Abstract
Background:
Clinical exome sequencing (CES) provides the advantage of assessing genetic variation across the human exome compared to a traditional stepwise diagnostic approach or multi-gene panels. Comparative effectiveness research methods offer an approach to better understand the patient-centered and economic outcomes of CES.
Purpose:
To evaluate CES compared to usual care (UC) in the diagnostic work-up of inherited colorectal cancer/polyposis (CRCP) in a randomized controlled trial (RCT).
Methods:
The primary outcome was clinical sensitivity for the diagnosis of inherited CRCP; secondary outcomes included psychosocial outcomes, family communication, and healthcare resource utilization. Participants were surveyed 2 and 4 weeks after results return and at 3-month intervals up to one year.
Results:
Evolving outcome measures and standard of care presented critical challenges. The majority of participants in the UC arm received multi-gene panels [94.73%]. Rates of genetic findings supporting the diagnosis of hereditary CRCP were 7.5% [7/93] vs. 5.4% [5/93] in the CES and UC arms, respectively (P=0.28). Differences in privacy concerns after receiving CRCP results were identified (0.88 in UC vs 0.38 in CES, P=0.05); however, healthcare resource utilization, family communication and psychosocial outcomes were otherwise similar between the two arms. More participants with positive results (17.7%) intended to change their life insurance one month after the first return visit compared to participants returned a VUS (variants of uncertain significance) (9.1%) or negative result (4.8%) (P=0.09).
Conclusion:
Our results suggest that CES provides similar clinical benefits to multi-gene panels in the diagnosis of hereditary CRCP.
Keywords: hereditary colorectal cancer/polyposis, clinical exome sequencing, comparative effectiveness
Introduction
Colorectal cancer (CRC) is the third most common cancer in the United States with an overall 5-year survival rate of 64% [1]. Survival rates significantly increase with early diagnosis [1] and screening colonoscopy is a successful preventative intervention [2]. Hereditary colorectal cancer (CRC), the majority of which is caused by pathogenic variants in genes associated with Lynch syndrome, accounts for approximately 3% of all newly diagnosed CRC cases [3–5]. Evaluation for Lynch syndrome has historically involved a multistep process beginning with family/personal history screening, followed by tumor tissue testing for mismatch repair (MMR) protein expression of the MLH1, MSH2, MSH6 and PMS2 MMR genes using immunohistochemistry (IHC)/microsatellite instability (MSI) and, finally, sequential germline genetic testing to establish a diagnosis [6]. This approach is relatively costly, requires multiple points of contact with patients, which is burdensome for patients/providers, has limited clinical sensitivity [7], and takes weeks to months before potentially reaching a diagnosis. Evaluation for other, rarer inherited CRC conditions, such as Familial Adenomatous Polyposis, causes by pathogenic variants in the APC gene, or MUTYH-associated Polyposis, caused by pathogenic variants in the MUTYH gene, includes discussion of personal/family history and directed germline genetic testing.
Massively parallel sequencing (MPS) is a transformative technology that has been rapidly integrated into the practice of medicine [8,9]. This technology has supported the development of multi-gene sequencing panels which allow simultaneous, cost-effective testing of many genes associated with the clinical indication of interest. Multi-gene panel testing [10] is replacing the sequential diagnostic approach for hereditary CRCP in clinical practice, though both considered as the current standard diagnostic approach for hereditary CRCP evaluation. Multi-gene panels need to be continually updated and re-validated with the discovery of additional disease-associated genes. Patients with negative results on one cancer gene panel might benefit from being tested again in the future with a different or updated panel that includes additional, often newly identified as associated, genes.
Clinical exome sequencing (CES) involves sequencing all genes in the exome and enables return of both diagnostic findings as well as medically actionable findings that are unrelated to the initial indication (SFs: secondary findings). CES is increasingly being adopted by clinical laboratories to diagnose genetic diseases, aid treatment decisions and provide prognostic information [11–16]. However, the impacts of CES on clinical diagnosis, patient-centered outcomes and economic outcomes are not clear, and are potentially challenging to evaluate.
Comparative effectiveness research (CER) generates and synthesizes evidence through comparisons of alternative methods for healthcare. It can help patients, clinicians and health policy makers to make informed decisions that will improve healthcare at both the individual and population levels. The objective of our study was to assess the clinically actionable findings identified by CES compared to UC (Usual Care) in the context of diagnosis of inherited CRCP, and to explore the effects of CES on psychosocial outcomes, family communication and healthcare resource utilization using CER methods.
Materials and methods
Study Design
We conducted a randomized controlled trial (RCT) to evaluate the comparative effectiveness of CES to UC in participants referred to medical genetics for evaluation for hereditary CRCP. The study design has been described in detail previously [17]. Eligible participants were unrelated adult patients referred to the University of Washington Medical Center (UWMC) Genetic Medicine Clinic, the Seattle Cancer Care Alliance (SCCA), or Kaiser Permanente Washington (formerly Group Health Cooperative) for genetic counseling for hereditary CRCP. All three of these institutions serve patients in King County in Washington State. The UWMC and SCCA also serve patients from the Washington, Wyoming, Alaska, Montana and Idaho (WWAMI) region. Most patients receiving care at these institutions have access to either private or federally funded health insurance. Informed consent of all participants was obtained and participant protection was assured under the University of Washington institutional review board.
A personal and/or family history of CRCP was the usual referral indication in these clinic populations. We excluded patients for whom single gene testing was indicated due to 1) a personal and/or family history consistent with a specific CRCP syndrome or 2) abnormal MMR protein expression from IHC testing suggesting a pathogenic variant in a single gene underlying their condition. After clinical UC genetic testing was ordered, informed consent and enrollment took place, and clinical and research blood volumes were drawn for all participants. Participants were then randomized to the UC or CES arm; participants in the CES arm also received UC genetic testing, after discussion with the University of Washington Institutional Review Board (IRB), to ensure that the research intervention of CES was not inferior to UC. This study was approved by the IRB at the University of Washington.
Usual Care
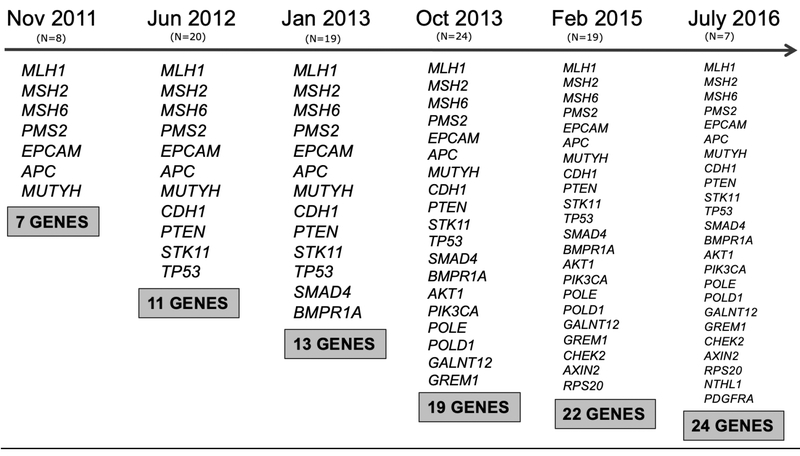
Based on participants’ CRCP related personal and family history, and clinician preference, two types of UC were offered: 1) tumor tissue testing for CRCP-related MMR gene protein expression with IHC and/or MSI and 2) genetic testing panels with variable numbers of hereditary CRCP-associated genes using MPS technology (BROCA [18], ColoSeq [19], and others). Clinical laboratories added genes to panels as new gene-disease association evidence was published; thus, the number of genes included on UC genetic testing panels was dynamic ranging from less than 10 to over 50. Figure A.1 presents the expansion of the genes included on the ColoSeq panel over the course of the study. UC results were restricted to findings from only the UC clinical test ordered for the patient at the time of enrollment. Some UC genetic testing panels also included genes associated with other hereditary cancer conditions (such as BRCA1/2 associated with hereditary breast and ovarian cancer). Clinicians ordered larger “pan-cancer” gene panels when indicated based on patient personal and/or family history. Thus, variants in non-CRCP hereditary cancer genes were returned to some participants from UC genetic testing panels in this study. We refer to variants detected by the UC approach that are not related to CRCP as non-CRCP diagnostic findings.
Clinical Exome Sequencing
All CES was conducted at the University of Washington, Northwest Clinical Genomics Laboratory (NCGL). Variants in a panel of hereditary CRCP genes were annotated. This hereditary CRCP gene panel included genes with a well-established association with hereditary CRCP and emerging hereditary CRCP genes. The number of CRCP-related genes annotated increased from 23 to 34 over the course of the study with the addition of genes with new evidence of association with hereditary CRCP. The original CES CRCP-related gene list included the following genes: APC, BMPR1A, EPCAM, GREM1, MLH1, MSH2, MSH6, MUTYH, PMS2, PTEN, SCG5, SMAD4, STK11, TP53, CDH1, FLCN, PTCH1, TGFBR2, MLH3, PMS1 and RET. The following genes were added to the CES CRCP-related gene list during the study period: POLD1, POLE, ATM, GALNT12, NBN, NTHL1, RINT1, RPS20, XRCC2, RNF43, LRP6, PTPN12, EMR3 and AXIN2.
Variants in a dynamic set of medically actionable, adult onset genes were also annotated as SFs from CES. This SFs gene list included the 55 genes associated with adult onset conditions on the American College of Medical Genetics and Genomics (ACMG) 59 actionable gene-disease pair list [13]. A description of the content and development of this SFs list, as well as the included and excluded genes, has been published elsewhere [20, 21]. Briefly, a formal return of results committee consisting of 14 practicing medical geneticists and 2 genetic counselors, as well as non-genetics providers, bioethicists and molecular laboratory representatives met regularly over the course of the study to develop and maintain the SFs gene list. Genes were included after unanimous agreement that they met the committee’s definition of actionability, which included the existence of specific, defined medical recommendations leading to significant, tangibly improved outcomes in terms of morbidity and mortality after their implementation. Genes associated with pediatric onset conditions and genes that did not meet the actionability threshold for inclusion were excluded. CRCP-related genes were excluded from our SFs gene list. Variants in both CRCP-related and SF genes were interpreted based on the ACMG guidelines for the interpretation of sequence variants [22] or our internal approach prior to the ACMG publication [23].
Select Clinical Pharmacogenetics Implementation Consortium (CPIC) recognized functional pharmacogenomic variants and pathogenic variants in genes associated with carrier status were also annotated. The decision to annotate carrier status genes for return was based on participant interest and to increase the number of participants receiving a positive test result. Carrier status genes were chosen primarily based on the frequency of the condition in European ancestry populations (the ancestry of most participants) and pharmacogenetic variants were chosen based on a high level of evidence of an established relationship with drug metabolism and the ability of CES to identify the variant (Table A.1). The number of pharmacogenomic variants annotated increased slightly over the study period as additional variant-drug metabolism relationships were evaluated by the study team. Of note, only a subset of participants underwent testing for carrier status genes, subsequent to focus groups determining interest in them.
Only variants in annotated genes were evaluated and interpreted for return to participants. Pathogenic (P) variants, likely pathogenic (LP) variants and variants of uncertain significance (VUS) were reported in CRCP associated genes, but only pathogenic (P) variants were reported in the adult onset, medically actionable SF and carrier status genes. The decision not to return LP variants in medically actionable SF genes was made prior to the publication of the ACMG recommendations which advocated for doing so, and was based on an attempt to minimize the potential harms associated with returning variants with a lower prior probability of causing disease in this research cohort. .
Return of Results
Participants in both arms received their UC diagnostic results either by phone or in person at a first return visit. Participants in the CES arm were additionally returned CRCP-related results from CES at this visit. Approximately two months later at the second return visit, medically actionable SFs, carrier status results and pharmacogenetic variants were returned to participants in the CES arm. Participants in the UC arm received further review and discussion of family history during this second return visit. A licensed, certified genetic counselor, often accompanied by a medical geneticist, conducted the return of UC and CES results visits.
Healthcare Resource Utilization
Participant surveys for healthcare resource utilization and participant behavior data were developed de novo for this study and administered at the initial visit either online or by postal mail, one month after the first return visit, and then at one, four, seven, and ten months after the second return visit. Participants responded to questions about their use of medical services, such as the number of visits to a genetic counselor and having CRCP or non-CRCP related medical procedures, as well as actual or intended changes to their life and health insurance. Survey instruments also asked participants how many first-degree family members they shared their genetic testing results with since their last visit. The instrument did not ask specifically about actions taken in response to participants receiving their genetic test results; we relied on differences between the study arms to estimate attribution.
Psychosocial Outcomes
Participants in both arms also completed the FACToR (Feelings About genomiC Testing Results) questionnaire, the Veterans RAND 12 Item Health Survey (VR-12) [24], the Generalized Anxiety Disorder 7-Item Scale (GAD-7) [25], the Brief Patient Health Questionnaire Mood Scale (PHQ-9) [26], and a five-item version of the Mental Health Inventory (MHI-5) [27] to measure psychosocial impacts of testing results. These instruments were completed two weeks after their first return visit and two weeks, and four, seven, and ten months after their second return visit. The development and validation of the FACToR questionnaire has been described elsewhere [28]. In brief, the FACToR questionnaire was created from open-ended qualitative interviews with patients based on categories from the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire and additional categories suggested by interview findings [29]. The FACToR questionnaire has four subscales measuring participants’ positive feelings (score range: 0–16), negative emotions (score range: 0–12), uncertainty (score range: 0–12) and privacy concerns (score range: 0–8) towards receiving genetic test results. For items that measure positive feelings, scores were first reversed before being summed into a total score. Higher scores on each subscale indicate greater functional impairment.
Statistical Analysis
We used descriptive statistics (frequencies, means, standard deviation (SD), proportions) to summarize participants’ demographic characteristics, CRCP history, and type of UC testing. With comparable UC received in both arms, we assumed a higher detection rate of CRCP P/LP variants in the CES arm due to the additional sequencing of CRCP related genes anticipated when the trial was originally designed. Thus, our pre-specified primary test was a one-sided standard two-sample test comparing the proportions of participants identified with CRCP P/LP variants in both arms. We also performed standard two sided two-sample tests of proportions for health resources utilization outcomes, and two-sided standard t-tests for mean scores of psychosocial outcomes between the two randomization arms. We did not compare results from the surveys to medical records because the majority of the patients also received care outside of our healthcare system. While collection of insurance claims data for individual patients was considered, this approach was not considered practical given these were secondary outcomes. For exploratory descriptive analyses of healthcare resource utilization and psychosocial impact, we stratified groups of participants with different primary CRCP genetic testing results (P/LP [positive] vs. VUS vs. Negative [absence of P, LP, or VUS variants]). We used a Chi-square statistic and Wald-based 95% confidence intervals (CIs) for a trend analysis for proportions. The statistical significance level was set at α=0.05. All statistical analyses were conducted in Stata 14 software (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Participants
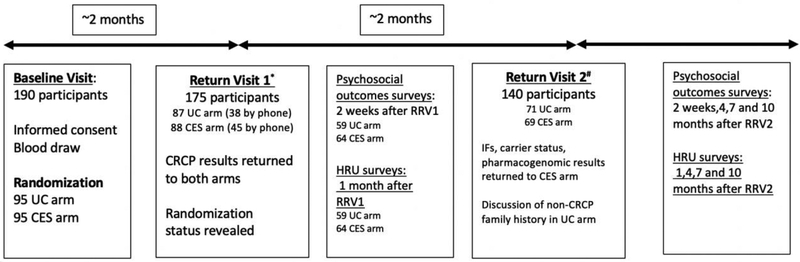
Figure A.2 show participant enrollment and study events throughout the project. From March 2012 to October 2016, 190 eligible participants were recruited and randomized to the UC and CES arms at the baseline visit. The majority of participants (179/190) were enrolled from the genetics clinics at the UWMC and SCCA. Expected UC testing was not performed for four participants (2 CES; 2 UC) after study enrollment, thus these participants were excluded from comparisons after the baseline visit. One hundred and seventy-five participants attended the first return visit for their diagnostic genetic testing results (88 CES, 87 UC). Sixty-nine participants in the CES arm attended a second return visit and received medically actionable SFs results, carrier status results and pharmacogenetic variant results, and 71 participants in the UC arm had further review of family history at this time.
Sociodemographic characteristics, participant CRCP personal/family history, and the type of UC test received by randomization status are presented in Table 1. The participant mean age was 52.9 years (SD:13.1), and 51.6% were female. Participants in the UC and CES arm were similar in their mean age, gender distribution, marital and employment status, education level, household income, and household size. Thirty-one (36.84%) participants in the UC arm had a personal history of CRC compared to 26 (33.68%) in the CES arm. The University of Washington ColoSeq™ hereditary CRC gene panel accounted for just over half of the UC tests conducted in both arms (53.68% vs 53.68%); notably, the number of genes included on ColoSeq™ increased several times over the course of the study (Figure A.1).
Table 1.
Participant characteristics by randomization arm at baselinea
| UC (N=95) | CES (N=95) | |
|---|---|---|
| Demographics | ||
| Annual Household Income >$100,000 | 40.00% | 36.84% |
| Mean Age, years (SD) | 51.8(14.0) | 53.4(12.5) |
| College & Graduate Degree | 55.79% | 57.90% |
| Female | 56.84% | 46.32% |
| Employed | 58.95% | 71.58% |
| Now Married | 63.16% | 69.47% |
| Household Size >=2 | 78.95% | 86.32% |
| Race (self-reported) | ||
| Hawaiian / Other Pacific | 1.53% | 0.00% |
| Islander | ||
| Black/African American | 0.00% | 1.53% |
| Asian | 2.11% | 8.42% |
| American Indian/Alaska | 10.53% | 8.42% |
| Native | ||
| White | 84.2% | 81.1% |
| CRCP History | ||
| CRC Diagnosis | 36.84% | 33.68% |
| Colon Polyps | 86.32% | 80.00% |
| UC Testing Receivedb | ||
| None | 2.11% | 2.11% |
| MSI and/or IHC | 3.16% | 5.26% |
| BROCA™ panelc. | 18.95% | 21.05% |
| Commercial paneld. | 22.11% | 21.05% |
| ColoSeq™ e. | 53.68% | 53.68% |
No statistically significant differences for any characteristics between the two arms
Numbers might not add up to 100% because some participants received more than one type of UC
The BROCA™ pan cancer gene panel included the following genes at the start of the study: APC, ATM, ATR, BABAM1, BAP1, BARD1, BMPR1A, BRCC36, BRIP1, CDH1, CDK4, CDKN2A, CHEK1, CHEK2, EPCAM, FAM175A, MLH1, MRE11A, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, PRSS1, PTEN, RAD50, RAD51B, RAD51C, RAD51D, RBBP8, RET, SMAD4, STK11, TP53, TP53BP1, UIMC1, VHL, XRCC2, and XRCC3. The following genes were added to the BROCA™ panel during the course of the study: AKT1, AXIN2, BRCA1, BRCA2, CTNNA1, FANCM, FH, FLCN, GALNT12, GEN1, GREM1, HOXB13, MEN1, MET, MITF, NF1, NTHL1, PALLD, PDGFRA, PIK3CA, POLD1, POLE POT1, PRKAR1A, PTCH1, RB1, RECQL, RINT1, RPS20, SDHB, SDHC, SDHD, SLX4 and SMARCA4. The following genes were removed from the BROCA™ panel during the course of the study: BABAM1, BRCC36, RAD50, RBBP8, STK11, TP53BP1, UIMC1 and XRCC3.
Other panels included colon cancer and pan-cancer panels from GeneDx (N = 35), Myriad Genetics (N = 2), Ambry Genetics (N = 1) and Invitae (N = 1)
See Figure A.1 for genes included on the ColoSeq™ panel throughout the course of the study.
Hereditary Cancer Diagnostic Results
Approximately half of the participants who received diagnostic hereditary cancer results did so over the phone (92/175, 52.6%). The other half received these results in person (83/175, 47.3%). Five (5.4%) participants in the UC arm and seven (7.5%) participants in the CES arm had a P or LP CRCP-related variant identified (P=0.28, Table 2.). Six of these 12 participants had a personal history of colorectal cancer. One CES participant had two pathogenic variants in MUTYH. Homozygosity or compound heterozygosity for two pathogenic variants in MUTYH is associated with autosomal recessive MUTYH associated polyposis and CRC. Fifteen (16.1%) participants in the UC arm and twenty-one (22.6%) in the CES arm had CRCP-related VUS identified (P=0.13). Participants having CES testing also had UC testing, as noted above. For the CRCP genes tested by both approaches there were no differences in the P/LP results returned to those participants. One participant had a VUS returned from UC that was not returned from CES. Five participants in the UC arm and three participants in the CES arm had a P variant in a non-CRCP related hereditary cancer gene returned at their first return visit. An additional two participants (one in the UC arm and one in the CES arm) had a VUS in a non-CRCP hereditary cancer related gene identified by their UC testing. The number and interpretation of CRCP and non-CRCP hereditary cancer related variants are shown by randomization arm in Table A2. Combining all CRCP-related variants in both arms, eight P/LP variants in Lynch syndrome related genes and seven P/LP variants in non-Lynch CRCP related genes were returned.
Table 2.
Number of participants with diagnostic results in hereditary cancer genes by randomization arm
| UC (N/%) Total = 93 |
CES (N/%) Total = 93 |
P-valued | |
|---|---|---|---|
| CRCP related | |||
| P/LP variant(s) | 5 (5.4%) | 7a (7.5%) | 0.28 |
| VUS(s) | 15 (16.1%) | 21b (22.6%) | 0.13 |
| Non-CRCPc related | |||
| P/LP variant(s) | 5(5.4%) | 3(3.2%) | 0.77 |
| VUS(s) | 1(1.1%) | 1(1.1%) | 0.50 |
One CES participant was compound heterozygous for 2 CRCP related P variants in the MUTYH gene
One CES participant had three CRCP related VUS findings (two in APC and one in AXIN2); one ES participants had two CRCP related VUS findings (one in APC, one in PTCH1); one CES participant had one CRCP related P variant (MSH2) and one CRCP related VUS finding (PMS2).
Non-CRCP related findings were due to non-CRCP genes on the UC multi-gene panels, not considered as SFs
P-value based on one-sided two-sample test of proportions
Return of Medically Actionable SFs, carrier status and pharmacogenetic variants
One participant (1.45%) in the CES arm was returned a P variant in the BRCA1 gene, which is associated with hereditary breast and ovarian cancer, at the second return visit as a medically actionable SFs. A second participant in the CES arm had a BRCA2 pathogenic variant identified by CES, but this participant did not return for a second visit. This participant had also received a UC test that reported this BRCA2 P variant; thus, it had been returned by his clinical providers at his first return visit. The majority of participants in the CES arm (60/69, 87%) had one or more pharmacogenomic variant returned, and 7 of the 23 participants (30%) who had consented for return of carrier status results had a P variant returned in one of these genes.
Healthcare Resources Utilization
One hundred twenty-three participants (59 in the UC arm, 64 in the CES arm) responded to the healthcare resources utilization survey administered one month after the first return visit. Selected healthcare resource utilization were similar between the UC vs. CES arms (Table 3). These analyses were not corrected for multiple comparisons. Healthcare resources included visits to medical providers, medical procedures and changes to health and life insurance. In terms of family communication, 14% more participants in the UC arm have shared their genetic test results with their first-degree family members than the CES arm (P=0.06). The non-responders did not differ significantly from the responders regarding their baseline characteristics and results status (results not shown here).
Table 3.
Participant healthcare resource utilization one month after the first return visit by randomization arm
| In the past month… | Randomization Arms | P-valuea | |
|---|---|---|---|
| UC (N=59) | CES (N=64) | ||
| n(%) | n(%) | ||
| No visits to a specialist/doctor | 40(67.8%) | 36(56.3%) | 0.19 |
| No visits to a genetic counselor | 39(66.1%) | 48(75.0%) | 0.28 |
| Had any medical procedures | 15(25.4%) | 19(29.7%) | 0.59 |
| Had any evaluation of cancer | 12(20.35) | 19(29.7%) | 0.23 |
| Have made changes to health insurance | 3(5.1%) | 4(6.3%) | 0.77 |
| Have made changes to life insurance | 2(3.4%) | 4(6.3%) | 0.46 |
| Have shared genetic test results with 1st-degree blood family member(s) | 50(84.7%) | 45(70.3%) | 0.06 |
P-value based on two-sided two-sample test of proportions
Psychosocial Outcomes
As shown in Table 4, the scores for the negative emotion, positive feelings, and uncertainty subscales on the FACToR survey were not statistically different by randomization arms two weeks after the receipt of CRCP-related test results. A small difference in the privacy concerns subscale scores was seen at this time point (0.88 in UC vs 0.38 in CES, P=0.05). However, this pattern did not persist at later visits. No statistically significant differences in any of the FACToR subscale scores between arms were seen in surveys administered at subsequent time points (Table 5). The mean total scores on VR-12, GAD-7, PHQ-9 and MHI-5 administered two weeks after the second return visit were also similar (Table 6). Analyses of psychosocial outcomes were also not corrected for multiple comparisons.
Table 4.
FACToR subscale scores two weeks after the first return visit by randomization arm
| Subscale | UC (n=59) | CES (n=64) | P-valuea |
|---|---|---|---|
| Mean(SD) | Mean(SD) | ||
| Negative Emotion, 0–12 | 0.56(0.93) | 0.84(1.81) | 0.28 |
| Positive Feelings, 0–16 | 7.20(4.60) | 6.45(3.92) | 0.33 |
| Uncertainty, 0–12 | 1.92(2.14) | 1.97(2.50) | 0.90 |
| Privacy Concerns, 0–8 | 0.88(1.81) | 0.38(0.93) | 0.05 |
P-values were based on two-sided t-test for two-sample comparison of means
Table 5.
FACToR subscale scores two weeks, four months and ten months after the second research return visit (RRV2) by randomization arm
| FACTOR | UC | CES | P-valuea |
|---|---|---|---|
| Mean(SD) | Mean(SD) | ||
| 2w after RRV2 | (n=47) | (n=48) | |
| Negative Emotion, 0–12 | 0.28(0.71) | 0.40(1.11) | 0.53 |
| Positive Feelings, 0–16 | 7.81(4.57) | 7.13(4.27) | 0.42 |
| Uncertainty, 0–12 | 1.09(1.90) | 1.13(1.94) | 0.92 |
| Privacy Concerns, 0–8 | 0.47(1.23) | 0.69(1.60) | 0.46 |
| 4m after RRV2 | (n=57) | (n=63) | |
| Negative Emotion, 0–12 | 0.40(1.03) | 0.41(0.99) | 0.96 |
| Positive Feelings, 0–16 | 10.14(4.832) | 9.60(4.52) | 0.53 |
| Uncertainty, 0–12 | 1.44(2.31) | 1.46(2.12) | 0.96 |
| Privacy Concerns, 0–8 | 0.53(1.23) | 0.29(0.77) | 0.19 |
| 10m after RRV2 | (n=51) | (n=52) | |
| Negative Emotion, 0–12 | 0.16(0.54) | 0.19(0.56) | 0.75 |
| Positive Feelings, 0–16 | 10.29(5.06) | 9.37(5.16) | 0.35 |
| Uncertainty, 0–12 | 1.18(1.85) | 1.15(2.24) | 0.96 |
| Privacy Concerns, 0–8 | 0.47(1.39) | 0.25(0.68) | 0.31 |
P-values were based on two-sided t-test for two-sample comparison of means
Table 6.
Results of other psychosocial scales two weeks after the second return visit by randomization arms
| Other Instruments | UC | CES | P-valuea | |
|---|---|---|---|---|
| Mean(SD) | Mean(SD) | |||
| VR-12 | (n=62) | (n=63) | ||
| Physical Component Score | 45.47(12.50) | 47.31(11.83) | 0.39 | |
| Mental Component Score | 50.13(10.00) | 50.11(9.07) | 0.99 | |
| GAD-7 total Score | (n=62) | (n=62) | ||
| 3.34(4.12) | 3.02(4.11) | 0.66 | ||
| PHQ-9 total Score | (n=61) | (n=63) | ||
| 4.11(4.29) | 3.71(4.55) | 0.62 | ||
| MHI-5 total Score | (n=62) | (n=63) | ||
| 76.69(16.79) | 77.86(17.22) | 0.70 | ||
P-values were based on two-sided t-test for two-sample comparison of means
Exploratory Analyses by CRCP Results (P/LP vs. VUS vs. Negative)
We also conducted exploratory stratified analyses of healthcare resource utilization and psychosocial outcome across groups of participants with different primary CRCP genetic testing results (P/LP vs. VUS vs. Negative).
During the month after the first return visit, similar percentages of participants had shared their CRCP-related results with at least one first-degree blood relative (Table A.3). There was no significant difference in the number of participants who made changes to their life insurance based on the type of result returned (P=0.13). A higher proportion of participants with positive (P/LP) results (17.7%) had thought about changing their life insurance one month after the first return visit compared to participants returned a VUS (9.1%) or negative result (4.8%); however, this observed trend was not statistically significant (P=0.09). Participants were not asked what specific type of insurance changes they thought about making. There were no other trends for other aspects of healthcare resource utilization based on type of CRCP-related result returned at this time point, including thoughts about or actual changes to health insurance or the average number of visits to a specialist doctor or genetic counselor. No obvious trends were seen at one, four or seven months after the second return visit.
The overall mean scores on the FACToR negative emotion subscale were all relatively low. Participants with positive (P/LP) CRCP-related results had a higher mean score on the FACToR negative emotion subscale (1.56) than participants returned VUS (0.60) or negative (0.57) results two weeks after the first return visit; however, this observed trend was not statistically significant (P=0.27; Table A.4). We observed the same pattern on the uncertainty, and privacy concern subscales at this time point as well. Participants with positive (P/LP) CRCP-related results had lower scores on the positive emotion subscale than those returned other types of results. There were no differences seen on the FACToR subscales two weeks, four months, and ten months after the second return visit (Table A.4). No differences were seen on the other psychosocial instruments based on type of variant returned. (Table A.5).
Discussion
We compared the clinically actionable findings from UC vs. CES in patients having clinical genetic testing for hereditary CRCP, and found similar proportions of participants with CRCP related P/LP variants and VUS in both arms. The implementation of CES in this context did not significantly increase the number of VUS returned to participants, which has been cited in the literature as a concern with expanded gene panel testing [30]. VUS rates from multi-gene panels and CES will depend on the number of genes annotated, variant interpretation criteria and lab specific policies for return. The CES approach does not currently appear to provide additional immediate diagnostic value in this context beyond multi-gene cancer panel tests. CES does allow future reinterpretation of genes newly associated with CRCP that were not on the UC panel test.
One participant was returned a P variant identified by CES in a gene (BRCA1) associated with an adult onset, medically actionable condition. An additional participant had a BRCA2 P variant identified on CES, but did not return to receive this result from the study team. This rate of medically actionable SFs identified is consistent with previous estimates [31].
The current National Comprehensive Cancer Network (NCCN) 2017 guidelines support universal screening of newly diagnosed CRC patients with IHC or MSI testing before gene-specific germline testing. The guideline also recommends multi-gene panel testing be offered in the context of genetic expertise and counseling, among selected populations such as those from familial, high-risk clinic-based populations or with early-onset CRC [6,32,33]. Recent studies have explored using multi-gene panels to identify variants of interest in cancer predisposition genes beyond those evaluated through IHC or MSI [34,35]. There are currently no recommendations for if or when to consider expanded or alternative multi-gene panel tests for patients being evaluated for hereditary cancer, and best practice recommendations for clinical laboratory policies on data reanalysis are needed [36]. Approximately half of the CRCP-related P/LP variants returned in this study were not in Lynch syndrome related genes, providing further evidence to support the consideration of larger multi-gene panels, rather than Lynch panels, as a first-line test in the evaluation for hereditary CRCP.
Concern regarding the potential psychological burden of pursuing and receiving results from genomic sequencing tests has been cited in the literature, as have questions regarding the downstream costs to healthcare systems of incorporating this testing into clinical practice [37–40]. In this study, we did not observe differences in psychosocial impacts, healthcare use or family communication outcomes between the UC and CES arms, or based on type of CRCP-related result returned. Due to the small sample size and the similarity in the number of CRCP-related results returned across arms, our study was likely underpowered to evaluate the impact of different CRCP findings on psychosocial outcomes, family communication and healthcare utilization. It is also possible that no difference in healthcare use was found because participants in both arms were already following a high risk cancer prevention screening program prior to testing or had not yet accessed healthcare resources when the surveys were administered. None the less, these findings are consistent with a similar study exploring genome sequencing in 100 healthy adults in a primary care setting that found no additional participant anxiety or depression related to genome sequencing when compared with standard family history assessment [41].
Limitations
The increased use of multi-gene panels for UC clinical testing, and the increasing number of CRCP-related genes included on these panels throughout the time period of the study, led to few expected differences in primary findings between the two arms. Our study also included relatively few participants from underserved or ethnically and sociodemographically diverse populations so may not be generalizable across all patient care settings. Standardized outcome measures for psychosocial consequences after genetic tests are still evolving and the reliability and validity of the FACToR needs to be re-confirmed among different populations with different clinical conditions. More studies evaluating the clinical significance of elevated scores of FACToR are also warranted. Finally, participant healthcare resource utilization questions were not specific to actions taken in response to receiving the test results, thus making attribution difficult given the relatively small sample size and number of findings returned. We recommend future studies inquire specifically about actions taken in response to test results, even though such attribution may be challenging for participants.
Conclusions
CES and UC testing, consisting primarily of gene panels, had similar rates of P/LP variants and VUS returned in patients being evaluated for hereditary CRCP. Evolving clinical practice of increasing the number of genes on the UC panel to include all associated CRCP genes presented a major challenge for the original comparative effectiveness design in this study. CES in the evaluation for hereditary CRCP currently does not add diagnostic value beyond UC testing at the time of the initial test, though the potential for reanalysis of newly associated genes is a noted benefit of CES. The current standard practice of MSI/IHC with subsequent germline testing or multi-gene panels is appropriate for hereditary CRCP evaluation until further evidence is available regarding the benefits of exome level data in this context. Future studies with larger sample sizes, that explore phenotypes for which there is a higher proportion of unknown genetic etiology and that include diverse and underserved patient populations to evaluate the psychosocial, familial communication and economic outcomes of CES using standardized, validated measures are needed.
Acknowledgments:
This work was supported by the National Human Genome Research Institute (Grants U01 HG0006507 and U01HG007307) and the Agency for Healthcare Research and Quality/University of Washington (grant number K12 HS021686, Patient-Centered Outcomes Research Career Development Program). We are also grateful to the participants and their families for providing samples and clinical histories.
app
Figure A.1.
Genes on the University of Washington, Coloseq™ hereditary CRC panel over course of study and number of participants who received each version of the panel.
Figure A.2.
Study Flow & Participant Enrollment Diagram
*Five participants declined results; five lost to follow-up; four did not have UC testing; one died before return visit 1.
#16 and 19 participants lost to follow-up in the UC arm and CES arm respectively between return visit 1 and 2.
Table A.1:
Select pharmacogenetic variants and carrier status genes with analyzed by CES at conclusion of the triala
| Association | |
|---|---|
| Pharmacogenetic Variants | |
| CYP2C19*2, *3 | Impaired responsiveness to Clopidogrel |
| CYP2C9*2, *3,*4,*5,*6,*9,*11 | Warfarin sensitivity |
| CYP4F2*3 | Warfarin resistance |
| DPYD*2A, c.496A>G/M166V | Dihydropyrimidine dehydrogenase deficiency |
| SCL01B1*5 | Statin induced myopathy |
| TPMT*2,*3A,*3B,*3C,*4 | 6-mercaptopurine sensitivity; Azathioprine sensitivity |
| Carrier Status Genes | |
| ACADM | Medium Chain Acyl-CoA Dehydrogenase Deficiency |
| BCHE | Pseudocholinesterase Deficiency |
| CFTR | Cystic fibrosis |
| G6PC | Glycogen Storage Disease, Type 1A |
| GBA | Gaucher disease |
| GJB2 | GJB2-related DFNB1 hearing loss |
| HBB | Beta thalassemia |
| HEXA | Tay-Sachs |
| PAH | Phenylalanine Hydroxylase Deficiency(PKU) |
| SERPINA1 | Alpha-1 Antitrypsin deficiency |
The pharmacogenetic variants list was dynamic throughout the course of the study and not all participants randomized to CES had carrier status genes annotated.
Table A2.
Number and interpretation of variants identified by UC and CES diagnostic testing and returned to participants in each randomization arm
| Randomization Arm | ||||
|---|---|---|---|---|
| UC | CES | |||
| P/LP | VUS | P/LP | VUS | |
| Lynch syndrome related | ||||
| MLH1 | 0 | 0 | 0 | 1 |
| MSH2 | 2 | 2 | 2 | 2 |
| MSH6 | 0 | 1 | 0 | 1 |
| PMS2 | 3 | 2 | 1 | 3 |
| Total | 5 | 5 | 3 | 7 |
| Non-Lynch syndrome related | ||||
| APC | 1 | 5 | 0 | 7 |
| AXIN2 | 0 | 1 | 1 | 1 |
| MUTYH | 1 | 1 | 3 | 0 |
| PMS1 | 0 | 0 | 0 | 2 |
| POLE | 0 | 0 | 0 | 2 |
| PTCH1 | 0 | 0 | 0 | 1 |
| SEMA4A | 0 | 0 | 0 | 2 |
| SMAD4 | 0 | 0 | 0 | 2 |
| STK11 | 0 | 1 | 0 | 0 |
| TP53 | 0 | 0 | 1 | 0 |
| XRCC2 | 0 | 0 | 0 | 1 |
| CDH1 | 0 | 1 | 0 | 1 |
| Total | 2 | 9 | 5 | 19 |
| Non-CRCPr elateda | ||||
| BRCA1 | 1 | 0 | 1 | 0 |
| BRCA2 | 0 | 0 | 1 | 0 |
| CHEK2 | 2 | 0 | 1 | 0 |
| H0XB13 | 2 | 0 | 0 | 0 |
| PALB2 | 0 | 0 | 0 | 1 |
| Total | 5 | 0 | 3 | 1 |
Non-CRCP findings at the first return visit in both arms were due to non-CRCP genes included on the UC multi-gene panels.
Table A.3.
Participant healthcare resource utilization one month after the first return visit by types of results returned
| In the past month | Genetic Testing Results1 | ||
|---|---|---|---|
| Positive (P/LP) N=17(%) |
VUS N=25(%) |
Negative N=84(%) |
|
| Number of visits to a specialist doctor | |||
| 0 | 13(74.5) | 14(63.6) | 49 (58.3) |
| 1 | 2(11.8) | 6(27.3) | 21(25.0) |
| 2 | 1(5.9) | 1(4.6) | 6(7.1) |
| 3 | 1(5.9) | 0(0) | 8(9.5) |
| Missing | 0(0) | 1(4.6) | 0(0) |
| Number of visits to a genetic counselor | |||
| 0 | 14(82.4) | 15(68.2) | 58(69.1) |
| 1 | 3(17.7) | 5(22.7) | 23(27.4) |
| 2 | 0(0) | 0(0) | 2(2.4) |
| 3 | 0(0) | 1(4.6) | 0(0) |
| Missing | 0(0) | 1(4.6) | 1(1.2) |
| Had any medical procedures | |||
| No | 14(82.4) | 18(84.8) | 56(66.7) |
| Yes | 3(17.7) | 4(18.2) | 27(32.1) |
| Missing | 0(0) | 0(0) | 1(1.2) |
| Had any evaluation of cancer | |||
| No | 15(88.2) | 17(77.3) | 60(71.4) |
| Yes | 2(11.8) | 5(22.7) | 24(28.6) |
| Health insurance changes | |||
| Have made | 0(0) | 2(9.1) | 5(6.0) |
| Intend to | 3(17.7) | 1(4.6) | 4(4.8) |
| Thought about | 0(0) | 2(9.1) | 3(3.6) |
| Have not | 14(82.4) | 17(77.3) | 72(85.7) |
| Life insurance changes | |||
| Have made | 0(0) | 0(0) | 6(7.1) |
| Intend to | 0(0) | 1(4.6) | 2(2.4) |
| Thought about | 3(17.7) | 2(9.1) | 4(4.8) |
| Have not | 14(82.4) | 19(86.4) | 72(85.7) |
| Shared genetic test | |||
| results with 1st-degree | |||
| blood family members | |||
| No | 3(17.7) | 5(22.7) | 20(23.8) |
| Yes | 14(82.4) | 17(77.3) | 64(76.2) |
Genetic testing results included both CRCP and non-CRCP findings
Table A.4.
FACToR subscale scores at each time point by type of result returned
| Positive (P/LP) |
VUS | Negative | |
|---|---|---|---|
| Mean(SD) | Mean(SD) | Mean(SD) | |
| 2w after first return visit | (n=16) | (n=25) | (n=82) |
| Negative Emotion, 0–12 | 1.56(2.56) | 0.60(1.32) | 0.57(1.14) |
| Positive Feelings, 0–16 | 9.88(3.76) | 7.60(4.04) | 5.98(4.13) |
| Uncertainty, 0–12 | 3.19(3.12) | 2.16(2.49) | 1.63(2.01) |
| Privacy Concerns, 0–8 | 0.88(1.75) | 0.56(1.76) | 0.59(1.28) |
| 2w after second return visit | (n=11) | (n=14) | (n=70) |
| Negative Emotion, 0–12 | 0.91(1.45) | 0.14(0.36) | 0.29(0.89) |
| Positive Feelings, 0–16 | 8.18(2.79) | 8.07(4.73) | 7.23(4.58) |
| Uncertainty, 0–12 | 1.18(1.99) | 1.00(1.96) | 1.11(1.92) |
| Privacy Concerns, 0–8 | 0.55(1.29) | 0.14(0.53) | 0.67(1.56) |
| 4m after second return visit | (n=13) | (n=20) | (n=87) |
| Negative Emotion, 0–12 | 1.00(1.35) | 0.65(1.18) | 0.26(0.89) |
| Positive Feelings, 0–16 | 9.77(2.35) | 11.35(4.42) | 9.53(4.93) |
| Uncertainty, 0–12 | 2.77(2.74) | 2.10(2.71) | 1.10(1.89) |
| Privacy Concerns, 0–8 | 0.23(0.83) | 0.30(0.80) | 0.45(1.08) |
| 10m after second return visit | (n=12) | (n=15) | (n=76) |
| Negative Emotion, 0–12 | 0.33(0.65) | 0.53(1.06) | 0.08(0.32) |
| Positive Feelings, 0–16 | 10.33(3.23) | 10.00(5.72) | 9.71(5.27) |
| Uncertainty, 0–12 | 1.00(1.41) | 2.40(3.22) | 0.95(1.77) |
| Privacy Concerns, 0–8 | 0.08(0.29) | 0.47(0.99) | 0.38(1.19) |
Table A.5.
Results of other instruments by genetic finding groups at two weeks after the first return visit
| Positive | VUS | Negative | |
|---|---|---|---|
| Mean(SD) | Mean(SD) | Mean(SD) | |
| VR-12 | (n=16) | (n=25) | (n=84) |
| Physical Component Score | 47.63(12.46) | 49.78(9.48) | 45.15(12.70) |
| Mental Component Score | 50.23(8.78) | 49.88(9.00) | 50.18(9.87) |
| GAD-7 total Score | (n=16) | (n=25) | (n=83) |
| 2.94(4.39) | 2.88(3.18) | 3.31(4.32) | |
| PHQ-9 total Score | (n=16) | (n=24) | (n=84) |
| 3.88(5.51) | 3.46(3.06) | 4.05(4.54) | |
| MHI-5 total Score | (n=16) | (n=25) | (n=84) |
| 74.38(19.05) | 79.00(13.99) | 77.32(17.45) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure: All authors declare no relevant financial interests in this manuscript.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- [2].Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on genetic evaluation and management of lynch syndrome: A consensus statement by the us multi-society task force on colorectal cancer. Gastroenterology 2014;147:502–26. doi: 10.1053/j.gastro.2014.04.001. [DOI] [PubMed] [Google Scholar]
- [4].Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005;352:1851–60. doi: 10.1056/NEJMoa1514204. [DOI] [PubMed] [Google Scholar]
- [5].Lynch HT, de la Chapelle A. Hereditary Colorectal Cancer. N Engl J Med 2003;348:919–32. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- [6].National Comprehensive Cancer Network. Genetic / Familial High-Risk Assessment: Colorectal 1.2016. NCCN Guidel 2016:81 https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf.
- [7].Kovacs ME, Papp J, Szentirmay Z, Otto S, Olah E. Deletions removing the last exon of TACSTD1 constitute a distinct class of mutations predisposing to lynch syndrome. Hum Mutat 2009;30:197–203. doi: 10.1002/humu.20942. [DOI] [PubMed] [Google Scholar]
- [8].Gonzaga-Jauregui C, Lupski JR, Gibbs RA. Human Genome Sequencing in Health and Disease. Annu Rev Med 2012;63:35–61. doi: 10.1146/annurev-med-051010-162644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gagan J, Van Allen EM. Next-generation sequencing to guide cancer therapy. Genome Med 2015;7:80. doi: 10.1186/s13073-015-0203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shah PD, Nathanson KL. Application of Panel-Based Tests for Inherited Risk of Cancer. Annu Rev Genomics Hum Genet 2017;18: 10.1146/annurev-genom-091416-035305. [DOI] [PubMed] [Google Scholar]
- [11].Bertier G, Hétu M, Joly Y. Unsolved challenges of clinical whole-exome sequencing: a systematic literature review of end-users’ views. BMC Med Genomics 2016;9:52. doi: 10.1186/s12920-016-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Majewski J, Schwartzentruber J, Lalonde E, Montpetit A, Jabado N. What can exome sequencing do for you? J Med Genet 2011;48:580–9. doi: 10.1136/jmedgenet-2011-100223. [DOI] [PubMed] [Google Scholar]
- [13].Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med 2017;19:249–55. doi: 10.1038/gim.2016.190. [DOI] [PubMed] [Google Scholar]
- [14].Powis Z, Espenschied CR, LaDuca H, Hagman KD, Paudyal T, Li S, et al. Clinical germline diagnostic exome sequencing for hereditary cancer: Findings within novel candidate genes are prevalent. Cancer Genet 2018;224–225:12–20. doi: 10.1016/j.cancergen.2018.04.002. [DOI] [PubMed] [Google Scholar]
- [15].O JM, Lee K, O’Daniel JM, Lee K. Whole-genome and whole-exome sequencing in hereditary cancer: impact on genetic testing and counseling. Cancer J 2012;18:287–92. doi: 10.1097/PPO.0b013e318262467e. [DOI] [PubMed] [Google Scholar]
- [16].Sokolenko AP, Suspitsin EN, Kuligina ES, Bizin IV., Frishman D, Imyanitov EN. Identification of novel hereditary cancer genes by whole exome sequencing. Cancer Lett 2015;369:274–88. doi: 10.1016/j.canlet.2015.09.014. [DOI] [PubMed] [Google Scholar]
- [17].Gallego CJ, Bennette CS, Heagerty P, Comstock B, Horike-Pyne M, Hisama F, et al. Comparative effectiveness of next generation genomic sequencing for disease diagnosis: Design of a randomized controlled trial in patients with colorectal cancer/polyposis syndromes. Contemp Clin Trials 2014;39:1–8. doi: 10.1016/j.cct.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].(University of Washington). UW Laboratory Medicine Clinical Test Information - BROCA - Cancer Risk Panel n.d. http://web.labmed.washington.edu/tests/genetics/BR (accessed September 28, 2017).
- [19].(University of Washington). UW Laboratory Medicine Clinical Test Information - ColoSeqTM and ColoSeqTM Tumor - Lynch and Polyposis Panel n.d. http://web.labmed.washington.edu/tests/genetics/COLOSEQ (accessed October 2, 2017).
- [20].Dorschner MO, Amendola LM, Turner EH, Robertson PD, Shirts BH, Gallego CJ, et al. Actionable, Pathogenic Incidental Findings in 1,000 Participants’ Exomes. Am J Hum Genet 2013;93:631–40. doi: 10.1016/j.ajhg.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Berg JS, Amendola LM, Eng C, Allen E Van, Gray SW, Wagle N, et al. Processes and preliminary outputs for identification of actionable genes as incidental findings in genomic sequence data in the Clinical Sequencing Exploratory Research Consortium. Genet Med 2013;15:860–7. doi: 10.1038/gim.2013.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–23. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Amendola LM, Dorschner MO, Robertson PD, Salama JS, Hart R, Shirts BH, et al. Actionable exomic incidental findings in 6503 participants: Challenges of variant classification. Genome Res 2015;25:305–15. doi: 10.1101/gr.183483.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kazis LE, Selim A, Rogers W, Ren XS, Lee A, Miller DR, et al. Veterans RAND 12 Item Health Survey (VR-12): A White Paper Summary. Unpubl Manuscr 2008. [Google Scholar]
- [25].Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- [26].Martin A, Rief W, Klaiberg A, Braehler E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry 2006;28:71–7. doi: 10.1016/j.genhosppsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [27].Berwick DM, Murphy JM, Goldman PA, Ware JE, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care 1991;29:169–76. [DOI] [PubMed] [Google Scholar]
- [28].Li M, Bennette CS, Amendola LM, Ragan Hart M, Heagerty P, Comstock B, et al. The Feelings About genomiC Testing Results (FACToR) Questionnaire: Development and Preliminary Validation. J Genet Couns 2018. doi: 10.1007/s10897-018-0286-9. [DOI] [PubMed] [Google Scholar]
- [29].Cella D, Hughes C, Peterman A, Chang CH, Peshkin BN, Schwartz MD, et al. A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Heal Psychol 2002. doi: 10.1037/0278-6133.21.6.564. [DOI] [PubMed] [Google Scholar]
- [30].Slavin TP, Niell-Swiller M, Solomon I, Nehoray B, Rybak C, Blazer KR, et al. Corrigendum: Clinical Application of Multigene Panels: Challenges of Next-Generation Counseling and Cancer Risk Management. Front Oncol 2015. doi: 10.1007/978-3-319-99365-2_41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dorschner MO, Amendola LM, Turner EH, Robertson PD, Shirts BH, Gallego CJ, et al. Actionable, pathogenic incidental findings in 1,000 participants’ exomes. Am J Hum Genet 2013;93:631–40. doi: 10.1016/j.ajhg.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN. Gene panel testing for inherited cancer risk. JNCCN J Natl Compr Cancer Netw 2014;12:1339–46. [DOI] [PubMed] [Google Scholar]
- [33].Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, et al. American society of clinical oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol 2015;33:3660–7. doi: 10.1200/JCO.2015.63.0996. [DOI] [PubMed] [Google Scholar]
- [34].Hansen MF, Johansen J, Sylvander AE, Bjørnevoll I, Talseth-Palmer BA, Lavik LAS, et al. Use of multigene-panel identifies pathogenic variants in several CRC-predisposing genes in patients previously tested for Lynch Syndrome. Clin Genet 2017;92:405–14. doi: 10.1111/cge.12994. [DOI] [PubMed] [Google Scholar]
- [35].DeRycke MS, Gunawardena S, Balcom JR, Pickart AM, Waltman LA, French AJ, et al. Targeted sequencing of 36 known or putative colorectal cancer susceptibility genes. Mol Genet Genomic Med 2017;5:553–69. doi: 10.1002/mgg3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Daniel JM, McLaughlin HM, Amendola LM, Bale SJ, Berg JS, Bick D, et al. A survey of current practices for genomic sequencing test interpretation and reporting processes in US laboratories. Genet Med 2017;19:575–82. doi: 10.1038/gim.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Janssens ACJW. The hidden harm behind the return of results from personal genome services: A need for rigorous and responsible evaluation. Genet Med 2015;17:621–2. doi: 10.1038/gim.2014.169. [DOI] [PubMed] [Google Scholar]
- [38].Anticipate Weiner C. and communicate: Ethical management of incidental and secondary findings in the clinical, research, and direct-to-consumer contexts (December 2013 Report of the Presidential Commission for the Study of Bioethical Issues). Am J Epidemiol 2014;180:562–4. doi: 10.1093/aje/kwu217. [DOI] [PubMed] [Google Scholar]
- [39].Wolf SM, Annas GJ, Elias S. Point-counterpoint. Patient autonomy and incidental findings in clinical genomics. Science 2013;340:1049–50. doi: 10.1126/science.1239119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Nimwegen KJM, Schieving JH, Willemsen MAAP, Veltman JA, Van Der Burg S, Van Der Wilt GJ, et al. The diagnostic pathway in complex paediatric neurology: A cost analysis. Eur J Paediatr Neurol 2015;19:233–9. doi: 10.1016/j.ejpn.2014.12.014. [DOI] [PubMed] [Google Scholar]
- [41].Vassy JL, Christensen KD, Schonman EF, Blout CL, Robinson JO, Krier JB, et al. The Impact of Whole-Genome Sequencing on the Primary Care and Outcomes of Healthy Adult Patients. Ann Intern Med 2017;167:159. doi: 10.7326/M17-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]