Abstract
Objective
To describe the burden of pneumococcal disease and associated risk factors in the Canadian adult population, delineate available pneumococcal vaccines and associated efficacy and effectiveness data, and review current pneumococcal vaccine recommendations and community-acquired pneumonia (CAP) prevention strategies in Canada.
Quality of evidence
Pneumococcal vaccination guidelines from the Canadian National Advisory Committee on Immunization in 2013 and 2016 constitute level III evidence for CAP prevention in the Canadian adult population.
Main message
It is recommended that immunosuppressed adults of all ages receive the 13-valent pneumococcal conjugate vaccine (PCV13) (grades A and B recommendations). In 2016, the National Advisory Committee on Immunization also recommended that all adults aged 65 years and older receive PCV13 (grade A recommendation) on an individual basis, followed by the 23-valent pneumococcal polysaccharide vaccine (grade B recommendation). This update is based on a large clinical study that demonstrated PCV13 efficacy against vaccine-type CAP in this population.
Conclusion
Physicians should focus on improving pneumococcal vaccination rates among adults, which remain low. Vaccination with PCV13 should also be considered for adults with chronic conditions, whose baseline risk is often higher than that for healthy individuals aged 65 years and older.
Résumé
Objectif
Décrire le fardeau des infections à pneumocoque et les facteurs de risque qui leur sont associés dans la population canadienne adulte, déterminer les vaccins disponibles contre le pneumocoque, cerner les données relatives à leur efficacité et à leur efficience réciproques, et examiner les recommandations actuelles sur le vaccin contre le pneumocoque, de même que les stratégies de prévention de la pneumonie d’origine communautaire (POC) au Canada.
Qualité des données
Les déclarations sur la vaccination contre le pneumocoque du Conseil consultatif national de l’immunisation du Canada en 2013 et en 2016 constituent une base de données probantes de niveau III pour la prévention de la POC dans la population adulte canadienne.
Message principal
Il est recommandé que les adultes en état d’immunodépression de tous les groupes d’âge reçoivent le vaccin conjugué 13-valent contre le pneumocoque (PCV13) (recommandations de grades A et B). En 2016, le Comité consultatif national de l’immunisation a aussi recommandé que tous les adultes de 65 ans et plus reçoivent le PCV13 (recommandation de grade A) sur une base individuelle, suivi du vaccin polysaccharidique 23-valent (recommandation de grade B). Cette mise à jour se fonde sur une étude clinique d’envergure qui a démontré l’efficacité dans cette population du PCV13 contre la POC due à un stéréotype contenu dans le vaccin.
Conclusion
Les médecins devraient aspirer à améliorer les taux de vaccination contre le pneumocoque chez les adultes, qui demeurent faibles. Le PCV13 devrait aussi être envisagé chez les adultes souffrant d’un problème chronique, dont le risque au départ est plus élevé que celui des personnes en santé de 65 ans et plus.
Case description
A 65-year-old woman visits your office to discuss possible new treatment options for her high blood pressure. As her family physician, you review her vaccination history and discover that she has received influenza, shingles, and tetanus, diphtheria, and acellular pertussis vaccinations over the past 3 years. What is missing?
Lower respiratory tract infections, including pneumonia, represented the fourth leading cause of death worldwide in 2016.1 Similarly, in Canada, chronic lower respiratory diseases were ranked fifth, while influenza and pneumonia were ranked sixth, as causes of death in 2017.2 Community-acquired pneumonia (CAP) in Canada is additionally associated with high hospitalization rates (50% to 77% for patients aged ≥ 60 years),3 high intensive care unit (ICU) admission rates (18%), and high mortality rates (11.4% within 30 days).4 It is important to note that patients with CAP are at increased risk of cardiovascular complications (eg, cardiovascular disease, heart failure)5–7—one study showed that within 30 days after hospitalization for pneumonia, patients had a more than 4-fold risk of developing cardiovascular complications compared with healthy controls7—and there is an association with increased mortality in the short term.8 Although no randomized clinical studies have reported effects of pneumococcal vaccination on cardiovascular disease, a meta-analysis of 8 observational studies found a significant reduction in acute coronary syndrome events among elderly patients who received pneumococcal polysaccharide vaccination (odds ratio [OR] of 0.83; 95% CI 0.71 to 0.97).9 However, it is important to note that such observational studies might be confounded by the “healthy user effect,” wherein patients seeking preventive therapy, such as vaccination, are also more likely to practise healthier habits in general.10
Streptococcus pneumoniae is the most frequently isolated bacterial pathogen in CAP11 and is a leading cause of CAP, meningitis, and bacteremia.12 It was the most common pathogen causing CAP in all sites of care (out-patients, hospitalized non-ICU patients, and hospitalized ICU patients) across multiple studies13 and was the most frequently identified pathogen among 3 of 4 sources of culture-positive specimens from Canadian CAP patients admitted to the ICU (2000 to 2002).14 Approximately 75% of pneumococcal pneumonia events are nonbacteremic,15 but noninvasive disease might become invasive (eg, pneumonia accompanied by bacteremia).12 In addition to invasiveness, which is more often associated with certain pneumococcal serotypes,16 antimicrobial resistance in S pneumoniae is an important problem in Canada that highlights the need for CAP prevention. Penicillin resistance among S pneumoniae isolates increased dramatically from 1988 to 200917 but decreased from 2011 (12%) to 2014 (9%).18 Serogroup 19 (19A, 19F), which is common across all age groups in Canada,19 particularly demonstrates a multidrug-resistant pattern.20,21
Community-acquired pneumonia has many established risk factors. For instance, patients 60 years of age or older have much higher incidence rates than other individuals22 and account for most cases and hospitalizations.23,24 Symptoms in older adults are often more subtle, potentially delaying diagnosis and treatment.25 Patients aged 65 or older hospitalized for CAP have a higher 1-year mortality rate than the general population or individuals hospitalized for other reasons.26 Frailty, a related CAP risk factor, is associated with higher 1-year mortality resulting from CAP27 and impaired immune responses to pneumococcal conjugate vaccines (PCVs).28 Behavioral factors (including cigarette smoking,29–31 second-hand smoke exposure in non-smokers aged older than 65 years,32 and high alcohol consumption32,33) and environmental factors (such as living in long-term care facilities34 and homelessness29) also increase CAP risk. Comorbidities (eg, heart disease, diabetes, chronic respiratory disease) also increase the risk of adult pneumococcal pneumonia.30,33 Finally, multiple underlying conditions30,31,35,36 or immunocom-promising conditions30 further increase risk. For example, compared with similarly aged healthy individuals, risk of CAP is approximately 3-fold higher in the at-risk (high-risk using terminology from the Canadian National Advisory Committee on Immunization [NACI]) population and 4- to 6-fold higher in immunosuppressed individuals (Table 1).30,37 Individuals with certain comorbidities, such as chronic lung disease, show baseline risk exceeding that of healthy individuals aged 65 years or older or some immunosuppressed individuals.
Table 1.
Rate ratios of all-cause pneumonia among high-risk and immunocompromised adults compared with healthy adults in the same age group: Baseline rates (per 100 000 person-years) are 363 for those aged 18–49 y, 651 for those aged 50–64 y, and 1874 for those aged ≥ 65 y.
| RISK GROUP | AGE GROUP, RATE RATIO (95% CI) | ||
|---|---|---|---|
|
| |||
| 18–49 Y | 50–64 Y | ≥ 65 Y | |
| At risk* | 3.2 (3.1–3.2) | 3.1 (3.1–3.1) | 3.0 (3.0–3.0) |
| • Alcoholism | 3.6 (3.5–3.8) | 5.0 (4.9–5.2) | 3.9 (3.8–4.1) |
| • Asthma | 3.8 (3.8–3.9) | 4.7 (4.6–4.7) | 4.6 (4.5–4.6) |
| • Chronic heart disease | 4.9 (4.9–5.0) | 4.3 (4.2–4.3) | 3.8 (3.8–3.8) |
| • Chronic liver disease† | 5.6 (5.4–5.9) | 5.6 (5.5–5.7) | 4.1 (4.0–4.3) |
| • Chronic lung disease | 8.6 (8.4–8.7) | 8.6 (8.5–8.7) | 6.6 (6.6–6.7) |
| • Chronic use of oral steroids | 2.4 (2.3–2.5) | 2.3 (2.2–2.4) | 2.0 (1.9–2.1) |
| • Diabetes | 3.1 (3.1–3.2) | 3.0 (3.0–3.0) | 2.8 (2.8–2.8) |
| • Neuromuscular or seizure disorders | 4.6 (4.5–4.8) | 4.8 (4.7–5.0) | 4.6 (4.5–4.7) |
| • Rheumatoid arthritis, Crohn disease, lupus | 4.1 (4.0–4.3) | 4.0 (3.9–4.0) | 3.5 (3.4–3.5) |
| • Smokers | 3.3 (3.2–3.3) | 4.0 (3.9–4.0) | 3.6 (3.5–3.6) |
| High risk† | 6.1 (6.0–6.2) | 5.5 (5.5–5.6) | 4.1 (4.0–4.1) |
| • Chronic renal failure | 11.1 (10.8–11.4) | 9.8 (9.6–10.0) | 6.3 (6.3–6.4) |
| • Cochlear implant | 3.9 (2.4–6.2) | 3.1 (2.1–4.5) | 2.4 (1.8–3.2) |
| • Congenital immunodeficiency | 11.9 (11.3–12.5) | 11.5 (11.1–11.9) | 7.9 (7.5–8.2) |
| • Diseases of white blood cells | 14.0 (14.1–14.6) | 12.0 (11.7–12.3) | 7.1 (6.9–7.3) |
| • Functional or anatomic asplenia | 18.2 (17.7–18.9) | 16.5 (16.1–16.9) | 8.5 (8.3–8.7) |
| • HIV | 5.7 (5.5–6.0) | 4.5 (4.4–4.7) | 3.4 (3.2–3.8) |
| • Immunosuppressive drugs or conditions | 6.0 (5.9–0.0) | 5.6 (5.5–5.6) | 3.9 (3.8–3.9) |
| Multiple at-risk conditions | |||
| • 1 chronic condition | 2.5 (2.5–2.5) | 2.2 (2.2–2.2) | 2.1 (2.0–2.1) |
| • 2 chronic conditions | 6.2 (6.1–6.3) | 4.9 (4.8–5.0) | 4.1 (4.1–4.2) |
| • ≥ 3 chronic conditions | 15.6 (15.3–16.0) | 11.9 (11.7–12.0) | 8.1 (8.1–8.2) |
Quality of evidence
Pneumococcal vaccination guidelines from NACI are based on level III evidence (ie, opinions or statements of expert authorities); these guidelines were updated in June 2016 regarding adults aged 65 years or older.38
Main message
Pneumococcal vaccines available in Canada.
Two pneumococcal vaccines are recommended for adults in Canada37: the 23-valent pneumococcal polysaccharide vaccine (PPSV23), which is $30.00 to $34.58 per dose,39–41 with antigens of serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F, 2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, and 33F42; and the 13-valent PCV (PCV13), which is $110.00 to $125.00 per dose,39–41,43 containing antigens of serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F conjugated to nontoxic diphtheria CRM197 protein.44 In Canada, 26% and 38% of cases of invasive pneumococcal disease (IPD) in 2014 were caused by PCV13 and PPSV23 serotypes, respectively.45 On average, between 7.0% and 14.8% of CAP cases in hospitalized patients in Canada were caused by PCV13 serotypes, depending on the number of diagnostic tests performed.46
Effectiveness of PPSV23.
Inconclusive evidence for PPSV23 vaccine efficacy against noninvasive (nonbacteremic) pneumococcal pneumonia in older adults has been acknowledged by regulatory and public health agencies, including the US Centers for Disease Control and Prevention47 and the European Medicines Agency.48 Table 2 summarizes findings from recent systematic reviews and meta-analyses, which are commonly regarded as providing the highest level of evidence, assessing PPSV23 effectiveness against IPD, pneumococcal CAP, and all-cause CAP.49–54
Table 2.
Summary of findings from recent meta-analyses regarding PPSV23 effectiveness against IPD, pneumococcal pneumonia, and all-cause pneumonia
| META-ANALYSIS | STUDIES INCLUDED, N | PATIENTS INCLUDED, N | INCIDENCE RATE IN THOSE VACCINATED WITH PPSV23, % | INCIDENCE RATE IN UNVACCINATED SUBJECTS, % | METRIC USED TO ESTIMATE EFFECTIVENESS | ESTIMATE (95% CI) | SIGNIFICANT STATISTICAL HETEROGENEITY |
|---|---|---|---|---|---|---|---|
| IPD | |||||||
| • Kraicer-Melamed et al, 2016, cohort studies49 | 8 | NA | NA | NA | Vaccine effectiveness* | 50% (21% to 69%) | Yes |
| • Kraicer-Melamed et al, 2016, case-control studies49 | 4 | NA | NA | NA | Vaccine effectiveness* | 54% (32% to 69%) | Yes |
| • Moberley et al, 201350 | 11 | 36 489 | 0.08 | 0.35 | Odds ratio | 0.26 (0.14 to 0.45) | No |
| Pneumococcal CAP | |||||||
| • Schiffner-Rohe et al, 201651 | 4 | 29 218 | 0.56 | 0.70 | Odds ratio | 0.72 (0.33 to 1.58) | Yes |
| • Diao et al, 201652 | 3 | 2293 | 2.90 | 5.03 | Relative risk | 0.54 (0.18 to 1.65) | Yes |
| • Moberley et al, 201350 | 10 | 35 483 | 0.08 | 0.35 | Odds ratio | 0.26 (0.15 to 0.46) | No |
| All-cause CAP | |||||||
| • Kraicer-Melamed et al, 2016, trials49,53 | 3 | NA | NA | NA | Vaccine efficacy* | −10% (−36% to 12%) | No |
| • Kraicer-Melamed et al, 2016, cohort studies49 | 9 | NA | NA | NA | Vaccine effectiveness* | 17% (−26% to 45%) | Yes |
| • Kraicer-Melamed et al, 2016, case-control studies49 | 7 | NA | NA | NA | Vaccine effectiveness* | 7% (−10% to 21%) | Yes |
| • Diao et al, 201652 | 7 | 156 010 | 0.54 | 0.61 | Relative risk | 0.87 (0.76 to 0.98) | No |
| • Moberley et al, 201350 | 16 | 47 734 | 4.32 | 6.17 | Odds ratio | 0.72 (0.56 to 0.93) | Yes |
CAP—community-acquired pneumonia, IPD—invasive pneumococcal disease, NA—not available, PPSV23—23-valent pneumococcal polysaccharide vaccine.
Vaccine effectiveness or efficacy is defined as a reduction in relative risk in the vaccinated population compared with the unvaccinated population.54
Three meta-analyses included in 2 different studies all found statistically significant effectiveness of PPSV23 against IPD.49,50 However, 2 of these were characterized by significant statistical heterogeneity, which indicates greater variation in study outcomes than can be attributed to chance alone, likely as a result of differences in the study parameters (eg, participant age, comorbidities, etc).55 For this reason, pooled results should be interpreted cautiously.
For pneumococcal CAP, 2 studies with significant statistical heterogeneity found that PPSV23 had no proven effectiveness for this outcome.51,52 Another study without heterogeneity concerns demonstrated significant effectiveness of PPSV23 against pneumococcal CAP (OR = 0.26; 95% CI 0.15 to 0.46), which was notably similar to findings for IPD (OR = 0.26; 95% CI 0.14 to 0.45), likely reflecting substantial overlap between the 2 analyses.50
Finally, there were 5 meta-analyses that included 3 studies evaluating PPSV23 effectiveness against all-cause CAP.49,50,52,53 Of these 5 meta-analyses, 3 (2 with significant statistical heterogeneity) found no effectiveness.49,53 The most recent Cochrane meta-analysis found that PPSV23 significantly reduced all-cause CAP (OR = 0.72; 95% CI 0.56 to 0.93), with pneumonia occurring in 4.32% of the vaccinated group compared with 6.17% of the placebo group.50 However, heterogeneity of the included studies was very high (85%), indicating that the pooled results are unreliable. Finally, a fifth study found a modest reduction in all-cause pneumonia (relative risk of 0.87; 95% CI 0.76 to 0.98), with those aged 65 years and older and those aged younger than 65 years who were at high risk showing particular benefit (relative risk of 0.72; 95% CI 0.69 to 0.94).52 Taken together, the results of these meta-analyses indicate that although PPSV23 is effective for IPD, there is inconclusive evidence of its effectiveness against pneumococcal or all-cause CAP.
Effectiveness of PCV13.
Pneumococcal conjugate vaccines have demonstrated effectiveness since their introduction in 2001.56 Specifically in Canada, hospitalization for all-cause and pneumococcal pneumonia has significantly declined in adults aged 65 years of age and older (P < .001), likely at least partially because of herd effects from the pediatric pneumococcal immunization program (which achieved 79.2% national coverage with 3 doses in 201357).22 More recently, the Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA), which randomized 84 496 vaccine-naïve subjects aged 65 years and older in the Netherlands to receive PCV13 or placebo, demonstrated PCV13 efficacy in preventing first episodes of pneumococcal CAP as well as vaccine-type pneumococcal CAP.58 In the modified intent-to-treat analysis, vaccine efficacy for PCV13 against first episodes of pneumococcal CAP was 22.4% (95% CI 2.3% to 38.5%), with incidence rates of 0.32% for PCV13 and 0.41% for placebo over a mean follow-up duration of approximately 4 years. Efficacy against first episodes of all-cause CAP (5.1%; 95% CI −5.1% to 14.2%; respective incidence rates of 1.77% and 1.86%) was not significant.58
The overall adverse event profile was consistent with findings of previous adult studies.58 Serious adverse events (the primary safety end point) occurred with similar frequencies across groups within both 1 month (all participants) and 6 months (safety subgroup) of vaccination; the frequencies of newly diagnosed chronic medical conditions (safety subgroup) and deaths (all participants) were also comparable across groups. The higher frequency of adverse events within 1 month of vaccination in the PCV13 group compared with the placebo group (18.7% vs 14.3% [safety subgroup]) was attributable to injection-site reactions and muscular pain.
The protective efficacy of PCV13 extended throughout the 4-year study. A recent report estimated that, based on data from this study, the number needed to vaccinate to prevent 1 case of all-cause CAP in those aged 65 years and older over 5 years was 234; it is important to note that this estimate used vaccine efficacy estimates for vaccine-type CAP in the per-protocol population (45.0%; 95.2% CI 14.2% to 65.3%) and the proportion of CAP caused by PCV13 serotypes as input parameters.58,59 Additionally, results from a post hoc analysis of CAPiTA data found that using additional screening to detect missed end points greatly reduced the calculated number need to vaccinate against vaccine-type CAP (from 1007 to 634)60; this overestimation likely relates to all study outcomes. Based on results from the CAPiTA trial, in July 2015, Health Canada approved PCV13 for active immunization of adults aged 18 years and older for preventing pneumonia and IPD caused by the PCV13 serotypes.61,62
Serotype replacement.
Widespread implementation of PCVs in pediatric vaccination programs, including in Canada, has led to increases in the prevalence of nonvaccine serotypes.19,63–65 In Canada, the proportion of IPD caused by PCV13 serotypes declined from 55% in 2010 (the year PCV13 was introduced) to 43% in 2012, but the proportion of nonvaccine types concomitantly increased from 20% to 25%.19 This phenomenon, termed serotype replacement, underscores the importance of monitoring changes in all-cause CAP and all-serotype IPD in both observational and interventional studies, as it is possible for vaccine-type CAP or IPD to decrease while morbidity and mortality remain unchanged owing to increasing prevalence of other serotypes or pathogens. For PCV13 in particular, it is important to note that serotype distributions following PCV13 introduction have been associated with statistically significant decreases in both invasiveness and antimicrobial resistance.63,66
Pneumococcal vaccination recommendations for select groups.
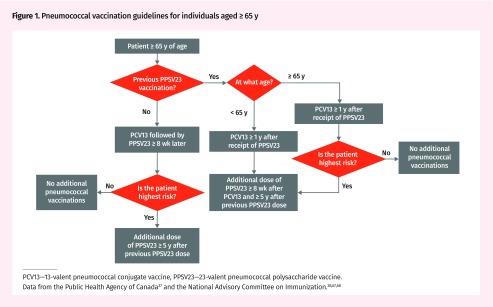
Pneumococcal vaccination guidelines in Canada have evolved over time. In 2013, NACI recommended PCV13 followed by PPSV23 8 or more weeks later for immunocompromised individuals.67 In 2016, NACI recommended PCV13 followed by PPSV23 for preventing CAP and IPD caused by PCV13 serotypes “on an individual basis” in immunocompetent pneumococcal vaccine-naïve individuals aged 65 or older (NACI recommendation grade A).38 Pneumococcal vaccination guidelines for individuals aged 65 years and older are illustrated in Figure 1.37,38,67,68 Immunocompetent individuals younger than 65 years of age with underlying medical conditions are generally recommended to receive PPSV23 alone.37 All individuals who received PPSV23 before age 65 should be revaccinated at age 65 years or older68; revaccination is also recommended for those of all ages at highest risk.37 Table 3 summarizes current NACI recommendations for pneumococcal vaccination by age and risk group.37,38,67,68
Figure 1.
Pneumococcal vaccination guidelines for individuals aged ≥65 y
PCV13—13-valent pneumococcal conjugate vaccine, PPSV23—23-valent pneumococcal polysaccharide vaccine.
Data from the Public Health Agency of Canada37 and the National Advisory Committee on Immunization.38,67,68
Table 3.
Pneumococcal vaccination recommendations by age group in Canada: Arrows indicate sequential administration of PCV13 followed by PPSV23 8 wk or more later. If PPSV23 has already been administered, wait 1 y before administering PCV13.
| AGE GROUP | INDICATED* | NACI RECOMMENDATIONS | ||
|---|---|---|---|---|
|
| ||||
| HEALTHY INDIVIDUALS | HIGH-RISK† INDIVIDUALS | IMMUNOCOMPROMISED‡§ INDIVIDUALS | ||
| 6 wk to < 12 mo | PCV13 | 3 or 4 doses of PCV13ǁ¶#** | 4 doses of PCV13¶ | 4 doses of PCV13¶ |
| 12 to < 24 mo | PCV13 | 2 doses of PCV13†† | 2 doses of PCV13†† | 2 doses of PCV13†† |
| 2 to < 5 y | PCV13, PPSV23 | PCV13# | PCV13# → PPSV23‡‡ | PCV13# → PPSV23‡‡ |
| 5 to 17 y | PCV13, PPSV23 | NA | PCV13# → PPSV23‡‡ | PCV13# → PPSV23‡‡ |
| 18 to 64 y | PCV13, PPSV23 | NA | PPSV23‡‡ | PCV13 → PPSV23‡‡ |
| ≥ 65 y | PCV13, PPSV23 | PCV13§§ → PPSV23 | PCV13 → PPSV23‡‡ǁǁ | PCV13 → PPSV23‡‡ǁǁ |
HSCT—hematopoietic stem cell transplant, NA—not applicable, NACI—National Advisory Committee on Immunization, PCV13—13-valent pneumococcal conjugate vaccine, PPSV23—23-valent pneumococcal polysaccharide vaccine.
Age indication licensed by Health Canada.
High risk includes chronic conditions of cerebral spinal fluid leak, neurologic conditions that impair clearance of oral secretions, heart disease, lung disease including asthma, diabetes, kidney disease, and liver disease; cochlear implants; alcoholism; smoking; homelessness; and residing in long-term care.
Includes sickle cell disease, asplenia, congenital immunodeficiency, immunocompromising therapy, HIV infection, HSCT, malignant neoplasms, nephrotic syndrome, and organ transplant.
Recipients of HSCT of all ages should receive 3 doses of PCV13 ≥ 4 wk apart 3 to 9 mo after HSCT. The PPSV23 should be administered 6 to 12 mo after the last PCV13 dose or when the HSCT recipient reaches 2 y of age, with a second dose of PPSV23 given 1 y later.
For the 3-dose schedule, doses should be given at 2, 4, and 12 mo of age.
For the 4-dose schedule, doses should be given at 2, 4, 6, and 12 to 15 mo of age.
Children who have not already received PCV13.
Dosing schedule from 2 to < 7 mo of age depends on the province.
Children who have received ≤ 1 dose of PCV13 at < 12 mo of age.
A second dose of PPSV23 after 5 y is indicated for those who are at highest risk, which includes individuals who are immunocompromised‡ or have either chronic liver disease including hepatic cirrhosis or chronic kidney disease including nephrotic syndrome.
The PCV13 should be considered on an individual basis for pneumococcal vaccine–naïve individuals.
Improving vaccination rates in adults.
Vaccination with PPSV23 in Canada is cost-effective,69 and implementing a schedule of PCV13 followed by PPSV23 for all currently recommended adult groups is predicted to be cost-effective.70 Currently, most Canadian provinces at least partially cover PCV13 for the highest-risk patients,71–83 and PPSV23 is covered for all indicated groups.84 However, pneumococcal vaccination rates are low, with individuals aged 65 years and older and 18 to 64 years with chronic conditions (other than asthma) having respective estimated PPSV23 vaccination rates of only 37% and 17% in 2014, respectively.85 A series of studies between 2010 and 2013 identified barriers to vaccination in both patients and practitioners and made appropriate recommendations for diminishing the effects of these barriers (Box 1).86–91
Box 1. Barriers to vaccination and recommendations for diminishing their effects.
Barriers to vaccination
Lack of awareness of invasive pneumococcal disease or pneumococcal disease86
Infrequent well-visit vaccination opportunities89
Lack of strong endorsement or recommendation by health care providers87
Views that vaccines without public funding are less important (personal observation)
Concerns about safety of newer vaccines (personal observation)
Beliefs that vaccine refusal is the healthiest choice (personal observation)
Recommendations
Vaccination campaigns88
Use of physician extenders (eg, nurse-led vaccination programs)90
Patient outreach including reminder cards tied to nonmedical events (eg, 65th birthday)87,90
Administering pneumococcal vaccines during the annual influenza vaccine visit87,91
Administering recommended vaccines at sick visits in addition to well visits87,89
Case resolution
Based on current evidence, our recommendation for your patient is that she should be offered PCV13 followed by PPSV23 at least 8 weeks later to maximize potential protection against both CAP and IPD.
Conclusion
Recent NACI recommendations advise using PCV13 followed by PPSV23 8 or more weeks later for all immunocompromised adults and immunocompetent adults aged 65 years and older. Revaccination with PPSV23 is recommended after 5 years for all adults at highest risk.38 Efforts should be intensified by family physicians and other health care providers to increase coverage in these groups.
The most recent PCV13 recommendations do not target certain adults at elevated CAP and IPD risk, such as those with chronic illnesses, smokers, and the homeless. It is currently recommended that high-risk individuals receive PPSV23, the cost of which remains covered across all indicated groups,84 despite inconclusive evidence of PPSV23 effectiveness against CAP. There is demonstrated efficacy of PPSV23 against IPD50; thus, PPSV23 use should continue for IPD prevention because it targets more serotypes than PCV13,38 especially given ongoing serotype replacement induced by widespread PCV13 vaccination.19,63–65 However, high-risk individuals often have a higher risk of pneumonia than healthy individuals aged 65 and older (Table 1)30,37 and have CAP episodes that are more costly to the health care system in both direct and, potentially, indirect medical costs.92,93 We therefore recommend consideration of PCV13 vaccination for high-risk individuals based on superior PCV immunogenicity compared with PPSV23 in these populations.94,95 Individuals with chronic lung disease have the highest risk of all-cause and pneumococcal pneumonia among those with chronic at-risk conditions (Table 1)30,37; vaccination is thus especially critical in this group. We also stress the importance of identifying patients with increased CAP risk based on the presence of specific comorbidities, lifestyle, and environmental factors. In general, prevention of CAP also decreases the need for antibiotic use and in turn slows the progression of antibiotic resistance.
The most recent NACI recommendations outline directions for future research, which include assessing risks of concomitant PCV13 and PPSV23 administration, determining PCV13 booster efficacy and effectiveness in the immunocompetent population aged 65 years of age and older, and conducting nationwide CAP and IPD surveillance by age and serotype.38
Acknowledgments
Editorial support for development of this manuscript was provided by Dr Judith Kandel at Complete Healthcare Communications, LLC, in West Chester, Pa, and was funded by Pfizer.
Editor’s key points
▸ In 2016, the Canadian National Advisory Committee on Immunization updated guidelines to recommend the 13-valent pneumococcal conjugate vaccine (PCV13) for adults 65 years of age and older, based on efficacy data against community-acquired pneumonia from a large clinical study. These new guidelines suggest that vaccine-naïve adults aged 65 and older should receive PCV13 on an individual basis, followed by the 23-valent pneumococcal polysaccharide vaccine, which offers broader serotype coverage. Additionally, PCV13 continues to be recommended for all adults aged 18 years or older with immunocompromising conditions.
▸ While not recommended in the current guidelines, the authors suggest that PCV13 and the 23-valent vaccine should also be considered in adults younger than 65 who are at increased risk of pneumococcal disease owing to medical, lifestyle, or environmental factors, as the risk of community-acquired pneumonia in this population might exceed that in the healthy elderly population.
▸ While the effectiveness data are mixed, pneumococcal vaccination rates among Canadian adults remain low. Physicians should engage in shared decision making with patients on an individual basis.
Points de repère du rédacteur
▸ En 2016, le Comité consultatif national de l’immunisation du Canada a mis à jour sa déclaration pour recommander le vaccin conjugué antipneumococcique 13-valent (PCV13) chez les adultes de 65 ans et plus, en se fondant sur des données étayant son efficacité contre la pneumonie d’origine communautaire, tirées d’une étude clinique d’envergure. Cette nouvelle déclaration préconise que les adultes de 65 ans et plus non vaccinés reçoivent le PCV13 sur une base individuelle, qui sera suivi du vaccin polysaccharidique 23-valent contre le pneumocoque, ce dernier offrant une protection plus large contre d’autres stéréotypes. De plus, le PCV13 continue d’être recommandé pour tous les adultes de 18 ans et plus qui sont immunodéprimés.
▸ Même si les lignes directrices actuelles n’en font pas la recommandation, les auteurs suggèrent d’envisager le PCV13 et le vaccin 23-valent chez les adultes de moins de 65 ans qui sont à risque accru d’infections à pneumocoque en raison de facteurs médicaux, environnementaux ou liés au mode de vie, puisque le risque d’une pneumonie d’origine communautaire au sein de cette population pourrait être supérieur à celui chez les personnes plus âgées en santé.
▸ Si les données sur l’efficacité sont mitigées, les taux de vaccination contre le pneumocoque au Canada restent faibles. Les médecins devraient s’engager dans une prise de décision partagée avec leurs patients sur une base individuelle.
Footnotes
Contributors
All authors contributed to the literature review and interpretation, and to preparing the manuscript for submission.
Competing interests
This review was sponsored by Pfizer. Dr Kaplan reports support from AstraZeneca, Becton Dickinson, Boehringer Ingelheim, CanniMed, GlaxoSmithKline, Grifols, Meda, Merck Frosst, Pfizer, Purdue, Johnson & Johnson, Novartis, Novo Nordisk, Trudell, Sanofi, and Teva. Dr Arsenault has served on advisory boards for Amgen, Lundbeck, Novo Nordisk, and Purdue, and has served as a speaker in meetings sponsored by Purdue, Astra, and Merck. Dr Aw reports continuing medical education (CME) sponsorships from Merck, Sanofi, and Pfizer. Dr Brown reports participation on advisory boards for Pfizer, Merck, GlaxoSmithKline, and Amgen, and on speakers bureaus for Pfizer, Merck, Amgen, and Valneva. Dr Fox reports receiving honoraria or hospitality from Actelion, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Roche. Dr Grossman reports support from AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis, Merck, GlaxoSmithKline, and Bayer Schering Pharma.Dr Jadavji reports receiving honoraria from Pfizer, Novartis, and Merck. Dr Laferrière was an employee of Pfizer Canada Inc. Dr Levitz has received support from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Pfizer, and Novartis. Dr Loeb reports support from Sanofi, Seqirus, and Pfizer. Dr McIvor has served on advisory boards for and received honoraria for CME from AstraZeneca, Boehringer Ingelheim, Merck, Novartis, Pfizer, and Trudell Medical, and is an employee of McMaster University and St Joseph’s Healthcare. Dr Mody has received speakers honoraria from Pfizer and was a site principal investigator for a clinical trial sponsored by Aradigm for which he received no personal compensation. Dr Poulin has served as a speaker for Pfizer. Dr Shapiro has served on advisory boards for Pfizer, Merck, GlaxoSmithKline, and Amgen, and has served on speakers bureaus for Merck, Pfizer, Amgen, and Novo Nordisk. Dr Tessier has served as a speaker for and received honoraria from Pfizer, Merck, Sanofi Pasteur, and Valneva. Dr Théorêt has received honoraria from Pfizer, Novartis, Knight, Merck, AstraZeneca, and Boehringer Ingelheim. Dr Weiss has received research funds and served on advisory boards for Pfizer. Dr Yaremko has received CME sponsorship from Merck, Pfizer, Sanofi, and Shire. Dr Zhanel has received research funding from Achaogen, Astellas, Basilea, Merck, Paratek, Pfizer, Sunovion, The Medicines Company, Tetraphase, and Zoetis.
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.World Health Organization. The top 10 causes of death worldwide. Geneva, Switz: World Health Organization; 2018. Available from: www.who.int/mediacentre/factsheets/fs310/en/#. Accessed 2018 Oct 14. [Google Scholar]
- 2.Statistics Canada. Leading causes of death, total population, by age group. All ages, both sexes. Ottawa, ON: Statistics Canada; 2018. Available from: www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/hlth36a-eng.htm. Accessed 2018 May 29. [Google Scholar]
- 3.Marrie TJ, Huang JQ. Epidemiology of community-acquired pneumonia in Edmonton, Alberta: an emergency department-based study. Can Respir J. 2005;12(3):139–42. doi: 10.1155/2005/672501. [DOI] [PubMed] [Google Scholar]
- 4.McNeil S, Andrews M, Ye L, Elsherif M, MacKinnon-Cameron D, Ambrose A, et al. Active surveillance for community acquired pneumonia (CAP) amongst hospitalized Canadian adults, 2011–2013: a Public Health Agency of Canada/Canadian Institutes of Health Research (PCIRN) Serious Outcomes Surveillance (SOS) Network study. Poster 47.; Poster presented at: Canadian Immunization Conference; 2014 Dec 2–4; Ottawa, ON. [Google Scholar]
- 5.Bergh C, Fall K, Udumyan R, Sjöqvist H, Fröbert O, Montgomery S. Severe infections and subsequent delayed cardiovascular disease. Eur J Prev Cardiol. 2017;24(18):1958–66. doi: 10.1177/2047487317724009. Epub 2017 Aug 1. [DOI] [PubMed] [Google Scholar]
- 6.Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Risk of heart failure after community acquired pneumonia: prospective controlled study with 10 years of follow-up. BMJ. 2017;356 doi: 10.1136/bmj.j413. j413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264–74. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation. 2012;125(6):773–81. doi: 10.1161/CIRCULATIONAHA.111.040766. Epub 2012 Jan 4. [DOI] [PubMed] [Google Scholar]
- 9.Ren S, Newby D, Li SC, Walkom E, Miller P, Hure A, et al. Effect of the adult pneumococcal polysaccharide vaccine on cardiovascular disease: a systematic review and meta-analysis. Open Heart. 2015;2(1):e000247. doi: 10.1136/openhrt-2015-000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26(5):546–50. doi: 10.1007/s11606-010-1609-1. Epub 2011 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–27. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Pneumococcal disease. In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington, DC: Public Health Foundation; 2015. pp. 279–96. [Google Scholar]
- 13.File TM. Community-acquired pneumonia. Lancet. 2003;362(9400):1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrie TJ, Shariatzadeh MR. Community-acquired pneumonia requiring admission to an intensive care unit: a descriptive study. Medicine (Baltimore) 2007;86(2):103–11. doi: 10.1097/MD.0b013e3180421c16. [DOI] [PubMed] [Google Scholar]
- 15.Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O’Brien KL, AGEDD Adult Pneumococcal Burden Study Team Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8(4):e60273. doi: 10.1371/journal.pone.0060273. Epub 2013 Apr 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190(7):1203–11. doi: 10.1086/423820. Epub 2004 Aug 25. [DOI] [PubMed] [Google Scholar]
- 17.Canadian Bacterial Surveillance Network. Streptococcus pneumoniae resistance data—2009. Toronto, ON: Mount Sinai Hospital; 2010. Available from: https://eportal.mountsinai.ca/Microbiology//data/sp/sp_2009.shtml#figure1. Accessed 2017 Nov 14. [Google Scholar]
- 18.Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System—report 2016. Ottawa, ON: Public Health Agency of Canada; 2016. Available from: www.canada.ca/content/dam/phac-aspc/documents/services/publications/drugs-health-products/antibiotic-resistance-antibiotique/antibiotic-resistance-antibiotique-2016-eng.pdf. Accessed 2017 Mar 2. [Google Scholar]
- 19.Demczuk WH, Martin I, Griffith A, Lefebvre B, McGeer A, Lovgren M, et al. Serotype distribution of invasive Streptococcus pneumoniae in Canada after the introduction of the 13-valent pneumococcal conjugate vaccine, 2010–2012. Can J Microbiol. 2013;59(12):778–88. doi: 10.1139/cjm-2013-0614. Epub 2013 Oct 21. [DOI] [PubMed] [Google Scholar]
- 20.Adam HJ, Gilmour M, Baxter M, Martin I, Scharikow L, Nichol KA, et al. Multi-drug resistance in the most prevalent invasive Streptococcus pneumoniae (SPN) serotypes (STs) post PCV-13 introduction in Canada.; Paper presented at: 52nd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2012 Sep 9–12; San Francisco, CA.. [Google Scholar]
- 21.Golden AR, Adam HJ, Baxter M, Nichol KA, Demczuk W, Gilmour M, et al. Antimicrobial resistance in Streptococcus pneumoniae isolated in Canada: comparison of blood and respiratory isolates, CANWARD 2007–2014.; Paper presented at: 55th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2015 Sep 18–21; San Diego, CA. [Google Scholar]
- 22.McNeil SA, Qizilbash N, Ye J, Gray S, Zanotti G, Munson S, et al. A retrospective study of the clinical burden of hospitalized all-cause and pneumococcal pneumonia in Canada. Can Respir J. 2016;2016:3605834. doi: 10.1155/2016/3605834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ewig S, Birkner N, Strauss R, Schaefer E, Pauletzki J, Bischoff H, et al. New perspectives on community-acquired pneumonia in 388 406 patients. Results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax. 2009;64(12):1062–9. doi: 10.1136/thx.2008.109785. Epub 2009 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnstone J, Eurich DT, Minhas JK, Marrie TJ, Majumdar SR. Impact of the pneumococcal vaccine on long-term morbidity and mortality of adults at high risk for pneumonia. Clin Infect Dis. 2010;51(1):15–22. doi: 10.1086/653114. [DOI] [PubMed] [Google Scholar]
- 25.Fung HB, Monteagudo-Chu MO. Community-acquired pneumonia in the elderly. Am J Geriatr Pharmacother. 2010;8(1):47–62. doi: 10.1016/j.amjopharm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan V, Clermont G, Griffin MF, Kasal J, Watson RS, Linde-Zwirble WT, et al. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163(3):317–23. doi: 10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 27.Ma HM, Yu RH, Woo J. Recurrent hospitalisation with pneumonia is associated with higher 1-year mortality in frail older people. Intern Med J. 2013;43(11):1210–5. doi: 10.1111/imj.12258. [DOI] [PubMed] [Google Scholar]
- 28.Ridda I, Macintyre CR, Lindley R, Gao Z, Sullivan JS, Yuan FF, et al. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009;27(10):1628–36. doi: 10.1016/j.vaccine.2008.11.098. Epub 2008 Dec 17. Erratum in: Vaccine 2009;27(47):6649. [DOI] [PubMed] [Google Scholar]
- 29.Plevneshi A, Svoboda T, Armstrong I, Tyrrell GJ, Miranda A, Green K, et al. Population-based surveillance for invasive pneumococcal disease in homeless adults in Toronto. PLoS One. 2009;4(9):e7255. doi: 10.1371/journal.pone.0007255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shea KM, Edelsberg J, Weycker D, Farkouh RA, Strutton DR, Pelton SI. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014;1(1):ofu024. doi: 10.1093/ofid/ofu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelton SI, Shea KM, Farkouh RA, Strutton DR, Braun S, Jacob C, et al. Rates of pneumonia among children and adults with chronic medical conditions in Germany. BMC Infect Dis. 2015;15:470. doi: 10.1186/s12879-015-1162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almirall J, Bolíbar I, Serra-Prat M, Roig J, Hospital I, Carandell E, et al. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J. 2008;31(6):1274–84. doi: 10.1183/09031936.00095807. Epub 2008 Jan 23. [DOI] [PubMed] [Google Scholar]
- 33.Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68(11):1057–65. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nace DA, Archbald-Pannone LR, Ashraf MS, Drinka PJ, Frentzel E, Gaur S, et al. Pneumococcal vaccination guidance for post-acute and long-term care settings: recommendations from AMDA’s Infection Advisory Committee. J Am Med Dir Assoc. 2017;18(2):99–104. doi: 10.1016/j.jamda.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelton SI, Weycker D, Farkouh RA, Strutton DR, Shea KM, Edelsberg J. Risk of pneumococcal disease in children with chronic medical conditions in the era of pneumococcal conjugate vaccine. Clin Infect Dis. 2014;59(5):615–23. doi: 10.1093/cid/ciu348. Epub 2014 May 13. [DOI] [PubMed] [Google Scholar]
- 36.Curcio D, Cané A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis. 2015;37:30–5. doi: 10.1016/j.ijid.2015.05.003. Epub 2015 May 19. [DOI] [PubMed] [Google Scholar]
- 37.Public Health Agency of Canada. Canadian immunization guide. Part 4—active vaccines. Pneumococcal vaccine. Ottawa, ON: Public Health Agency Canada; 2016. Available from: www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines.html. Accessed 2017 Nov 14. [Google Scholar]
- 38.National Advisory Committee on Immunization (NACI). Update on the use of 13-valent pneumococcal conjugate vaccine (PNEU-C-13) in addition to 23-valent pneumococcal polysaccharide vaccine (PNEU-P-23) in immunocompetent adults 65 years of age and older—interim recommendation. Ottawa, ON: Public Health Agency of Canada; 2016. Available from: www.healthycanadians.gc.ca/publications/healthy-living-vie-saine/update-pneu-c-13-andpneu-p-23-mise-a-jour-2016/index-eng.php. Accessed 2017 Feb 28. [Google Scholar]
- 39.Daw J. Canadians! Making travel plans? Plan your travel vaccinations too. Toronto, ON: Intrepid 24/7.; Available from: www.intrepid247.com/tips-resources/plan-your-travel-vaccinations. Accessed 2018 May 29. [Google Scholar]
- 40.Vancouver Coastal Health Travel Clinic [website]. Vaccines. Vancouver, BC: Vancouver Coastal Health; 2018. Available from: http://travelclinic.vch.ca/services/vaccines. Accessed 2018 Jun 1. [Google Scholar]
- 41.Prepared Traveller Travel Clinic [website]. Vaccine price list in alphabetical order of disease state. Calgary, AB: Prepared Traveller Travel Clinic; 2016. Available from: www.preparedtraveller.ca/travel-clinic. Accessed 2018 Jun 1. [Google Scholar]
- 42.Merck & Co. Pneumovax 23® (pneumococcal vaccine polyvalent). Full prescribing information. Whitehouse Station, NJ: Merck & Co, Inc; 2015. [Google Scholar]
- 43.Middlesex-London Ontario Health Unit [website]. Paid vaccine price list. London, ON: Middlesex-London Ontario Health Unit; 2017. Available from: www.healthunit.com/uploads/vpd-paid-vaccine-price-list.pdf. Accessed 2018 Jun 1. [Google Scholar]
- 44.Pfizer, Inc. Prevnar 13® (pneumococcal 13-valent conjugate vaccine [diphtheria CRM protein]). Full prescribing information. Collegeville, PA: Pfizer, Inc; 2016. [Google Scholar]
- 45.Streptococcus and STI Unit, Bacteriology and Enteric Diseases Program, National Microbiology Laboratory, Public Health Agency of Canada. National laboratory surveillance of invasive streptococcal disease in Canada. Annual summary 2013. Available from: www.healthycanadians.gc.ca/publications/drugs-products-medicaments-produits/2013-streptococcus/index-eng.php. Accessed 2017 Nov 14.
- 46.LeBlanc JJ, ElSherif M, Ye L, MacKinnon-Cameron D, Li L, Ambrose A, et al. Burden of vaccine-preventable pneumococcal disease in hospitalized adults: a Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) network study. Vaccine. 2017;35(29):3647–54. doi: 10.1016/j.vaccine.2017.05.049. Epub 2017 May 26. [DOI] [PubMed] [Google Scholar]
- 47.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822–5. [PMC free article] [PubMed] [Google Scholar]
- 48.European Medicines Agency. Assessment report. Prevenar 13. Pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed). London, UK: European Medicines Agency; 2011. [Google Scholar]
- 49.Kraicer-Melamed H, O’Donnell S, Quach C. The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: a systematic review and meta-analysis. Vaccine. 2016;34(13):1540–50. doi: 10.1016/j.vaccine.2016.02.024. Epub 2016 Feb 17. Erratum in: Vaccine 2016;34(34):4083–4. [DOI] [PubMed] [Google Scholar]
- 50.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;(1):CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiffner-Rohe J, Witt A, Hemmerling J, von Eiff C, Leverkus FW. Efficacy of PPV23 in preventing pneumococcal pneumonia in adults at increased risk—a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146338. doi: 10.1371/journal.pone.0146338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diao WQ, Shen N, Yu PX, Liu BB, He B. Efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing community-acquired pneumonia among immunocompetent adults: a systematic review and meta-analysis of randomized trials. Vaccine. 2016;34(13):1496–503. doi: 10.1016/j.vaccine.2016.02.023. Epub 2016 Feb 17. [DOI] [PubMed] [Google Scholar]
- 53.Kraicer-Melamed H, O’Donnell S, Quach C. Corrigendum to “The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: a systematic review and meta-analysis” [Vaccine 34 (2016) 1540–1550]. Vaccine. 2016;34(34):4083–4. doi: 10.1016/j.vaccine.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. Principles of epidemiology in public health practice. An introduction to applied epidemiology and biostatistics. 3rd ed. Atlanta, GA: Centers for Disease Control and Prevention; 2012. Available from: www.cdc.gov/csels/dsepd/ss1978/SS1978.pdf. Accessed 2019 Aug 12. [Google Scholar]
- 55.Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–9. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 56.Pillai DR, Shahinas D, Buzina A, Pollock RA, Lau R, Khairnar K, et al. Genome-wide dissection of globally emergent multi-drug resistant serotype 19A Streptococcus pneumoniae. BMC Genomics. 2009;10:642. doi: 10.1186/1471-2164-10-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Public Health Agency Canada. Vaccine coverage in Canadian children: results from the 2013 Childhood National Immunization Coverage Survey (CNICS). Revised edition. Ottawa, ON: Public Health Agency Canada; 2017. Available from: http://publications.gc.ca/collections/collection_2017/aspc-phac/HP40-156-2017-eng.pdf. Accessed 2018 May 29. [Google Scholar]
- 58.Bonten MJM, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 59.McLaughlin JM, Swerdlow DL, Isturiz RE, Jodar L. Rethinking number-needed-to-vaccinate for pneumococcal conjugate vaccines in older adults: current and future implications. Vaccine. 2017;35(40):5360–5. doi: 10.1016/j.vaccine.2017.08.028. Epub 2017 Aug 31. [DOI] [PubMed] [Google Scholar]
- 60.Van Werkhoven CH, Huijts SM, Paling FP, Bonten MJ. The scrutiny of identifying community-acquired pneumonia episodes quantified bias in absolute effect estimation in a population-based pneumococcal vaccination trial. J Clin Epidemiol. 2016;69:185–92. doi: 10.1016/j.jclinepi.2015.07.004. Epub 2015 Jul 18. [DOI] [PubMed] [Google Scholar]
- 61.Health Canada. Regulatory decision summary for PREVNAR 13. Ottawa, ON: Health Canada; 2015. Available from: https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00040. Accessed 2018 Oct 24. [Google Scholar]
- 62.Pfizer Canada, Inc. Product monograph: Prevnar 13. Kirkland, QC: Pfizer Canada, Inc; 2015. [Google Scholar]
- 63.Desai AP, Sharma D, Crispell EK, Baughman W, Thomas S, Tunali A, et al. Decline in pneumococcal nasopharyngeal carriage of vaccine serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in children in Atlanta, Georgia. Pediatr Infect Dis J. 2015;34(11):1168–74. doi: 10.1097/INF.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 64.Gladstone RA, Jefferies JM, Tocheva AS, Beard KR, Garley D, Chong WW, et al. Five winters of pneumococcal serotype replacement in UK carriage following PCV introduction. Vaccine. 2015;33(17):2015–21. doi: 10.1016/j.vaccine.2015.03.012. Epub 2015 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535–43. doi: 10.1016/S1473-3099(15)70044-7. Epub 2015 Mar 20. Erratum in: Lancet Infect Dis 2015;15(6):629. Epub 2015 May 17. [DOI] [PubMed] [Google Scholar]
- 66.Varon E, Cohen R, Béchet S, Doit C, Levy C. Invasive disease potential of pneumococci before and after the 13-valent pneumococcal conjugate vaccine implementation in children. Vaccine. 2015;33(46):6178–85. doi: 10.1016/j.vaccine.2015.10.015. Epub 2015 Oct 21. [DOI] [PubMed] [Google Scholar]
- 67.National Advisory Committee on Immunization (NACI). Statement on the use of conjugate pneumococcal vaccine—13 valent in adults (PNEU-C-13) Can Commun Dis Rep. 2013;39:1–52. doi: 10.14745/ccdr.v39i00a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.National Advisory Committee on Immunization (NACI). Re-Immunization with polysaccharide 23-valent pneumococcal vaccine (Pneu-P-23). Ottawa, ON: Public Health Agency of Canada; 2015. Available from: www.canada.ca/en/public-health/services/publications/healthy-living/re-immunization-with-polysaccharide-23-valent-pneumococcal-vaccinepneu-p-23.html#a8. Accessed 2017 Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Public Health Agency Canada. Canadian immunization guide. 7th ed. Ottawa, ON: Public Health Agency of Canada; 2006. [Google Scholar]
- 70.Weycker D, Atwood M, Sato R, Beausoleil L, McNeil S, Laferriere C. Cost-effectiveness of alternative strategies for use of 13-valent pneumococcal conjugate vaccine (PCV13) in Canadian adults.; Paper presented at: IDWeek 2015; 2015 Oct 7–11; San Diego, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alberta Health, Public Health and Compliance. Immunization of specific populations (immunosuppressed and chronic health conditions). Edmonton, AB: Government of Alberta; 2018. Available from: https://open.alberta.ca/dataset/58d31634-61d9-469d-b95f-f714719b923e/resource/8a92b77b-351b-4f1e-9d4d-31aaa9effdb3/download/AIP-Specific-Populations-Immunocompromised.pdf. Accessed 2018 Oct 25. [Google Scholar]
- 72.BC Centre for Disease Control. Communicable disease control manual. Chapter 2: immunization. Part 4: Biological products (vaccines and immune globulins). Vancouver, BC: BC Centre for Disease Control.; Available from: www.bccdc.ca/resource-gallery/Documents/Guidelines%20and%20Forms/Guidelines%20and%20Manuals/Epid/CD%20Manual/Chapter%202%20-%20Imms/SectionVII_BiologicalProducts.pdf. Accessed 2017 Mar 2. [Google Scholar]
- 73.Prince Edward Island Canada, Health PEI. Adults need immunization too! Recommended adult vaccines in PEI. Charlottetown, PE: Health PEI; 2016. Available from: www.princeedwardisland.ca/sites/default/files/publications/adult_immunization_schedule_ef_0.pdf. Accessed 2017 Mar 2. [Google Scholar]
- 74.Government of the Northwest Territories. Vaccine information sheet: pneumococcal conjugate 13 (Pneu-C-13). Yellowknife, NT: Government of the Northwest Territories; 2017. Available from: www.hss.gov.nt.ca/sites/www.hss.gov.nt.ca/files/pneu-c-13.pdf. Accessed 2017 Mar 2. [Google Scholar]
- 75.Yukon Health and Social Services. Community health programs: Yukon immunization program: section 8—biological products. Whitehorse, YT: Yukon Health and Social Services; 2018. Available from: www.hss.gov.yk.ca/pdf/im_manual_section8.pdf. Accessed 2018 Oct 25. [Google Scholar]
- 76.Newfoundland and Labrador Canada, Department of Health and Community Services. Newfoundland and Labrador immunization manual. Section 5: immunization programs for high risk groups. St John’s, NL: Newfoundland and Labrador Canada, Department of Health and Community Services; 2018. Available from: www.health.gov.nl.ca/health/publichealth/cdc/im_section5.pdf. Accessed 2018 Oct 25. [Google Scholar]
- 77.Manitoba Health, Seniors and Active Living. Manitoba’s immunization program: vaccines offered free-of-charge (eligibility criteria for publicly-funded vaccines). Winnipeg, MB: Manitoba Health, Seniors and Active Living; 2018. Available from: www.gov.mb.ca/health/publichealth/cdc/vaccineeligibility.html. Accessed 2018 Oct 25. [Google Scholar]
- 78.Government of Saskatchewan. Pneumococcal conjugate 13 vaccine. Regina, SK: Government of Saskatchewan; 2017. Available from: http://publications.gov.sk.ca/documents/13/108097-Pneu-C-13%20April%202017.pdf. Accessed 2017 Mar 2. [Google Scholar]
- 79.Ontario Ministry of Health and Long-Term Care. Publicly funded immunization schedules for Ontario—December 2016. Toronto, ON: Ontario Ministry of Health and Long-Term Care; 2016. Available from: www.health.gov.on.ca/en/pro/programs/immunization/docs/immunization_schedule.pdf. Accessed 2017 Mar 2. [Google Scholar]
- 80.Biologics and Genetic Therapies Directorate. Standard 3.3—eligibility criteria for publicly funded vaccines/biologics. Ottawa, ON: Health Canada; 2018. Available from: www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/en/CDC/HealthProfessionals/NBIPG-standard3-3-e.pdf. Accessed 2017 Mar 2. [Google Scholar]
- 81.Nova Scotia Health and Wellness. Publicly funded vaccine eligibility for individuals at high risk of acquiring vaccine preventable diseases. Halifax, NS: Nova Scotia Health and Wellness; 2017. Available from: http://novascotia.ca/dhw/cdpc/documents/Vaccine-Eligibility-for-High-Risk-Conditions.pdf. Accessed 2017 Mar 2. [Google Scholar]
- 82.Government of Nunavut. Routine immunization schedule 2010. Adults previously immunized. Iqaluit, NU: Government of Nunavut; 2010. Available from: http://gov.nu.ca/sites/default/files/files/Approved%20Immunization%20Schedules(adult)(1).pdf. Accessed 2017 Mar 2. [Google Scholar]
- 83.Santé et services sociaux du Québec. Protocole d’immunisation du Québec. Vaccins. Pneu-C: vaccin conjugué contre le pneumocoque. Quebec, QC: Santé et services sociaux du Québec; 2018. Available from: www.msss.gouv.qc.ca/professionnels/vaccination/piq-vaccins/pneu-c-vaccin-conjugue-contre-le-pneumocoque/. Accessed 2018 Nov 1. [Google Scholar]
- 84.Public Health Agency Canada. Vaccine coverage amongst adult Canadians: results from the 2012 adult National Immunization Coverage (aNIC) survey. Ottawa, ON: Government of Canada; 2014. Available from: www.phac-aspc.gc.ca/im/nics-enva/vcac-cvac-eng.php. Accessed 2017 Nov 14. [Google Scholar]
- 85.Government of Canada. Vaccine uptake in Canadian adults: results from the 2014 adult National Immunization Coverage survey. Ottawa, ON: Government of Canada; 2016. Available from: www.canada.ca/en/public-health/services/publications/healthy-living/vaccine-uptake-canadian-adults-results-2014-adult-national-immunization-coverage-survey.html. Accessed 2017 Feb 20. [Google Scholar]
- 86.Lode H, Ludwig E, Kassianos G. Pneumococcal infection—low awareness as a potential barrier to vaccination: results of a European study. Adv Ther. 2013;30(4):387–405. doi: 10.1007/s12325-013-0025-4. [DOI] [PubMed] [Google Scholar]
- 87.Rehm SJ, File TM, Metersky M, Nichol KL, Schaffner W, National Foundation for Infectious Diseases Pneumococcal Disease Advisory Board Identifying barriers to adult pneumococcal vaccination: an NFID task force meeting. Postgrad Med. 2012;124(3):71–9. doi: 10.3810/pgm.2012.05.2550. [DOI] [PubMed] [Google Scholar]
- 88.Badertscher N, Morell S, Rosemann T, Tandjung R. General practitioners’ experiences, attitudes, and opinions regarding the pneumococcal vaccination for adults: a qualitative study. Int J Gen Med. 2012;5:967–74. doi: 10.2147/IJGM.S38472. Epub 2012 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gannon M, Qaseem A, Snooks Q, Snow V. Improving adult immunization practices using a team approach in the primary care setting. Am J Public Health. 2012;102(7):e46–52. doi: 10.2105/AJPH.2012.300665. Epub 2012 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lau D, Hu J, Majumdar SR, Storie DA, Rees SE, Johnson JA. Interventions to improve influenza and pneumococcal vaccination rates among community-dwelling adults: a systematic review and meta-analysis. Ann Fam Med. 2012;10(6):538–46. doi: 10.1370/afm.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krueger P, St Amant O, Loeb M. Predictors of pneumococcal vaccination among older adults with pneumonia: findings from the Community Acquired Pneumonia Impact Study. BMC Geriatr. 2010;10:44. doi: 10.1186/1471-2318-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Polsky D, Bonafede M, Suaya JA. Comorbidities as a driver of the excess costs of community-acquired pneumonia in U.S. commercially-insured working age adults. BMC Health Serv Res. 2012;12:379. doi: 10.1186/1472-6963-12-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ryan M, Suaya JA, Chapman JD, Stason WB, Shepard DS, Thomas CP. Incidence and cost of pneumonia in older adults with COPD in the United States. PLoS One. 2013;8(10):e75887. doi: 10.1371/journal.pone.0075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dransfield MT, Harnden S, Burton RL, Albert RK, Bailey WC, Casaburi R, et al. Long-term comparative immunogenicity of protein conjugate and free polysaccharide pneumococcal vaccines in chronic obstructive pulmonary disease. Clin Infect Dis. 2012;55(5):e35–44. doi: 10.1093/cid/cis513. Epub 2012 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schmoele-Thoma B, Jackson LA, Greenberg RN, Frenck R, Gurtman A, Sundaraiyer V, et al. Immunogenicity of 13-valent pneumococcal conjugate vaccine in immunocompetent older adults with stable underlying medical conditions. J Vaccines Immun. 2015;3(2):7–12. Epub 2015 Jul 15. [Google Scholar]