Abstract
Objective
To raise awareness about degenerative cervical myelopathy (DCM) and to help family physicians identify, diagnose, and manage DCM more effectively.
Sources of information
A PubMed search was conducted for articles published between 1970 and October 2017, using the terms cervical myelopathy and degenerative spinal cord injury with family medicine or primary care.
Main message
Owing to limited knowledge of DCM in primary care, along with the large variability of the disease, the diagnosis of DCM is often missed or delayed. The natural course of DCM presents as a stepwise decline, with symptoms ranging from muscle weakness to complete paralysis. All individuals with signs and symptoms should be referred to a spine surgeon for consideration of surgery; those with mild DCM might be offered conservative treatment but should receive a surgical evaluation and opinion nonetheless. Asymptomatic patients with evidence of cord compression on magnetic resonance imaging might need to be referred for assessment; however, surgery is not advised. It is critical to closely monitor asymptomatic individuals or those with mild DCM for neurologic deterioration.
Conclusion
Degenerative cervical myelopathy is the most common cause of spinal cord dysfunction in adults. This review helps streamline its diagnosis in primary care, allowing for improved chances of early diagnosis and prevention of further neurologic decline among patients.
Case description
Mrs Cole is a 52-year-old woman who comes into the family practice with complaints of numbness in both hands. She is finding that she has problems with the coordination of her hands, has difficulty doing up buttons, and is dropping things. She believes this is because her carpal tunnel syndrome that she was diagnosed with in the past is worsening. She also reports that she has had some neck pain and stiffness recently. She has continued working at her factory job, but is finding it more difficult.
Sources of information
A PubMed search was conducted for articles published between 1970 and October 2017, using the terms cervical myelopathy and degenerative spinal cord injury with family medicine or primary care.
Main message
Spinal cord injury (SCI) is a devastating condition that portends considerable morbidity (eg, pain, spasticity, neurogenic bowel or bladder, autonomic dysreflexia).1 Beyond this, there are social, emotional, and economic consequences for patients, their families, and society at large (eg, employment, relationships, community access, isolation).2 The lifetime costs of traumatic SCI in Canada are estimated at $2 billion.3 The prototypical SCI is still commonly viewed as a traumatic injury caused by a motor vehicle accident or fall; young male patients make up 80% of new cases of traumatic SCI.4 However, in actuality, the leading cause of SCI is now degenerative cervical myelopathy (DCM).5,6
Degenerative cervical myelopathy occurs when age-related osteoarthritic changes cause narrowing of the cervical spinal canal, leading to chronic spinal cord compression and resultant neurologic disability. A 2017 study has estimated the prevalence of DCM to be 1120 per 1 million people in Canada, with an incidence of hospitalizations at 4 per 100 000 person-years.7 However, the precise prevalence of DCM is not known owing to nonuniform definition and the lack of large population-based studies, and thus true prevalence is thought to be higher.8 Patient presentation can vary broadly, with symptoms ranging from mild dysfunction, such as numbness or dexterity problems, to severe dysfunction, such as quadraparesis and incontinence, as later findings.5,9–12 It is important to note that paresthesia in the extremities is often the first sign, and because it might be mild, it can be easily overlooked by patients and providers.
Studies have shown that early diagnosis and surgical management might improve neurologic and overall outcomes13 and prevent further deterioration.14 More important, the literature indicates that the most common initial point of contact for those developing DCM is the primary care provider.13 For this reason, as the population ages, family physicians and health care professionals will be confronted with an increase in the number of patients presenting with a wide spectrum of symptoms relating to various stages of DCM.9 However, owing to the variability of clinical presentation, DCM can be very difficult to diagnose, with delay times of up to 2 years before a diagnosis is made.13 Behrbalk et al found that delayed diagnosis was due to a lack of knowledge within the primary care setting.13 Our own experience with medical learners in primary care is that most of them can easily recite the signs and symptoms of cauda equina syndrome, having been taught this repeatedly during their medical education, but often are not aware of or able to describe the signs and symptoms for cord compression in the cervical spine.
There exists an important opportunity to affect the outcomes of this potentially devastating condition. The objective of this clinical review is to raise awareness of DCM and to help primary care practitioners identify, diagnose, and manage DCM more effectively and efficiently.
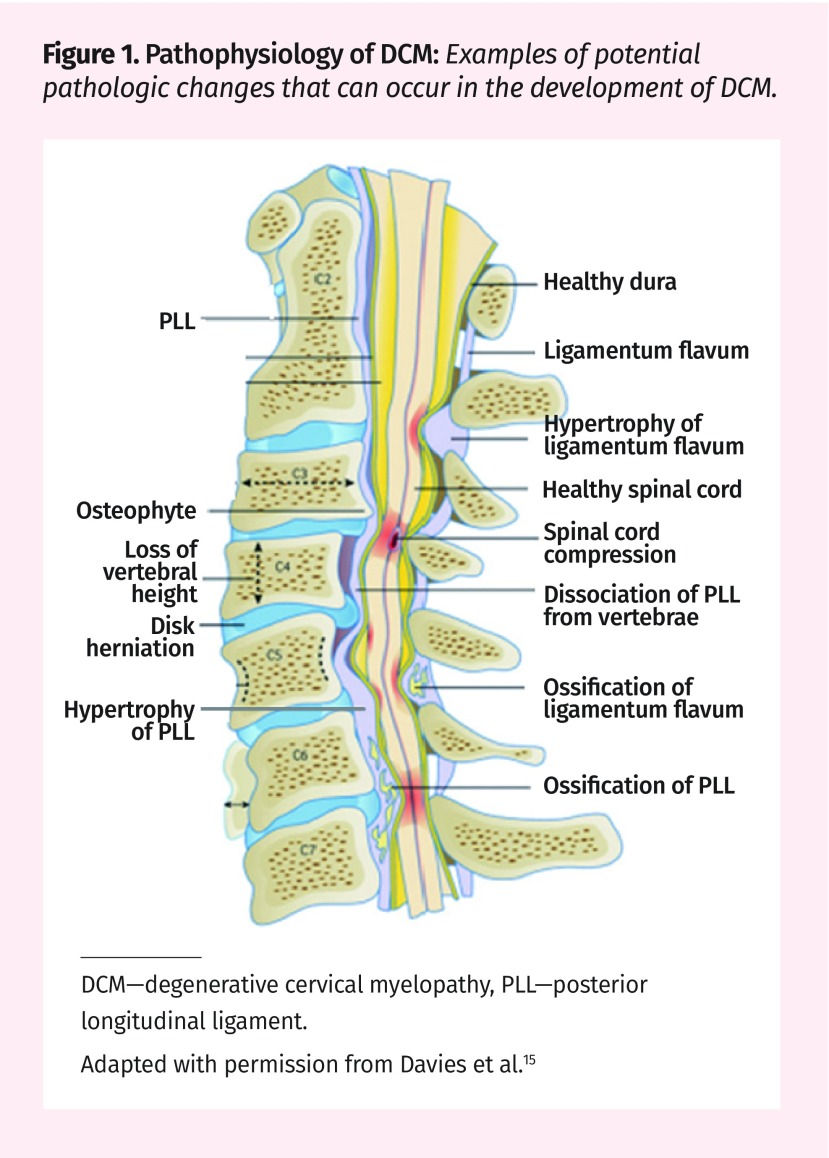
Pathophysiology of DCM.
Degenerative changes to the components of the spine occur as a part of healthy aging (Figure 1).5,15 The pathogenesis of the disease can be divided into 3 main components: static, dynamic, and histopathologic.
Figure 1.
Pathophysiology of DCM: Examples of potential pathologic changes that can occur in the development of DCM.
DCM—degenerative cervical myelopathy, PLL—posterior longitudinal ligament.
Adapted with permission from Davies et al.15
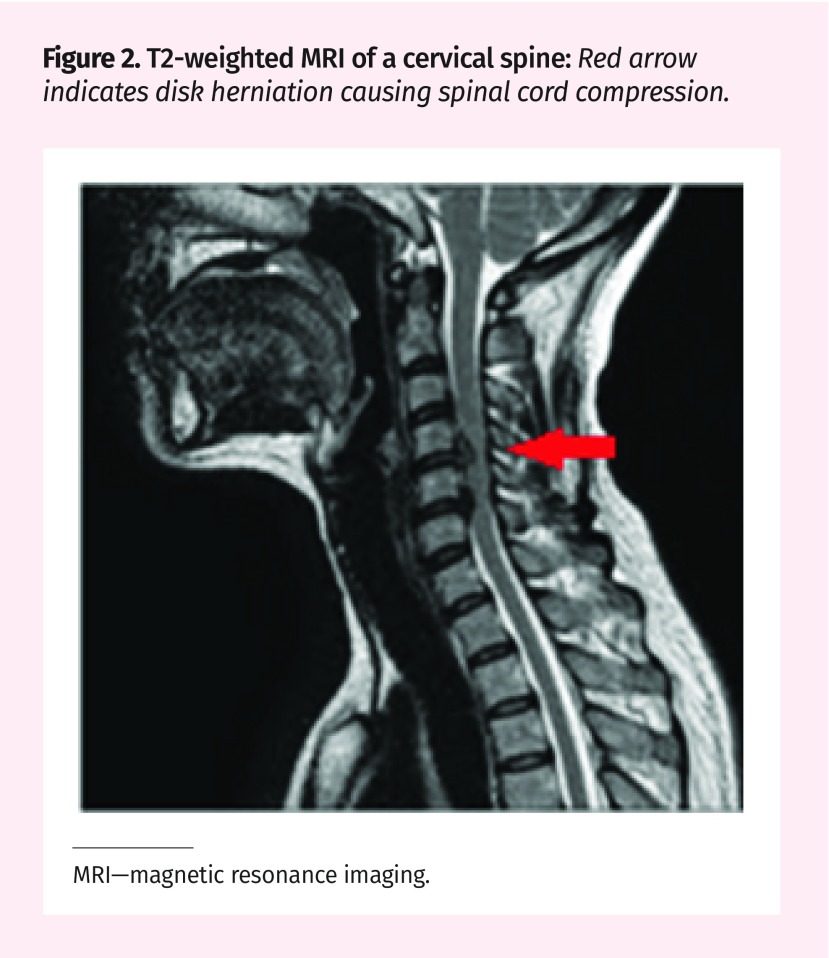
Static: Static factors are structural factors that cause canal narrowing. The degenerative cascade of DCM typically begins with the deterioration of the intervertebral disk.9,16,17 The disk collapses and bulges posteriorly causing a narrowing of the spinal canal (Figure 2). Decreased disk height causes the spinal column to shorten, leading to abnormal spine biomechanics.5,9,11,18 The ligamentum flavum can also cause compression by thickening and buckling into the spinal canal. Ossification of the posterior longitudinal ligament can also lead to DCM by direct compression of the cord.9,16,17 These changes often cause a stiffening of the affected structures. To compensate for the decreased motion at the affected levels, adjacent regions of the spine become hypermobile.19
Figure 2.
T2-weighted MRI of a cervical spine: Red arrow indicates disk herniation causing spinal cord compression.
MRI—magnetic resonance imaging.
Dynamic: Dynamic factors refer to abnormal repetitive movement of the cervical spine during flexion and extension causing spinal cord irritation and compression. Flexion might compress the spinal cord against anterior osteophytes and intervertebral disks.9,16,17,19 Hyperextension might lead to cord pinching between the posterior margins of the vertebral body anteriorly and the hypertrophied buckled ligamentum flavum posteriorly.9,16,17,19
Histopathologic: Mechanical compression of the cord leads to vascular changes causing ischemia and inflammation.16 Chronic cord compression can lead to neuronal cell loss, degeneration of the posterior columns and anterior horn cells, and endothelial damage resulting in a compromised blood–spinal cord barrier, which all accumulate to the functional decline of the patient.9,16,19 The effects of cord compression vary depending on the degree of compression; however, they conclude in a range of motor and sensory impairments, as detailed below. As DCM is a progressive, degenerative condition, it is estimated that 20% to 60% of those with myelopathic symptoms will further deteriorate over time.14,20,21
Signs and symptoms.
At its earliest stage, DCM often presents as numbness and tingling of 1 or more extremities. Primary care providers need to have a high degree of suspicion in evaluating patients who present with paresthesia (eg, if the patient reports symptoms in 1 hand, specifically ask about symptoms in other extremities and other associated signs and symptoms). Patients might complain of “clumsiness” such as having difficulty doing up buttons or changes in their handwriting. For individuals presenting to primary care clinics with all or some of upper limb or neck pain, sensory (parasthesia) or motor (weakness, clumsiness) complaints in the extremities (arms, legs, or both), and unsteady gait, DCM should be considered as part of the differential diagnosis. Upper limb radicular pain is the most prevalent symptom in patients with DCM (86% of patients), with neck pain only present for 60% of patients.13
Degenerative cervical myelopathy is often a slow stepwise deterioration, with symptoms of gait abnormalities, weakness, sensory changes, and dexterity problems. Bowel and bladder problems can occur, but are rare and often an indicator of severe cord injury.9,13,22 The most common physical signs upon examination include motor weakness, particularly of the intrinsic hand muscles, hyperactive reflexes, ankle or patellar clonus, spasticity, and abnormal Babinski and Hoffmann signs (Table 1).9,12,23 The finger escape sign can be highly indicative of cervical cord dysfunction. During this test, the patient holds his or her fingers extended and closed together (in adduction). If the ulnar digits drift away (into abduction or flexion), cervical cord damage might be present.12 In addition, quantifying walking times (eg, performing the 30-m walk test24 or tandem gait) might be useful to assess gait abnormalities. It is very important to examine the cervical spine (performing movements in all planes to determine if there is reproduction of symptoms) in any patient presenting with radicular or myelopathic symptoms; quite often, tests of the neck (eg, cervical extension) might reproduce the paresthesia in the extremities thereby indicating the cause.
Table 1.
Clinical signs and tests for DCM
| CLINICAL SIGNS AND TESTS | DESCRIPTION |
|---|---|
| Clonus | Muscular spasm involving repeated, often rhythmic, contractions |
| Spasticity | Velocity-dependent exaggeration of stretch reflexes resulting in increased muscle tone |
| Babinski sign | Testing of the plantar reflex by stroking the lateral sole of the foot from heel to ball and moving medially to the great toe; extension of the great toe and fanning of the rest is considered an abnormal finding (positive Babinski sign); a normal finding is flexion of all toes (negative Babinski sign) |
| Finger escape sign | Abduction of flexion of the ulnar digits upon holding the hand out, fingers closed |
| Hoffmann sign | Flexion of the thumb or index finger upon flicking the nail of the middle or ring finger |
| Spurling test | With the neck in extension, lateral flexion with axial compression reproduces symptoms in the extremities |
Diagnosis of DCM is challenging especially in mild cases, as signs and symptoms might be transient and less severe. Urgent magnetic resonance imaging (MRI) should be ordered for those with suspected DCM, and those with considerable neurologic signs and symptoms should be referred immediately for MRI and consultation with a spine surgeon or sent to the emergency department.
Diagnosis of DCM.
Diagnosis is determined by 1 or more symptoms (hand clumsiness, gait imbalance, numbness, weakness, and bladder dysfunction) and signs (fine motor dysfunction of the hands, hyperreflexia, gait ataxia, sensory deficits, and focal weakness) that are attributable to the cervical spinal cord, as well as the presence of spinal cord compression on MRI (Figure 2).8
Magnetic resonance imaging is the current criterion standard for diagnosis, visualizing the spinal cord and nerve roots in relation to the cerebrospinal fluid. T2-weighted images have the greatest contrast. The presence of cord signal change should be assessed; compression might be reflected by any deformation of the spinal cord and should be assessed clinically. Cord compression might also be seen on incidental imaging; asymptomatic cord compression is estimated at 8% to 57%.8 Computed tomography and radiographs do not visualize the spinal cord and therefore are not accurate for diagnosis of DCM; however, they can provide useful information about dynamic changes, bone quality, and alignment that can be used to guide surgical intervention.8 Electromyography is rarely useful in adding to the diagnosis of DCM; however, it does hold value in excluding other neurologic disorders such as peripheral neuropathy, amyotrophic lateral sclerosis, and multiple sclerosis.25 Somatosensory evoked potential might contribute to the diagnosis of DCM, as it provides a more direct assessment of spinal cord dysfunction.12,25
Box 1 describes the clinical approach to diagnosis of DCM at the office.9,12,23
Box 1. Clinical approach to DCM: Consider the following approach when a patient presents with sensory or motor symptoms in the upper extremities, with or without unsteady gait or neck pain.
Question his or her history of*
other extremity symptoms (numbness, paresthesia, pain, and sensory or motor dysfunction in arms and legs)
bowel and bladder dysfunction (might be a late finding)
saddle paresthesia (might be a late finding if present)
gait disturbances
Physical examination should include
cranial nerves
cervical ROM
attention to the loss of ROM and reproduction of symptoms with cervical spine testing or movements (ie, if cervical extension reproduces symptoms in extremities)
upper extremity ROM testing
myotomes and dermatomes (consider lower extremity and sacral myotomes and dermatomes if symptoms are present)
Spurling test, deep tendon reflexes, tone, spasticity, clonus, Babinski sign, and Hoffmann sign (Table 1)9,12,23
gait, tandem gait
digital rectal examination (if symptoms require assessment of tone)
DCM—degenerative cervical myelopathy, ROM—range of motion.
*Patients who present with bilateral neurologic upper extremity symptoms should be questioned and examined for a more central cause such as DCM (eg, DCM has often been misdiagnosed as bilateral carpal tunnel syndrome).
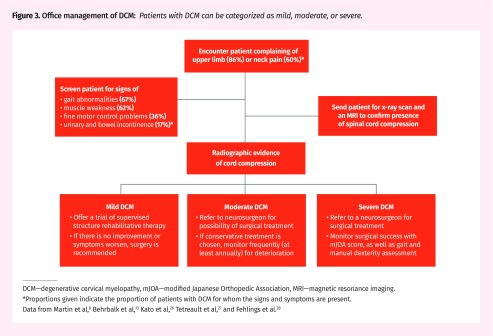
Office management of DCM.
Patients with DCM can be categorized into 3 groups: mild, moderate, and severe, often classified by the modified Japanese Orthopedic Association scale (mJOA) (Figure 3).8,13,26–28 We will also discuss individuals who have evidence of cord compression on imaging but do not have myelopathic symptoms.
Figure 3.
Office management of DCM: Patients with DCM can be categorized as mild, moderate, or severe.
DCM—degenerative cervical myelopathy, mJOA—modified Japanese Orthopedic Association, MRI—magnetic resonance imaging.
*Proportions given indicate the proportion of patients with DCM for whom the signs and symptoms are present.
Data from Martin et al,8 Behrbalk et al,13 Kato et al,26 Tetreault et al,27 and Fehlings et al.28
There is a paucity of high-quality studies relating to the optimal management of patients with mild DCM. However, in the absence of robust evidence, Fehlings and colleagues recommend offering a trial of supervised, structured rehabilitative therapy as a conservative treatment measure.22 If there is no improvement or there is worsening with conservative treatment, surgical treatment is recommended.22,23 Conservative treatment might also be indicated owing to patient preference or unacceptable surgical risk. Examples of conservative treatment include structured, careful physiotherapy, a soft neck collar, massage, and medication; however, there is a lack of evidence-based approaches to conservative treatment.5,9,27,29 Cervical manipulative therapy and cervical traction should be avoided in order to prevent complications.30 It is also recommended that patients stay away from activities that have high impact on the neck (contact sports, skydiving, etc).
An important group of patients includes those who are found to have cervical cord compression on MRI but no signs and symptoms of myelopathy. Fehlings and colleagues28 recommend following these patients with regular clinic visits but no treatment. However, a caveat to this is that if a patient has radiculopathy with evidence of cord compression on MRI, these individuals have a higher risk of progressing to myelopathy; therefore, surgery might be offered. A systematic review by Wilson et al reported that only 8% of patients with evidence of spinal cord compression but who exhibited no myelopathic signs or symptoms had developed myelopathy a year later.31 These patients should be monitored thoroughly and frequently with repeat MRI and physical examination.32 Owing to the lack of consistent, evidence-based information on the natural history, recommendations for treatment must be largely determined on an individual basis.22,33
There are no studies examining the frequency of repeat clinical and imaging examinations for those treated conservatively8; however, these patients need education regarding signs and symptoms that represent deterioration, as well as close clinical monitoring and repeat MRI depending on clinical examination findings. Surgical intervention is reserved for those who fail to respond to conservative treatment and whose symptoms progressively worsen.
Differential diagnosis.
The wide variability and lack of consistency in the pattern of onset of DCM can make diagnosis clinically challenging. There are also other neurologic disorders that might present in a similar manner (Table 2).9,10,25,34 Carpal tunnel syndrome is a common mistaken diagnosis for a more central cause such as DCM; attention should be paid to proper diagnosis based on clinical presentation and testing, especially if there are bilateral symptoms.35
Table 2.
Differential diagnoses that might present similarly to DCM, with some possible signs and symptoms that might differentiate from DCM
| DIFFERENTIAL DIAGNOSES | DIFFERENTIATING SIGNS AND SYMPTOMS |
|---|---|
| Amyotrophic lateral sclerosis | |
| Multiple sclerosis |
|
| Peripheral nerve entrapments (ulnar neuropathy, carpal tunnel syndrome)10 |
|
| Intracranial pathology (eg, brain neoplasm) |
|
| Normal pressure hydrocephalus34 |
|
| Vitamin B deficiency9,10 |
|
DCM—degenerative cervical myelopathy.
Surgical treatment.
Surgery is highly recommended for those displaying moderate (mJOA score of 12 to 14) to severe (mJOA score of <11) symptoms of DCM.11,28 Research has shown that the positive outcomes of surgery far outweigh possible negative complications of surgery (worsening myelopathy, hematoma, dysphagia).11,36,37 The goal of surgery is to decompress the spinal cord, stabilize the spinal column, and prevent any further neurologic damage. Research has shown variable outcomes of surgery. Successful surgery occurs in a third of individuals, 40% show no change, and 25% show signs of worsening.5,11,38 The literature is still unclear as to why some individuals get better and others continue to decline after surgery; however, a 2015 study suggests that patients are more likely to improve (based on mJOA score) following surgery if they are younger, have milder symptoms of shortened duration preoperatively, do not smoke, have fewer comorbidities, and do not present with gait dysfunction.39 Despite treatment, many patients might have residual spinal cord deficits such as neurogenic bladder or bowel, spasticity, and pain; the primary care provider will need to monitor and manage these.
Case resolution
On further questioning, Mrs Cole mentions she has also noticed some mild tingling in her legs and feels her balance is not as good as it once was. She denies bowel and bladder symptoms. Physical examination findings reveal a decreased cervical range of motion, with an increase in extremity symptoms with cervical extension. She has increased (grade 3+) upper extremity reflexes, a positive Hoffmann sign bilaterally, and decreased coordination in her hands. She is unable to perform tandem gait and has a positive Babinski sign. An urgent MRI of the cervical spine and brain is ordered. The MRI reveals a C5–C6 osteochondral bar with moderate mass effect on the thecal sac, moderate to severe spinal canal stenosis, and myelomalacia within the cord. At C6–C7 there is moderate diffuse disk bulging with mass effect on the thecal sac, moderate to severe spinal canal stenosis, and myelomalacia within the cord. Mrs Cole is referred urgently to neurosurgery and ultimately undergoes C5–C6 and C6–C7 anterior discectomy and fusion.
Conclusion
Degenerative cervical myelopathy is the most common form of spinal cord dysfunction in adults. Family physicians need to be aware of the condition and the associated clinical examinations leading to timely diagnosis and management. All individuals with signs and symptoms should be referred to a spine surgeon for consideration of surgery; those with mild DCM might be offered conservative treatment but should receive a surgical evaluation and opinion nonetheless. Asymptomatic patients with evidence of cord compression on MRI might need to be referred for assessment; however, surgery is not advised. It is important to educate asymptomatic individuals and those with mild DCM about the signs and symptoms that represent deterioration, as well as to closely monitor these patients for neurologic deterioration.
Acknowledgments
We thank the Ontario Neurotrauma Foundation for its support of this review.
Editor’s key points
▸ Degenerative cervical myelopathy (DCM) is the most common form of spinal cord dysfunction in adults. It occurs when age-related osteoarthritic changes cause narrowing of the cervical spinal canal, leading to chronic spinal cord compression and neurologic disability. Owing to the variability of clinical presentation, DCM can be very difficult to diagnose.
▸ Degenerative cervical myelopathy is often a slow stepwise deterioration. Consider DCM as a differential diagnosis in individuals presenting with all or some of the following: upper limb or neck pain, sensory (paresthesia) or motor (weakness, clumsiness) complaints in the extremities (arms, legs, or both), and unsteady gait.
▸ For patients with suspected DCM, urgent magnetic resonance imaging should be ordered; those with considerable neurologic signs and symptoms should be referred immediately for magnetic resonance imaging and consultation with a spine surgeon or sent to the emergency department.
Footnotes
Contributors
All authors contributed to the literature review and interpretation, and to preparing the manuscript for submission.
Competing interests
None declared
This article is eligible for Mainpro+ certified Self-Learning credits. To earn credits, go to www.cfp.ca and click on the Mainpro+ link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de septembre 2019 à la page e379.
References
- 1.Johnson RL, Gerhart KA, McCray J, Menconi JC, Whiteneck GG. Secondary conditions following spinal cord injury in a population-based sample. Spinal Cord. 1998;36(1):45–50. doi: 10.1038/sj.sc.3100494. [DOI] [PubMed] [Google Scholar]
- 2.Anson CA, Stanwyck DJ, Krause JS. Social support and health status in spinal cord injury. Paraplegia. 1993;31(10):632–8. doi: 10.1038/sc.1993.102. [DOI] [PubMed] [Google Scholar]
- 3.A look at traumatic spinal cord injury in Canada: Rick Hansen Spinal Cord Registry (RHSCIR). J Spinal Cord Med. 2017;40(6):870–1. doi: 10.1080/10790268.2017.1387124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Spinal Cord Injury Statistical Center. Spinal cord injury. Facts and figures at a glance. Birmingham, AL: University of Alabama at Birmingham; 2017. Available from: www.nscisc.uab.edu/Public/Facts%20and%20Figures%20-%202017.pdf. Accessed 2019 Jul 19. [Google Scholar]
- 5.Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409–21. doi: 10.1177/1073858412467377. Epub 2012 Nov 30. [DOI] [PubMed] [Google Scholar]
- 6.Witiw CD, Fehlings MG. Degenerative cervical myelopathy. CMAJ. 2017;189(3):E116. doi: 10.1503/cmaj.151478. Epub 2016 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhsheshian J, Mehta VA, Liu JC. Current diagnosis and management of cervical spondylotic myelopathy. Global Spine J. 2017;7(6):572–86. doi: 10.1177/2192568217699208. Epub 2017 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin AR, Tadokoro N, Tetreault L, Arocho-Quinones EV, Budde MD, Kurpad SN, et al. Imaging evaluation of degenerative cervical myelopathy: current state of the art and future directions. Neurosurg Clin N Am. 2018;29(1):33–45. doi: 10.1016/j.nec.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 9.De Oliveira Vilaça C, Orsini M, Leite MA, de Freitas MR, Davidovich E, Fiorelli R, et al. Cervical spondylotic myelopathy: what the neurologist should know. Neurol Int. 2016;8(4):6330. doi: 10.4081/ni.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HJ, Tetreault LA, Massicotte EM, Arnold PM, Skelly AC, Brodt ED, et al. Differential diagnosis for cervical spondylotic myelopathy: literature review. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S78–88. doi: 10.1097/BRS.0b013e3182a7eb06. [DOI] [PubMed] [Google Scholar]
- 11.Kato S, Fehlings M. Degenerative cervical myelopathy. Curr Rev Musculoskelet Med. 2016;9(3):263–71. doi: 10.1007/s12178-016-9348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course and diagnosis. Neurosurgery. 2007;60(1 Suppl 1):S35–41. doi: 10.1227/01.NEU.0000215383.64386.82. [DOI] [PubMed] [Google Scholar]
- 13.Behrbalk E, Salame K, Regev GJ, Keynan O, Boszczyk B, Lidar Z. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg Focus. 2013;35(1):E1. doi: 10.3171/2013.3.FOCUS1374. [DOI] [PubMed] [Google Scholar]
- 14.Badhiwala JH, Wilson JR. The natural history of degenerative cervical myelopathy. Neurosurg Clin N Am. 2018;29(1):21–32. doi: 10.1016/j.nec.2017.09.002. Epub 2017 Oct 27. [DOI] [PubMed] [Google Scholar]
- 15.Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. BMJ. 2018;360:k186. doi: 10.1136/bmj.k186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(Suppl 6):190S–7. doi: 10.1016/j.spinee.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine (Phila Pa 1976) 1998;23(24):2730–7. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 18.Toledano M, Bartleson JD. Cervical spondylotic myelopathy. Neurol Clin. 2013;31(1):287–305. doi: 10.1016/j.ncl.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Lebl DR, Hughes A, Cammisa FP, O’Leary PF. Cervical spondylotic myelopathy: pathophysiology, clinical presentation, and treatment. HSS J. 2011;7(2):170–8. doi: 10.1007/s11420-011-9208-1. Epub 2011 Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tetreault L, Palubiski LM, Kryshtalskyj M, Idler RK, Martin AR, Ganau M, et al. Significant predictors of outcome following surgery for the treatment of degenerative cervical myelopathy: a systematic review of the literature. Neurosurg Clin N Am. 2018;29(1):115–27.e35. doi: 10.1016/j.nec.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976) 2015;40(12):E675–93. doi: 10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 22.Fehlings MG, Tetreault LA, Wilson JR, Aarabi B, Anderson P, Arnold PM, et al. A clinical practice guideline for the management of patients with acute spinal cord injury and central cord syndrome: recommendations on the timing (≤24 hours versus >24 hours) of decompressive surgery. Global Spine J. 2017;7(Suppl 3):195S–202. doi: 10.1177/2192568217706367. Epub 2017 Sep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser JA, Curé JK, Bailey KL, Morrow DL. Cervical spinal cord compression and the Hoffman sign. Iowa Orthop J. 2001;21:49–52. [PMC free article] [PubMed] [Google Scholar]
- 24.Bohm PE, Fehlings MG, Kopjar B, Tetreault LA, Vaccaro AR, Anderson KK, et al. Psychometric properties of the 30-m walking test in patients with degenerative cervical myelopathy: results from two prospective multicenter cohort studies. Spine J. 2017;17(2):211–7. doi: 10.1016/j.spinee.2016.08.033. Epub 2016 Aug 31. [DOI] [PubMed] [Google Scholar]
- 25.Young WF. Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician. 2000;62(5):1064–70. 1073. [PubMed] [Google Scholar]
- 26.Kato S, Oshima Y, Oka H, Chikuda H, Takeshita Y, Miyoshi K, et al. Comparison of the Japanese Orthopaedic Association (JOA) score and modified JOA (mJOA) score for the assessment of cervical myelopathy: a multicenter observational study. PLoS One. 2015;10(4):e0123022. doi: 10.1371/journal.pone.0123022. Erratum in: PLoS One 2015;10(5):e0128392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tetreault LA, Rhee J, Prather H, Kwon BK, Wilson JR, Martin AR, et al. Change in function, pain, and quality of life following structured nonoperative treatment in patients with degenerative cervical myelopathy: a systematic review. Global Spine J. 2017;7(Suppl 3):42S–52. doi: 10.1177/2192568217700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fehlings MG, Tetreault LA, Riew KD, Middleton JW, Aarabi B, Arnold PM, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 2017;7(Suppl 3):70S–83. doi: 10.1177/2192568217701914. Epub 2017 Sep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhee JM, Shamji MF, Erwin WM, Bransford RJ, Yoon ST, Smith JS, et al. Nonoperative management of cervical myelopathy: a systematic review. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S55–67. doi: 10.1097/BRS.0b013e3182a7f41d. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara T. Neurologic complications in managing degenerative cervical myelopathy: pathogenesis, prevention, and management. Neurosurg Clin N Am. 2018;29(1):129–37. doi: 10.1016/j.nec.2017.09.008. Epub 2017 Oct 27. [DOI] [PubMed] [Google Scholar]
- 31.Wilson JR, Fehlings MG, Kalsi-Ryan S, Shamji MF, Tetreault LA, Rhee JM, et al. Diagnosis, heritability and outcome assessment in cervical myelopathy: a consensus statement. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S76–7. doi: 10.1097/BRS.0b013e3182a7f4bf. [DOI] [PubMed] [Google Scholar]
- 32.Edwards CC, 2nd, Riew KD, Anderson PA, Hilibrand AS, Vaccaro AF. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003;3(1):68–81. doi: 10.1016/s1529-9430(02)00566-1. [DOI] [PubMed] [Google Scholar]
- 33.Karadimas SK, Erwin WM, Ely CG, Dettori JR, Fehlings MG. Pathophysiology and natural history of cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2013;38(22 Suppl 1):S21–36. doi: 10.1097/BRS.0b013e3182a7f2c3. [DOI] [PubMed] [Google Scholar]
- 34.Shprecher D, Schwalb J, Kurlan R. Normal pressure hydrocephalus: diagnosis and treatment. Curr Neurol Neurosci Rep. 2008;8(5):371–6. doi: 10.1007/s11910-008-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witt JC, Stevens JC. Neurologic disorders masquerading as carpal tunnel syndrome: 12 cases of failed carpal tunnel release. Mayo Clin Proc. 2000;75(4):409–13. doi: 10.4065/75.4.409. [DOI] [PubMed] [Google Scholar]
- 36.Fehlings MG, Wilson JR, Kopjar B, Yoon ST, Arnold PM, Massicotte EM, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95(18):1651–8. doi: 10.2106/JBJS.L.00589. [DOI] [PubMed] [Google Scholar]
- 37.Fehlings MG, Ibrahim A, Tetreault L, Albanese V, Alvarado M, Arnold P, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine (Phila Pa 1976) 2015;40(17):1322–8. doi: 10.1097/BRS.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 38.Moussellard HP, Meyer A, Biot D, Khiami F, Sariali E. Early neurological recovery course after surgical treatment of cervical spondylotic myelopathy: a prospective study with 2-year follow-up using three different functional assessment tests. Eur Spine J. 2014;23(7):1508–14. doi: 10.1007/s00586-014-3315-x. Epub 2014 Apr 29. [DOI] [PubMed] [Google Scholar]
- 39.Tetreault L, Kopjar B, Côté P, Arnold P, Fehlings MG. A clinical prediction rule for functional outcomes in patients undergoing surgery for degenerative cervical myelopathy: analysis of an international prospective multicenter data set of 757 subjects. J Bone Joint Surg Am. 2015;97(24):2038–46. doi: 10.2106/JBJS.O.00189. [DOI] [PubMed] [Google Scholar]