Abstract
Periodically throughout history developments from adjacent fields of science and technology reach a tipping point where together they produce unparalleled advances, such as Allen Brain Atlas1 and the Human Genome Project2. Today, research focused at the interface between the nervous system and electronics is not only leading to advances in fundamental neuroscience, but also unlocking the potential of implants capable of cellular-level therapeutic targeting. Ultimately, these personalized electronic therapies will provide new treatment modalities for neurodegenerative and neuropsychiatric illness, powerful control of prosthetics for restorative function in degenerative diseases, trauma and amputation, and even augmentation of human cognition. Overall, we believe that emerging advances in tissue-like electronics will enable minimally invasive devices capable of establishing a stable long-term cellular neural interface and providing long-term treatment for chronic neurological conditions.
Neurotechnologies that interface directly with the human nervous system have reached a tipping point—one that could open new applications for electronic implants in neuroscience and medicine. Decades of research and clinical applications of therapeutic electrical stimulation1–3, as well as the development of neural probes for neuroscientific exploration4–6, provide a strong foundation for this future. However, despite this positive trajectory, we argue here that current neural interfaces are only a stop gap until basic structural, mechanical, and topological mismatches between electrical probes and the cellular networks comprising the brain are resolved7. In this Perspective, we highlight the need for truly stable and minimally invasive brain–electronic interfaces that mimic the natural properties of neural tissues and their constitutive cells. Approaches that allow stable mapping and modulation of the same individual neurons and neural circuits over extended periods of time promise to unlock new avenues for delivering personalized therapy to individuals with complex neurological and psychiatric disorders, as well as powerful control of prosthetics for restorative function in degenerative diseases, trauma and amputation— what we term here ‘precision electronic medicine’. The key components of precision electronic medicine are as follows: (1) stable recording and tracking of the same individual neurons that comprise neural circuits over the time, where most current technologies do not have this capability8,9; (2) stable modulation of the individual neurons in neural circuits based on changes in recorded signals monitored in (1), where current technologies can only modulate regions of the brain comprising thousands of neurons1; (3) closed-loop feedback and control based on the stable tracking and stable modulation of individual neurons in neural circuits; and ultimately, (4) monitoring and modulation at the level of specific neuron subtypes.
In this Perspective, we suggest that a central component for achieving these breakthroughs will require development and adoption of ‘tissue-like’ neural technologies capable of producing a stable interface at the cellular to subcellular level in the brain over extended periods of time. We first outline our vision of precision electronic medicine, essential pieces needed for implementation of this vision, and areas where it might impact in terms of basic science and therapeutics. Next, we step back to discuss briefly the current state-of-the-art in neural implant technologies both for medical and research applications. We highlight substantial advances made in front-end integration where implants connect to the brain and backend input/output connectivity and data processing, as well as highlight fundamental mechanical, structural, and biochemical mismatches between implants and cellular networks within neural tissues that ultimately limit the ability to have ‘precise communication’ with the same neurons over the life of an implant and more sophisticated biological functionality. We then describe how applying concepts of biomimicry have yielded ‘tissue- and neuron-like’ electronics with immune-privileged characteristics capable of stably integrating and recording from the brain over long periods of time. Lastly, we discuss developments that could produce a cell-type specific bidirectional electrical interface, modification of the tissue-like implants to enable cellular development for neural (or tissue) healing, as well as limitations that must be overcome to realize precision electronic medicine.
Trends in neural recording and neuromodulation
The three key components of neuromodulation and neuroprosthetic systems are sensing, control, and processing (Fig. 1). Among the diverse technologies used in these three areas, there are commonalities that can both help assess the advantages and disadvantages of existing and emerging neural devices and provide a framework to contextualize our vision for precision electronic medicine. When referring to sensing, we consider signals of activity recorded directly from the brain (e.g., surface or implanted electrode arrays), as well as from devices used, for instance, to detect external visual or audio signals. Signals that provide control are those that can be delivered to a part of the brain or peripheral nervous system via implanted electrodes, or for example, to a prosthetic limb. The last component, the processor, we define as the signals that are sent to a control device or nervous tissue. In many commercial implanted stimulators, the processor and control electrodes form an open loop—without direct sensory feedback— although the processor can be adjusted and/or subsequently optimized to maximize effectiveness based on observed patient response. Current and future trends point to closed-loop systems in which feedback signals, especially from the brain or nervous system, are used directly in the processor to optimize the control signals in real-time to maximize effectiveness10,11. Ultimately, this would allow for more precise targeting and control of neural biomarkers directly related to symptom relief, thus improving therapeutic efficacy and reducing unwanted side-effects.
Figure 1:
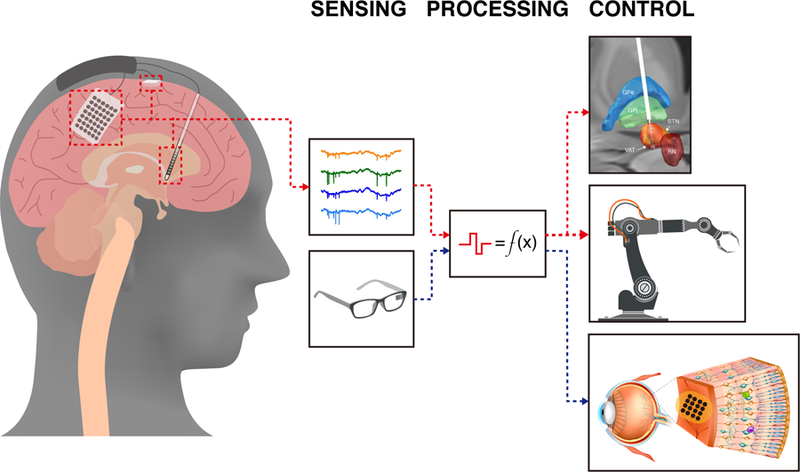
Unidirectional and bidirectional neurostimulation approaches. To date, the vast majority of commercially available neurostimulation devices are unidirectional capable of singularly recording or stimulating. For example, unidirectional recording devices (red dashed-line) like the motor cortical prosthetics decode motor intention from motor cortical networks to actuate a robotic arm and restore movement12,13. Similarly, unidirectional stimulation devices (blue dashed-line), such as retinal prosthetics map visual-spatial information from cameras to create visual percepts by stimulating retinal receptive fields15,16. Bidirectional neurostimulation devices are capable of both sensing and stimulating in a real-time and adaptive manner, thus creating new opportunities leveraging closed-loop approaches.
To date, the vast majority of neural devices are unidirectional—capable of singularly recording or stimulating neural activity. A unidirectional recording device, such as a motor prosthetic, can decode motor intention from cortical neural activity and actuate a robotic arm in order to restore movement in a paralyzed individual12,13. Similarly, unidirectional stimulation devices have yielded successes in modifying and augmenting brain function for therapy or support, including cochlear implants14, deep brain stimulation1, and motor12 and visual prosthetics15,16. Technological and scientific constraints, including relatively small numbers of electrodes in multi-site neural stimulators, a lack of stable neural interfaces making difficult stable tracking of neural activity necessary for feedback, limitations in computational processing, as well as insufficient understanding of the underlying neural code, have limited progress in expanding beyond unidirectional prosthetics, though this is slowly changing17–19.
Neural stimulation systems.
The first account on the clinical application of electrical brain stimulation can be traced to a Roman physician, Scribonius Largus in A.D. 46, which detailed the application of a bioelectric fish, Torpedo ocellata, to the cranial surface for the treatment of headache and gout20. Today, though we have access to more modern technologies, the same open-loop electrical stimulation concept is used in devices implanted on the surface or deeper tissue of the brain (Figure 1). These approaches, which include deep brain stimulation (DBS), are being used for treatment of movement disorders and neurological and neuropsychiatric disorders, including Parkinson’s disease, obsessive-compulsive disorder, depression, epilepsy, and Alzheimer’s disease (for review, see ref. 1).
Specifically, these neural stimulation approaches are relatively ‘brute-force’ therapeutic interventions involving widespread modulation of neural activity through implantation of large low-impedance stimulating electrodes. For example, reduction of motor symptoms such as tremor, bradykinesia, and rigidity with bilateral subthalmic nucleus DBS treatment of Parkinson’s disease with implantable electrodes is well established, but is prone to the following limitations (e.g., see p.XXXBROWN)21: first, DBS electrode sizes and corresponding estimated stimulation volumes encompass large numbers of distinct types of neurons and different functional pathways22–24, which has the potential for unwanted side-effects, and precludes therapeutic applications of higher precision; second, stimulation is typically applied without feedback, except for adjustments made by the neurologist to optimize effectiveness post-implantation through iterative and periodic patient observation, limiting the efficiency of therapy; and third, the continuous mode of operation and large implant designs limit the effective lifetime of implants in terms of the battery life25–27 and adverse tissue immune response to the implants28, respectively.
Several of these limitations are being addressed by efforts focused on improving clinical implants. To provide finer control of the effective stimulation volume commercial designs that segment and increase the number of addressable electrodes are being implemented29,30. However, the typical sizes of these segmented electrodes remain large with respect to individual neurons and finite element models suggest they may have limited ability to steer therapeutic stimulation currents beyond the four radial electrodes of common DBS electrode design31. Demonstrations of closed-loop stimulation in research studies17,32 where brain activity is monitored through local-field potentials (LFPs) have led to the implementation of upgraded DBS systems for clinical evaluation33,34. Real-time recordings from DBS electrodes or tandemly placed brain surface electrodes can provide feedback-controlled neural stimulation, adjusting stimulation parameters such as voltage and timing through embedded algorithms35. Although advances in closed-loop stimulation delivery have immediate applications for the improved treatment of diseases, such as Parkinson’s, Tourette’s Syndrome36,37, and epilepsy38, they do not overcome fundamental limitations of selective circuit-to-neuron group pairings necessary in precision electronic medicine. Ultimately, these approaches remain low resolution in terms of indiscriminately stimulating large numbers of different types of neurons and distinct neuronal pathways, are unable to provide detailed feedback information from neuronal spiking circuit activity, and do not address the intrinsic mismatch of implants with tissue that result in an immune response and ultimately limit electrode stability and lifetime.
The development of improved neural interfaces39,40, including the engineering and manufacture of fully implantable neural recording systems capable of large-scale high-bandwidth recordings and stimulation as well as algorithms capable of real-time closed-loop therapy and prosthetic control41,42 have also been an emphasis of large research programs in USA, Europe, and Asia43–45,46. The primary objective of these programs is growth of neurotechnologies that bridge the spatial-temporal gap between the sub-millisecond functioning of networks with the micron-scale connectivity of neurons. For example, these programs have promoted high-density integration (discussed in the next section) and have led to substantial advances in integrated chips for processing between the ‘sensing’ and ‘control’ components (Fig. 1). Recently, a fully implanted closed-loop device was produced capable of recording LFPs, as well as performing on device single-unit detection on raw neural signals43,45. The availability of custom chip design allows configurable recording and stimulation mosaics based on implant location, duration, and physiological function43. Although these advances represent important milestones in demonstrating proof-of-principle in technological hardware capable of supporting bidirectional neural applications, these approaches are fundamentally limited by the use of non-precise and relatively rigid neural interfaces that preclude stable interfaces to cellular and sub-cellular neural elements, and correspondingly, prevent stable monitoring and modulation of the same neurons relevant to precise therapy and prosthetic control.
High-resolution neural interfaces.
In parallel with developments in neural stimulation systems, substantial effort has focused on increasing electrode density of neural recording probes (sensing in Figure 1) to record spiking activity from greater and greater numbers of individual neurons5,47,48 (see p. AnikeevaXXX). These efforts have been motivated by prior contributions to fundamental neuroscience that identified the role of single neurons in behaviors, such as the discovery of simple cells in visual encoding49, place/grid cells in spatial encoding50,51, and motor population coding52. In addition, advances in the fabrication technology for electronics have allowed the production of devices capable of simultaneous recording on the order of 1000 neurons. Immediate opportunities to, for example, improve brain–machine interfaces for prosthetic control, are possible by increasing the number of simultaneously recorded neurons because larger numbers allow additional degrees of freedom for more natural control53–55.
Emerging large-scale electrode technologies56–58 have enabled the generation of neural recording datasets of unprecedented size across functionally connected networks in different regions of the brain, but not without limitation. Foremost, micromotion and the foreign body response elicited by rigid neural probes makes difficult the tracking of single neuronal activity over extended periods of times as would be necessary for a precision therapeutic implant6. Many factors contribute to chronic inflammatory response, including the physical size, mechanical properties and biochemical composition of the probe48,59–61. For example, activated microglia and reactive astrocytes attempt to sequester the probe, which they recognize as a foreign body, eventually forming a multinucleated dense encapsulation layer between the probe and parenchyma. This barrier reduces probe signal-to-noise, inhibits local axonal growth, and results in neural atrophy4. The fundamental mismatch of the sizes and mechanical properties of probes and their impact on the foreign body response have been reviewed48,62 (see Fig. 2 for a summary with respect to the brain and effective probe lifetime). Biomimicry and biocompatibility approaches have been explored with varying degrees of efficacy, including biomolecular coatings to promote neural density and to reduce immunoreactivity near the probe (for comprehensive reviews, see refs 4–6). Although these approaches can improve the performance of rigid neural probes, they do not address the fundamental size and mechanical mismatches with host cells and tissues.
Figure 2:
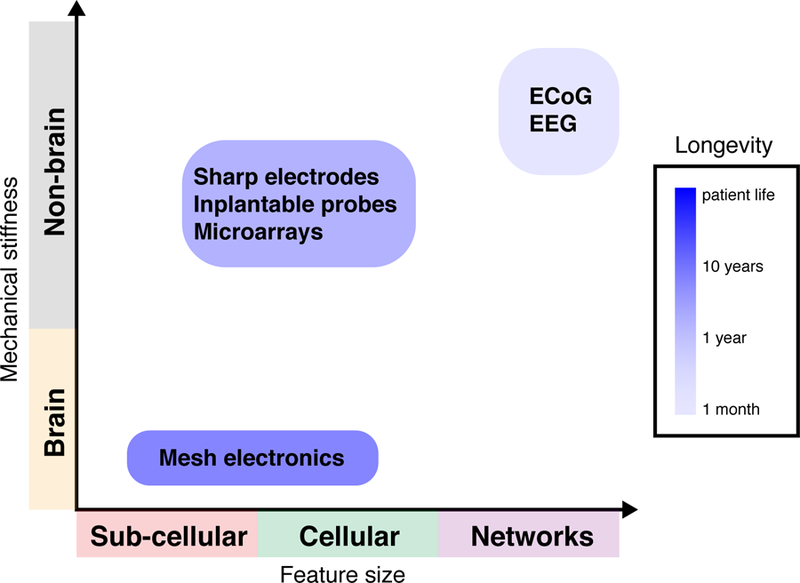
Challenges with current neural interfaces. Mismatches in structural, mechanical, and topological features between the brain and interface lead to micromotion and a prolonged chronic immune response limiting the longevity of conventional neural recording probes6. Similarly, factors including the physical, chemical, and mechanical composition of the electrode influence probe features such as diameter, shape, cross-sectional area, and size of recording surfaces governing the spatial resolution of the interface5,84. Mesh electronics optimize the neural interface design for structural, mechanical, and topological similarity between the implant and neural substrate in order to create an interface that “looks” and “feels” like the cellular and sub-cellular networks comprising the brain62.
Neural probes tailored to the brain architecture
From the perspective of probe function, the different elements that make up nervous tissue span a wide range of sizes: synapses can vary from 20–40 nanometers, whereas neuron cell bodies and glia can span 4–100 microns63. Microwire and conventional silicon array-based technologies remain larger than the scale of neural elements, particularly in high-density probes 64. In terms of probe flexibility, neural tissue is soft, and the subcellular structures of neurons, such as axons, are even softer65,66. Moreover, neural tissue undergoes periodic motion due to blood flow and periodic pressure changes associated with beating of the heart 67 as well as motion of the brain within the skull during locomotion and head movements47. Mechanically, conventional neural probes, such as silicon, carbon, or so-called flexible polyimide probes, have bending stiffnesses at least two-three orders-of-magnitude greater than that of brain tissue, which is between 10–4 to 10–1 nN m per unit width for a 20–100 µm thick brain slice68, with the values for axons being several orders of magnitude smaller65,66. The size and mechanical differences between the probe and brain are at the root of chronic inflammatory responses and gliosis, which result in scarring and degradation of the neural interface. Mechanical stiffness mismatch, which readily leads to micromotion, also makes it difficult to track the same individual neurons and neural circuits over time. Finally, the topology of standard probes is different from the interconnected open three-dimensional (3D) structure of the brain with neurons, astrocytes, and glia69. Without adopting such a topology, probes not only exclude cells from their occupied volume, but also perhaps more importantly, preclude formation/re-formation of connections across the excluded space and inhibit the free diffusion of molecules that maintain homeostasis70.
To develop probes that more closely resemble brain properties our group (C.M.L.)8,9 has developed mesh electronics, which optimize the neural interface design for structural, mechanical, and topological similarity between the implant and neural substrate. The idea is to create an interface that resembles the cellular networks comprising the brain9,48,62 (Fig. 2). The mesh is fabricated with cellular to sub-cellular sized components within a two-dimensional (2D) ultra-flexible scaffold with a bending stiffness comparable to neural tissue. This ultra-flexible macroporous interconnected arrangement of the mesh allows deep integration and interpenetration of neurons and glia without disruption to the local cytoarchitecture9,71.
Mesh electronics can be introduced into the brain using a conventional syringe72 similar to many biological ‘therapeutics’, allowing ease of implantation for less conventional targets, such as the eye73. The ultra-flexible 3D structure elicits only a minor foreign body response measured up to one year post-implantation in mice74, enabling long-term stable recordings from a set of approximately 200 neurons in a single mouse71, stable tracking of the activity of the same neurons and local neural circuits for over 8 months75. Despite these promising results in research there are areas that must be addressed with respect to human translation, including development of connection to interface cable or controller chip that is compatible with constraints of neurosurgery, demonstration of the stability and safety on time scales longer than year and approaching projected maximum timescale of patient treatment, and increasing substantially the number of addressable electrodes similar to the advances being made with high-density silicon probes.
Tissue-like implants enabling precision electronic medicine
New developments in neurotechnology promise to fundamentally shift proof-of-concept studies into applications in basic research and ultimately medical therapy (Fig. 3). For example, the functionalization of neural probes such that individual electrodes promote interactions with specific cell-surface protein markers, could allow precise measurement and control of direct and indirect pathway activity. In models of Parkinson’s disease, theoretical and computational models have postulated the role of neural subtypes such as basal ganglia D1 and D2 dopamine receptor subtypes80; designing neural probes to interact with these cells may have a beneficial therapeutic effect by promoting selective targeting of underlying neural circuits such as the direct and indirect basal ganglia pathways. Development of neural devices to incorporate both electrophysiological recording and biochemical sensing (e.g., of dopamine and/or glutamate) through functionalized field-effect transistors76,77 may also contribute towards the dynamic application of precision electronic medicine for Parkinson’s disease, in which neurotransmitters, such as dopamine, play an extensive role78. For example, the dissociable roles of neural synchronization and dopamine release following electrical stimulation can be used to promote the therapeutic effects on motor symptoms while reducing any unwanted side-effects related to excessive dopamine release.
Figure 3.
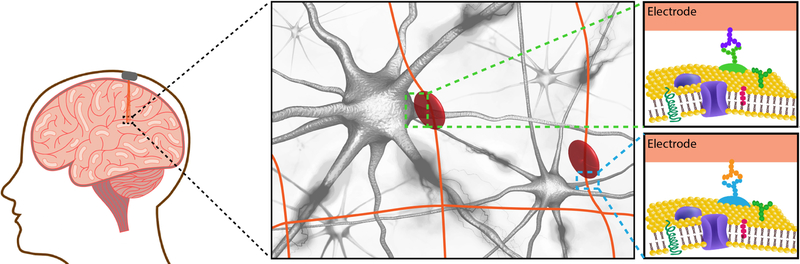
Schematic representation of syringe-injectable mesh electronic implant into a human brain. Mesh electronics directly address the structural, mechanical, and topological mismatch between probe and host tissue. The mesh is fabricated with subcellular-sized nanowire field-effect transistor detectors which maintain measurement sensitivity and allow for highly localized sampling, formation of artificial synapses, and minimally invasive intracellular recordings and single-neuron stimulation (stimulation pad depicted by red circle)76,85–87. The cellular and sub-cellular sized components are incorporated onto 3D ultra-flexible scaffold with a bending stiffness similar to the brain71, and the macroporous interconnected arrangement of the mesh allow deep integration and interpenetration of neurons and glia without disruption to the local cytoarchitecture71. Schematic representation of mesh electronics implanted in region of heterogenous neuronal sub-types (green and blue dashed-squares). Identifying unique cell-surface protein patterns may enable targeted cellular recording/stimulation interfaces through, for example, expression of complementary antibodies or aptamers on the mesh electronics electrode surfaces and/or polymer-encapsulated mesh structure.
Work from our laboratory (C.M.L.)86 has shown that cells adhere and migrate along the electronically-active scaffold of mesh electronics. Along these lines, it may prove useful to modify neural surfaces to promote interactions with neural progenitor cells and tissue remodeling factors, as this would potentially create an opportunity to build interfaces that address both structural and functional components of neuromodulation. It could also provide a means of a unique active regenerative therapy to the hippocampus and adjacent cortical regions that are often sites of early neural neurodegeneration in diseases, such as Alzheimer’s disease79. Additionally, emerging evidence suggests that electrical stimulation in the hippocampus induces neurogenesis in the dentate gyrus layer of the hippocampus80. Multi-pronged therapeutic strategies in which temporally interleaved stimulation could induce neurogenesis, allow directed migration and differentiation of new nerve cells on the electronic scaffold, and functionally incorporate these cells into existing neural circuits through electrical stimulation81,82. Such approaches would enable novel and exciting applications in precision electronic medicine.
The encouraging prospects for the creation of a long-term stable neural interface, we believe, will result in the enhancement of emerging approaches to neuromodulation therapies as well as open new approaches previously unconsidered. We envisage that future neurotechnologies will continue to blur the mechanical, structural, and biochemical dissimilarities between probe and tissue. However, developments are needed in two major areas to realize the full potential of the technology.
First, applying advances in semiconductor fabrication and microscale chip design for signal multiplexing, which have been demonstrated for high-density silicon probes56, will allow increases in local recording density and volume over larger swaths of the brain and thus sould be capable of improved capture of the intrinsic network activity observed throughout the human brain over long periods of time. Additional benefits, such as the need for template-based spike sorting, will be obviated by the implementation of more robust signal triangulation methods following attainment of a critical density of recording contacts83. Similarly, increases in sensor density will provide high-fidelity control over targeted neurons and circuits through directed exposure to electrical stimulation.
Second, advances in neural implants capable of handling high-density sensing and stimulation will be required to enable next-generation applications of implants that match the brain and are capable of stably recording from populations of neurons over extended periods of time. Challenges relating to mechanical and biocompatibility of high-density connectors, cables, and housing along with issues relating to battery longevity and recharge cycles will pose substantial barriers to the implementation of precision electronic medicine.
Conclusions
The field of electronic neural implants is poised to usher in a new era of basic research, therapeutic intervention, and other applications in neuroscience. This perspective outlines some of the constraints for realizing a vision of precision electronic medicine, and highlights the importance of a natural tissue-electronics interface for long-term implants that are immune-privileged and free of the foreign body response to enable precise recording and stimulation of the same individual neurons72 and neural circuits over extended time periods. Given the importance for both fundamental neuroscience research and human translation we expect to see efforts focused on fabrication of highly flexible probes such as mesh electronics, which do not exhibit an immune response, with high densities of recording and stimulation electrodes in the near term. Moreover, we expect that interfacing such ultraflexible implants with mature silicon-based processor chips will see increased emphasis as it could allow for efficient handling of expected large data streams as well as would being central to closed-loop controllers.
With these technology advances emerging, we envision that high-density immune privileged interfaces will rapidly expand our ability to study the brain unlike ever before, building the foundation of understanding necessary to unlock the potential for seamless neural-electronic systems. In the not too distant future, we posit that our level of technological and scientific insight will be sufficient to stably interface with the human brain in a manner that mirrors the organization of the brain itself. This will first arise in the form of enhanced therapeutic approaches to treating some of the most challenging neurodegenerative and neuropsychiatric disease, such as Alzheimer’s disease, depression, and obsessive-compulsive disorder. Subsequent applications will likely involve interfacing the brain in the absence of disease, to enhance or prevent the decline of cognitive capacities and expanding the exploration of what is possible with such a novel interface. Given that the brain is the very organ that makes us human, careful consideration of ethical issues will be required, although it is our opinion that the opportunity to develop powerful treatments for neurodegenerative and neuropsychiatric diseases as well as enhancing restorative function in trauma and amputation mandates that these efforts should proceed forward aggressively.
Acknowledgements
S.R.P. is supported by the Cure Alzheimer’s Fund and the Henry and Allison McCance Center. C.M.L. acknowledges support of this work by the Air Force Office of Scientific Research (FA9550–14-1–0136) and a National Institutes of Health Director’s Pioneer Award (1DP1EB025835–01).
Footnotes
Data availability
Not applicable.
Competing financial interests
S.R.P. has no competing financial interests. C.M.L. is a co- inventor on patents and patent applications relating to the article that have been filed by the authors’ institution (Harvard University) as follows: ‘Scaffolds comprising nanoelectronic components, tissues, and other applications’, inventors C.M.L., J. Liu, B. Tian, T. Dvir, R. S. Langer and D. S. Kohane; US9,457,128 (issued); describes nanoscale transistors for cell recording. ‘Systems and methods for injectable devices’, inventors C.M.L., J. Liu, Z. Cheng, G.H., T.-M. Fu and T. Zhou; 61/975,601 (pending), PCT/US2015/024252 (pending) and 15/301,792 (pending); describes injectable mesh electronics. ‘Techniques and systems for injection and/or connection of electrical devices’, inventors C.M.L., G.H., T.-M. Fu and J. Huang; 62/209,255 (pending), PCT/US2016/045587 (issued) and 15/749,617 (pending); describes injection method of mesh electronics. The authors are not involved in efforts related to commercialization of this intellectual property
References
- 1.Herrington TM, Cheng JJ & Eskandar EN Mechanisms of deep brain stimulation. J. Neurophysiol 115, 19–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miocinovic S, Somayajula S, Chitnis S & Vitek JL History, Applications, and Mechanisms of Deep Brain Stimulation. JAMA Neurol 70, 163–171 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Kringelbach ML, Jenkinson N, Owen SLF & Aziz TZ Translational principles of deep brain stimulation. Nat. Rev. Neurosci 8, 623–635 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Kook G, Lee S, Lee H, Cho I-J & Lee H Neural Probes for Chronic Applications. Micromachines 7, 179 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellman SM et al. A Materials Roadmap to Functional Neural Interface Design. Advanced Functional Materials 28, 1701269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fattahi P, Yang G, Kim G & Abidian MR A Review of Organic and Inorganic Biomaterials for Neural Interfaces. Advanced Materials 26, 1846–1885 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong G, Viveros RD, Zwang TJ, Yang X & Lieber CM Tissue-like Neural Probes for Understanding and Modulating the Brain. Biochemistry 57, 3995–4004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong G, Yang X, Zhou T & Lieber CM Mesh electronics: a new paradigm for tissue-like brain probes. Current Opinion in Neurobiology 50, 33–41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X et al. Bioinspired neuron-like electronics. Nat Mater 1 (2019). doi: 10.1038/s41563-019-0292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun FT & Morrell MJ Closed-loop neurostimulation: the clinical experience. Neurotherapeutics 11, 553–563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parastarfeizabadi M & Kouzani AZ Advances in closed-loop deep brain stimulation devices. J NeuroEngineering Rehabil 14, 79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilja V et al. Clinical translation of a high-performance neural prosthesis. Nature Medicine 21, 1142–1145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aflalo T et al. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 348, 906–910 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng F-G, Rebscher S, Harrison W, Sun X & Feng H Cochlear implants: system design, integration, and evaluation. IEEE Rev Biomed Eng 1, 115–142 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadjinicolaou AE, Meffin H, Maturana MI, Cloherty SL & Ibbotson MR Prosthetic vision: devices, patient outcomes and retinal research. Clinical and Experimental Optometry 98, 395–410 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Lewis PM, Ackland HM, Lowery AJ & Rosenfeld JV Restoration of vision in blind individuals using bionic devices: A review with a focus on cortical visual prostheses. undefined 1595, 51–73 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Rosin B et al. Closed-Loop Deep Brain Stimulation Is Superior in Ameliorating Parkinsonism. Neuron 72, 370–384 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Lo MC, Psychiatry, A. W. I. R. O.2017. Closed-loop neuromodulation systems: next-generation treatments for psychiatric illness. Taylor & Francis 29, 191–204 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezzyat Y et al. Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat Comms 9, 365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kellaway P The part played by electric fish in the early history of bioelectricity and electrotherapy. Bull Hist Med 20, 112–137 (1946). [PubMed] [Google Scholar]
- 21.Ramirez-Zamora A et al. Evolving Applications, Technological Challenges and Future Opportunities in Neuromodulation: Proceedings of the Fifth Annual Deep Brain Stimulation Think Tank. Frontiers in Neuroscience 11, 343 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Dijk KJ et al. A novel lead design enables selective deep brain stimulation of neural populations in the subthalamic region. J Neural Eng 12, 046003 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Lein ES et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007). [DOI] [PubMed] [Google Scholar]
- 24.McIntyre CC, Chaturvedi A, Shamir RR & Lempka SF Engineering the next generation of clinical deep brain stimulation technology. Brain Stimulation 8, 21–26 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher B et al. Battery Longevity Comparison of Two Commonly Available Dual Channel Implantable Pulse Generators Used for Subthalamic Nucleus Stimulation in Parkinson’s Disease. Stereotact Funct Neurosurg 96, 151–156 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Park K et al. Battery Life Matters in Deep Brain Stimulation. Stereotact Funct Neurosurg 96, 65–66 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Helmers AK et al. Comparison of the Battery Life of Nonrechargeable Generators for Deep Brain Stimulation. Neuromodulation : journal of the International Neuromodulation Society 21, 593–596 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Cicchetti F & Barker RA The glial response to intracerebrally delivered therapies for neurodegenerative disorders: is this a critical issue? Front. Pharmacol 5, e598 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buhlmann J, Hofmann L, Tass PA & Hauptmann C Modeling of a Segmented Electrode for Desynchronizing Deep Brain Stimulation. Front. Neuroeng 4, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alonso F, Latorre MA, Göransson N, Zsigmond P & Wårdell K Investigation into Deep Brain Stimulation Lead Designs: A Patient-Specific Simulation Study. Brain Sci 6, 39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teplitzky BA, Zitella LM, Xiao Y & Johnson MD Model-Based Comparison of Deep Brain Stimulation Array Functionality with Varying Number of Radial Electrodes and Machine Learning Feature Sets. Front. Comput. Neurosci 10, e74462 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little S et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry 87, 717–721 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Starr PA Totally Implantable Bidirectional Neural Prostheses: A Flexible Platform for Innovation in Neuromodulation. Frontiers in Neuroscience 12, 3743.e3–5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maling N, Lempka SF, Blumenfeld Z, Bronte-Stewart HM & McIntyre CC Biophysical Basis of Subthalamic Local Field Potentials Recorded from Deep Brain Stimulation Electrodes. J. Neurophysiol 25, 8505–1944 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swann NC et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J Neural Eng 15, 046006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina R et al. Report of a patient undergoing chronic responsive deep brain stimulation for Tourette syndrome: proof of concept. J. Neurosurg 129, 308–314 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shute JB et al. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. NeuroImage: Clinical 12, 165–172 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergey GK et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 84, 810–817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miranda RA et al. DARPA-funded efforts in the development of novel brain-computer interface technologies. J. Neurosci. Methods 244, 52–67 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Neely RM, Piech DK, Santacruz SR, Maharbiz MM & Carmena JM Recent advances in neural dust: towards a neural interface platform. Current Opinion in Neurobiology 50, 64–71 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Heelan C, Nurmikko AV & Truccolo W FPGA implementation of deep-learning recurrent neural networks with sub-millisecond real-time latency for BCI-decoding of large-scale neural sensors (104 nodes). Conf Proc IEEE Eng Med Biol Soc 2018, 1070–1073 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Shenoy KV & Carmena JM Combining Decoder Design and Neural Adaptation in Brain-Machine Interfaces. Neuron 84, 665–680 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Wheeler JJ et al. An implantable 64-channel neural interface with reconfigurable recording and stimulation in 7837–7840 (IEEE). doi: 10.1109/EMBC.2015.7320208 [DOI] [PubMed]
- 44.Hamilton L et al. Neural signal processing and closed-loop control algorithm design for an implanted neural recording and stimulation system in 7831–7836 (IEEE). doi: 10.1109/EMBC.2015.7320207 [DOI] [PubMed]
- 45.Bjune CK et al. Package architecture and component design for an implanted neural stimulator with closed loop control in 7825–7830 (IEEE). doi: 10.1109/EMBC.2015.7320206 [DOI] [PubMed]
- 46.Reardon S Worldwide brain-mapping project sparks excitement — and concern. Nature 537, 597–597 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Patil AC & Thakor NV Implantable neurotechnologies: a review of micro- and nanoelectrodes for neural recording. Med Biol Eng Comput 54, 23–44 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Hong G & Lieber CM Novel electrode technologies for neural recordings. Nat. Rev. Neurosci 19, 199 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubel DH & Wiesel TN Receptive fields of single neurones in the cat’s striate cortex. J. Physiol. (Lond.) 148, 574–591 (1959). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Keefe J & Dostrovsky J The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research 34, 171–175 (1971). [DOI] [PubMed] [Google Scholar]
- 51.Hafting T, Fyhn M, Molden S, Moser M-B & Moser EI Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Georgopoulos AP, Schwartz AB & Kettner RE Neuronal population coding of movement direction. Science 233, 1416–1419 (1986). [DOI] [PubMed] [Google Scholar]
- 53.Normann RA & Fernández E Clinical applications of penetrating neural interfaces and Utah Electrode Array technologies. J Neural Eng 13, 061003 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Hochberg LR et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aflalo T et al. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 348, 906–910 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jun JJ et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raducanu BC et al. Time Multiplexed Active Neural Probe with 1356 Parallel Recording Sites. Sensors (Basel) 17, 2388 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stringer C et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, 255–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lacour SP, Courtine G & Guck J Materials and technologies for soft implantable neuroprostheses. Nature Reviews Materials 2017 2:2 1, 16063 (2016). [Google Scholar]
- 60.Chen R, Canales A & Anikeeva P Neural recording and modulation technologies. Nature Reviews Materials 2017 2:2 2, 16093 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polikov VS, Tresco PA & Reichert WM Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Hong G, Yang X, Zhou T & Lieber CM Mesh electronics: a new paradigm for tissue-like brain probes. Current Opinion in Neurobiology 50, 33–41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kandel E Principles of Neural Science, Fifth Edition. (McGraw Hill Professional, 2013). [Google Scholar]
- 64.Ghane-Motlagh B & Sawan M Design and Implementation Challenges of Microelectrode Arrays: A Review. Materials Sciences and Applications 04, 483–495 (2013). [Google Scholar]
- 65.Garcia JA, Pena JM, McHugh S & Jerusalem A A model of the spatially dependent mechanical properties of the axon during its growth. Computer Modeling in Engineering and Sciencs 87, 411–432 (2012). [Google Scholar]
- 66.Wang SSH et al. Functional trade-offs in white matter axonal scaling. - PubMed - NCBI. Journal of Neuroscience 28, 4047–4056 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terem I et al. Revealing sub-voxel motions of brain tissue using phase-based amplified MRI (aMRI). Magn. Reson. Med 80, 2549–2559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tyler WJ The mechanobiology of brain function. Nat. Rev. Neurosci 13, 867 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Kasthuri N et al. Saturated Reconstruction of a Volume of Neocortex. Cell 162, 648–661 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Saxena T & Bellamkonda RV Implantable electronics: A sensor web for neurons. Nat Mater 14, 1190–1191 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Fu T-M, Hong G, Viveros RD, Zhou T & Lieber CM Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc. Natl. Acad. Sci. U.S.A 114, E10046–E10055 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong G et al. Syringe Injectable Electronics: Precise Targeted Delivery with Quantitative Input/Output Connectivity. Nano Lett 15, 6979–6984 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Hong G et al. A method for single-neuron chronic recording from the retina in awake mice. Science 360, 1447–1451 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou T et al. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl. Acad. Sci. U.S.A 114, 5894–5899 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu T-M et al. Stable long-term chronic brain mapping at the single-neuron level. Nat Meth 13, 875–882 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Cui Y, Wei Q, Park H & Lieber CM Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 293, 1289–1292 (2001). [DOI] [PubMed] [Google Scholar]
- 77.Gao N et al. General strategy for biodetection in high ionic strength solutions using transistor-based nanoelectronic sensors. Nano Lett 15, 2143–2148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Calabresi P, Picconi B, Tozzi A, Ghiglieri V & Di Filippo M Direct and indirect pathways of basal ganglia: a critical reappraisal. Nature Neuroscience 17, 1022–1030 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Choi SH et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 361, eaan8821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mann A et al. Chronic deep brain stimulation in an Alzheimer’s disease mouse model enhances memory and reduces pathological hallmarks. Brain Stimulation 11, 435–444 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Boulanger-Weill J et al. Functional Interactions between Newborn and Mature Neurons Leading to Integration into Established Neuronal Circuits. Curr. Biol 27, 1707–1720.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spitzer NC Electrical activity in early neuronal development. Nature 444, 707–712 (2006). [DOI] [PubMed] [Google Scholar]
- 83.Moore-Kochlacs C et al. Principles of high–fidelity, high–density 3–d neural recording. BMC Neurosci 15, P122 (2014). [Google Scholar]
- 84.Guo L The Pursuit of Chronically Reliable Neural Interfaces: A Materials Perspective. Frontiers in Neuroscience 10, 2329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tian B et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 830–834 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patolsky F et al. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 313, 1100–1104 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Qing Q et al. Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions. Nat Nanotechnol 9, 142–147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]