonists, such as cocaine and amphetamine (Pontieri et al. 1995), than that obtained with non-psychostimulant drugs, such as morphine or delta-9-THC (Pontieri et al. 1995, Tanda et al. 1997). Thus, it is possible that similarities of mechanism and time-course between cocaine and selected H1 antagonists could contribute, respectively, to the abuse of cocaine/heroin combinations and H1-antagonist/ opioid combinations (“T’s and Blues,” “Blue Velvet”) (Schnoll et al. 1985),
Virtually all drugs abused by humans, including psychostimulants like cocaine and amphetamine, elicit a preferential increase in DA transmission in the shell as compared to the core of the NAc after systemic administration (Pontieri et al. 1995, Pontieri et al. 1996, Tanda et al. 1997). The H1 receptor antagonists that significantly stimulated DA levels in the present report have been shown to elicit psychostimulant-like effects in behavioral studies (e.g. Bergman & Spealman 1988, McKearney 1982, Suzuki et al. 1997, Suzuki et al. 1999) that are in agreement with the cocaine-like activities, both in vivo and in vitro, described in this study. The present experiments show that selected antagonists at H1 histamine receptors increase DA neurotransmission in mesolimbic areas with a pattern of activation that overlaps that showed for cocaine and other drugs abused by humans, and provides a strong neurobiological basis underlying the cocaine-like behavioral effects observed with these compounds, and the occasional misuse of over-the-counter antihistamine medications.
ACKNOWLEDGEMENTS
Work was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services. Tsutomu Suzuki kindly helped locate a supplier of (−)-chlorpheniramine.
List of nonstandard abbreviations
- DA
dopamine
- DAT
dopamine transporter
- NS
nonsignificant
- NAc
nucleus accumbens
- OTC
over the counter
- SERT
serotonin transporter
- SEM
standard error of the mean
REFERENCES
- Banerji S and Anderson IB (2001) Abuse of Coricidin HBP cough & cold tablets: episodes recorded by a poison center. Am J Health Syst Pharm, 58, 1811–1814. [DOI] [PubMed] [Google Scholar]
- Barsoum A, Kolivakis TT, Margolese HC and Chouinard G (2000) Diphenhydramine (Unisom), a central anticholinergic and antihistaminic: abuse with massive ingestion in a patient with schizophrenia. Canadian journal of psychiatry, 45, 846–847. [PubMed] [Google Scholar]
- Beardsley PM and Balster RL (1992) The intravenous self-administration of antihistamines by rhesus monkeys. Drug and alcohol dependence, 30, 117–126. [DOI] [PubMed] [Google Scholar]
- Bergman J (1990) Psychomotor stimulant effects of the stereoisomers of chlorpheniramine. Psychopharmacology, 100, 132–134. [DOI] [PubMed] [Google Scholar]
- Bergman J and Spealman RD (1986) Some behavioral effects of histamine H1 antagonists in squirrel monkeys. The Journal of pharmacology and experimental therapeutics, 239, 104–110. [PubMed] [Google Scholar]
- Bergman J and Spealman RD (1988) Behavioral effects of histamine H1 antagonists: comparison with other drugs and modification by haloperidol. The Journal of pharmacology and experimental therapeutics, 245, 471–478. [PubMed] [Google Scholar]
- Campbell VC, Kopajtic TA, Newman AH and Katz JL (2005) Assessment of the influence of histaminergic actions on cocaine-like effects of 3alpha-diphenylmethoxytropane analogs. The Journal of pharmacology and experimental therapeutics, 315, 631–640. [DOI] [PubMed] [Google Scholar]
- Chang RS, Tran VT and Snyder SH (1979) Heterogeneity of histamine H1-receptors: species variations in [3H]mepyramine binding of brain membranes. Journal of neurochemistry, 32, 1653–1663. [DOI] [PubMed] [Google Scholar]
- Chen C, Hanson E, Watson JW and Lee JS (2003) P-glycoprotein limits the brain penetration of nonsedating but not sedating H1-antagonists. Drug metabolism and disposition: the biological fate of chemicals, 31, 312–318. [DOI] [PubMed] [Google Scholar]
- Cheng Y and Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochemical pharmacology, 22, 3099–3108. [DOI] [PubMed] [Google Scholar]
- Cox D, Ahmed Z and McBride AJ (2001) Diphenhydramine dependence. Addiction (Abingdon, England), 96, 516–517. [PubMed] [Google Scholar]
- Di Chiara G and Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America, 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Bassareo V, Pontieri F, Acquas E, Fenu S, Cadoni C and Carboni E (1999) Drug addiction as a disorder of associative learning. Role of nucleus accumbens shell/extended amygdala dopamine. Annals of the New York Academy of Sciences, 877, 461–485. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Frau R and Carboni E (1993) On the preferential release of dopamine in the nucleus accumbens by amphetamine: further evidence obtained by vertically implanted concentric dialysis probes. Psychopharmacology, 112, 398–402. [DOI] [PubMed] [Google Scholar]
- Dinndorf PA, McCabe MA and Frierdich S (1998) Risk of abuse of diphenhydramine in children and adolescents with chronic illnesses. The Journal of pediatrics, 133, 293–295. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, de Souza-Silva MA, Schwarting RK and Huston JP (1998) Increased levels of extracellular dopamine in neostriatum and nucleus accumbens after histamine H1 receptor blockade. Naunyn-Schmiedeberg’s archives of pharmacology, 358, 423–429. [DOI] [PubMed] [Google Scholar]
- Estelle F and Simons R (1999) H1-receptor antagonists: safety issues. Ann Allergy Asthma Immunol, 83, 481–488. [DOI] [PubMed] [Google Scholar]
- Galosi R, Lenard L, Knoche A, Haas H, Huston JP and Schwarting RK (2001) Dopaminergic effects of histamine administration in the nucleus accumbens and the impact of H1-receptor blockade. Neuropharmacology, 40, 624–633. [DOI] [PubMed] [Google Scholar]
- Graham JH and Balster RL (1993) Cocaine-like discriminative stimulus effects of procaine, dimethocaine and lidocaine in rats. Psychopharmacology, 110, 287–294. [DOI] [PubMed] [Google Scholar]
- Green SM, Rothrock SG and Gorchynski J (1994) Validation of diphenhydramine as a dermal local anesthetic. Annals of emergency medicine, 23, 1284–1289. [DOI] [PubMed] [Google Scholar]
- Haaksma EE, Leurs R and Timmerman H (1990) Histamine receptors: subclasses and specific ligands. Pharmacology & therapeutics, 47, 73–104. [DOI] [PubMed] [Google Scholar]
- Halpert AG, Olmstead MC and Beninger RJ (2003) Dimenhydrinate produces a conditioned place preference in rats. Pharmacology, biochemistry, and behavior, 75, 173–179. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Guzman NA and Hoebel BG (1991) Bidirectional microdialysis in vivo shows differential dopaminergic potency of cocaine, procaine and lidocaine in the nucleus accumbens using capillary electrophoresis for calibration of drug outward diffusion. Psychopharmacology, 105, 264–268. [DOI] [PubMed] [Google Scholar]
- Hughes GF, McElnay JC, Hughes CM and McKenna P (1999) Abuse/misuse of non-prescription drugs. Pharm World Sci, 21, 251–255. [DOI] [PubMed] [Google Scholar]
- Jun JH, Thorndike EB and Schindler CW (2004) Abuse liability and stimulant properties of dextromethorphan and diphenhydramine combinations in rats. Psychopharmacology, 172, 277–282. [DOI] [PubMed] [Google Scholar]
- Kim YS, Shin YK, Lee C and Song J (2000) Block of sodium currents in rat dorsal root ganglion neurons by diphenhydramine. Brain research, 881, 190–198. [DOI] [PubMed] [Google Scholar]
- Koob GF (2003) Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol, 13, 442–452. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Kopajtic TA, Katz JL and Newman AH (2006) Comparative structure-activity relationships of benztropine analogues at the dopamine transporter and histamine H(1) receptors. Bioorganic & medicinal chemistry, 14, 3625–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Huang RC and Lou BS (2000) Inhibition of Na(+) current by diphenhydramine and other diphenyl compounds: molecular determinants of selective binding to the inactivated channels. Molecular pharmacology, 57, 135–143. [PubMed] [Google Scholar]
- Leri F, Bruneau J and Stewart J (2003) Understanding polydrug use: review of heroin and cocaine co-use. Addiction (Abingdon, England), 98, 7–22. [DOI] [PubMed] [Google Scholar]
- McKearney JW (1982) Stimulant actions of histamine H1 antagonists on operant behavior in the squirrel monkey. Psychopharmacology, 77, 156–158. [DOI] [PubMed] [Google Scholar]
- Munzar P, Tanda G, Justinova Z and Goldberg SR (2004) Histamine h3 receptor antagonists potentiate methamphetamine self-administration and methamphetamine-induced accumbal dopamine release. Neuropsychopharmacology, 29, 705–717. [DOI] [PubMed] [Google Scholar]
- NIDA/NIH (October 2006) Community Epidemiology Work Group, Epidemiologic trends in drug abuse Vol. NIH publication # 06–5878A National Institute on Drug Abuse, NIH/DHHS [Google Scholar]
- Orhan ME, Yuksel U, Bilgin F and Dogrul A (2007) Comparison of the local anesthetic effects of chlorpheniramine, midazolam, lidocaine, and normal saline after intradermal injection. Med Sci Monit, 13, PI7–11. [PubMed] [Google Scholar]
- Paxinos G and Watson C (1987) The Rat Brain in Stereotaxic Coordinates. Academic Press, Sydney. [Google Scholar]
- Pontieri FE, Tanda G and Di Chiara G (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America, 92, 12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F and Di Chiara G (1996) Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature, 382, 255–257. [DOI] [PubMed] [Google Scholar]
- Reith ME, Allen DL, Sershen H and Lajtha A (1984) Similarities and differences between high-affinity binding sites for cocaine and imipramine in mouse cerebral cortex. Journal of neurochemistry, 43, 249–255. [DOI] [PubMed] [Google Scholar]
- Ritz MC and Kuhar MJ (1989) Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. The Journal of pharmacology and experimental therapeutics, 248, 1010–1017. [PubMed] [Google Scholar]
- Roth FE and Govier WM (1958) Comparative pharmacology of chlorpheniramine (Chlor-Trimeton) and its optical isomers. The Journal of pharmacology and experimental therapeutics, 124, 347–349. [PubMed] [Google Scholar]
- Schnoll SH, Chasnoff IJ and Glassroth J (1985) Pentazocine and tripelennamine abuse: T’s and Blues. Psychiatric medicine, 3, 219–231. [PubMed] [Google Scholar]
- Shishido S, Oishi R and Saeki K (1991) In vivo effects of some histamine H1-receptor antagonists on monoamine metabolism in the mouse brain. Naunyn-Schmiedeberg’s archives of pharmacology, 343, 185–189. [DOI] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR and Tanda G (2006) Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. Journal of neurochemistry, 98, 408–419. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mori T, Tsuji M, Misawa M and Onodera K (1997) Generalization of D-, L- and DL-chlorpheniramine and zolantidine to the discriminative stimulus effects of cocaine and methamphetamine. Behavioural pharmacology, 8, 718–724. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mori T, Tsuji M, Nomura M, Misawa M and Onodera K (1999) Evaluation of the histamine H1-antagonist-induced place preference in rats. Japanese journal of pharmacology, 81, 332–338. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs A, Newman AH and Katz JL (2005) Effects of 4’-chloro-3 alpha-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. The Journal of pharmacology and experimental therapeutics, 313, 613–620. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH and Katz JL (2007) Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. The Journal of pharmacology and experimental therapeutics, 321, 334–344. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE and Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science (New York, N.Y, 276, 2048–2050. [DOI] [PubMed] [Google Scholar]
- Wang Z and Woolverton WL (2007) Self-administration of cocaine-antihistamine combinations: super-additive reinforcing effects. European journal of pharmacology, 557, 159–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM and Howell LL (2005) In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse (New York, N.Y, 58, 220–228. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA and Woolverton WL (1999) Comparison between dopamine transporter affinity and self-administration potency of local anesthetics in rhesus monkeys. European journal of pharmacology, 367, 175–181. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Compton DM, Balster RL and Martin BR (1995) In vitro and in vivo effects of cocaine and selected local anesthetics on the dopamine transporter. European journal of pharmacology, 277, 7–13. [DOI] [PubMed] [Google Scholar]
- Young CS, Mason R and Hill SJ (1988) Inhibition by H1-antihistamines of the uptake of noradrenaline and 5-HT into rat brain synaptosomes. Biochemical pharmacology, 37, 976–978. [DOI] [PubMed] [Google Scholar]
- Zacny JP (1989) Discriminative stimulus effects of H(1)-anti-histamines in cocaine-trained pigeons. Behavioural pharmacology, 1, 261–265. [DOI] [PubMed] [Google Scholar]