Abstract
Background
The halophyte Suaeda aralocaspica performs complete C4 photosynthesis within individual cells (SCC4), which is distinct from typical C4 plants, which require the collaboration of 2 types of photosynthetic cells. However, despite SCC4 plants having features that are valuable in engineering higher photosynthetic efficiencies in agriculturally important C3 species such as rice, there are no reported sequenced SCC4 plant genomes, limiting our understanding of the mechanisms involved in, and evolution of, SCC4 photosynthesis.
Findings
Using Illumina and Pacific Biosciences sequencing platforms, we generated ∼202 Gb of clean genomic DNA sequences having a 433-fold coverage based on the 467 Mb estimated genome size of S. aralocaspica. The final genome assembly was 452 Mb, consisting of 4,033 scaffolds, with a scaffold N50 length of 1.83 Mb. We annotated 29,604 protein-coding genes using Evidence Modeler based on the gene information from ab initio predictions, homology levels with known genes, and RNA sequencing–based transcriptome evidence. We also annotated noncoding genes, including 1,651 long noncoding RNAs, 21 microRNAs, 382 transfer RNAs, 88 small nuclear RNAs, and 325 ribosomal RNAs. A complete (circular with no gaps) chloroplast genome of S. aralocaspica 146,654 bp in length was also assembled.
Conclusions
We have presented the genome sequence of the SCC4 plant S. aralocaspica. Knowledge of the genome of S. aralocaspica should increase our understanding of the evolution of SCC4 photosynthesis and contribute to the engineering of C4 photosynthesis into economically important C3 crops.
Keywords: Suaeda aralocaspica, genome, single-cell C4, photosynthesis, long noncoding RNAs, halophyte
Background
Carbon loss through photorespiration and water loss through transpiration are common in C3 plants, especially in warm or dry environments, and they result in significant decreases in growth, water use efficiency, and harvestable yields [1]. These problems are overcome in C4 and crassulacean acid metabolism (CAM) plant families [2], which perform evolved CO2-concentrating mechanisms (C4 cycle) and Calvin cycle (C3 cycle) using spatial (Kranz structure) and temporal (day to night switch) separations, respectively. Both C4 and CAM plants can outperform C3 plants, especially under photorespiratory conditions, and increase their water use efficiency [2], which has created considerable interest in implementing the C4 cycle in C3 crops such as rice to improve yields and stress tolerance [3–6].
Among eudicots, C4 photosynthesis most frequently occurs in the Amaranthaceae of Caryophyllales [7–9]. Four Amaranthaceae species (3 Bienertia and 1 Suaeda) can perform both C4 and C3 cycles within individual photosynthetic cells (single-cell C4[SCC4]) [10–13]. Suaeda contains species that utilize all types of C4, C3, and SCC4 mechanisms for CO2 fixation and, thus, represents a unique genus to study the evolution of C4 photosynthesis [14]. Mechanistically, the spatially separated chloroplasts in SCC4 contain different sets of nuclear-encoded proteins that are related to specific functions in the C4 and C3 cycles, which biochemically and functionally resemble mesophyll and bundle sheath cells in chloroplasts of Kranz C4 plant species [10, 11, 15–18]. These findings indicate that the key enzymes in photosynthesis are conserved and that both C3 and C4 enzymes work in the same cells in SCC4 plants during the daytime, which is different from both C4 and CAM plants.
At present, most of the knowledge of SCC4 photosynthesis has come from studies of Bienertia sinuspersici, which has 2 types of chloroplasts distributed in the central and peripheral parts of the cell [16, 18–29]. Studies on Suaeda aralocaspica (NCBI:txid224144) have focused on the germination of dimorphic seeds [30–34]. S. aralocaspica has elongated photosynthetic cells with 2 types of chloroplasts distributed at the opposite ends of the cell. This is analogous to the Kranz anatomy but lacks the intervening cell wall [35]. This cellular feature indicates that S. aralocaspica conducts C4 and C3 photosynthesis within a single cell, perhaps retaining the photosynthetic characteristics of both C4 and C3 cycles and representing an intermediate model of the evolutionary process from C3 to C4 [35,36]. S. aralocaspica is a hygro-halophyte that grows in temperate salt deserts with low night temperatures in areas ranging from the northeast of the Caspian lowlands eastwards to Mongolia and western China [35]. Therefore, it is important to sequence the genome of S. aralocaspica, which should aid the study of C4 evolution under stressful growth conditions and accelerate the engineering of C4 photosynthesis into C3 crops for adaptation to high-saline growth conditions.
In the present study, we sequenced the genome of S. aralocaspica collected from a cold desert in the Junggar Basin, Xinjiang, China. Using an integrated assembly strategy that combined shotgun Illumina sequencing and single-molecule real-time sequencing technology from Pacific Biosciences (PacBio), we generated a reference genome assembly of S. aralocaspica using protocols established in other plant species [37–40]. To our knowledge, this is the first sequenced SCC4 genome. These genomic resources provide a platform for advancing basic biological research and gene discovery in SCC4 plants, as well as for engineering C4 functional modules into C3 crops to increase yields and to adapt to high-salt conditions.
Data Description
Plant material
Seeds were first collected from a healthy specimen of S. aralocaspica (Fig. 1). The selected plant measured ∼40 cm in height and was located within a natural stand close to Fu-kang County, Xinjiang Uygur Autonomous Region, China (44 14 N latitude, E 87 40 E longitude, 445 m elevation). The seeds were placed in 0.1% potassium permanganate, washed clean for 5 min with ultrapure water, and then spread in sterilized petri dishes. After a week of 30°C shaded culturing, the seeds germinated. After seed germination, leaves were collected as tissue sources for whole-genome sequencing. In addition, 6 other healthy S. aralocaspica (collected from the same location as the plant used for seed collection) were chosen as tissue (mature leaf, stem, root, and fruit) sources for RNA sequencing (RNA-seq). The samples were frozen in liquid nitrogen immediately after being collected and then stored at −80°C until DNA/RNA extraction. All the samples were collected with permission from and under the supervision of the local forestry bureau.
Figure 1:

Example of S. aralocaspica.
DNA extraction and genome sequencing
Genomic DNA was extracted from leaves using a General AllGen Kit (Tiangen Biotech, Beijing, China) according to its manufacturer's instructions. Genomic DNA isolated from S. aralocaspica was used to construct multiple types of libraries, including short insert size (350, 500, and 800 bp) libraries, mate-paired (2, 5, 10, and 20 kb) libraries, and PacBio single-molecule real-time cell libraries. The purified libraries were quantified and stored at −80°C before sequencing. Then, the S. aralocaspica genome was sequenced on Illumina HiSeq 2000 (Illumina Inc., San Diego, CA, USA) and PacBio RS II platform (Pacific Biosciences of California, Menlo Park, CA, USA) using 8 libraries with different insert sizes. This generated 370 Gb raw Illumina HiSeq data and 10 Gb (∼21× genome coverage) PacBio reads (Supplemental Table 1).
To reduce the effects of sequencing errors on the assembly, a series of stringent filtering steps were used during read generation. We cleaned Illumina reads using the following steps: (1) Cut off adaptors. For the mate-paired library data, reads without Nextera adaptors longer than 10 bp on both end1 and end2 were removed; (2) Remove tail bases with quality score <20; (3) Remove reads harboring >20% bases with quality scores <20; (4) Remove reads with lengths <30 nucleotides (nt) for DNA-seq; and (5) Remove duplicated paired-end reads from DNA-seq that represent potential PCR artifacts. In total, 1053,309 raw subreads were produced by Pacbio. Then, reads with lengths <1 kb were filtered, and 935,509 reads were retained. Next, 46 Gb of Illumina clean reads with 100-bp read lengths was used to correct the PacBio raw reads using Proovread (Proovread, RRID:SCR_017331) [41] (v2). This yielded 632,805 corrected PacBio reads. After the quality control and filtering steps, 195 Gb clean Illumina reads and 6.9 clean PacBio reads were retained, resulting in a 433× fold coverage of the genome (Supplemental Table 1).
Estimation of genome size
GCE (GCE, RRID:SCR_017332) [42] (v1.0.0) was used to estimate the genome size and heterozygosity. The term k-mer refers to a sequence with a length ofk bp, and each unique k-mer within a genome dataset can be used to determine the discrete probability distributions of all possible k-mers and their frequencies of occurrence. Genome size can be calculated using the total length of sequencing reads divided by sequencing depth. To estimate the sequencing depth of the S. aralocaspica genome, we counted the copy number of a certain k-mer (e.g., 17-mer) present in the sequence reads and plotted the distribution of the copy numbers. The peak value of the frequency curve represents the overall sequencing depth. We used the algorithm N × (L − K + 1)/D = G, where N represents the total sequence read number, L represents the average length of sequence reads, and K represents the k-mer length, which was defined here as 17 bp. G denotes the genome size, and D represents the overall depth estimated from the k-mer distribution. Based on this method, the estimated genome size of S. aralocaspica was 467 Mb (Supplemental Fig. 1) and the heterozygosity was 0.16%.
Genome assembly
The primary assembled genome was generated by SOAPdenovo (SOAPdenovo2, RRID:SCR_014986) [43] (version 2.04-r240) and contained 17,302 initial contigs (N50, ∼49.2 kb) and 4,184 scaffolds (N50, ∼1.44 Mb) spanning 445.6 Mb, with 96.1 Mb (21.56%) of the total size being intra-scaffold gaps (Supplemental Table 2). Then, we used all of the reads from the short insert libraries to fill gaps using GapCloser (GapCloser, RRID:SCR_015026) [44] (v1.12), and 74.7% of the total gaps were filled. This resulted in a genome size of 424.5 Mb, with 5.92% gaps, which was calculated using the total length of Ns divided by the total length of the assembly. Then, PBJelly (PBJelly, RRID:SCR_012091) [45] (v15.8.24) was used for the second round of gap filling using the polished PacBio data. This finally yielded a ∼452-Mb genome assembly with 4,033 scaffolds (N50, 1.83 Mb) (Table 1, Supplemental Table 2). The assembly spanned 96.8% of the S. aralocaspica genome (467 Mb) estimated by the k-mer spectrum (Supplemental Fig. 1).
Table 1.
Summary of S. aralocaspica genome assembly
| Assembly | Illumina | Illumina + PacBio |
|---|---|---|
| Total assembly size | 424 Mb | 452 Mb |
| Number of scaffolds (≥500 bp) | 4,184 | 4,033 |
| Longest scaffold | 9.29 Mb | 9.98 Mb |
| N50 contig (size/number) | 49.21 kb/2,464 | |
| N50 scaffold (size/number) | 1.44 Mb/80 | 1.83 Mb/67 |
| N90 scaffold (size/number) | 306.62 kb/332 | 363.87 kb/282 |
| % of N | 5.78% | 2.98% |
| Annotation | ||
| Number of protein-coding genes | 29,604 | |
| Number of small RNAs | 816 | |
| Number of long noncoding genes | 1,982 |
RNA preparation and sequencing
RNA-seq was performed for genome annotation. Different tissues (mature leaf, stem, root, and fruit) of 6 S. aralocaspica specimens were used for RNA extraction. Tissues were ground in liquid nitrogen. After homogenizing the samples in a guanidine thiocyanate extraction buffer, sodium acetate and chloroform/isoamyl alcohol (24:1) were added. The solution was shaken vigorously, placed on ice for 15 min, and centrifuged (13,200 rpm) at 4°C to separate a clear upper aqueous layer, from which RNA was precipitated with isopropanol. The precipitated RNA was washed with 75% ethanol to remove impurities and then resuspended with diethyl pyrocarbonate–treated water. Total RNA was treated with RQ1 DNase (Promega) to remove DNA. The quality and quantity of the purified RNA were determined by measuring the absorbance at 260 nm/280 nm (A260/A280) using smartspec plus (BioRad). RNA integrity was further verified by 1.5% agarose gel electrophoresis. RNAs were then equally mixed for RNA-seq library preparation. Polyadenylated messenger RNAs (mRNAs) were purified and concentrated with oligo(dT)-conjugated magnetic beads (Invitrogen) before directional RNA-seq library preparation. Purified mRNAs were fragmented at 95°C, followed by end repair and 5′ adaptor ligation. Reverse transcription was performed using an RT primer harboring a 3′ adaptor sequence and a randomized hexamer. The complementary DNAs (cDNAs) were purified and amplified, and PCR products corresponding to 200–500 bp were purified, quantified, and stored at −80°C before sequencing. Transcriptomic libraries were sequenced using Illumina HiSeq X Ten (Illumina Inc., San Diego, CA, USA) for paired-end 150-nt reads. As a result, we generated 30 Gb of RNA-seq data (Supplemental Table 3).
To further annotate transcriptional start and termination sites, we also sequenced cap analysis of gene expression and deep sequencing (CAGE) and polyadenylation site sequencing (PAS) data. In brief, 20 μg of total RNA of mature leaves was used for CAGE-seq library preparation. Polyadenylated mRNAs were purified and concentrated with oligo (dT)-conjugated magnetic beads (Invitrogen). After treating with FastAP (Invitrogen) for 1 h at 37°C and subsequently with tobacco acid pyrophosphatase (Ambion) for 1 h at 37°C, the decapped full-length mRNA was ligated to the Truseq 5′ RNA adaptor (Illumina) for 1 h at 37°C and purified with oligo (dT)-conjugated magnetic beads (Invitrogen). Following fragmentation at 95°C, first-strand cDNA was synthesized using an RT primer harboring the Truseq 3′ adaptor sequence (Illumina) and a randomized hexamer. The cDNAs were purified and amplified using Truseq PCR primers (Illumina), and products corresponding to 200–500 bp were purified, quantified, and stored at −80°C until sequencing. CAGE-seq libraries were sequenced with Illumina Nextseq 500 (Illumina Inc., San Diego, CA, USA) for paired-end 150-nt reads. Finally, 16 Gb of CAGE-seq data were generated (Supplemental Table 3). In addition, 10 μg of total RNA of mature leaves was used for PAS-seq library preparation. In brief, polyadenylated mRNAs were purified using oligo (dT)-conjugated magnetic beads (Invitrogen). Purified RNA was fragmented and then reverse transcription was performed using a PAS-RT primer (a modified Truseq 3′ adaptor harboring dT18 and 2 additional anchor nucleotides at the 3′ terminus). DNA was then synthesized with Terminal-Tagging oligo cDNA using a ScriptSeq™cv2 RNA-Seq Library Preparation Kit (Epicentre). The cDNAs were purified and amplified, and PCR products corresponding to 300–500 bp were purified, quantified, and stored at −80°C before sequencing. PAS-seq libraries were sequenced with Illumina Nextseq 500 (Illumina Inc., San Diego, CA, USA) for single-end 300-nt reads. Finally, 28.5 Gb of PAS-seq data were generated (Supplemental Table 3).
To annotate microRNA, a total of 3 μg of mixed total RNA was the template for a small RNA cDNA library preparation using Balancer NGS Library Preparation Kit for small/microRNA (GnomeGen), following the manufacturer's instructions. Briefly, RNAs were ligated to 3′ and 5′ adaptors sequentially, reverse transcribed to cDNA, and PCR amplified. The whole library was applied to 10% native polyacrylamide gel electrophoresis, and bands corresponding to microRNA insertions were cut and eluted. After ethanol precipitation and washing, the purified small RNA libraries were quantified using a Qubit Fluorometer (Invitrogen) and stored at −80°C until sequencing. The small RNA library was sequenced with Illumina GA IIx (Illumina Inc., San Diego, CA, USA) for 33-nt reads. Finally, 4.5 Gb of small RNA data were generated (Supplemental Table 3).
Genome quality evaluation
Different methods and data were used to check the completeness of the assembly. Using BWA (BWA, RRID:SCR_010910) [46], we found that 87.08–90.63% of DNA-paired end reads (350, 500, and 800 bp) could be properly mapped to the final assembled genome (Supplemental Table 4, Supplemental Fig. 2). We evaluated the completeness of the gene regions in our assembly using BUSCO (BUSCO, RRID:SCR_015008) [47] (v3.0.2). In total, 89.5% of the 1,440 single-copy orthologs presented in the plant lineage was completely identified in the genome (Supplemental Fig. 3).
Furthermore, Trinity (Trinity, RRID:SCR_013048) [48] (r20140413p1) was used to assemble the RNA-seq reads sequenced from the mixed S. aralocaspica RNA library into 157,521 unigenes. Then, these unigenes were aligned to the genome assembly by BLASTN with default parameter. We found that 94.5% of the unigenes could be aligned to the genome assembly, and 76.3% of the unigenes could cover 90% of the sequence length of 1 scaffold. For unigenes longer than 1 kb, 99.5% of the unigenes could be aligned to the genome assembly, and 92.8% of the unigenes could cover 90% of the sequence length of 1 scaffold (Supplemental Table 5).
Gene and functional annotations
The genome of S. aralocaspica was annotated for protein-coding genes (PCGs), repeat elements, noncoding genes, and other genomic elements. In detail, MAKER (MAKER, RRID:SCR_005309) [49] (v2.31.9) was used to generate a consensus gene set based on 3 different types of evidence, ab initio, protein homologues, and the transcripts. De novo predictions were processed by AUGUSTUS (AUGUSTUS, RRID:SCR_008417) [50] (v3.2.1). Nonredundant protein sequences of 7 sequenced plants (Arabidopsis thaliana, Oryza sativa, Beta vulgaris, Chenopodium quinoa, Glycine max, Spinacia oleracea, and Vitis vinifera) provided homology evidence. The S. aralocaspica RNA-seq data generated from this study and a published transcriptome of the seed [51] were assembled into unigenes by Trinity [48] as the transcript evidence. We predicted 29,064 PCGs, with an average transcript length of 4,462 bp, coding sequence size of 1,112 bp, and a mean of 4.76 exons per transcript (Supplemental Tables 6 and 7). Of the annotated PCGs, 97.2% were functionally annotated by the InterPro, GO, KEGG, SwissProt, or NR databases (Supplemental Figs 4 and 5, Supplemental Table 8), and ∼91% were annotated with protein or transcript support (Supplemental Table 9). The transcriptional start and termination sites of most of the annotated genes were supported by sequencing reads from CAGE-seq and PAS-seq (Supplemental Figs 6 and 7).
In addition, 1,651 long noncoding RNAs were predicted following a previously published method [52]. In total, 382 transfer RNAs (tRNAs) were predicted using tRNAscan-SE (tRNAscan-SE, RRID:SCR_010835) [53] (v1.3.1). Additionally, 21 miRNAs, 88 small nuclear RNAs, and 325 ribosomal RNAs were identified by using the CMscan tool from INFERNAL (Infernal, RRID:SCR_011809) [54] (v1.1.2) to search the Rfam database with option –cut_ga (Supplemental Table 10, Supplemental Fig. 8).
Repeat annotation
To annotate the repeat sequences of the S. aralocaspica genome, a combination of de novo and homology-based approaches was used [55,56]. For homology-based identification, we used RepeatMasker (RepeatMasker, RRID:SCR_012954) [57] (open-4.0.5) to search the protein database in Rebase against the S. aralocaspica genome and identify transposable elements (TEs). The Rebase database [58] was used to identify TEs. Parameters of RepeatMasker were set to “-species Viridiplantae -pa 30 -e rmblast”. In the de novo approach, PILER (PILER, RRID:SCR_017333) [59] (v1.0) was used to build the consensus repeat database. PILER software requires PALS, FAMS, and PILER to construct the consensus library. The default parameters of PILER were used. Then, the predicted consensus TEs were classified using RepeatClassifer implemented in the RepeatModeler package (RepeatModeler, RRID:SCR_015027) [60] (Version 1.0.11). We used RepeatMasker to search the TEs within the database constructed by PILER. Finally, we combined the de novo and homolog predictions of repeat elements according to their coordination in the genome, and detected 173.5 Mb repeat elements, which constituted 38.41% of the genome (Supplemental Table 11). As observed in other sequenced genomes [61], long terminal repeats [62] in S. aralocaspica occupied the majority (48.5%) of the repeated sequences (Supplemental Table 12).
Phylogenetic placement of S. aralocaspica
The OrthoFinder (OrthoFinder, RRID:SCR_017118) [63] (v2.3.3) clustering method was used to perform orthologous group analyses with complete annotated protein sequences of 18 sequenced plant genomes: 8 C3 species (Solanum tuberosum, S. oleracea, B. vulgaris, C. quinoa, A. thaliana, O. sativa, Musa acuminata, and Physcomitrella patens), 8 C4 species (S. aralocaspica, Amaranthus hypochondriacus, Sorghum bicolor, Setaria italica, Zea mays, Saccharum spp., Panicum hallii, and Pennisetum glaucum), and 2 CAM species (Ananas comosus and Phalaenopsis equestris). The longest proteins encoded by each gene in all species were selected as input for OrthoFinder with default parameters. In total, 19,324 orthogroups, containing ≥2 genes, were circumscribed, 11,768 of which contained ≥1 gene from S. aralocaspica (Supplemental Table 13). Of the 29,604 annotated S. aralocaspica genes, 23,112 (89%) were classified into orthogroups. In total, 3,895 orthogroups (172,107 genes) were shared among all the genomes analyzed. A total of 70 orthogroups (351 genes) were specific to the assembled S. aralocaspica genome when compared with the other 17 genomes.
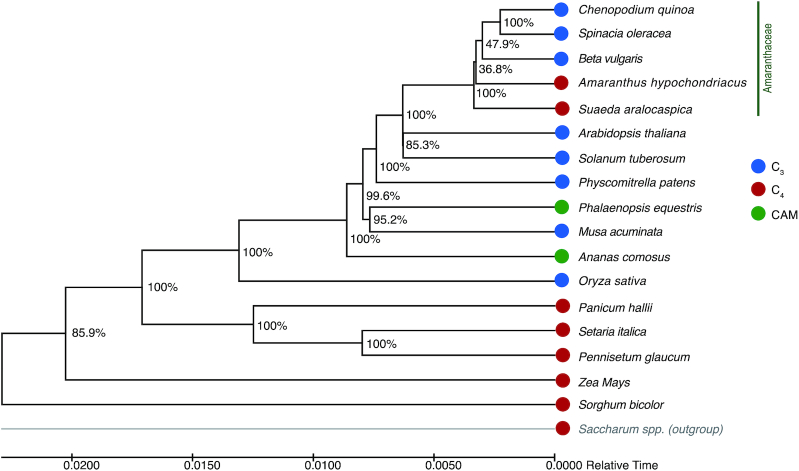
With OrthoFinder, 15 single-copy orthologous genes, shared across 18 species, were identified and were aligned with MUSCLE (MUSCLE, RRID:SCR_011812) [58] (v3.8.31), using default settings (see Supplementary File 1 for commands and settings). The concatenated amino acid sequences were trimmed using trimAI (trimAI, RRID:SCR_017334) [64] (trimal -gt 0.8 -st 0.001 -cons 60) (v1.2rev59) and were further used by ModelFinder to select the best model (JTTDCMut+F+I+G4). Then, the phylogenetic trees were constructed using IQ-Tree (IQ-TREE, RRID:SCR_017254) [65] (v1.6.10). The aLRT method was used to perform 1,000 bootstrap analyses to test the robustness of each branch. Then, a timetree was inferred using the Realtime method [66,67] and ordinary least-squares estimates of branch lengths. This analysis involved 18 amino acid sequences. There were 4,489 positions in the final dataset. The timetree was constructed using MEGA X (MEGA Software, RRID:SCR_000667) [68]. The resulting phylogenetic tree showed that all 5 Amaranthaceae species were placed in the same clade, among which A. hypochondriacus (C4) was placed as a sister subclade to the other 3 C3 species (Fig. 2). Moreover, S. aralocaspica (SCC4) was the sister clade of 4 other species from the Amaranthaceae including A. hypochondriacus (C4) (Fig. 2). Our results of phylogenetic analyses were consistent with a previous study on the evolution of C. quinoa [69]. Inside of the Amaranthaceae, the close phylogenetic distance between S. aralocaspica (SCC4) and A. hypochondriacus (C4), away from all other C3 relatives, suggests that these SCC4 and C4 photosynthesizers might have had independently evolved. Outside of the Amaranthaceae, S. aralocaspica (SCC4) is more closely related to the C3 than C4 plants. These findings do not fully support the existing model that S. aralocaspica would be a C3–C4 intermediate and was on the road toward the C4 plants [35,36].
Figure 2:
Phylogenetic tree of S. aralocaspica with other C3/C4/CAM plants. Bootstrap values were obtained from 1,000 bootstrap replicates and are reported as percentages.
Assembly of the S. aralocaspica chloroplast genome
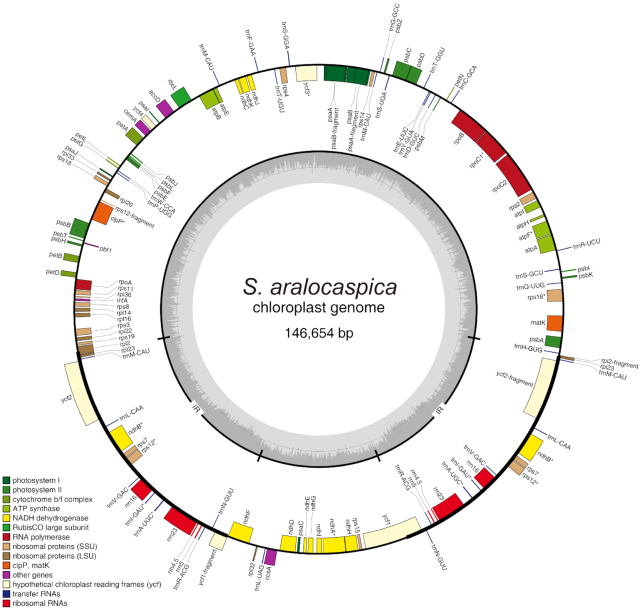
Using the short insert size (350 bp) data, a complete (circular with no gaps) chloroplast genome of S. aralocaspica was assembled at 146,654 bp in length using NOVOPlasty (NOVOPlasty, RRID:SCR_017335) [70] (v2.7.2). The Rubisco-bis-phosphate oxygenase (RuBP) subunit of C. quinoa (GenBank:KY419706.1) was selected as a seed sequence. An initial gene annotation of the genome was performed using GeSeq (GeSeq, RRID:SCR_017336) [71]. The circular chloroplast genome maps were drawn using the OrganellarGenome DRAW tool (OGDraw, RRID:SCR_017337) [72], with subsequent manual editing (Fig. 3).
Figure 3:
Gene map of the S. aralocaspica chloroplast genome. Genes shown outside the outer circle are transcribed clockwise, and those inside are transcribed counterclockwise. Genes belonging to different functional groups are color coded. The dashed area in the inner circle indicates guanine-cytosine content of the chloroplast genome.
Conclusion
Using the Illumina and Pacbio platforms, we successfully assembled the genome of S. aralocaspica, the first sequenced genome of a SCC4 plant. The final genome assembly was 452 Mb in size and consisted of 4,033 scaffolds, with a scaffold N50 length of 1.83 Mb. We annotated 29,604 protein-coding genes and noncoding genes including 1,651 long noncoding RNAs, 21 miRNAs, 382 tRNAs, 88 small nuclear RNAs, and 325 ribosomal RNAs. The phylogenetic tree placed SCC4 in a clade more closely related to the C3 than the C4 plants, not fully supporting the hypothesis that SCC4 is a C3–C4 intermediate that independently evolved from the C3 ancestors. A complete (circular with no gaps) chloroplast genome of S. aralocaspica was also assembled, and was 146,654 bp in size. The available genome assembly, together with transcriptomic data of S. aralocaspica, provides a valuable resource for investigating C4 evolution and mechanisms. We anticipate that future studies of S. aralocaspica will greatly facilitate the process of engineering crops, especially C3 species, including rice, with higher photosynthetic efficiencies and saline tolerance.
Availability of supporting data and materials
Raw sequencing data are deposited in the NCBI SRA with accession number SRP128359. The NCBI Bioproject accession is PRJNA428881. Further supporting data and materials are available in the GigaScience GigaDB database [73].
Additional files
Supplemental Figure 1: k-mer distribution of sequencing reads.
Supplemental Figure 2: Size distribution of inserts in sequenced paired-end DNA reads.
Supplemental Figure 3: Integrity comparison of genome assemblies of S. aralocaspica with BUSCO. For S. aralocaspica, assemblies in each step were analyzed respectively.
Supplemental Figure 4: Annotated genes supported by different evidence.
Supplemental Figure 5: Gene ontology distribution of S. aralocaspica protein-coding genes.
Supplemental Figure 6: Transcription start site (TSS) annotation with CAGE-seq.
Supplemental Figure 7: Transcription terminal site (TTS) annotation with PAS-seq.
Supplemental Figure 8: Noncoding RNAs classification in S. aralocaspica.
Supplemental Table 1: Summary of sequencing data obtained for genome assembly.
Supplemental Table 2: The assembly statistics of the S. aralocaspica genome.
Supplemental Table 3: Information of different types of RNA libraries.
Supplemental Table 4: Mapping efficiency of short insert library reads
Supplemental Table 5: Assessment of sequence coverage of S. aralocaspica genome assembly using unigenes.
Supplemental Table 6: Gene prediction in the S. aralocaspica genome.
Supplemental Table 7: Comparison of the gene structure among S. aralocaspica and some other species.
Supplemental Table 8: Summary of S. aralocaspica gene annotation based on homology or functional classification.
Supplemental Table 9: Number of S. aralocaspica genes with protein or unigene support.
Supplemental Table 10: Noncoding RNA genes in the S. aralocaspica genome.
Supplemental Table 11: Repeat elements in the S. aralocaspica genome. Repeat elements were identified by different methods and then combined into a final repeat set.
Supplemental Table 12: Repeat elements in S. aralocaspica genome.
Supplemental Table 13: Orthogroups clustered by OrthoFinder in 18 species.
Robert van Buren -- 3/19/2019 Reviewed
John Lovell -- 4/9/2019 Reviewed
John Lovell -- 7/24/2019 Reviewed
Abbreviations
bp: base pairs; BUSCO: Benchmarking Universal Single-Copy Orthologs; BWA: Burrows-Wheeler Aligner; CAGE: cap analysis of gene expression and deep sequencing; CAM: crassulacean acid metabolism; cDNA: complementary DNA; Gb: gigabase pairs; GCE: Genomic Character Estimator; GO: Gene Ontology; kb: kilobase pairs; KEGG: Kyoto Encyclopedia of Genes and Genomes; Mb: megabase pairs; miRNA: microRNA; mRNA: messenger RNA; NCBI: National Center for Biotechnology Information; nt: nucleotide; PacBio: Pacific Biosciences; PAS: polyadenylation site sequencing; PCG: protein-coding gene; RNA-seq: RNA sequencing; SCC4: single-cell C4 photosynthesis; SRA: Sequence Read Archive; TE: transposable element; tRNA: transfer RNA; TTS: transcription terminal site.
Competing interests
The authors declare that they have no competing interests.
Funding
This research was supported by the Key Research and Development Program of Xinjiang province (2018B01006–4), the National Natural Science Foundation of China (31770451), the National Key Research and Development Program (2016YFC0501400), and ABLife ( ABL2014–02028).
Authors' contributions
C.T., L.W., Yi Z., and S.M. initiated the project and designed the study. L.W., H.W., L.J., Z.Z., and K.Z. prepared experimental materials and performed experiments for data collection. G.M., C.C., Yu Z., H.W., L.J., and K.Z. assembled the genome, analyzed the data, and generated the graphs. Yi Z., W.Q., C.T., L.W., C.C., and X.W. wrote the manuscript.
References
- 1. Walker BJ, VanLoocke A, Bernacchi CJ, et al.. The costs of photorespiration to food production now and in the future. Annu Rev Plant Biol. 2016;67(1):107–29. [DOI] [PubMed] [Google Scholar]
- 2. Yamori W, Hikosaka K, Way DA. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynth Res. 2014;119(1–2):101–17. [DOI] [PubMed] [Google Scholar]
- 3. Hibberd JM, Sheehy JE, Langdale JA. Using C4 photosynthesis to increase the yield of rice-rationale and feasibility. Curr Opin Plant Biol. 2008;11(2):228–31. [DOI] [PubMed] [Google Scholar]
- 4. von Caemmerer S, Quick WP, Furbank RT. The development of C4 rice: current progress and future challenges. Science. 2012;336(6089):1671–2. [DOI] [PubMed] [Google Scholar]
- 5. Gu J-F, Qiu M, Yang J-C. Enhanced tolerance to drought in transgenic rice plants overexpressing C4 photosynthesis enzymes. Crop J. 2013;1(2):105–14. [Google Scholar]
- 6. Betti M, Bauwe H, Busch FA, et al.. Manipulating photorespiration to increase plant productivity: recent advances and perspectives for crop improvement. J Exp Bot. 2016;67(10):2977–88. [DOI] [PubMed] [Google Scholar]
- 7. Akhani H, Trimborn P, Ziegler H. Photosynthetic pathways in Chenopodiaceae from Africa, Asia and Europe with their ecological, phytogeographical and taxonomical importance. Plant Syst Evol. 1997;206(1):187–221. [Google Scholar]
- 8. Sage RF, Li M, Monson RK. The taxonomic distribution of C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 Plant Biology. San Diego, CA, USA: Academic Press; 1999:551–84. [Google Scholar]
- 9. Jacobs SWL. Review of leaf anatomy and ultrastructure in the Chenopodiaceae (Caryophyllales). J Torrey Bot Soc. 2001;128(3):236–53. [Google Scholar]
- 10. Voznesenskaya EV, Franceschi VR, Kiirats O, et al.. Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature. 2001;414(6863):543–6. [DOI] [PubMed] [Google Scholar]
- 11. Voznesenskaya EV, Franceschi VR, Kiirats O, et al.. Proof of C4 photosynthesis without Kranz anatomy in Bienertia cycloptera (Chenopodiaceae). Plant J. 2002;31(5):649–62. [DOI] [PubMed] [Google Scholar]
- 12. Akhani H, Barroca J, Koteeva N, et al.. Bienertia sinuspersici (Chenopodiaceae): a new species from southwest Asia and discovery of a third terrestrial C4 plant without Kranz anatomy. Syst Bot. 2005;30(2):290–301. [Google Scholar]
- 13. Akhani H, Chatrenoor T, Dehghani M, et al.. A new species of Bienertia (Chenopodiaceae) from Iranian salt deserts: a third species of the genus and discovery of a fourth terrestrial C4 plant without Kranz anatomy. Plant Biosyst. 2012;146(3):550–9. [Google Scholar]
- 14. Schütze P, Freitag H, Weising K. An integrated molecular and morphological study of the subfamily Suaedoideae Ulbr. (Chenopodiaceae). Plant Syst Evol. 2003;239(3):257–86. [Google Scholar]
- 15. Voznesenskaya EV, Edwards GE, Kiirats O, et al.. Development of biochemical specialization and organelle partitioning in the single-cell C4 system in leaves of Borszczowia aralocaspica (Chenopodiaceae). Am J Bot. 2003;90(12):1669–80. [DOI] [PubMed] [Google Scholar]
- 16. Voznesenskaya EV, Koteyeva NK, Chuong SD, et al.. Differentiation of cellular and biochemical features of the single-cell C4 syndrome during leaf development in Bienertia cycloptera (Chenopodiaceae). Am J Bot. 2005;92(11):1784–95. [DOI] [PubMed] [Google Scholar]
- 17. Offermann S, Okita TW, Edwards GE. Resolving the compartmentation and function of C4 photosynthesis in the single-cell C4 species Bienertia sinuspersici. Plant Physiol. 2011;155(4):1612–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Offermann S, Friso G, Doroshenk KA, et al.. Developmental and subcellular organization of single-cell C4 photosynthesis in Bienertia sinuspersici determined by large-scale proteomics and cDNA assembly from 454 DNA sequencing. J Proteome Res. 2015;14(5):2090–108. [DOI] [PubMed] [Google Scholar]
- 19. Wimmer D, Bohnhorst P, Shekhar V, et al.. Transit peptide elements mediate selective protein targeting to two different types of chloroplasts in the single-cell C4 species Bienertia sinuspersici. Sci Rep. 2017;7:41187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jurić I, González-Pérez V, Hibberd JM, et al.. Size matters for single-cell C4 photosynthesis in Bienertia. J Exp Bot. 2017;68(2):255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stutz SS, Edwards GE, Cousins AB. Single-cell C4 photosynthesis: efficiency and acclimation of Bienertia sinuspersici to growth under low light. New Phytol. 2014;202(1):220–32. [DOI] [PubMed] [Google Scholar]
- 22. Lung SC, Yanagisawa M, Chuong SD. Protoplast isolation and transient gene expression in the single-cell C4 species, Bienertia sinuspersici. Plant Cell Rep. 2011;30(4):473–84. [DOI] [PubMed] [Google Scholar]
- 23. Leisner CP, Cousins AB, Offermann S, et al.. The effects of salinity on photosynthesis and growth of the single-cell C4 species Bienertia sinuspersici (Chenopodiaceae). Photosynth Res. 2010;106(3):201–14. [DOI] [PubMed] [Google Scholar]
- 24. Uzilday B, Ozgur R, Yalcinkaya T, et al.. Changes in redox regulation during transition from C3 to single cell C4 photosynthesis in Bienertia sinuspersici. J Plant Physiol. 2017;220:1–10. [DOI] [PubMed] [Google Scholar]
- 25. Koteyeva NK, Voznesenskaya EV, Berry JO, et al.. The unique structural and biochemical development of single cell C4 photosynthesis along longitudinal leaf gradients in Bienertia sinuspersici and Suaeda aralocaspica (Chenopodiaceae). J Exp Bot. 2016;67(9):2587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosnow J, Yerramsetty P, Berry JO, et al.. Exploring mechanisms linked to differentiation and function of dimorphic chloroplasts in the single cell C4 species Bienertia sinuspersici. BMC Plant Biol. 2014;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park J, Knoblauch M, Okita TW, et al.. Structural changes in the vacuole and cytoskeleton are key to development of the two cytoplasmic domains supporting single-cell C(4) photosynthesis in Bienertia sinuspersici. Planta. 2009;229(2):369–82. [DOI] [PubMed] [Google Scholar]
- 28. Lara MV, Offermann S, Smith M, et al.. Leaf development in the single-cell C4 system in Bienertia sinuspersici: expression of genes and peptide levels for C4 metabolism in relation to chlorenchyma structure under different light conditions. Plant Physiol. 2008;148(1):593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chuong SD, Franceschi VR, Edwards GE. The cytoskeleton maintains organelle partitioning required for single-cell C4 photosynthesis in Chenopodiaceae species. Plant Cell. 2006;18(9):2207–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L, Huang Z, Baskin CC, et al.. Germination of dimorphic seeds of the desert annual halophyte Suaeda aralocaspica (Chenopodiaceae), a C4 plant without Kranz anatomy. Ann Bot. 2008;102(5):757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L, Baskin JM, Baskin CC, et al.. Seed dimorphism, nutrients and salinity differentially affect seed traits of the desert halophyte Suaeda aralocaspica via multiple maternal effects. BMC Plant Biol. 2012;12:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao J, Lv XY, Chen L, et al.. Effects of salinity on the growth, physiology and relevant gene expression of an annual halophyte grown from heteromorphic seeds. AoB Plants. 2015;7:plv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang HL, Tian CY, Wang L. Germination of dimorphic seeds of Suaeda aralocaspica in response to light and salinity conditions during and after cold stratification. PeerJ. 2017;5:e3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang L, Wang HL, Yin L, et al.. Transcriptome assembly in Suaeda aralocaspica to reveal the distinct temporal gene/miRNA alterations between the dimorphic seeds during germination. BMC Genomics. 2017;18:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Edwards GE, Voznesenskaya EV. C4 photosynthesis: Kranz forms and single-cell C4 in terrestrial plants. In: Raghavendra AS, Sage RF, eds. C4 Photosynthesis and Related CO2 Concentrating Mechanisms. Dordrecht, Netherlands: Springer; 2011:29–61. [Google Scholar]
- 36. Sharpe RM, Offermann S. One decade after the discovery of single-cell C4 species in terrestrial plants: what did we learn about the minimal requirements of C4 photosynthesis?. Photosynth Res. 2014;119(1–2):169–80. [DOI] [PubMed] [Google Scholar]
- 37. Badouin H, Gouzy J, Grassa CJ, et al.. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature. 2017;546(7656):148–52. [DOI] [PubMed] [Google Scholar]
- 38. Jarvis DE, Ho YS, Lightfoot DJ, et al.. The genome of Chenopodium quinoa. Nature. 2017;542:307. [DOI] [PubMed] [Google Scholar]
- 39. Zhang GQ, Liu KW, Li Z, et al.. The Apostasia genome and the evolution of orchids. Nature. 2017;549(7672):379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao G, Zou C, Li K, et al.. The Aegilops tauschii genome reveals multiple impacts of transposons. Nat Plants. 2017;3:946–55. [DOI] [PubMed] [Google Scholar]
- 41. Hackl T, Hedrich R, Schultz J, et al.. proovread: large-scale high-accuracy PacBio correction through iterative short read consensus. Bioinformatics. 2014;30(21):3004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu B, Shi Y, Yuan J, et al.. Estimation of genomic characteristics by analyzing k-mer frequency in de novo genome projects. arXiv. 2013:1308.2012. [Google Scholar]
- 43. Li R, Zhu H, Ruan J, et al.. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20(2):265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. The-Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 2012;485:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. English AC, Richards S, Han Y, et al.. Mind the gap: upgrading genomes with Pacific Biosciences RS long-read sequencing technology. PLoS One. 2012;7(11):e47768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simao FA, Waterhouse RM, Ioannidis P, et al.. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–2. [DOI] [PubMed] [Google Scholar]
- 48. Grabherr MG, Haas BJ, Yassour M, et al.. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cantarel BL, Korf I, Robb SM, et al.. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 2008;18(1):188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stanke M, Morgenstern B. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005;33(Web Server issue):W465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang L, Wang HL, Yin L, et al.. Transcriptome assembly in Suaeda aralocaspica to reveal the distinct temporal gene/miRNA alterations between the dimorphic seeds during germination. BMC Genomics. 2017;18(1):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cabili MN, Trapnell C, Goff L, et al.. Integrative annotation of human large intergenic noncoding RNAs reveals global properties and specific subclasses. Genes Dev. 2011;25(18):1915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nawrocki EP, Kolbe DL, Eddy SR. Infernal 1.0: inference of RNA alignments. Bioinformatics. 2009;25(10):1335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Iorizzo M, Senalik DA, Grzebelus D, et al.. De novo assembly and characterization of the carrot transcriptome reveals novel genes, new markers, and genetic diversity. BMC Genomics. 2011;12:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang L, Yu S, Tong C, et al.. Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol. 2014;15(2):R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tarailo-Graovac M, Chen N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr Protoc Bioinform. 2009:Chap 4:Unit 4.10. [DOI] [PubMed] [Google Scholar]
- 58.Repbase. 2001. http://www.girinst.org/server/RepBase/index.php. Accessed 1st April 2019 [Google Scholar]
- 59. Edgar RC, Myers EW. PILER: identification and classification of genomic repeats. Bioinformatics. 2005;21(Suppl 1):i152–i8. [DOI] [PubMed] [Google Scholar]
- 60. Rao SK, Fukayama H, Reiskind JB, et al.. Identification of C4 responsive genes in the facultative C4 plant Hydrilla verticillata. Photosynth Res. 2006;88(2):173–83. [DOI] [PubMed] [Google Scholar]
- 61. Vlasova A, Capella-Gutierrez S, Rendon-Anaya M, et al.. Genome and transcriptome analysis of the Mesoamerican common bean and the role of gene duplications in establishing tissue and temporal specialization of genes. Genome Biol. 2016;17:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wicker T, Sabot F, Hua-Van A, et al.. A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 2007;8(12):973–82. [DOI] [PubMed] [Google Scholar]
- 63. Emms DM, Kelly S. OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015;16:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25(15):1972–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nguyen LT, Schmidt HA, von Haeseler A, et al.. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tamura K, Tao Q, Kumar S. Theoretical foundation of the RelTime method for estimating divergence times from variable evolutionary rates. Mol Biol Evol. 2018;35(7):1770–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tamura K, Battistuzzi FU, Billing-Ross P, et al.. Estimating divergence times in large molecular phylogenies. Proc Natl Acad Sci U S A. 2012;109(47):19333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kumar S, Stecher G, Li M, et al.. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zou C, Chen A, Xiao L, et al.. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017;27:1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dierckxsens N, Mardulyn P, Smits G. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017;45(4):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tillich M, Lehwark P, Pellizzer T, et al.. GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017;45(W1):W6–W11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lohse M, Drechsel O, Kahlau S, et al.. OrganellarGenomeDRAW–a suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013;41(Web Server issue):W575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang L, Ma G, Wang H, et al.. Supporting data for “A draft genome assembly of halophyte Suaeda aralocaspica, a plant that performs C4 photosynthesis within individual cells.". GigaScience Database. 2019. 10.5524/100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Robert van Buren -- 3/19/2019 Reviewed
John Lovell -- 4/9/2019 Reviewed
John Lovell -- 7/24/2019 Reviewed